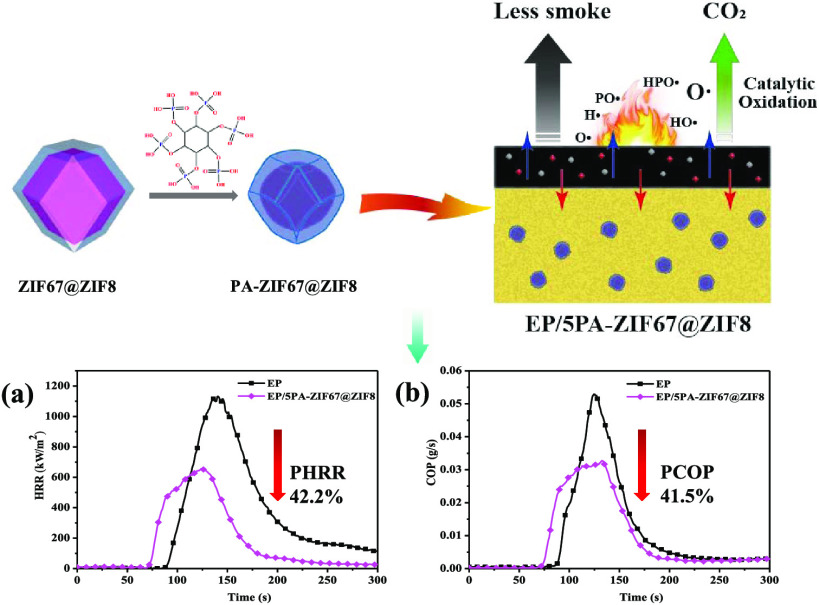
Abstract
Despite many important industrial applications, epoxy resin (EP) suffers from high flammability and toxicity emission, extremely hampering their applications. To circumvent the problem, core–shell structured ZIF67@ZIF8 is successfully synthesized and further functionalized with phytic acid (PA) to obtain PA-ZIF67@ZIF8 hybrids. Then, it is used as an efficient flame retardant to reduce the fire risk of EP. The fire test results show a significant reduction in heat and smoke production. Compared with EP, incorporating 5.0 wt % PA-ZIF67@ZIF8 into EP, the peak heat release rate, total heat release, and peak carbon monoxide production are dramatically reduced by 42.2, 33.0, and 41.5%, respectively. Moreover, the EP/PA-ZIF67@ZIF8 composites achieve the UL-94 V-0 rating and the limiting oxygen index increases by 29.3%. These superior fire safety properties are mainly attributed to the excellent dispersion and the catalytic effect of metal oxide and phosphorus-containing compounds. This work provides an efficient strategy for preparing a promising ZIF-based flame retardant for enhancing flame retardancy and smoke toxicity suppression of EP.
1. Introduction
Epoxy resin (EP) is one of the promising thermosetting polymers. It has been extensively used in many fields, such as painting, building construction, and composite applications, owing to its high thermal stability, outstanding mechanical properties, and excellent chemical resistance.1−4 However, its inflammability severely poses many disadvantages for its applications.5,6 Hence, it is imperative to improve the fire-retardant performance of EP.
Metal–organic frameworks (MOFs) are a kind of porous material consisting of metal ions (or metal clusters) and organic linkers.7 They have been widely used in the fields of gas separation, drug delivery, proton conductivity, etc.8,9 Recently, MOFs have attracted increasing attention in fire retardancy studies.10−13 Compared with traditional flame retardants (FRs), MOFs exhibit superior compatibility with polymers due to the existence of organic linkers.14−16 Here, it should be mentioned that the zeolitic imidazolate frameworks (ZIFs) are a subset of MOFs, which are composed of metal ions and imidazolate ligands.17,18 The ZIFs commonly used as FRs are the Co2+-containing zeolitic imidazolate framework (ZIF67) and Zn2+-containing zeolitic imidazolate framework (ZIF8), which contain abundant flame-retardant elements. The metallic element can work with the nitrogen element to improve the fire safety of polymers together.11,14,19 For example, Xu et al.20 added a ZIF8/reduced graphene oxide hybrid in the EP matrix to decrease the peak heat release rate (PHRR) and total smoke production (TSP) of EP by 65 and 36.8%, respectively. In addition, Li et al.21 designed a ZIF67@MgAl-LDH hybrid to improve the performance of EP with a decrease in the total heat release rate of 39.3% and TSP of 38%.
However, it is difficult to achieve the desired flame-retardant effect by alternatively adding ZIF67 or ZIF8 to the polymer matrix. To solve the limitations of ZIF67 and ZIF8 flame retardance, bimetallic (Zn and Co) ZIF derivatives are prepared to improve the flame retardancy of polymeric materials. For example, Hou et al.22 used bimetallic ZIF derivatives (ZIF67 and ZIF8) as sacrificial agents to synthesize Co–Ni LDHs on the surfaces of GO or CNTs, and the UPR composites exhibit excellent flame retardancy as expected. At a loading amount of 2 wt %, UPR composites exhibit 27.7 and 22.7% decreases in the value of TSP. Zhang et al.14 synthesized Zn and Co bimetallic ZIFs on the surface of GO to improve the fire safety of EP. With the addition of 2 wt % MOF@GO, the PHRR of EP nanocomposites decreased by 29.4%. However, according to previous reports, the sample consisting of two different nanoparticles by physical mixing shows a poor flame-retardant effect than that of corresponding core–shell structured nanohybrid particles. For instance, EP/6ZHS@NCH shows a higher LOI value than EP/6(ZHS + NCH).23 The EP/MOF@GO and EP/MOF-GO show the same results.14 Hence, core–shell structured ZIF67@ZIF8 was synthesized and prepared.24,25 Until now, the utilization of ZIF67@ZIF8 and its derivatives as flame retardants has not been reported.
To further improve the flame retardancy of ZIF67@ZIF8, it is necessary to functionalize ZIF67@ZIF8. Among all halogen-free flame retardants, phosphorus-containing flame retardants are one of the most promising candidates because of their low toxicity, high efficiency, and multiple flame retardancy mechanisms.26−29 Phytic acid (PA), as an environmentally friendly and biocompatible organic acid, has gained increasing interest as an effective flame retardant due to the abundance of phosphorus (28% P).30−32
In this study, phytic acid was employed to functionalize ZIF67@ZIF8 to form core–shell structured ZIF67@ZIF8-PA hybrids and ZIF67@ZIF8-PA hybrids were then added to the EP matrix via a physical blending method. The thermal stabilities and fire hazards of the EP composites were investigated systematically. Furthermore, the flame retardancy mechanism of EP composites was proposed.
2. Experimental Section
2.1. Materials
Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) and 2-methylimidazole (2-MIM) were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Zinc nitrate hexahydrate (Zn(NO3)2·6H2O) was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Diamino diphenylmethane (DDM) and phytic acid (50% in H2O) were provided by Aladdin Chemistry Co., Ltd. (Shanghai, China). EP (E-51) was obtained from Petrochemical Co., Ltd. (Yueyang, Hunan).
2.2. Preparation of ZIF67@ZIF8
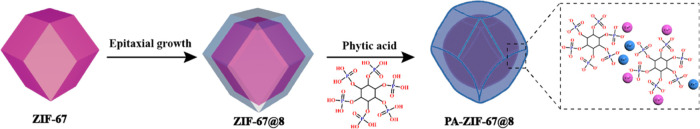
Co(NO3)2·6H2O (10.92 g) and 2-methylimidazole (12.32 g) were dissolved in 100 mL of methanol and 150 mL of methanol, respectively. Then, the Co(NO3)2·6H2O solution was added dropwise to the 2-methylimidazole solution under ultrasonication. After that, Zn(NO3)2·6H2O (12.32 g) in 100 mL of methanol was slowly added to the mixture and exposed to ultrasonic treatment for another 1 h, followed by stirring for 12 h at room temperature. Finally, the crude was washed with methanol and dried at 80 °C for 12 h. The preparation diagram of ZIF67@ZIF8 and PA-ZIF67@ZIF8 is displayed in Scheme 1.
Scheme 1. Synthesis Route of ZIF67@ZIF8 and PA-ZIF67@ZIF8.
2.3. Preparation of PA-ZIF67@ZIF8
In a typical procedure, 1 g of ZIF67@ZIF8 was dispersed into 150 mL of deionized water under sonication. PA solution (2.5 g) was added slowly to the suspensions above. The reaction would occur for 24 h with constant stirring at room temperature. Subsequently, the products were washed thoroughly with distilled water several times and then dried at 80 °C for 10 h.
2.4. Preparation of EP Composites
The fabrication procedure of EP composites containing 2 wt % ZIF67@ZIF8 was performed as follows: 1.2 g of ZIF67@ZIF8 and 11.76 g of DDM were dispersed into acetone by sonication. Then, 47.04 g of EP was added to the suspension with mechanical stirring for 2 h to obtain a uniform system. Whereafter, the mixture was transferred into a vacuum oven at 50 °C for 2 h to remove the acetone. Finally, the mixture was poured into a prepared Teflon mold and cured at 100 and 150 °C for 2 h, respectively. The EP composite was successfully prepared after natural cooling to room temperature, where EP composites containing 2.0 and 5.0 wt % ZIF67@ZIF8 were designated as EP/2ZIF67@ZIF8 and EP/5ZIF67@ZIF8, respectively; they could be fabricated using the same procedure. To make a comparison, EP/2PA-ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 with 2.0 and 5.0 wt % PA-ZIF67@ZIF8 were prepared by the same approach.
2.5. Characterization
The detailed characterization is provided in the Supporting information.
3. Results and Discussion
3.1. Characterization of ZIF67@ZIF8 and PA-ZIF67@ZIF8
Fourier transform infrared (FTIR) analysis provides the structural information of ZIF67@ZIF8 and PA-ZIF67@ZIF8. The FTIR spectra of ZIF67@ZIF8 and PA-ZIF67@ZIF8 are shown in Figure 1a. According to the FTIR spectrum of ZIF67@ZIF8, the typical peaks at 423 and 419 cm–1 are attributed to the stretching vibrations of Co–N and Zn–N, respectively.16,20 The peak in the range of 500–1600 cm–1 is assigned to the typic stretching vibration of 2-methimidazole.20 For PA-ZIF67@ZIF8, two newly emerged bands at 1130 and 1068 cm–1 are allocated to the stretching vibration of P–O and P=O, respectively,32 while the peaks at 620 and 520 cm–1 are ascribed to the characteristic stretching vibrations of Co–O and Zn–O, respectively.23 The crystal structures of ZIF67@ZIF8 and PA-ZIF67@ZIF8 are examined by the X-ray diffraction (XRD) technique. As shown in Figure 1b, the diffraction peaks of ZIF67@ZIF8 show a very close agreement with the XRD patterns of ZIF8 and ZIF67 as reported in the literature,24,25 which is caused by the similar unit cell parameters of ZIF67 and ZIF8. However, after ZIF67@ZIF8 is functionalized with PA, the characteristic peaks of ZIF7@ZIF8 are not found because PA gradually breaks the coordination bonds of ZIF67@ZIF8.
Figure 1.
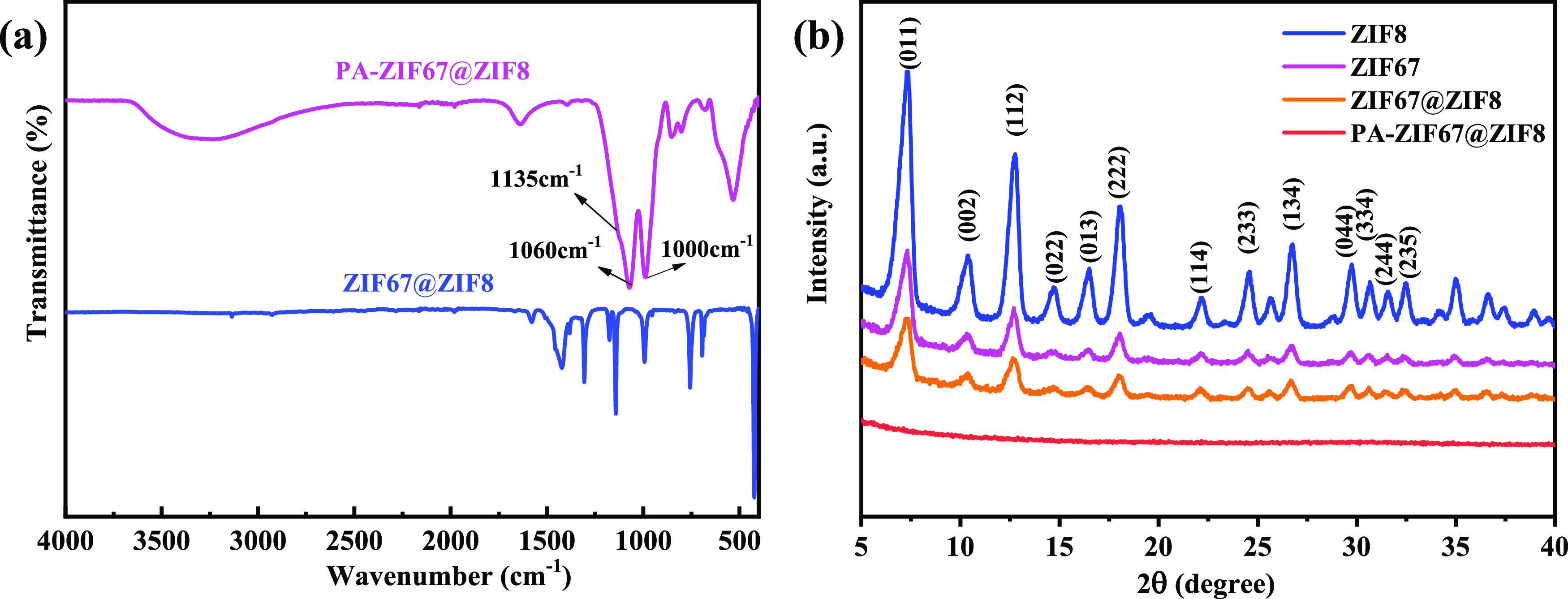
(a) FTIR spectra and (b) XRD patterns of ZIF67@ZIF8 and ZIF67@ZIF8-PA.
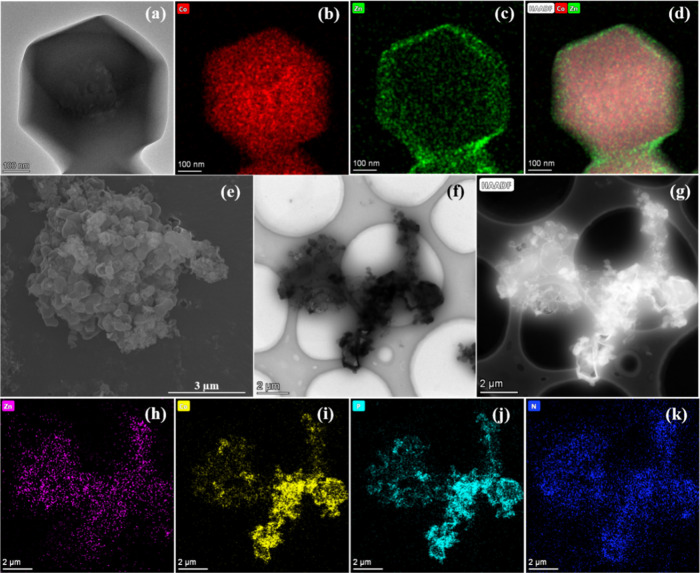
The morphologies of ZIFs are studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Figure 2, ZIF67@ZIF8 exhibits a rhombic dodecahedral morphology and a diameter of ca. 500 nm. To further determine the core–shell structure of ZIF67@ZIF8, elemental analysis is performed by energy-dispersive X-ray spectroscopy (EDS) elemental mapping. It is observed that Co is situated at the center and Zn is distributed over the whole sample, indicating that the core–shell structure of ZIF67@ZIF8 is successfully synthesized. After further modification with PA, its morphology is quite distinct from that of ZIF67@ZIF8 (Figure 2e,f). From Figure 2h–k, the EDS results of PA-ZIF67@ZIF8 exhibit the target elements of Co, Zn, N, and P that are distributed homogeneously, which is consistent with the results of XPS analysis (Figure 3a).
Figure 2.
(a) TEM image of ZIF67@ZIF8 and corresponding elemental mapping of (b) Co, (c) Zn, and (d) the layered image of panels a–c, (e) SEM image of PA ZIF67@ZIF8, (f, g) TEM image of PA-ZIF67@ZIF8 and corresponding elemental mapping of (h) Zn, (i) Co, (j) P, and (k) N.
Figure 3.
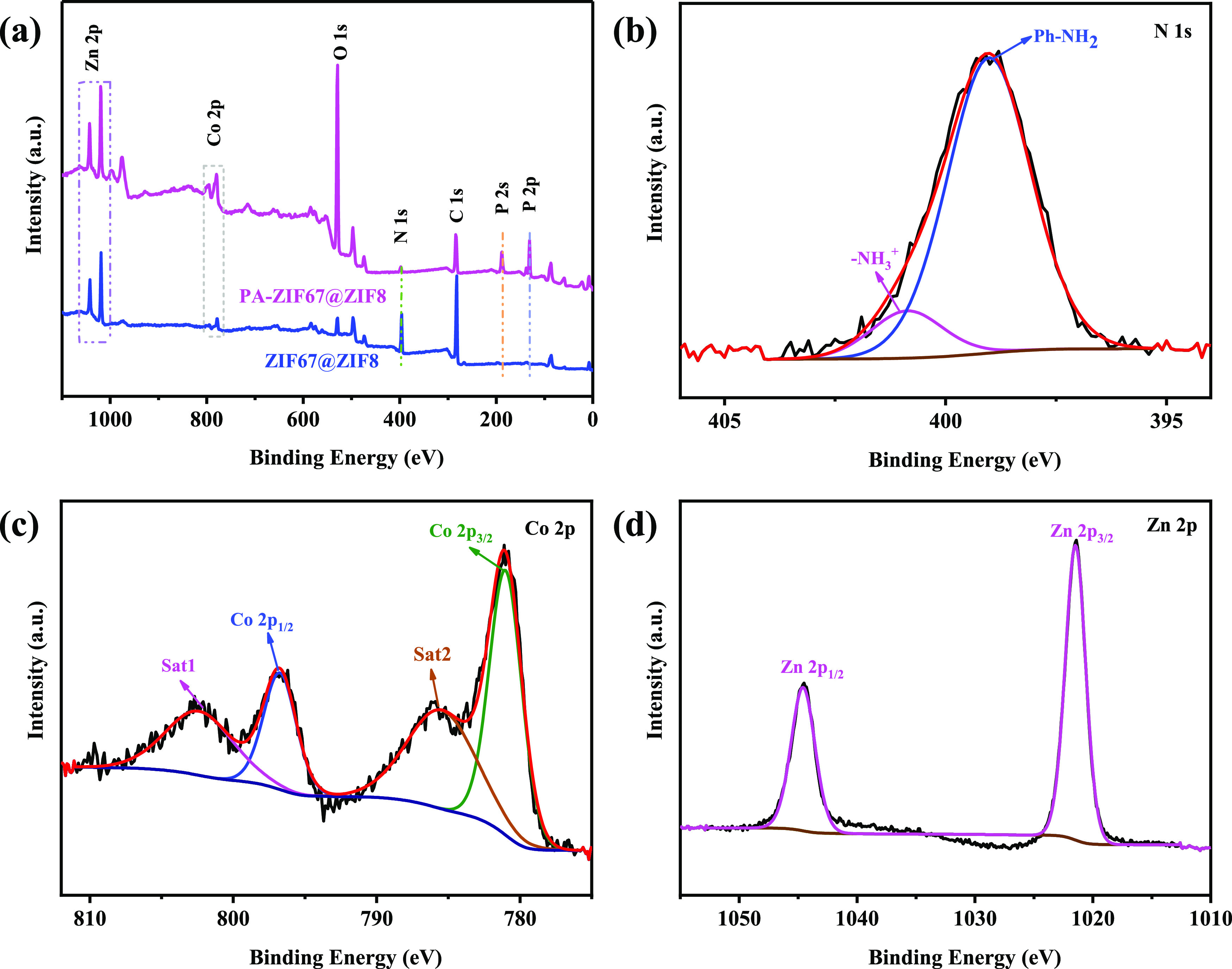
(a) XPS patterns of ZIF67@ZIF8 and PA-ZIF67@ZIF8 and high-resolution XPS spectra of (b) P 2p, (c) Co 2p, and (d) Zn 2p.
XPS spectra are utilized to determine the chemical states and elemental compositions of ZIF67@ZIF8 and PA-ZIF67@ZIF8. As depicted in Figure 3a, the XPS spectrum of ZIF67@ZIF8 reveals the Co 2p, Zn 2p, and N 1s peaks. In comparison, PA-ZIF67@ZIF8 exhibits two new sharp peaks of P 2p (133.0 eV) and P 2s (189.0 eV).32 Meanwhile, the peak of N 1s is significantly decreased, which is due to part of amine groups reacting with PA. In addition, the high-resolution N 1s, Co 2p, and Zn 2p XPS spectra of PA-ZIF67@ZIF8 are presented in Figure 3b,d. As for the N 1s spectrum, the peaks at 399 and 409.1 eV are assigned to Ph–NH2 and −NH3+, respectively.32 In the case of the Co 2p spectrum, besides two peaks centered at 781.1 eV (Co 2p3/2) and 797.1 eV (Co 2p1/2), there are two satellite peaks appearing at 786.1 eV (Co 2p3/2) and 803.1 eV (Co 2p1/2).16 In the case of the Zn 2p spectrum, Zn 2p1/2 and Zn 2p3/2 are located at 1044.2 and 1021.2 eV, respectively.23 These results confirm that the ZIF67@ZIF8 has been functionalized by PA.
3.2. Dispersion of PA-ZIF67@ZIF8 in the EP Matrix
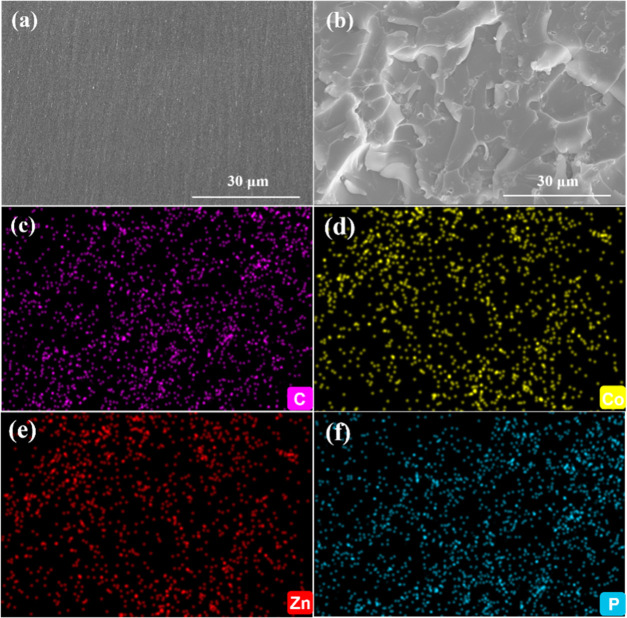
The dispersion of EP/5PA-ZIF67@ZIF8 in the EP matrix is investigated by SEM. As shown in Figure 4a, the fractured surface of EP is smooth, while the fractured surface of EP/5PA-ZIF67@ZIF8 is relatively rough (Figure 4b). To further study the dispersion of EP/5PA-ZIF67@ZIF8, elemental analysis of the fracture surfaces is characterized by EDS. The corresponding mapping images of the fracture surface are shown in Figure 4c–f. The main C, P, Co, and Zn elements are evenly distributed on the fracture surface, demonstrating that 5 wt % PA-ZIF67@ZIF8 exhibits good dispersion in the epoxy matrix. Meanwhile, the dispersion of ZIF67@ZIF8 and PA-ZIF67@ZIF8 in EP is further characterized by TEM. TEM images of EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 are shown in Figure S1. Obviously, ZIF67@ZIF8 and PA-ZIF67@ZIF8 are dispersed uniformly without aggregation in the EP matrix.
Figure 4.
(a) SEM images of the fractured surfaces for (a) EP and (b) EP/5PA-ZIF67@ZIF8 and corresponding elemental mapping images of (c) C, (d) P, (e) Zn, and (f) Co.
3.3. Thermal Behavior and Mechanical Properties of EP and Its Composites
The thermal property of EP composite samples in a N2 atmosphere is displayed in Figure 5, and the corresponding data are listed in Table 1, where T5% and Tmax are defined as the temperature at 5 wt % weight loss and the maximum weight loss, respectively. As shown in Figure 5a, all EP composite samples display one-step mass loss in the N2 atmosphere. The T5% of EP/2ZIF67@ZIF8 and EP/5ZIF67@ZIF8 is reduced slightly to that of EP due to the catalytic decomposition of ZIF67@ZIF8. However, the T5% of EP/2PA-ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 is increased by 16 and 31 °C, respectively. Compared to that of EP, the Tmax of the EP composites decreases slightly. Furthermore, compared with that of EP, the char yields of EP composites are increased. Particularly, the addition of 5 wt % PA-ZIF67@ZIF8 to EP improves the char residues from 17.0 to 29.2 wt %, indicating that the coordination of 5 wt % PA-ZIF67@ZIF8 can accelerate the formation of residual char. Figure 5b displays the derivative thermogravimetric analysis (DTG) curves of all EP composite samples; the maximum mass loss rates of EP composites are lower relative to those of EP, indicating that the thermal stability of EP composites is improved. The mechanical properties of EP and its composites are further studied by DMA. Figure S2 presents the storage modulus and tan delta of EP and EP composites as a function of temperature. With the addition of 5 wt % fillers, the storage modulus of EP composites at 40 °C is higher than that of EP. In particular, the storage modulus of EP/5PA-ZIF67@ZIF8 increases from 2340 to 2503 MPa. The glass transition temperature of each sample decreases compared with that of pure EP.
Figure 5.
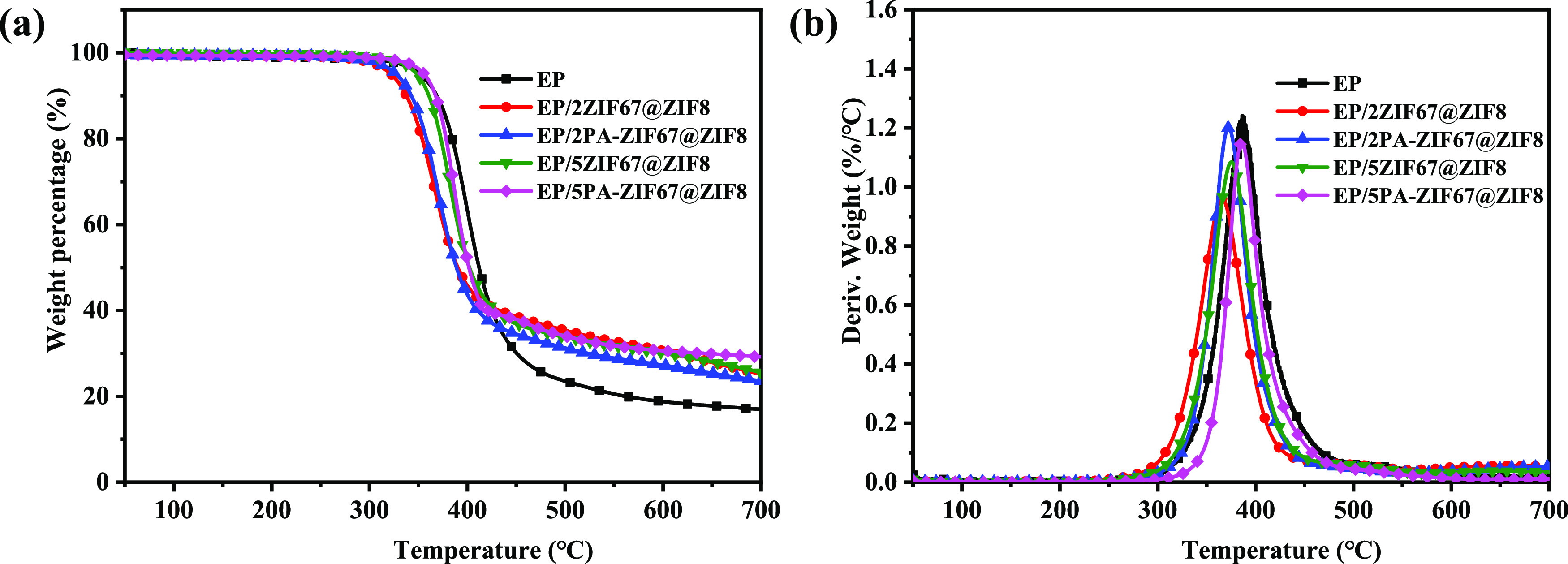
(a) TGA and (b) DTG curves of EP and its composites under a nitrogen atmosphere.
Table 1. TGA Data of EP Composite Samples.
| samples | T5% (°C) | Tmax (°C) | residue at 700 °C (%) |
|---|---|---|---|
| EP | 320 | 387 | 17.0 ± 0.8 |
| EP/2ZIF67@ZIF8 | 316 | 365 | 25.1 ± 0.7 |
| EP/2PA-ZIF67@ZIF8 | 336 | 369 | 24.8 ± 0.4 |
| EP/5ZIF67@ZIF8 | 319 | 377 | 26.1 ± 0.3 |
| EP/5PA-ZIF67@ZIF8 | 351 | 385 | 29.2 ± 1.5 |
3.4. Fire Performance of EP and Its Composites
The relative combustibility of EP and its composites is assessed by the limiting oxygen index (LOI) and UL-94 vertical test, and the relevant data are summarized in Table 2. The LOI value of EP is 25.4% and no rating (NR) in the vertical UL-94 test. With the introduction of 2 wt % of ZIF67@ZIF8 and PA-ZIF67@ZIF8 into EP, the LOI values increase to 28.1 and 27.7%, respectively. In particular, when the content of ZIF67@ZIF8 and PA-ZIF67@ZIF8 increases to 5 wt %, the LOI values of EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 enhance to 28.5 and 29.3%, respectively, and both samples pass the V-0 rating.
Table 2. Related Data of EP and Its Composites from UL-94, LOI, and CCT at 35 kW/m2.
| sample | UL-94 test | LOI (%) | PHRR (kW/m2) | THR (kW/m2) | TSP (m2) | PCOP (g/s) | av-EHC (MJ/K) | Char (wt %) |
|---|---|---|---|---|---|---|---|---|
| EP | NR | 25.4 ± 0.2 | 1133.0 ± 52 | 92.2 ± 2.0 | 20.6 ± 1.2 | 0.053 ± 0.002 | 27.5 ± 1.5 | 11.4 ± 0.5 |
| EP/2ZIF67@ ZIF8 | V-1 | 28.1 ± 0.1 | 958.3 ± 41 | 73.8 ± 1.8 | 16.7 ± 0.7 | 0.039 ± 0.002 | 26.2 ± 0.8 | 13.5 ± 1.2 |
| EP/2PA-ZIF67@ZIF8 | V-1 | 27.7 ± 0.3 | 1078.9 ± 31 | 80.2 ± 0.7 | 17.2 ± 0.8 | 0.038 ± 0.001 | 26.9 ± 1.2 | 13.8 ± 2.1 |
| EP/5ZIF67@ ZIF8 | V-0 | 28.5 ± 0.2 | 700.6 ± 41 | 67.8 ± 1.2 | 15.4 ± 0.5 | 0.032 ± 0.002 | 24.7 ± 2.0 | 22.9 ± 1.7 |
| EP/5PA-ZIF67@ZIF8 | V-0 | 29.3 ± 0.4 | 651.5 ± 37 | 61.8 ± 3.2 | 15.0 ± 0.7 | 0.029 ± 0.002 | 23.2 ± 1.9 | 25.4 ± 1.3 |
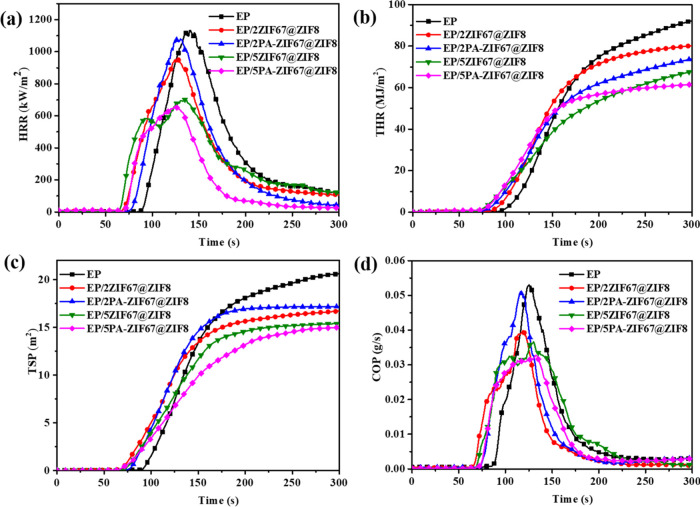
Based on LOI and UL-94 results, the fire behavior of EP and its composites is further studied by a cone calorimeter test at 35 kW/m2, and the detailed data are listed in Table 2. As shown in Figure 6a, EP burns fiercely and reaches a very high PHRR of 1133 kW m–2 in a short time, indicating that EP is highly flammable. When incorporating ZIF67@ZIF8 and PA-ZIF67@ZIF8, the PHRR values are reduced relative to pure EP. In addition, among all samples, EP/5PA-ZIF67@ZIF8 exhibits the lowest PHRR value, only 651.5 kW m–2, which is decreased by 42.2%, demonstrating that PA can improve the retardancy of EP composites compared with that of only 5 wt % ZIF67@ZIF8. However, the fire behavior of EP/2PA-ZIF67@ZIF8 is worse than that of EP/2ZIF67@ZIF8, which is probably because phytic acid can lead to a positive effect on the flame-retardant properties of EP composites when it raises to a certain extent. As a result, the EP/5PA-ZIF67@ZIF8 exhibits a better fire performance than that of EP/5ZIF67@ZIF8. Figure 6b shows the THR curves for EP and its composites; the trend of THR is similar to that of HRR. The THR values are reduced from 92.2 MJ/m2 of EP to 61.6 MJ/m2 of EP/5PA-ZIF67@ZIF8 (33.2%). As mentioned above, the early decomposition of PA-ZIF67@ZIF8 contributes to accelerating the formation of a stable char layer, which serves as a physical barrier to protect the underlying matrix, thus resulting in the decrease of PHRR and THR. As shown in Table 2, EP/5PA-ZIF67@ZIF8 shows a lower average effective heat of combustion (av-EHC). The reduction of the EHC means fuel dilution and lower combustion heat generated during the burning.
Figure 6.
(a) HRR, (b) TSP, (c) TSP, and (d) COP curves of EP and its composites.
Smoke and toxic fumes (e.g., CO) that are released are the other major causes of fatality in a real fire. Figure 6c,d displays the total smoke production (TSP) and carbon monoxide production (COP) curves of EP and its composites. As can be seen from Figure 6c, the TSP of EP reaches up to 20.6 m2/s, while that of EP/5PA-ZIF67@ZIF8 reduces by 27.2% relative to EP. The peak carbon monoxide production (PCOP) of EP/5PA-ZIF67@ZIF8 is decreased by 41.5% compared with that of EP. Total CO production (TCOP) curves of the EP and its composites are shown in Figure S3; the TCOP of EP reaches up to 22.6 g, while EP/5PA-ZIF67@ZIF8 reduces by 37.7% relative to EP. Additionally, the char residue rate of EP with the combustion time of 600 s is 11.4%, and the char residue rate of EP/5PA-ZIF67@ZIF8 is increased to 25.4%, which is ca. 2.3 times the weight of EP. This is mainly because of the phosphorous moieties and metal oxides generated in the combustion process of PA-ZIF67@ZIF8, which can promote the formation of residue in the condensed phase.30,33
3.5. Analysis of Char Residues
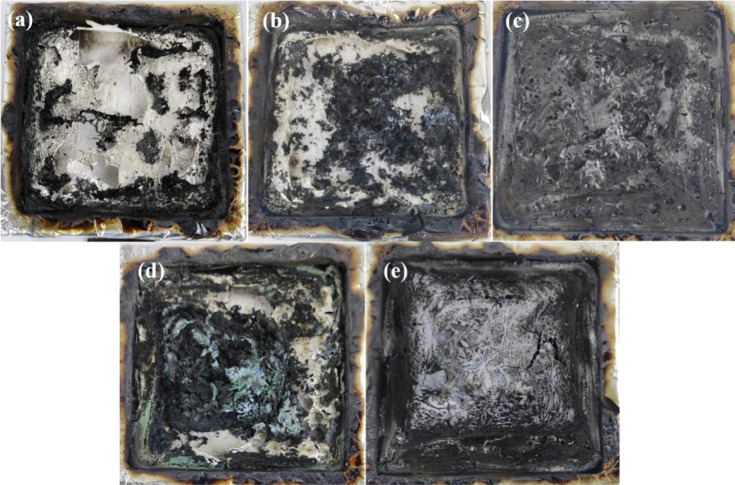
To explore the flame retardancy mechanism, the residual char generated after the cone calorimeter is investigated. The char residues are not the fire residues during burning, and the residue does not show the carbonaceous char properly. However, it can explain the relationship between the char layer and the fire retardancy mechanism to a certain extent. The digital photos of the char residues for EP and its composites are taken with a digital camera and shown in Figure 7. EP is almost burnt out, and enormous pores form on the char surface, which was unable to serve as a protective layer. With the addition of 2 wt % ZIF67@ZIF8 and 2 wt % PA-ZIF67@ZIF8, the char yields are gradually increased compared with EP. Meanwhile, the pores on the char surface are fewer and smaller. Although EP/2PA-ZIF67@ZIF8 shows more char residue than EP/2ZIF67@ZIF8, the char residue for EP/2PA-ZIF67@ZIF8 displays a relatively fluffy structure with larger holes on the surface, that is why EP/2PA-ZIF67@ZIF8 exhibits worse fire behavior. In comparison, EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 display a higher amount of char residues than EP/2ZIF67@ZIF8 and EP/2PA-ZIF67@ZIF8. Notably, EP/5PA-ZIF67@ZIF8 has the highest amount of char residue than that of the other samples; the char residue of EP/5PA-ZIF67@ZIF8 appears more continuous and shows a dense char layer than other samples.
Figure 7.
Digital images of the char residues for (a) EP, (b) EP/2ZIF67@ZIF8, (c) EP/2PA-ZIF67@ZIF8, (d) EP/5ZIF67@ZIF8, and (e) EP/5PA-ZIF67@ZIF8.
The structure of char residues after the cone calorimetry test is investigated by Raman spectra. As shown in Figure 8, two strong characteristic peaks at ca. 1365 and 1600 cm–1 are assigned to the D (vibration of amorphous carbon) and G (vibration of crystalline carbon) bands.34 Usually, the ratio of the band intensity of the D to G band (ID/IG) is used to estimate the graphitization degree of the char residues, and a lower value corresponds to a higher graphitization degree.35 From Figure 8a, the ID/IG value of EP is the highest (3.17), whereas the ID/IG values of EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 are lower than that of EP. Particularly, EP/5PA-ZIF67@ZIF8 exhibits the lowest ID/IG (2.35). It demonstrates that a more graphitized structure in char residue is formed during the combustion of EP/5PA-ZIF67@ZIF8, which can improve the insulation of fuel and heat transfer.
Figure 8.
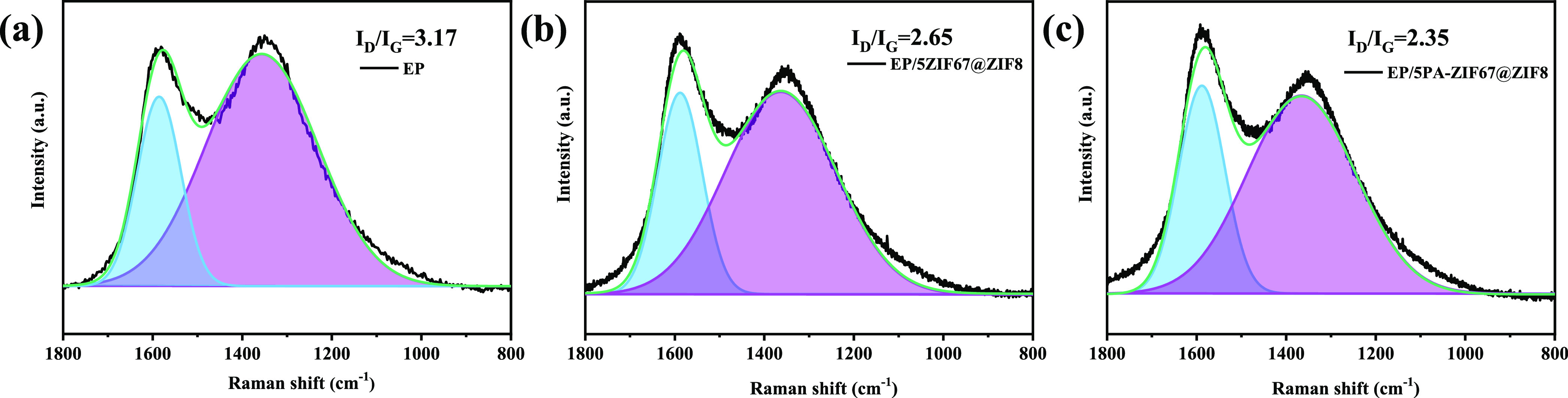
Raman spectra of char residues for (a) EP, (b) EP/5ZIF67@ZIF8, and (c) EP/5PA-ZIF67@ZIF8.
To further elucidate the formation of the char layer, the residual char for EP and its composite thermosets after cone calorimeter tests are examined by the XPS spectra and XRD spectra, as shown in Figures 9 and S2, respectively. The full XPS survey spectrum presents the existence of C, O, and N elements in the char residues for all of the samples. Besides these elements, the elements of Co and Zn are discovered in the char residues of EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8, whereas the P element only appears in the char layer of EP/5PA-ZIF67@ZIF8 due to the existence of PA in EP/5PA-ZIF67@ZIF8. The high-resolution XPS spectra of EP/5PA-ZIF67@ZIF8 are shown in Figure 9b–d. In the P 2p spectrum, the peaks at 133.8 and 135.4 eV correspond to P–O and P–O–Co/P–O–Zn, respectively.30Figure 9c shows the Co 2p spectrum; besides two satellite peaks of Co 2p3/2 (786.1 eV) and Co 2p1/2 (803.1 eV), two peaks appear at 781.1 and 797.1 eV, which are assigned to Co 2p3/2 and Co 2p1/2, respectively.36 In the Zn 2p spectrum, two major peaks at 1022.1 and 1045.4 eV are attributed to the Zn–O bond.13 These results demonstrate that Co3O4, ZnO, and phosphoric acid or polyphosphoric acid generated during the burning process of PA-ZIF67@ZIF8 can catalyze the formation of the char layer during burning. That is why the char layer of EP/5PA-ZIF67@ZIF8 is denser than other samples. XRD spectra of the char layer for EP/PA-ZIF67@ZIF8 are shown in S4, which can further prove the existence of Co3O4 and ZnO in the char layer.
Figure 9.
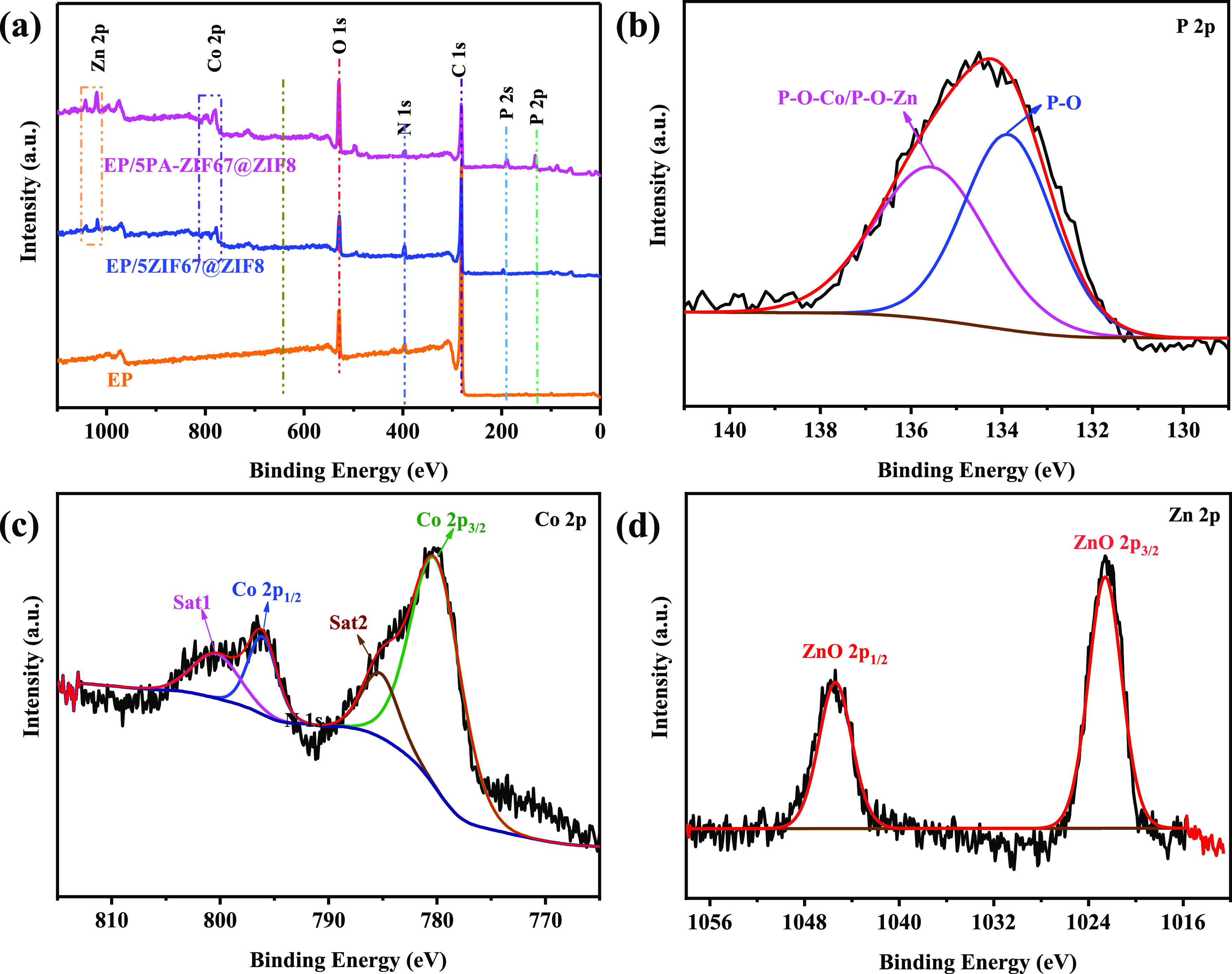
XPS spectra of the char residue for (a) EP, EP/5ZIF67@ZIF8 and EP/5PA-ZIF67@ZIF8 and high-resolution spectra of (b) P 2p, (c) Co 2p, and (d) Zn 2p in EP/5PA-ZIF67@ZIF8.
3.6. Analysis of Volatile Gases
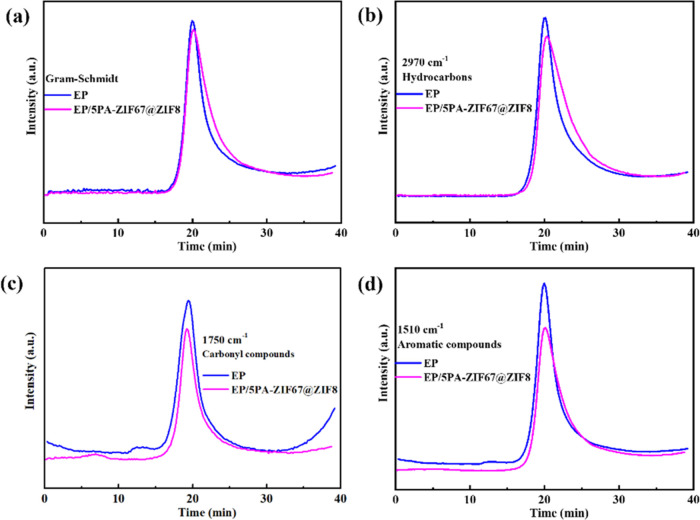
To study the influence of PA-ZIF67@ZIF8 on the pyrolysis gaseous products during the thermal decomposition process, TG-FTIR is performed to characterize the evolved gas products of EP and EP/5PA-ZIF67@ZIF8. The spectra at the maximum degradation rate of EP and EP/5PA-ZIF67@ZIF8 are presented in Figure 10. The characteristic peaks at 3650, 2971, 2360, 2180, 1730, and 1520 cm–1 are associated with −OH, aliphatic compound, CO2, CO, carbonyl compounds, and aromatic compounds, respectively. As shown in Figure 11a–d, the absorbance intensity of pyrolysis products for EP/5PA-ZIF67@ZIF8 is decreased in comparison with that of EP, including aliphatic compounds, carbonyl compounds, and aromatic compounds. In particular, aliphatic compounds, carbonyl compounds, and aromatic compounds are the fuel supplies for combustion. The absorbance intensity of these compounds decreased, demonstrating that the fuel supplied for burning decreased. These organic compounds remained in the condensed phase, protecting the underlying polymers. It demonstrates that the incorporation of PA-ZIF67@ZIF8 can reduce the yield of gaseous products and thus enhance the fire retardancy of the polymeric matrix.
Figure 10.
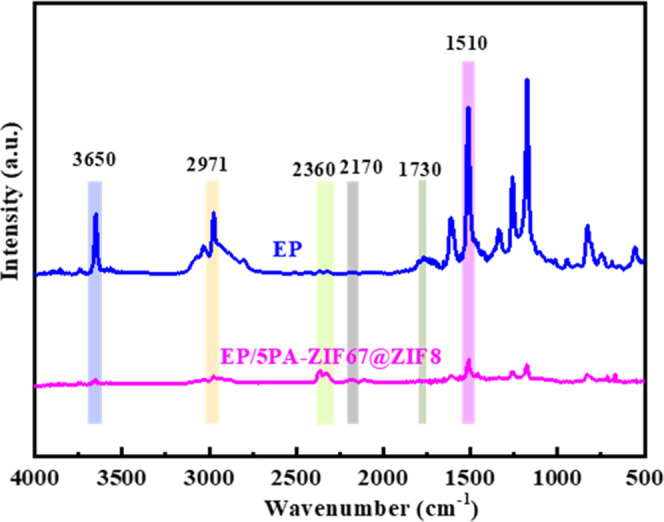
FTIR spectra of volatile gas emitted from EP and EP/5PA-ZIF67@ZIF8 at the maximum degradation rate.
Figure 11.
Intensity curves of (a) Gram–Schmidt, (b) hydrocarbons, (c) carbonyl compounds, and (d) aromatic compounds for EP and EP/5PA-ZIF67@ZIF8.
3.7. Flame Retardancy Mechanism
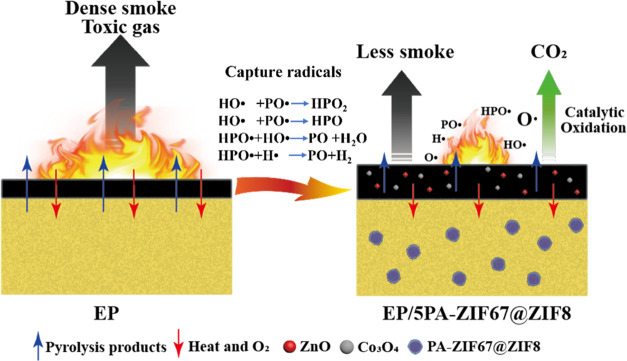
Based on the results of the study, the possible flame retardancy mechanism of PA-ZIF67@ZIF8 is proposed in Scheme 2. PA-ZIF67@ZIF8 generates phosphorous radicals (PO• and HPO•) to capture the highly active free radicals (H• and HO•) to interrupt the burning process, thus suppressing flame development in the gas phase.27,29,32 Meanwhile, phosphoric acid derivatives, ZnO, and Co3O4 are generated in the solid phase, produced by early-stage pyrolysis of EP/5PA-ZIF67@ZIF8, and promote dehydration and carbonization of the matrix to improve the yield and quality of residual carbon in the thermoset.27,33 The generated compact residual carbon can exert the flame-retardant effect by isolating the matrix from heat and oxygen.
Scheme 2. Proposed Fire Retardancy Mechanism of EP/5PA-ZIF67@ZIF8.
4. Conclusions
In this work, ZIF67@ZIF8-PA hybrids with core–shell structures are successfully synthesized. Then, ZIF67@ZIF8-PA hybrids as a flame retardant nanofiller are added to the EP matrix by a physical blending method. With the introduction of 5.0 wt % of ZIF67@ZIF8-PA into EP, the PHRR, TSP, and PCOP are dramatically decreased by 42.2, 27.2, and 41.5% compared with that of neat EP, respectively. Meanwhile, the LOI value is improved from 25.4 to 29.3%. This is mainly due to the catalytic charring performance of Co3O4, ZnO, and phosphorus-containing compounds generated in the burning process of EP/5PA-ZIF67@ZIF8. This work proposed halogen-free flame retardants to effectively reduce the fire hazard of EP.
Acknowledgments
This work is supported by the Aeronautical Science Foundation of China (No. 2018ZF53065), the Key Project of Shaanxi Provincial Natural Science Foundation (No. 2021JZ-09), and the National Undergraduate Training Program for Innovation and Entrepreneurship (No. 201910699113). The authors also thank the Analytical & Testing Center of NPU for SEM, XPS, and TEM testing.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01545.
Detailed characterization, TEM images of ZIF67@ZIF8 and PA-ZIF67@ZIF8 (Figure S1), storage modulus and tan delta of EP and its composites as a function of temperature (Figure S2), TCOP curves of EP and its nanocomposites (Figure S3), and XRD spectra of char residue of EP/5PA-ZIF67@ZIF8 (Figure S4) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu S.; Chevali V. S.; Xu Z.; Hui D.; Wang H. A review of extending performance of epoxy resins using carbon nanomaterials. Composites, Part B 2018, 136, 197–214. 10.1016/j.compositesb.2017.08.020. [DOI] [Google Scholar]
- Zhou Y.; Feng J.; Peng H.; Qu H. Q.; Hao J. W. Catalytic pyrolysis and flame retardancy of epoxy resins with solid acid boron phosphate. Polym. Degrad. Stab. 2014, 110, 395–404. 10.1016/j.polymdegradstab.2014.10.009. [DOI] [Google Scholar]
- Müller P.; Morys M.; Sut A.; Jager C.; Illerhaus B.; Schartel B. Melamine poly (zinc phosphate) as flame retardant in epoxy resin: decomposition pathways, molecular mechanisms and morphology of fire residues. Polym. Degrad. Stab. 2016, 130, 307–319. 10.1016/j.polymdegradstab.2016.06.023. [DOI] [Google Scholar]
- Hu X.; Yang H.; Jiang Y.; He H.; Liu H.; Huang H.; Wan C. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793 10.1016/j.jhazmat.2019.120793. [DOI] [PubMed] [Google Scholar]
- Tran P.; Nguyen Q. T.; Lau K. T. Fire performance of polymer-based composites for maritime infrastructure. Composites, Part B 2018, 155, 31–48. 10.1016/j.compositesb.2018.06.037. [DOI] [Google Scholar]
- Wang X.; Xing W. Y.; Feng X. M.; Yu B.; Lu H. D.; Song L.; Hu Y. The effect of metal oxide decorated graphene hybrids on the improved thermal and the reduced smoke toxicity in epoxy resins. Chem. Eng. J. 2014, 250, 214–220. 10.1016/j.cej.2014.01.106. [DOI] [Google Scholar]
- Wang H. W.; Qiao H.; Guo J.; Sun J.; Li H. F.; Zhang S.; Gu X. Y. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Composites, Part B 2020, 182, 107498 10.1016/j.compositesb.2019.107498. [DOI] [Google Scholar]
- Zhou H. C.; Long J. R.; Yaghi O. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Hou Y. B.; Hu W. Z.; Gui Z.; Hu Y. Preparation of metal-organic frameworks and their application as flame retardants for polystyrene. Ind. Eng. Chem. Res. 2017, 56, 2036–2045. 10.1021/acs.iecr.6b04920. [DOI] [Google Scholar]
- Sai T.; Ran S. Y.; Guo Z. H.; Fang Z. P. A Zr-based metal organic frameworks towards improving fire safety and thermal stability of polycarbonate. Composites, Part B 2019, 176, 107198 10.1016/j.compositesb.2019.107198. [DOI] [Google Scholar]
- Guo W. W.; Nie S. B.; Kalali E. N.; Wang X.; Wang W.; Cai W.; Song L.; Hu Y. Construction of SiO2@UiO-66 core-shell microarchitectures through covalent linkage as flame retardant and smoke suppressant for epoxy resins. Composites, Part B 2019, 176, 107261 10.1016/j.compositesb.2019.107261. [DOI] [Google Scholar]
- Pan Y. T.; Zhang Z.; Yang R. The rise of MOFs and their derivatives for flame retardant polymeric materials: A critical review. Composites, Part B 2020, 199, 108265 10.1016/j.compositesb.2020.108265. [DOI] [Google Scholar]
- Zhang M.; Ding X. Q.; Zhan Y. X.; Wang Y. T.; Wang X. L. Improving the flame retardancy of poly (lactic acid) using an efficient ternary hybrid flame retardant by dual modification of graphene oxide with phenylphosphinic acid and nano MOFs. J. Hazard. Mater. 2020, 384, 121260 10.1016/j.jhazmat.2019.121260. [DOI] [PubMed] [Google Scholar]
- Jing Z.; Zhi L.; Qi X. L.; Zhang W.; Wang D.-Y. Size tailored bimetallic metal-organic framework (MOF) on graphene oxide with sandwich-like structure as functional nano-hybrids for improving fire safety of epoxy. Composites, Part B 2020, 188, 107881 10.1016/j.compositesb.2020.107881. [DOI] [Google Scholar]
- Wang X. G.; Wang S. H.; Wang W. J.; Li H. F.; Liu X. D.; Gu X. Y.; Bourbigot S.; Wang Z. W.; Wang Z.; Sun J.; Zhang S. The flammability and mechanical properties of poly(lactic acid) composites containing Ni-MOF nanosheets with polyhydroxy groups. Composites, Part B 2020, 183, 107568 10.1016/j.compositesb.2019.107568. [DOI] [Google Scholar]
- Xu W. Z.; Wang X. L.; Wu Y.; Li W.; Chen C. Y. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2019, 363, 138–151. 10.1016/j.jhazmat.2018.09.086. [DOI] [PubMed] [Google Scholar]
- Li A. J.; Xu W. Z.; Chen R.; Liu Y. C.; Li W. Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin. Composites, Part A 2018, 112, 558–571. 10.1016/j.compositesa.2018.07.001. [DOI] [Google Scholar]
- Pan Y. T.; Wan J. T.; Zhao X. L.; Li C.; Wang D. Y. Interfacial growth of MOF-derived layered double hydroxide nanosheets on graphene slab towards fabrication of multifunctional epoxy nanocomposites. Chem. Eng. J. 2017, 330, 1222–1231. 10.1016/j.cej.2017.08.059. [DOI] [Google Scholar]
- Zhang J.; Li Z.; Qi X. L.; Wang D. Y. Recent progress on metal–organic framework and its derivatives as novel fire retardants to polymeric materials. Nano-Micro Lett. 2020, 12, 173 10.1007/s40820-020-00497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B. L.; Xu W. Z.; Wang G. S.; Liu L. C.; Xu J. Zeolitic imidazolate frameworks- 8 modified graphene as a green flame retardant for reducing the fire risk of epoxy resin. Polym. Adv. Technol. 2018, 29, 1733–1743. 10.1002/pat.4278. [DOI] [Google Scholar]
- Li A. J.; Xu W. Z.; Chen R.; Liu Y. C.; Li W. Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin. Composites, Part A 2018, 112, 558–571. 10.1016/j.compositesa.2018.07.001. [DOI] [Google Scholar]
- Hou Y. B.; Qiu S. L.; Hu Y.; Kundu C.; Zhou G.; Hu W. Z. Construction of bimetallic ZIF-derived Co-Ni LDHs on the surfaces of GO or CNTs with a recyclable method: toward reduced toxicity of gaseous thermal decomposition products of unsaturated polyester resin. ACS Appl. Mater. Interfaces 2018, 10, 18359–18371. 10.1021/acsami.8b04340. [DOI] [PubMed] [Google Scholar]
- Zhang Z. D.; Li X. L.; Yuan Y. S.; Pan Y. T.; Wang D. Y.; Yang R. J. Confined dispersion of zinc Hydroxystannate nanoparticles into layered bimetallic hydroxide nanocapsules and its application in flame-retardant epoxy nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 40951–40960. 10.1021/acsami.9b15393. [DOI] [PubMed] [Google Scholar]
- Zhao J. J.; Quan X.; Chen S.; Liu Y. M.; Yu H. T. Cobalt nanoparticles encapsulated in porous carbons derived from core-Shell ZIF67@ZIF8 as efficient electrocatalysts for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 28685–28694. 10.1021/acsami.7b10138. [DOI] [PubMed] [Google Scholar]
- Zhao H. Y.; Wang Y.; Zhao L. Magnetic nanocomposites derived from hollow ZIF-67 and core-shell ZIF-67@ZIF-8: synthesis, properties, and adsorption of rhodamine B. Eur. J. Inorg. Chem. 2017, 2017, 4110–4116. 10.1002/ejic.201700587. [DOI] [Google Scholar]
- Huo S. Q.; Wang J.; Yang S.; Wang J. P.; Zhang B.; Zhang B.; Chen X.; Tang Y. S. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene. Polym. Degrad. Stab. 2016, 131, 106–113. 10.1016/j.polymdegradstab.2016.07.013. [DOI] [Google Scholar]
- Chi Z. Y.; Guo Z. W.; Xu Z.; Zhang M. J.; Li M.; Shang L.; Ao Y. H. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151 10.1016/j.polymdegradstab.2020.109151. [DOI] [Google Scholar]
- Qu Z. C.; Kun W.; Meng W. H.; Nan B. F.; Hu Z. R.; Xu C. -A.; Tan Z. Y.; Zhang Q.; Meng H. F.; Shi J. Surface coordination of black phosphorene for excellent stability, flame retardancy and thermal conductivity in epoxy resin. Chem. Eng. J. 2020, 397, 125416 10.1016/j.cej.2020.125416. [DOI] [Google Scholar]
- Qiu S. L.; Ma C.; Wang X.; Zhou X.; Feng X. M.; Yuen R. K. K.; Hu Y. Melamine-containing polyphosphazene wrapped ammonium polyphosphate: A novel multifunctional organic-inorganic hybrid flame retardant. J. Hazard. Mater. 2018, 344, 839–848. 10.1016/j.jhazmat.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Wang J. L.; Zhang D. C.; Zhang Y.; Cai W.; Yao C. X.; Hu Y.; Hu W. Z. Construction of multifunctional boron nitride nanosheet towards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Hazard. Mater. 2019, 362, 482–494. 10.1016/j.jhazmat.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Qiu S. L.; Hou Y. B.; Xing W. Y.; Ma C.; Zhou X.; Liu L. X.; Kan Y. C.; Yuen R. K. K.; Hu Y. Self-assembled super molecular aggregate supported on boron nitride nanoplatelets for flame retardant and friction application. Chem. Eng. J. 2018, 349, 223–234. 10.1016/j.cej.2018.05.053. [DOI] [Google Scholar]
- Zhang J.; Li Z.; Zhang L.; Yang Y. X.; Wang D. Y. Green synthesis of biomass phytic acid-functionalized UiO-66-NH2 hierarchical hybrids toward fire safety of epoxy resin. ACS Sustainable Chem. Eng. 2020, 8, 994–1003. 10.1021/acssuschemeng.9b05658. [DOI] [Google Scholar]
- Nabipour H.; Wang X.; Song L.; Hu Y. Metal organic frameworks for flame retardants polymers application: a critical review. Composites, Part A 2020, 139, 106113 10.1016/j.compositesa.2020.106113. [DOI] [Google Scholar]
- Sai T.; Ran S. Y.; Guo Z. H.; Yan H.; Zhang Y.; Song P. G.; Zhang T.; Wang H.; Fang Z. P. Deposition growth of Zr-based MOFs on cerium phenylphosphonate lamella towards enhanced thermal stability and fire safety of polycarbonate. Composites, Part B 2020, 197, 108064 10.1016/j.compositesb.2020.108064. [DOI] [Google Scholar]
- Xiong Z. Q.; Zhang Y.; Du X. Y.; Song P. G.; Fang Z. P. Green and scalable fabrication of core–shell biobased flame retardants for reducing flammability of polylactic acid. ACS Sustainable Chem. Eng. 2019, 7, 8954–8963. 10.1021/acssuschemeng.9b01016. [DOI] [Google Scholar]
- Xu W. Z.; Wang X. L.; Liu Y. C.; Li W.; Chen R. Improving fire safety of epoxy filled with graphene hybrid incorporated with zeolitic imidazolate framework/layered double hydroxide. Polym. Degrad. Stab. 2018, 154, 27–36. 10.1016/j.polymdegradstab.2018.05.022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.