Abstract
Fanconi anemia complementation group I (FANCI) is a critical protein for maintaining DNA stability. However, the exact role of FANCI in tumors remains to be elucidated. The present study aimed to explore the role and potential mechanism of action of FANCI in non-small cell lung cancer (NSCLC). To quantify the expression levels of FANCI and ubiquitin-conjugating enzyme E2T (UBE2T) in NSCLC tissues, reverse-transcription quantitative PCR and western blotting were employed. Cell Counting Kit-8, wound healing and Transwell assays along with flow cytometry analysis and tumor xenograft were used to investigate the biological effects of FANCI in NSCLC in vitro and in vivo. The binding of FANCI with UBE2T was confirmed using a co-immunoprecipitation assay. Epithelial-to-mesenchymal transition (EMT) protein markers were quantified via western blotting. The results showed that FANCI expression level was higher in NSCLC tumor tissues, compared with adjacent tissues. In A549 and H1299 cells, knockdown of FANCI inhibited cell proliferation, migration, invasion, cell cycle and EMT in vitro. Tumor growth was repressed in vitro, upon downregulation of FANCI expression. UBE2T was observed to directly bind to FANCI and regulate its monoubiquitination. Overexpression of UBE2T reversed the effects induced by FANCI knockdown in NSCLC cells. Furthermore, it was noted that FANCI interacted with WD repeat domain 48 (WDR48). Overexpression of WDR48 reversed the effects of FANCI on cell proliferation, migration and EMT. In conclusion, FANCI was identified to be a putative oncogene in NSCLC, wherein FANCI was monouniubiquitinated by UBE2T to regulate cell growth, migration and EMT through WDR48. The findings suggested that FANCI could be used as a prognostic biomarker and therapeutic target for NSCLC.
Keywords: Fanconi anemia complementation group I, non-small cell lung cancer, ubiquitin-conjugating enzyme E2T, monoubiquitination, epithelial-mesenchymal transition
Introduction
Lung cancer is responsible for one fifth of cancer-related deaths worldwide (1). Non-small cell lung cancer (NSCLC) is the commonest type of lung cancer (2,3). Some targeted therapies against epidermal growth factor receptor, anaplastic lymphoma kinase and c-ros oncogene 1 receptor tyrosine kinase have been confirmed to provide survival benefits to NSCLC patients. However, a considerable proportion of NSCLC remains incurable, mainly due to late diagnosis and drug resistance (4–6). Therefore, it is necessary to understand the pathogenesis of NSCLC for proper diagnosis and development of new treatment modalities. Fanconi anemia (FA) is a rare recessive disorder characterized by anemia and bone marrow failure. It is caused by mutations in the FA family proteins (7,8). The FA family consists of ~19 genes associated with cell cycle progression, regulation and DNA damage repair (9,10). FANCI is an FA family protein; its gene is located on the long arm of chromosome 15. FANCI forms a complex with its molecular chaperone FANCD2 to participate in DNA repair and ribosome biosynthesis. The complex also regulates the cell cycle in the S and G2 phases (11–13). The FA core complex, which is an E3 ubiquitin ligase, monoubiquitinates FANCD2 and FANCI when DNA is damaged and stabilizes both proteins (14,15). Recently, FANCI was reported to be involved in the occurrence and development of several tumors. For example, FANCI is associated with susceptibility to familial prostate cancer (16). It has also been used as a novel marker for hepatitis B virus-associated hepatocellular carcinoma (17). In lung adenocarcinoma (LUAD), it co-operates with inosine monophosphate dehydrogenase type II to promote tumor growth (18). However, the understanding of the role of FANCI in tumor development and progression and the corresponding mechanism involved is still very limited, especially in the case of NSCLC.
Ubiquitin-conjugating enzyme E2T (UBE2T) is an E2 ubiquitin ligase involved in the FA pathway. It binds to FANCL and facilitates the monoubiquitination of FANCD2/FANCI to promote DNA interstrand cross-link (ICL) repair (19,20). Increasing evidence demonstrates that UBE2T is involved in multiple cancers. It is reported to decrease BRCA1 expression and promote breast cancer progression (21). It has been identified as a putative biomarker of bladder cancer (22). In addition, UBE2T-mediated monoubiquitination of H2AX induces hepatocellular carcinoma radioresistance by activating checkpoint kinase 1 (23). Based on the cancer genome atlas database, UBE2T was found to be associated with a poor prognosis in NSCLC (24). It also promotes epithelial-mesenchymal transition (EMT) and accelerates NSCLC cell proliferation, migration and invasion (25). In the EMT, epithelial cells gain migratory and invasive properties, owing to the loss of apico-basal polarity and cell-cell adhesion, to become mesenchymal stem cells. EMT is closely related to tumors, depending on the biological processes of wound healing, cell migration and proliferation (26–28). Although the aforementioned studies have found that UBE2T is associated with NSCLC, the role of UBE2T in the carcinogenesis of NSCLC is yet to be explored in depth.
In the current study, FANCI was found to be overexpressed and to serve a critical role in NSCLC progression by promoting EMT. The pathogenesis mechanism involved the UBE2T-mediated stabilization and monoubiquitination of FANCI.
Materials and methods
Sample collection
A total of 32 pairs of fresh NSCLC and adjacent (~5 cm) non-cancerous lung tissue samples were collected from patients (all >18 years old) who underwent surgery in Fujian Provincial Hospital between January 2015 and January 2018. The clinical information (shown in Table I) of all the patients was collected by the informed consent. This study was approved by the Medical Ethics Committee of Fujian Provincial Hospital (approval no. K2018-015-11) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Table I.
Correlation of lncRNA FANCI expression with clinical variables in NSCLC patients.
| FANCI expression | ||||
|---|---|---|---|---|
|
|
||||
| Clinicopathological feature | n | High | Low | P-value |
| Sex | ||||
| Male | 22 | 10 | 12 | 0.7043 |
| Female | 10 | 6 | 4 | |
| Age (years) | ||||
| <60 | 19 | 11 | 8 | 0.4725 |
| ≥60 | 13 | 5 | 8 | |
| Tumor size (cm) | ||||
| <3 | 17 | 5 | 12 | 0.032 |
| ≥3 | 15 | 11 | 4 | |
| Lymphatic metastasis | ||||
| N0 | 18 | 4 | 14 | 0.001 |
| N1-N3 | 14 | 12 | 2 | |
| Distant metastasis | ||||
| M0 | 17 | 5 | 12 | 0.032 |
| M1 | 15 | 11 | 4 | |
NSCLC, non-small cell lung cancer; FANCI, Fanconi anemia complementation group I.
Cell culture and transfection
A total of five NSCLC cell lines (H1650, H1975, HCC827, A549 and H1299) and a human normal epithelial (HBE) cell line were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. RPMI-1640 (cat. no. 11875093; Thermo Fisher Scientific, Inc.) medium containing 10% fetal bovine serum (cat. no. A3160901; Gibco; Thermo Fisher Scientific, Inc.) was used to culture cells at 37°C with 5% CO2 in a humidified chamber and were used for transfection when they reached 60–70% confluence. FANCI-targeting short hairpin (sh)RNA (ATGTAAGCTCGGAGCTAATAT) and scrambled negative control (NC1 (sh-FANCI); ACGUGACACGUUGGAGAAT), sh-UBE2T (TTGTCTGGATGTTCTCAAATT) and negative control (NC2 (sh-UBE2T); UUCUCCGAACGUGUCACGUTT), as well as the pEX-UBE2T and pEX-WDR48 overexpression and the empty vectors (pEX-1) were synthesized by Shanghai GenePharma Co., Ltd. and 1 µg/µl was used for transfection with Lipofectamine® 3000 (cat. no. L3000015; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 24 h, according to the manufacturer's protocol. Subsequent experiments were performed 24 h after transfection.
Reverse transcription-quantitative (RT-q) PCR
Total RNA was isolated from NSCLC tissues and cultured cells (at 80% density) using TRIzol® reagent (cat. no. 15596018; Thermo Fisher Scientific, Inc.) Nanodrop 2000 ultramicro spectrophotometer was applied for RNA purity and quantification. The cDNA Synthesis SuperMix for qPCR (cat. no. 11141ES10; Shanghai Yeasen Biotechnology Co., Ltd.) was used to synthesize cDNA according to the manufacturer's instructions. For RT-qPCR, SYBR Premix Ex Taq II (cat. no. RR420A; Takara Biotechnology Co., Ltd.) was used. RT-qPCR was conducted using a Roche Light Cycler 480 system (Roche Diagnostics, GmbH), according to the manufacturer's protocol. The following thermocycling conditions were used for qPCR: initial denaturation at 95°C for 5 min; followed by 40 cycles at 95°C for 10 sec of denaturation, 60°C for 20 sec of annealing and elongation; and 72°C for 20 sec of final extension. The 2−ΔΔCq method was used to calculate the relative expression of FANCI and UBE2T (29). β-Actin served as an internal control. Primer sequences used in this study are listed in Table II.
Table II.
Primer sequences (5′-3′) for reverse-transcription quantitative PCR.
| Gene | Primer sequences (5′-3′) |
|---|---|
| FANCI F | CCACCTTTGGTCTATCAGCTTC |
| FANCI R | CAACATCCAATAGCTCGTCACC |
| UBE2T F | TTGATTCTGCTGGAAGGATTTG |
| UBE2T R | CAGTTGCGATGTTGAGGGAT |
| β-actin F | AGGGGCCGGACTCGTCATACT |
| β-actin R | GGCGGCACCACCATGTACCCT |
FANCI, Fanconi anemia complementation group I; UBE2T, ubiquitin-conjugating enzyme E2T; F, forward; R, reverse.
Western blotting (WB)
Precooled RIPA buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) was used to extract total protein from cells and tissues. The BCA Protein Assay kit (cat. no. P0012S; Beyotime Institute of Biotechnology) was used to determine protein concentrations. Proteins (60 µg per lane) were separated on 10% SDS-PAGE. Subsequently, the proteins were transferred onto polyvinylidene fluoride membranes (cat. no. 32031602; MilliporeSigma). Then, the membranes were blocked with 5% fat-free milk at room temperature for 2 h. Following blocking, the membranes were incubated with primary antibodies (1:1,000, anti-FANCI, cat. no. ab74332; 1:1,000, anti-FANCD2, cat. no. ab108928; cat. no. Abcam) 1:1,000, anti-UBE2T, cat. no. ab179802; 1:1,000, anti-WDR48, cat. no. ab230645; 1:1,000, anti-E-cadherin, cat. no. ab40772; 1:1,000, anti-N-cadherin, cat. no. ab76011; and 1:1,000, anti-Vimentin, cat. no. ab92547) purchased from Abcam at 4°C overnight, followed by incubation with corresponding secondary antibodies for 1 h at room temperature. β-actin (1:2,000, cat. no. ab8226; Abcam) was used as an internal reference protein. The membranes were visualized using ECL solution (E411-04, Vazyme Biotech Co., Ltd.). Image Lab (version 3.0; Bio-Rad Laboratories, Inc.) was used for densitometry.
Cell proliferation
Cell proliferation was detected using a Cell counting kit-8 (CCK-8) assay (cat. no. CK04; Dojindo Laboratories, Inc.). Cells (~4×103) were seeded into each well of a 96-well plate and maintained for the indicated times. Next, 10 µl of CCK-8 reagent was added to each well for another 2 h of incubation. Finally, the absorbance was measured at 450 nm.
Cell migration
Cell migration was tested using Transwell and wound healing assays. For the Transwell assay, ~5×105 cells, mixed in 200 µl basal medium, were added to the upper and lower chambers of the Transwell. They were then supplemented with 500 µl medium. After incubation for 24 h at 37°C, cells were fixed using 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet for 30 min at room temperature (cat. no. C0121; Beyotime Institute of Biotechnology) and then observed under a light microscope (Olympus Corporation). For the scratch assay (wound healing assay), cells were first seeded into a 6-well plate and incubated for 24 h at 37°C using RPMI-1640 (without FBS). Then, a scratch was created with a 10 µl pipette tip in a cell monolayer. The images were captured upon scratching and after 24 h of scratching.
Flow cytometry
Cell cycle and apoptosis were determined via flow cytometry using the Annexin V-FITC/PE cell apoptosis and cell cycle detection kit (cat. no. C1052; Beyotime Institute of Biotechnology) at room temperature for 30 min according to the manufacturer's protocols (30). The cell apoptosis percentage was calculated as Q2+Q4. A flow cytometer (BeamCyte; BEAMDIAG) was used for the flow cytometry. The flow cytometric data were then analyzed with CytoSYS 1.0 software (BEAMDIAG).
Immunoprecipitation (IP)
IP was performed using the Crosslink Magnetic IP/Co-IP kit (cat. no. 88804; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Briefly, after cell lysis, 10–20 µl of lysis solution was collected as an input for further detection. The rest of the cell lysate was treated with an anti-UBE2T antibody (cat. no. ab179802; 1:1,000; Abcam) at 4°C for 12–16 h, Then, protein A/G magnetic beads (cat. no. HY-K0202; MedChemExpress) were added to the lysate. After incubation of ~4 h, they were collected and washed thrice to obtain the co-precipitated proteins. Subsequently, WB was conducted with anti-FANCI (1:200; cat. no. ab74332; cat. no. Abcam) and anti-FANCD2 (1:200; cat. no. ab108928; Abcam) antibodies, as aforementioned.
Mouse tumor xenografts
BALB/c nude mice (n=12; age, 6 weeks; female) were purchased from GemPharmatech Co. Ltd. A549/sh-NC cells or A549/sh-FANCI cells (~5×106) were injected into the mice in the right flanks subcutaneously (n=6 per group). Tumor volume was recorded every week. The mice were sacrificed by using pentobarbital sodium (200 mg/kg) via injection into the caudal vein. Mortality was confirmed by the stopping of the heart and breathing rate (5 min), as well as the disappearance of the foot withdrawal reflex. The tumor tissues were collected for subsequent experiments. The xenograft mice in the present study survived until to sacrifice (five weeks later). Appropriate humane end points were set, including the tumor burden should be <10% of body weight; tumors should be ≥20 mm; tumor rapid growth causing ulceration, necrosis or infection, interference with eating or ability to walk. The Animal Care and Use Committee of the Fujian Provincial Hospital (approval no. A2018-016-12) approved the in vivo experiments.
Immunohistochemistry (IHC)
IHC staining was performed on paraffin-embedded mouse tissue sections. The sections were treated with primary anti-FANCI (1:1,000; cat. no. ab74332; Abcam), anti-Ki67 (1:1,000; cat. no. ab16667; Abcam) and anti-cleaved-Caspase-3 (1:1,000; cat. no. 9661; CST) antibodies overnight at 4°C. After washing with PBS, samples were incubated with corresponding secondary antibodies for 30 min at 37°C and DAPI for 10 min at room temperature. By comparing the entire tissue area at ×10 magnification, the positive staining score was defined as 0, 1, 2, 3 and 4 for 0, 1–25, 26–50, 51–75 and 76–100% staining, respectively. If the staining score was ≥ 3, the protein was considered to be highly expressed.
Statistical analysis
The data were analyzed using GraphPad Prism 6.01 software (GraphPad, USA). The differences between NSCLC tumor tissues and paired adjacent tissues were assessed using paired Student's t-test. The differences between other two groups were assessed using unpaired Student's t-test and differences between more than two groups were assessed using one-way ANOVA with a Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
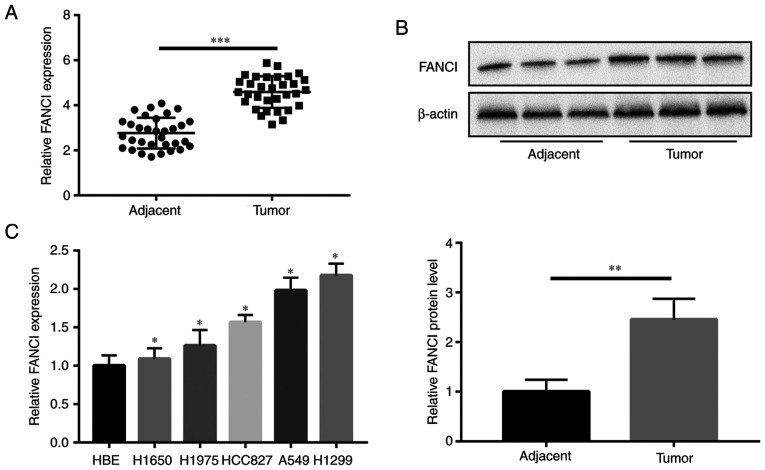
FANCI is highly expressed in NSCLC tissues and cell lines
Compared with the expression of FANCI in paired adjacent tissues, all 32 NSCLC tumor tissues showed higher expression of FANCI, as per results of RT-qPCR (Fig. 1A). As shown in Table I, significant correlations were found between high levels of FANCI expression and tumor size (P=0.032), lymphatic metastasis (P=0.001) and distant metastasis (P=0.032). WB revealed the upregulation of FANCI in the tumors (Fig. 1B), as well as in the NSCLC cell lines (H1650, H1975, HCC827, A549 and H1299), compared with its expression in HBE cells. Among all NSCLC cell lines, A549 and H1299 cells had the highest expression of FANCI (Fig. 1C). Therefore, H1299 and A549 cells were chosen for subsequent experiments.
Figure 1.
FANCI expression in NSCLC tissues and cell lines. (A and B) At mRNA and protein levels, FANCI expression was higher in NSCLC tumor tissues than in adjacent tissues. (C) FANCI was remarkably overexpressed in NSCLC cell lines, compared with its expression in HBE cells. *P<0.05, **P<0.01, ***P<0.001. FANCI, Fanconi anemia complementation group I; NSCLC, non-small cell lung cancer; HBE, human bronchial epithelial.
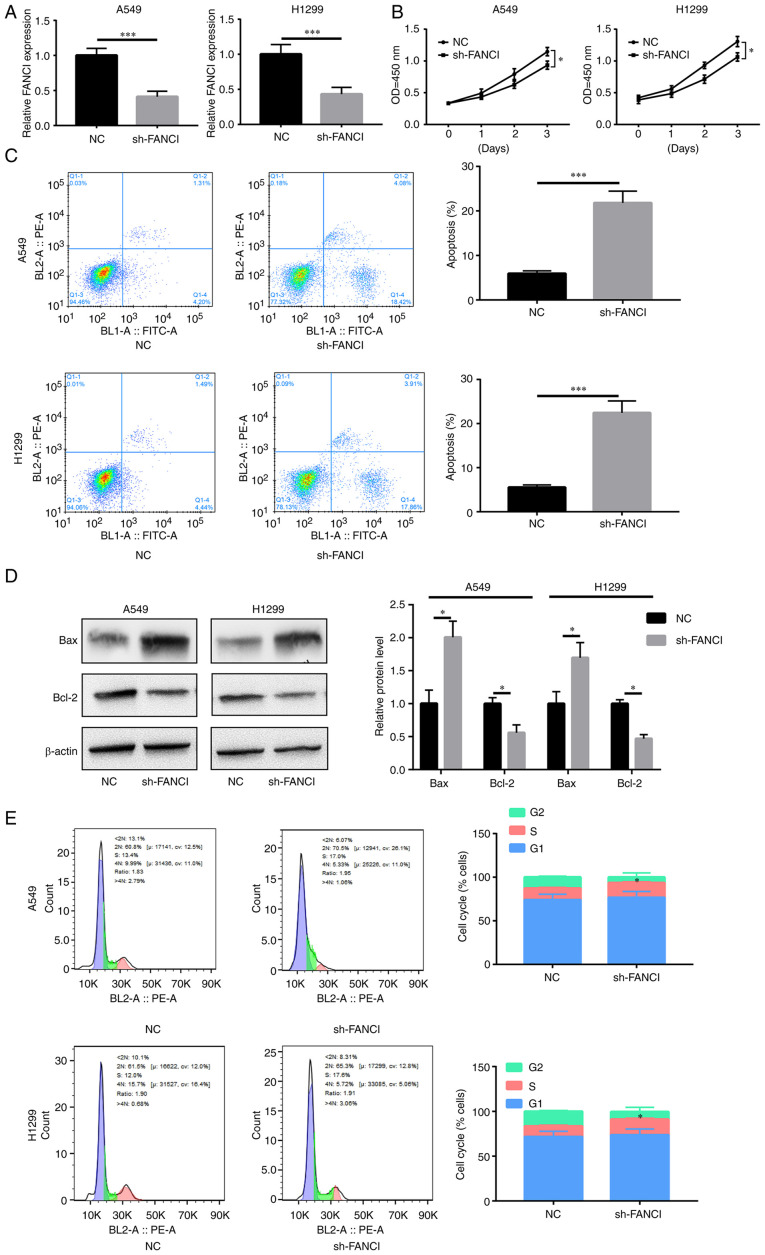
Downregulation of FANCI inhibits NSCLC cell proliferation
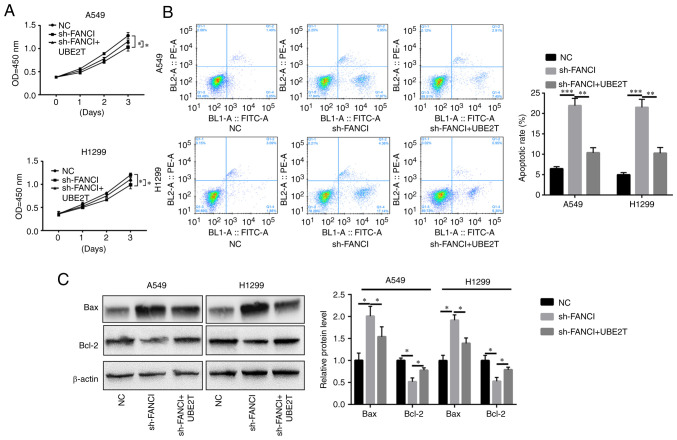
FANCI was knocked down in A549 and H1299 cells to evaluate its role in NSCLC. RT-qPCR showed that sh-FANCI effectively decreased FANCI expression (Fig. 2A). The results of the CCK-8 assay indicated that FANCI knockdown decreased proliferation of cancer cells, compared with the control group (Fig. 2B). Flow cytometry analysis revealed an elevated rate of apoptosis upon FANCI knockdown (Fig. 2C). The level of pro-apoptotic protein Bax increased, while that of anti-apoptotic protein Bcl-2 decreased (Fig. 2D). Furthermore, the cell cycle was blocked upon gene silencing (Fig. 2E). The above data showed that knockdown of FANCI abated cell proliferation by promoting apoptosis and cell cycle arrest in NSCLC cells.
Figure 2.
Effects of FANCI knockdown on NSCLC cell viability. (A) sh-FANCI silenced the expression of FANCI. FANCI silencing (B) inhibited A549 and H1299 cell proliferation, (C) promoted cell apoptosis (as revealed by flow cytometry), (D) upregulated Bax expression and downregulated Bcl-2 expression and (E) blocked the cell cycle in A549 and H1299 cells. *P<0.05, ***P<0.001. FANCI, Fanconi anemia complementation group I; NSCLC, non-small cell lung cancer; sh, short hairpin; NC, negative control.
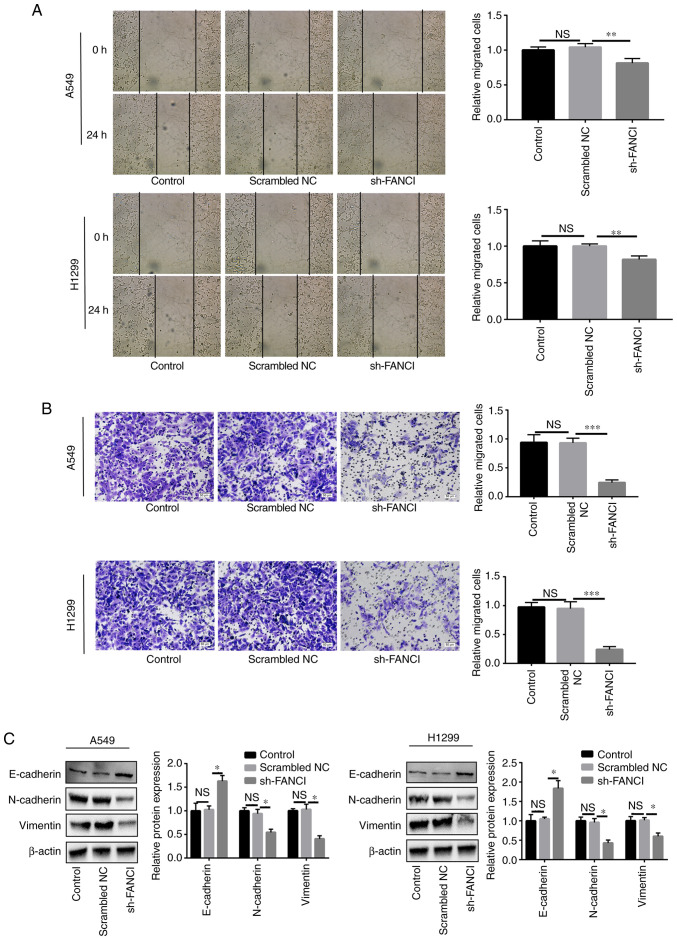
FANCI regulates migration and EMT in NSCLC cells
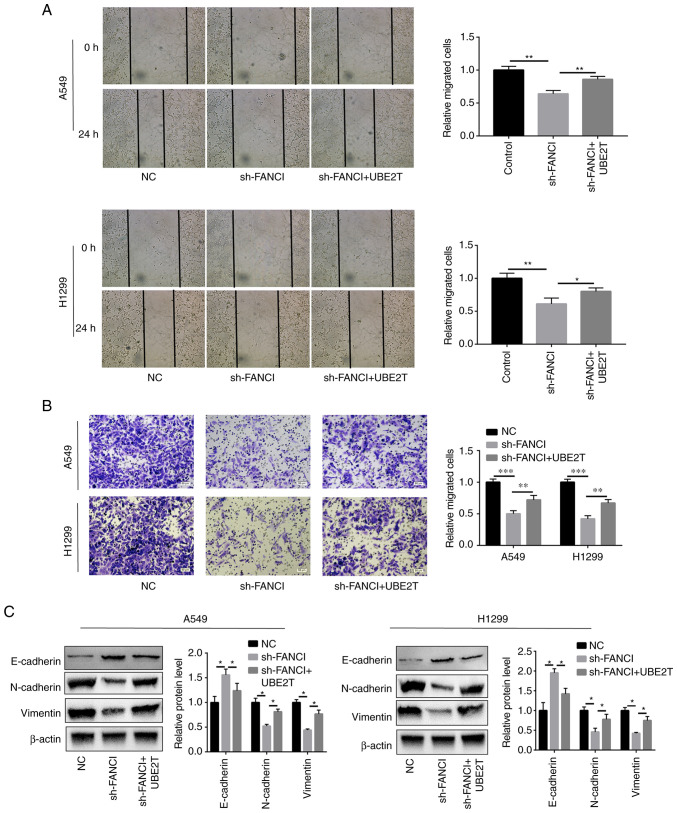
Cell migration and invasion were attenuated, compared with those of the scrambled NC group, when FANCI expression was downregulated (Fig. 3A and B). Furthermore, to determine the effect of FANCI on EMT, the EMT markers including cadherin (E-cadherin and N-cadherin) and vimentin were evaluated in A549 and H1299 cells. As the WB results showed, when FANCI was knocked down, the E-cadherin expression level was raised, but that of N-cadherin and vimentin was decreased (Fig. 3C), indicating FANCI could regulate the expression of cadherin. The above findings demonstrated that FANCI affects EMT in NSCLC by regulating the expression of cadherin and vimentin.
Figure 3.
Effects of FANCI knockdown on EMT in NSCLC cells. (A and B) Wound-healing (magnification, ×10) and Transwell assays (magnification, ×20) showed that downregulation of FANCI expression significantly inhibited cell migration and invasion in A549 and H1299 cells. (C) Knockdown of FANCI increased the level of EMT marker E-cadherin but reduced those of N-cadherin and vimentin. *P<0.05, **P<0.01, ***P<0.001. FANCI, Fanconi anemia complementation group I; EMT, epithelial-mesenchymal transition; NSCLC, non-small cell lung cancer; NS, not significant; NC, negative control.
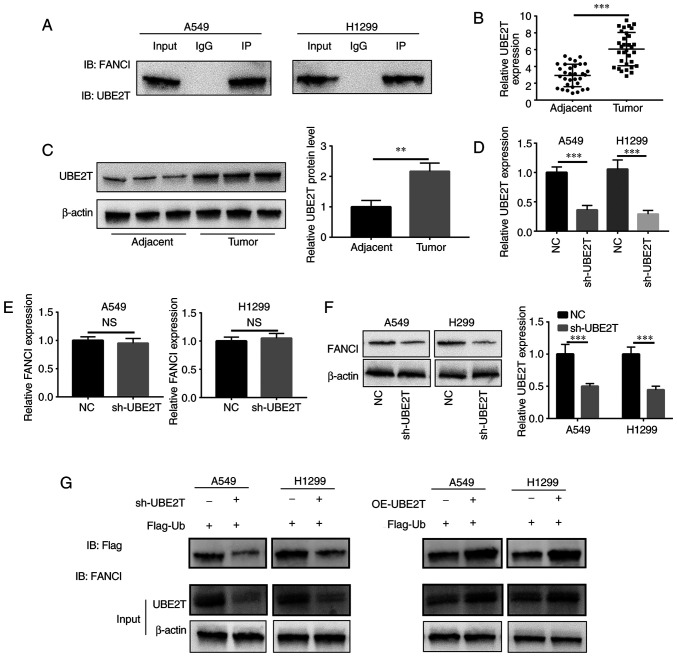
UBE2T contributes to monoubiquitination of FANCI in NSCLC
UBE2T induced monoubiquitination of FANCI to activate a downstream pathway. IP revealed that UBE2T bound to FANCI in A549 and H1299 cells (Fig. 4A). In NSCLC, the mRNA and protein expression of UBE2T in tumor tissues was higher compared with that in adjacent tissues (Fig. 4B and C). sh-UBE2T-mediated knockdown of UBE2Twas confirmed via RT-qPCR (Fig. 4D). Upon downregulation ofUBE2Texpression, FANCI mRNA level did not change significantly (Fig. 4E), but its protein level decreased (Fig. 4F), suggesting that UBE2T might regulate the stability of FANCI by binding to it. To explore the monoubiquitination of FANCI by UBE2T, co-IP was conducted and ubiquitin content was examined. The results showed that the ubiquitin content in A549/sh-UBE2T and H1299/sh-UBE2T cells was downregulated, compared with ubiquitin content in A549/NC and H1299/NC cells (Fig. 4G). By contrast, the ubiquitin content was higher inA549 and H1299/UBE2Tcells than in A549 and H1299/Vector cells (Fig. 4G), indicating that UBE2T mediated monoubiquitination of FANCI in NSCLC. As FANCI is a paralog of FANCD2, the monoubiquitination of FANCD2 by UBE2T was also detected. It was found that the knockdown of UBE2T decreased the monoubiquitination of FANCD2, while the overexpression of UBE2T increased it (Fig. S1). However, the knockdown of FANCD2 did not have any effect on cell growth (Fig. S2).
Figure 4.
Detection of UBE2T-mediated ubiquitination of FANCI. (A) IP assay showed that UBE2T could bind to FANCI in A549 and H1299 cells. UBE2T exhibited higher (B) mRNA and (C) protein levels in NSCLC tumor tissues, compared with its levels in adjacent non-cancerous tissues. (D) Transfection efficiency of sh-UBE2T in NSCLC cells. (E) FANCI mRNA level did not change significantly upon UBE2T silencing. (F) Downregulation of UBE2T expression reduced FANCI levels. (G) The total FANCI protein from A549 and H1299 cells was obtained using the IP method. Compared with the ubiquitin content of the control group, the enrichment of ubiquitin increased after UBE2T downregulation. **P<0.01, ***P<0.001. UBE2T, ubiquitin-conjugating enzyme E2T; FANCI, Fanconi anemia complementation group I; IP, immunoprecipitation; sh, short hairpin; NS, not significant; NC, negative control.
UBE2T mitigates the inhibitory effects of FANCI downregulation
To explore whether UBE2T could alleviate the effects of FANCI knockdown in NSCLC cells, UBE2T was overexpressed in FANCI-knockdown cells. It was found that overexpression of UBE2T promoted the proliferation of, and repressed apoptosis in, A549 and H1299 cells when FANCI was knocked down (Fig. 5A-C). Furthermore, cell migration and invasion were also enhanced upon upregulation of UBE2T expression (Fig. 6A and B). The levels of EMT markers cadherin and vimentin were quantified using WB. Although FANCI silencing increased E-cadherin expression level and decreased N-cadherin and vimentin expression levels, the overexpression of UBE2T partly reversed this effect (Fig. 6C). The above results revealed that the functions of FANCI in NSCLC are regulated by UBE2T.
Figure 5.
UBE2T-mediated alleviation of the effects of FANCI knockdown on NSCLC cells. (A) Overexpression of UBE2T reversed the inhibitory effect of FANCI knockdown on cell proliferation. (B and C) Flow cytometry and WB showed that upregulation of UBE2T expression protected cells from the apoptosis induced by FANCI knockdown *P<0.05, **P<0.01, ***P<0.001. UBE2T, ubiquitin-conjugating enzyme E2T; FANCI, Fanconi anemia complementation group I; NSCLC, non-small cell lung cancer; sh, short hairpin; NC, negative control; NS, not significant.
Figure 6.
UBE2T-mediated alleviation of the effects of FANCI knockdown on EMT. (A) Wound-healing and (B) Transwell assays showed that overexpression of UBE2T reversed the FANCI knockdown-induced inhibition of cell invasion and migration. (C) Upregulation of E-cadherin expression and downregulation of N-cadherin and vimentin expression, caused by FANCI knockdown, were reversed when UBE2T was overexpressed. *P<0.05, **P<0.01, ***P<0.001. UBE2T, ubiquitin-conjugating enzyme E2T; FANCI, Fanconi anemia complementation group I; EMT, epithelial-mesenchymal transition; NC, negative control.
FANCI functions via binding to WDR48
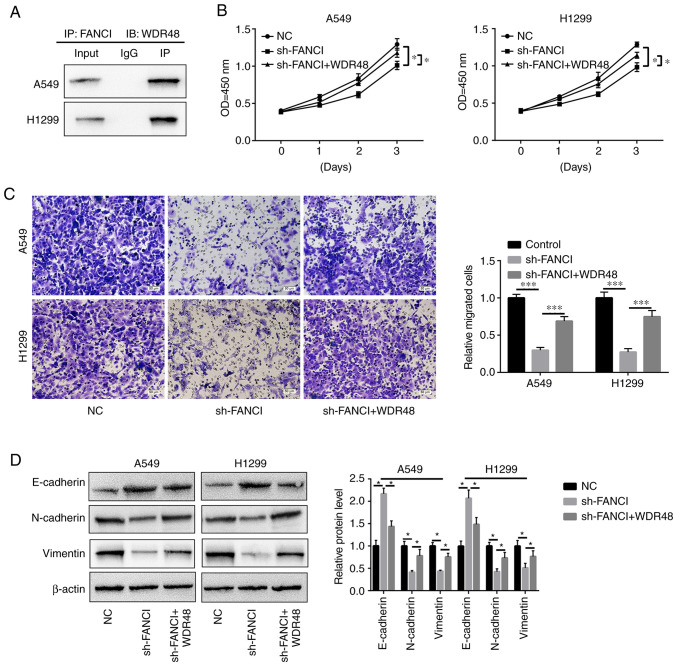
Through in silico analysis (String: http://cn.string-db.org/), it was found that WDR48, which is related to EMT and cell growth (31), might be a downstream factor of FANCI. IP assay confirmed that FANCI could bind to WDR48 in A549 and H1299 cells (Fig. 7A). The upregulation of WDR48 reversed the inhibitory effects of FANCI silencing on cell proliferation and migration (Fig. 7B and C) and EMT. It downregulated E-cadherin expression and upregulated that of N-cadherin and vimentin (Fig. 7D). The above findings suggest that FANCI might function by binding to WDR48.
Figure 7.
Binding of FANCI to WDR48. (A) IP assay confirmed the binding of FANCI to WDR48 in A549 and H1299 cells. Upregulation of WDR48 expression relieved the inhibitory effect of FANCI silencing on (B) cell proliferation and (C) migration. (D) Overexpression of WDR48 reversed the upregulation of E-cadherin expression and downregulation of N-cadherin and vimentin expression, which were caused by the silencing of FANCI expression. *P<0.05, ***P<0.001. FANCI, Fanconi anemia complementation group I; WDR48, WD repeat domain 48; IP, immunoprecipitation; NC, negative control; sh, short hairpin.
Knockdown of FANCI inhibits tumor growth in vivo
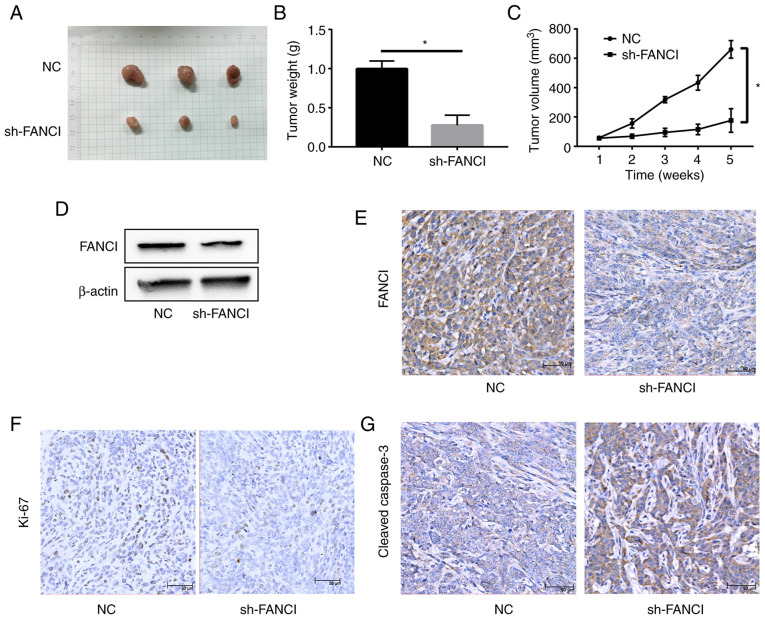
A549/sh-NC and A549/sh-FANCI cells were injected subcutaneously into nude mice to establish tumor xenografts. The tumor size and weight significantly reduced in the sh-FANCI group, compared with the sh-NC group. The tumor volume showed a similar pattern (Fig. 8A-C). WB and IHC revealed low FANCI expression in the sh-FANCI group (Fig. 8D and E). In addition, the expression of proliferation marker Ki67 was lower, whereas that of cleaved-caspase 3 was higher, in the sh-FANCI group (Fig. 8F and G). These data suggested that knockdown of FANCI significantly inhibited tumor growth in vivo.
Figure 8.
Effect of FANCI knockdown on tumor growth in vivo. (A and B) Tumor weights and (C) volumes significantly decreased in the sh-FANCI group compared with those in the sh-NC group. (D) WB and (E) IHC results in tumor tissues showed that FANCI expression was significantly reduced in the sh-FANCI group. IHC revealed (F) decreased expression of Ki-67 and (G) increased expression of cleaved-caspase-3 in the sh-FANCI group. *P<0.05. FANCI, Fanconi anemia complementation group I; sh, short haisrpin; WB, western blotting; IHC, immunohistochemistry; NC, negative control.
Discussion
Despite remarkable efforts to treat NSCLC, such as surgery, chemotherapy, radiotherapy and targeted therapy, the 5-year overall survival rate remains unsatisfactory (32,33). Elucidating the underlying mechanisms of NSCLC tumorigenesis requires intensive and consistent exploration. An ICL refers to the covalent bond between complementary bases of double-stranded DNA, which is one of the most toxic types of DNA damage (34). The FA pathway is responsible for repairing ICLs in the S phase and maintaining genomic stability (35). FANCL, an E3 ubiquitin ligase, serves an irreplaceable role in FA pathway activation and ICL repair. When ICLs occur on DNA, the FA core complex is recruited to the stagnated replication fork. FANCL, the E3 ubiquitin ligase subunit in the FA-core complex, interacts specifically with UBE2T and then, FANCL and UBE2T promote the monoubiquitination of the FANCI-FANCD2 heterodimer. The ubiquitinated FANCI-FANCD2 complex recruits downstream endonucleases to cut DNA. Then, the downstream proteins undergo cross-injury synthesis and homologous recombination repair to finally complete the repair of ICLs (36). FANCI was also revealed as a biomarker for the poor prognosis of LUAD (18); however, a previous study found a tumor suppressive role of FANCI, wherein it acted as a negative factor for the Akt pathway by regulating PHLPP phosphatases (37). Therefore, the role of FANCI in tumors remains unclear and further studies are needed to explore it properly. The present study discovered that FANCI is overexpressed in NSCLC tumor tissues. Knockdown of FANCI inhibited EMT, proliferation, migration, invasion and tumor growth in NSCLC cells. Although FANCI is a paralog of FANCD2 and UBE2T regulates the monoubiquitination of FANCD2 in NSCLC cells, we found that downregulation of FANCD2 did not influence cell growth. Based on these results, we propose that FANCI, but not FANCD2, functions as an oncogene in NSCLC. Thus, FANCI is a novel potential therapeutic target for NSCLC.
UBE2T is an E2 enzyme that is widely dysregulated in numerous cancers and functions as an oncoprotein (38,39). Recently, UBE2T was reported to promote β-catenin nuclear translocation in HCC through the MAPK/ERK axis (40). In addition, UBE2T promotes the progression of LUAD by regulating autophagy through the p53/AMPK/mTOR signaling pathway (41). As a member of the E2 family, UBE2T enhances DNA crosslinking-induced damage repair by monoubiquitinating the FANCI-FANCD2 complex (42). Some studies have indicated that FANCD2 is a preferred substrate for ubiquitination in the FANCI-FANCD2 complex (15,43), but it becomes a poor substrate for ubiquitination when FANCI is absent (44). Compared with FANCD2, free FANCI is more efficiently ubiquitinated by the FA-core complex (44,45). It has also been reported that the abundance of FANCI in U2OS cells is nearly 10 times more than that of FANCD2, indicating that FANCI may also be ubiquitinated alone in cells to a certain degree (46). The present study found that UBE2T expression was upregulated in NSCLC tumor tissues. Therefore, in terms of mechanism, it was hypothesized that FANCI might be regulated by UBE2T during the development and progression of NSCLC. According to the co-IP and WB assays, UBE2T interacted with FANCI and stabilized the FANCI level. Ubiquitin-like protein 5 (UBL5) has also been reported to bind FANCI and promote its stability (47). The present study discovered that overexpression of UBE2T increased the monoubiquitination level of FANCI in NSCLC cells. The foregoing results suggested that the function of FANCI in NSCLC was probably modulated by UBE2T-mediated monoubiquitination. However, it is still not well known how the FA-core complex and UBE2T specifically regulate FANCI monoubiquitination and this requires further research. In addition, the rescue experiments showed that overexpression of UBE2T partly reversed the FANCI knockdown-induced inhibition of EMT, cell growth, migration and invasion, indicating that UBE2T regulates the function of FANCI in NSCLC.
WDR48 is critical for EMT. The inhibition of endogenous WDR48 expression is reported to significantly decrease TGF-β-induced EMT, migration and invasion in tumor cells (47). The present study predicted the binding of FANCI and WDR48 using in silico analysis and confirmed it via IP assay. Furthermore, rescue experiments showed that the upregulation of WDR48 also reversed the FANCI knockdown-induced inhibition of EMT and cell growth. These findings indicate the probable role of WDR48 in FANCI function in NSCLC.
In conclusion, the present study revealed the oncogenic role of FANCI in promoting cell growth and EMT in NSCLC. This function of FANCI is mediated by WDR48 and regulated by UBE2T-induced monoubiquitination of FANCI. In the present study, FANCI emerged as a potential target for NSCLC therapy that may also serve as a biomarker for predicting its poor prognosis.
Supplementary Material
Acknowledgments
Not applicable.
Glossary
Abbreviations
- CCK-8
Cell Counting Kit-8
- IP
immunoprecipitation
- EMT
epithelial-mesenchymal transition
- FANCD2
Fanconi anemia complementation group D2
- FANCI
Fanconi anemia complementation group I
- FANCL
Fanconi anemia complementation group L
- HBE
human bronchial epithelial
- ICL
interstrand cross-link
- IP
immunoprecipitation
- LUAD
lung adenocarcinoma
- NSCLC
non-small cell lung cancer
- RT-qPCR
reverse-transcription quantitative PCR
- shRNA
short hairpin RNA
- UBE2T
ubiquitin-conjugating enzyme E2T
- WB
western blotting
- WDR48
WD repeat domain 48
Funding Statement
The present study was funded by the National Natural Science Foundation of China (grant no. 82074189), Fujian Natural Science Foundation (grant no. 2021J01380) and Science and Technology Planning Project of Fujian Provincial Health Commission (grant no. 2021zylc31).
Availability of data and materials
The datasets in the present study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and XL designed the study. JWa and JZ performed the experiments. XL and JW prepared the figures. JZ and XL confirm the authenticity of all the raw data. JWu, JH and ZL contributed to the drafting of the manuscript and the final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Fujian Provincial Hospital (approval no. K2018-015-11) and written informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, Swanton C. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet. 2016;388:1002–1011. doi: 10.1016/S0140-6736(16)31340-X. [DOI] [PubMed] [Google Scholar]
- 5.Del Re M, Crucitta S, Gianfilippo G, Passaro A, Petrini I, Restante G, Michelucci A, Fogli S, de Marinis F, Porta C, et al. Understanding the mechanisms of resistance in EGFR-positive NSCLC: From tissue to liquid biopsy to guide treatment strategy. Int J Mol Sci. 2019;20:3951. doi: 10.3390/ijms20163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doval DC, Desai CJ, Sahoo TP. Molecularly targeted therapies in non-small cell lung cancer: The evolving role of tyrosine kinase inhibitors. Indian J Cancer. 2019;56((Suppl)):S23–S30. doi: 10.4103/ijc.IJC_449_19. [DOI] [PubMed] [Google Scholar]
- 7.Kelaidi C, Makis A, Petrikkos L, Antoniadi K, Selenti N, Tzotzola V, Ioannidou ED, Tsitsikas K, Kitra V, Kalpini-Mavrou A, et al. Bone marrow failure in Fanconi anemia: Clinical and genetic spectrum in a cohort of 20 pediatric patients. J Pediatr Hematol Oncol. 2019;41:612–617. doi: 10.1097/MPH.0000000000001549. [DOI] [PubMed] [Google Scholar]
- 8.Engel NW, Schliffke S, Schüller U, Frenzel C, Bokemeyer C, Kubisch C, Lessel D. Fatal myelotoxicity following palliative chemotherapy with cisplatin and gemcitabine in a patient with stage IV cholangiocarcinoma linked to post mortem diagnosis of Fanconi anemia. Front Oncol. 2019;9:420. doi: 10.3389/fonc.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsuki Y, Takata M. Defects in homologous recombination repair behind the human diseases: FA and HBOC. Endocr Relat Cancer. 2016;23:T19–T37. doi: 10.1530/ERC-16-0221. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. Update of the human and mouse Fanconi anemia genes. Hum Genomics. 2015;9:32. doi: 10.1186/s40246-015-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493:356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo W, Xu G, Persky NS, Smogorzewska A, Rudge DG, Buzovetsky O, Elledge SJ, Pavletich NP. Structure of the FANCI-FANCD2 complex: Insights into the Fanconi anemia DNA repair pathway. Science. 2011;333:312–316. doi: 10.1126/science.1205805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sondalle SB, Longerich S, Ogawa LM, Sung P, Baserga SJ. Fanconi anemia protein FANCI functions in ribosome biogenesis. Proc Natl Acad Sci USA. 2019;116:2561–2570. doi: 10.1073/pnas.1811557116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 15.Smogorzewska A, Matsuoka S, Vinciguerra P, McDonald ER, III, Hurov KE, Luo J, Ballif BA, Gygi SP, Hofmann K, D'Andrea AD, Elledge SJ. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulo P, Maia S, Pinto C, Pinto P, Monteiro A, Peixoto A, Teixeira MR. Targeted next generation sequencing identifies functionally deleterious germline mutations in novel genes in early-onset/familial prostate cancer. PLoS Genet. 2018;14:e1007355. doi: 10.1371/journal.pgen.1007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie S, Jiang X, Zhang J, Xie S, Hua Y, Wang R, Yang Y. Identification of significant gene and pathways involved in HBV-related hepatocellular carcinoma by bioinformatics analysis. PeerJ. 2019;7:e7408. doi: 10.7717/peerj.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng P, Li L. FANCI cooperates with IMPDH2 to promote lung adenocarcinoma tumor growth via a MEK/ERK/MMPs pathway. Onco Targets Ther. 2020;13:451–463. doi: 10.2147/OTT.S230333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machida YJ, Machida Y, Chen Y, Gurtan AM, Kupfer GM, D'Andrea AD, Dutta A. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol Cell. 2006;23:589–596. doi: 10.1016/j.molcel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Longerich S, San Filippo J, Liu D, Sung P. FANCI binds branched DNA and is monoubiquitinated by UBE2T-FANCL. J Biol Chem. 2009;284:23182–23186. doi: 10.1074/jbc.C109.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueki T, Park JH, Nishidate T, Kijima K, Hirata K, Nakamura Y, Katagiri T. Ubiquitination and downregulation of BRCA1 by ubiquitin-conjugating enzyme E2T overexpression in human breast cancer cells. Cancer Res. 2009;69:8752–8760. doi: 10.1158/0008-5472.CAN-09-1809. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Li T, Niu X, Chen L, Ge C. Identification of UBE2T as an independent prognostic biomarker for gallbladder cancer. Oncol Lett. 2020;20:44. doi: 10.3892/ol.2020.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Zhu Z, Li W, Shen M, Cao C, Sun Q, Guo Z, Liu L, Wu D. UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation. J Exp Clin Cancer Res. 2020;39:222. doi: 10.1186/s13046-020-01734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu ZH, Zhang YJ, Sun HY. High ubiquitin conjugating enzyme E2 T mRNA expression and its prognostic significance in lung adenocarcinoma: A study based on the TCGA database. Medicine (Baltimore) 2020;99:e18543. doi: 10.1097/MD.0000000000018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin H, Wang X, Zhang X, Zeng Y, Xu Q, Wang W, Zhou F, Zhou Y. UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation. Cancer Lett. 2020;494:121–131. doi: 10.1016/j.canlet.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 27.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Sun H, Kong H, Chen Z, Chen B, Zhou M. The lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell proliferation, migration and EMT phenotype formation through sponging Mir-382. Cell Physiol Biochem. 2017;42:2145–2158. doi: 10.1159/000479990. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Yin H, Zhang H, Hu J, Lu H, Li C, Cao M, Yan S, Cai L. NF-κB-driven improvement of EHD1 contributes to erlotinib resistance in EGFR-mutant lung cancers. Cell Death Dis. 2018;9:418. doi: 10.1038/s41419-018-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han D, Wang L, Chen B, Zhao W, Liang Y, Li Y, Zhang H, Liu Y, Wang X, Chen T, et al. USP1-WDR48 deubiquitinase complex enhances TGF-β induced epithelial-mesenchymal transition of TNBC cells via stabilizing TAK1. Cell Cycle. 2021;20:320–331. doi: 10.1080/15384101.2021.1874695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao J, Yuan P, Wang Y, Xu J, Yuan X, Wang Z, Lv W, Hu J. Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg. 2018;105:1483–1491. doi: 10.1016/j.athoracsur.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Giaj-Levra N, Borghetti P, Bruni A, Ciammella P, Cuccia F, Fozza A, Franceschini D, Scotti V, Vagge S, Alongi F. Current radiotherapy techniques in NSCLC: Challenges and potential solutions. Expert Rev Anticancer Ther. 2020;20:387–402. doi: 10.1080/14737140.2020.1760094. [DOI] [PubMed] [Google Scholar]
- 34.Rozelle AL, Cheun Y, Vilas CK, Koag MC, Lee S. DNA interstrand cross-links induced by the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Nat Commun. 2021;12:1897. doi: 10.1038/s41467-021-22273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Guo T, Liu R, Ke H, Xu W, Zhao S, Qin Y. FANCL gene mutations in premature ovarian insufficiency. Hum Mutat. 2020;41:1033–1041. doi: 10.1002/humu.23997. [DOI] [PubMed] [Google Scholar]
- 36.Ceccaldi R, Sarangi P, D'Andrea AD. The Fanconi anaemia pathway: New players and new functions. Nat Rev Mol Cell Biol. 2016;17:337–349. doi: 10.1038/nrm.2016.48. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Lu X, Akhter S, Georgescu MM, Legerski RJ. FANCI is a negative regulator of Akt activation. Cell Cycle. 2016;15:1134–1143. doi: 10.1080/15384101.2016.1158375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Zhang Y, Yang Z, Liu X, Yang P, Wang J, Hu K, He X, Zhang X, Jing H. High expression of UBE2T predicts poor prognosis and survival in multiple myeloma. Cancer Gene Ther. 2019;26:347–355. doi: 10.1038/s41417-018-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu LP, Yang M, Peng QZ, Li MY, Zhang YS, Guo YH, Chen Y, Bao SY. UBE2T promotes hepatocellular carcinoma cell growth via ubiquitination of p53. Biochem Biophys Res Commun. 2017;493:20–27. doi: 10.1016/j.bbrc.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 40.Lioulia E, Mokos P, Panteris E, Dafou D. UBE2T promotes β-catenin nuclear translocation in hepatocellular carcinoma through MAPK/ERK-dependent activation. Mol Oncol. 2022;16:1694–1713. doi: 10.1002/1878-0261.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Ao H, Liu M, Cao K, Ma J. UBE2T promotes autophagy via the p53/AMPK/mTOR signaling pathway in lung adenocarcinoma. J Transl Med. 2021;19:374. doi: 10.1186/s12967-021-03056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nepal M, Che R, Ma C, Zhang J, Fei P. FANCD2 and DNA damage. Int J Mol Sci. 2017;18:1804. doi: 10.3390/ijms18081804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato K, Toda K, Ishiai M, Takata M, Kurumizaka H. DNA robustly stimulates FANCD2 monoubiquitylation in the complex with FANCI. Nucleic Acids Res. 2012;40:4553–4561. doi: 10.1093/nar/gks053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Wang R, Peralta C, Yaseen A, Pavletich NP. Structure of the FA core ubiquitin ligase closing the ID clamp on DNA. Nat Struct Mol Biol. 2021;28:300–309. doi: 10.1038/s41594-021-00568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Twest S, Murphy VJ, Hodson C, Tan W, Swuec P, O'Rourke JJ, Heierhorst J, Crismani W, Deans AJ. Mechanism of ubiquitination and deubiquitination in the Fanconi anemia pathway. Mol Cell. 2017;65:247–259. doi: 10.1016/j.molcel.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R. The quantitative proteome of a human cell line. Mol Syst Biol. 2011;7:549. doi: 10.1038/msb.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oka Y, Bekker-Jensen S, Mailand N. Ubiquitin-like protein UBL5 promotes the functional integrity of the Fanconi anemia pathway. EMBO J. 2015;34:1385–1398. doi: 10.15252/embj.201490376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets in the present study are available from the corresponding author on reasonable request.