Abstract
Transdermal drug delivery systems have been successful for decades. Now these devices can be further scaled down, and their applications have been extended to wide selections of cargo, ranging from natural molecules (e.g., insulin and glucose) to bioengineered molecules (e.g., nanoparticles and vaccines). Some emerging nanopatches show promise for precise single-cell gene transfection in vivo and have advantages over conventional tools in terms of delivery efficiency, safety, and controllability of delivered dose. In this review, we discuss recent technical advances in wearable micro/nano devices with unique capabilities or potential for single-cell biosensing and transfection in the skin or other organs, and we suggest future directions for these fields.
Keywords: Wearable Electronics, Biosensors, Single Cell, Drug Delivery, Gene Transfection
Introduction
The field of wearable electronics and devices has advanced quickly over the past decade, with a focus on sensing physical and chemical properties and delivering stimulation/substances via direct contact with the skin [1–4]. With the integration of nanomaterials and rapid advancement of fabrication technologies, new devices functioning at the cellular level will lead to improved efficiency, safety, and non-invasiveness [5]. Miniaturizing these new devices to the scale comparable to a single cell could significantly increase the precision in cellular diagnosis and treatment that could not be achieved within bulk environments [6–8]. By miniaturizing the sensitive module to the submicron or nanoscale, a wearable biosensor interacting with skin or organ enables the capture of targeting molecules from single cells, which results in significantly increased sensitivity, shorter response times, and precision for spatial-temporal measurement. Skin-patch devices with micro/nano features accurately guide external forces (e.g. penetration, electroporation, etc.) onto the cell membrane while actively injecting cargo into cells. Wearable healthcare devices have been widely discussed in the past few years [9–13].
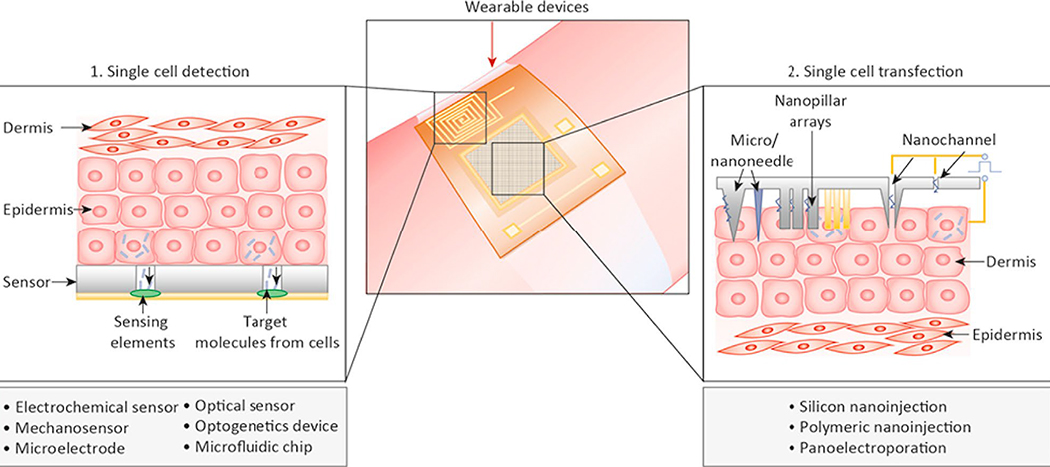
This paper primarily reviews the latest developments of unique wearable biosensors and delivery nanodevices aiming for single-cell level interaction. These developments include device designs, fabrication techniques, working principles and comparisons between these wearable devices and conventional devices. Specifically, this review is organized by first reviewing the wearable devices for single-cell sensing (Section 2), then reviewing the wearable devices for single-cell transfection (Section 3), as summarized in Figure 1, and finally offering concluding remarks and future perspectives (Section 4) in brief.
Figure 1: Overview of wearable single cell sensors and single cell transfection devices.
Wearable Devices for Single-cell Sensing
In this section, we describe various parameters from the body that can be measured by soft and wearable micro/nanosensors, from the organ and tissue levels down to the single-cell scale. Some emerging devices based on novel nanomaterials or nanofabrication have shown potential for wearable single cell detection in scenarios including cancer cell screening, cardiomyocyte detection, and optogenetics.
Current Flexible and Wearable Sensors
Wearable sensors with mechanical flexibility provide advantages for measuring physiological parameters and monitoring therapeutic responses in patients. These wearable sensors are likely to offer excellent sensitivities for detecting biomolecules that are low-abundant or have a short half-life in the human body [14]. Through exocytosis, cells release numerous molecules, including hormones, peptides, and metabolites that mediate cell-cell interactions. These molecules frequently indicate cellular states and provide clinicians with ample information about patients’ health conditions [14, 15]. Many flexible and wearable sensors have been developed to enable the detection and measurement of biomarkers at the interface of the skin and internal organs. For instance, the content of water, glucose, inorganic ions, lactic acid, and urea in sweat can be detected using skin-patch sensors, as illustrated in Figure 2a [2]. Certain sensors can detect vital signals generated by the nervous system, blood vessels, and muscle tissues under a skin barrier (Figure 2b, c)[16]. These sensors have been used for monitoring the normality of cardiovascular functions, brain activities, rehabilitation, wound healing, sleep conditions, blood pressure, and metabolism [16–30]. In addition to those sensors, integrated wearable systems have been developed; these systems can communicate wirelessly via near-field communication (NFC), utilize optoelectronics for simultaneous monitoring of vascular disease and UV exposure, and/or perform multiplexed sweat analysis of various biomarkers [19, 31]. Devices that can offer both sensing and therapeutic functions are also of great interest [32, 33]. However, most of these sensors and systems barely have the capability to enable detection and measurement with single-cell resolution.
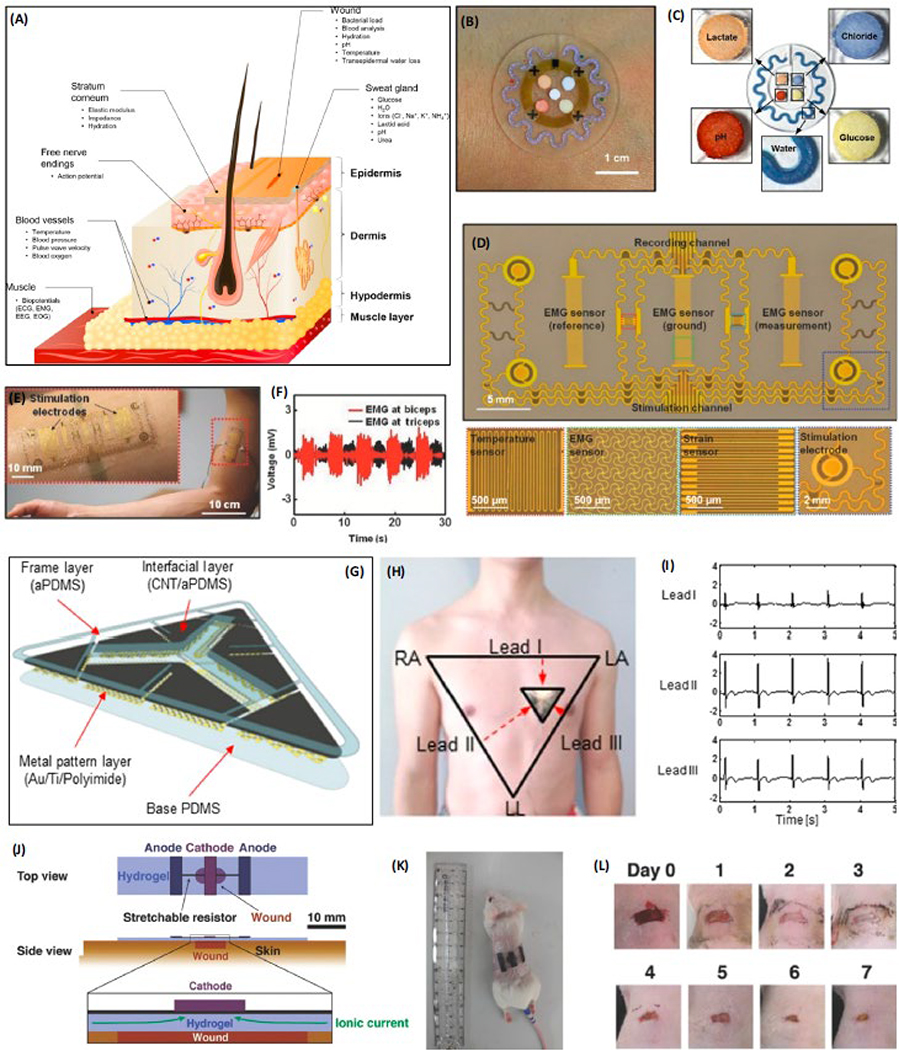
Figure 2: Wearable biosensors on the skin.
(a) Representative signals measured from the surface and below the skin. Reproduced with the permission from Ref. [2]. (b) A wearable microfluidic device for monitoring sweat. (c) Detection of lactate, chloride, glucose, water, and pH with microfluidic sensor using colorimetric reservoirs. Reproduced with the permission from Ref. [19]. (d) Multifunctional device for prosthetic control showing color coded EMG, temperature, strain sensors, and stimulation electrodes [34]. (e), (f) EMG activity at the biceps and triceps, corresponds to extension and flexion of robotic arm. (g) ECG electrodes based on CNT/aPDMS electrode structure. (h) Electrode on the chest of a subject showing a conventional method of ECG measurement. (i) Signals recorded from Lead I, II, and III with the ECG electrodes. Reproduced with the permission from Ref. [35]. (j) Schematic of the wearable wound healing device. (k) Device on the skin of living mouse. (l) Wound healing over 7 days with the application of the device. Reproduced with the permission from Ref. [36].
For amputees, the nerve endings at the remaining portion of an amputated limb continue to be electrically active and are often used as the source of signals to control robotic actuators. Typically, electrode patches are placed on muscle and skin sites at which the nerves can be reinnervated [34]. However, conventional electrodes have large dimensions, which compromise their function during simultaneous recording and stimulation and hinder their capability to detect at the single-cell level. Xu and co-workers recently developed a multifunctional epidermal sensor (Figure 2d) for recording electromyogram (EMG) signals from muscles while stimulating them [35]. The device was fabricated using a lithographic process in order to achieve an appropriate dimension for the stimulation of biceps and triceps muscles. Although this device can stimulate flexor/extensor muscle groups in a human subject to operate a gripper (Figure 2e, f), the electrical stimulation induced by this device may not be specifically directed to a single cell. In addition, the EMG sensor, which is in a size of 24 mm2, was too large to specifically detect the response of a single cell to the stimulation, and instead, detects signals from a population of cells. In another study, wearable devices also demonstrate the ease for daily monitoring of cardiac functions of patients with arrhythmias and atrial fibrillation [36]. For instance, a carbon nanotube (CNT)- and adhesive polydimethylsiloxane (PDMS)-based sensor for electrocardiogram (ECG) measurement was reported (Figure 2g, h). This sensor functions wirelessly while permitting repeated adhesion and normal operation under water (Figure 2i) [36]. Yet, the electrodes are, again, too large to detect signals at the single-cell resolution. In both of the aforementioned studies, signals are acquired from the surface of the skin, which primarily contains epithelial cells. For the cells at the skin level, it is preferable for the sensor to be designed in a low-modulus format for compliant interfacing. Although some existing wearable technologies lack the specifications required to sense at the single-cell level, they have the potential to realize this capability.
The skin forms a physical barrier between internal organs and the environment. It has many important functions in maintaining the normal physiology of the human body. Thus, proper wound healing is crucial for restoring the integrity of the skin when it is damaged. The quantitative measurement of wound healing often requires invasive procedures. To overcome this challenge, Hattori and coworkers developed a wearable device that can be used to monitor skin wound healing (Figure 2j) [37]. This device aimed to discriminate the wounded and normal skin from human subjects based on the temperature and thermal conductivities. Results showed increased temperature and thermal conductivity near the wounded and normal skin of mouse. Moreover, stimulating the wounded mouse skin with stretchable electrodes fabricated on a hydrogel substrate remarkably accelerated the healing (Figures 2k, l). This technology showcases the potential for single cell therapy from a wearable electronic device as the stimulation mobilizes many individual epithelial cells and fibroblasts to close the wound [38]. As the relevant theories and technologies continue to advance, more devices capable of sensing biomolecules and/or offering therapy at single-cell resolution could be developed to improve disease diagnosis and treatment.
Wearable sensing devices at single-cell resolution
The dimensions of a sensor or sensing elements primarily determine if it is able to perform detection at the single-cell level [15]. By positioning the sensing element of a device in a submicron to micron distance away from individual cells, molecules released by cells can be effectively captured by a sensor positioned in a limited extracellular volume, which essentially allows for precise measurement of cells at single-cell resolution [15]. This tiny interface is analogous to the synaptic cleft. By miniaturizing the size of the biosensing element to the size of a cell (10–100 μm in diameter, depending on the cell type), a greater surface area of the sensor can be in contact with a cell [14]. The enhanced contact between the sensor and cells results in an increased signal to noise ratio (SNR) and is critical for determining the limits of detection and response time for the sensor [15]. Numerous investigations of single-cell sensors are ongoing, some of which are summarized in Table 1. The existing single-cell sensors can be improved for 1) higher sensitivity (e.g. ultra-low detection limit), 2) high throughput (e.g. array of sensors), and 3) better mechanical compatibility with the tissues [14, 15, 39]. When the devices are placed on body, these benefits will presumably maintain the reliability and prolong the working time of the devices. For example, implantable electrodes with improved mechanical compatibilities with surrounding tissues could effectively reduce the immune responses for mechanosensing individual glial cells [7, 8, 39].
Table 1.
Single cell sensing devices
| Sensing/Stimulation Material | Device features and capabilities | Cell Type | Observed Property | Results | Reference |
|---|---|---|---|---|---|
| Au | CMOS device fabricated on Si Wafer for ECG detection with Au electrodes and optical detection of cardiomyocytes using photodiodes | Rat cardiomyocytes | Contraction and relaxation of cardiomyocytes | Real-time action potential and light intensity detection from cardiomyocytes | [40] |
| Au | Ion-sensitive field effect transistor (ISFET) fabricated on glass with gate electrode dimensions on the micron scale for cellular adhesion impedance sensing | Human lung adenocarcinoma epithelial cells and Human Embryonic Kidney cells | Impedance of cells attached to the substrate of the ISFET | Impedance spectra and membrane capacitance of single cells adhered to the substrate were obtained | [41] |
| Au | Optofluidic system comprised of an Au nanohole array on for detecting cytokines and pneumatic valves for controlling the flow of cells through an isolated microchamber | EL4 lymphoma cells | Label-free IL-2 secretion detection | Captured IL-2 cytokine secretion to show the real-time functional state of single cells | [42] |
| Au | Au nanodisk electrodes dip coated in Nafion for detecting neurotransmitters inside single cells | PC12 cells | Concentration of dopamine | Nanodisks can detect dopamine inside the vesicles of chemically stimulated cells | [43] |
| Carbon fiber | Carbon-fiber microelectrodes in the form of conical nanotips for detecting catecholamine neurotransmitters | PC12 cells | Concentration of catecholamines | Nanotips can detect the concentration of catecholamine neurotransmitters inside the vesicles | [44] |
| Fe-Porphyrin functionalized reduced graphene oxide | Nitric oxide (NO) monitoring using a field effect transistor (FET) fabricated on Si wafer | Human umbilical vein endothelial cells (HUVECs) | Concentration of NO | Monitoring of NO in cultured HUVECs stimulated with L-Arg can be performed in real-time | [45] |
| High-temperature reduced graphene oxide | Optical flow cytometer utilizing a laser and PDMS microfluidic chip | Jurkat (leukemia) cells | Identification of cancer cells | Successful detection of a few cancer cells amongst healthy cells | [46] |
| InP nanowires (NWs) | InP NW array on InP substrate for ex vivo force measurements of single bacterial cells | X. fastidiosa cells | Horizontal and vertical direction force mapping of attached cells on NWs | Increased cell adhesion strength present on surfaces coated with the adhesin XadA1 | [47] |
| Polypyrrole (PPy) | FET based on carbon nanotube electrodes and PPy as the channel material functionalized with various biological receptor molecules | Melanocytes and cardiomyocytes | pH changes from melanocytes and adenosine triphosphate (ATP) concentration for cardiomyocytes | Drain-source current is controlled by the pH value and hexokinase modified FET sensors successfully detect ATP | [48] |
The substrate material is another important factor to consider for building single cell sensors. Conventionally, the sensitive modules are functionalized on rigid substrates that are not comfortable to wear [45]. However, it is possible to adapt the sensing modalities on soft flexible substrates to improve their wearability [49]. Xie and co-workers fabricated a field-effect transistor (FET) based on a composite of reduced graphene oxide (rGO) and a metalloporphyrin (Fe (III) meso-tetra(4-carboxyphenyl) porphyrin, FeTCP), as illustrated in Figure 3a [45]. This composite material is capable of detecting nitric oxide (NO), an important neurotransmitter, with the detection limit as low as 1 pM in phosphate-buffered saline (PBS). The response time was found to be close to 500 ms, sufficient for monitoring NO released by growing single human umbilical vein endothelial cells on the FET, as shown in Figure 3b [45]. Further modification of the sensitive modules onto soft substrates would allow these single-cell sensors to be interfaced with internal organs and skin [50].
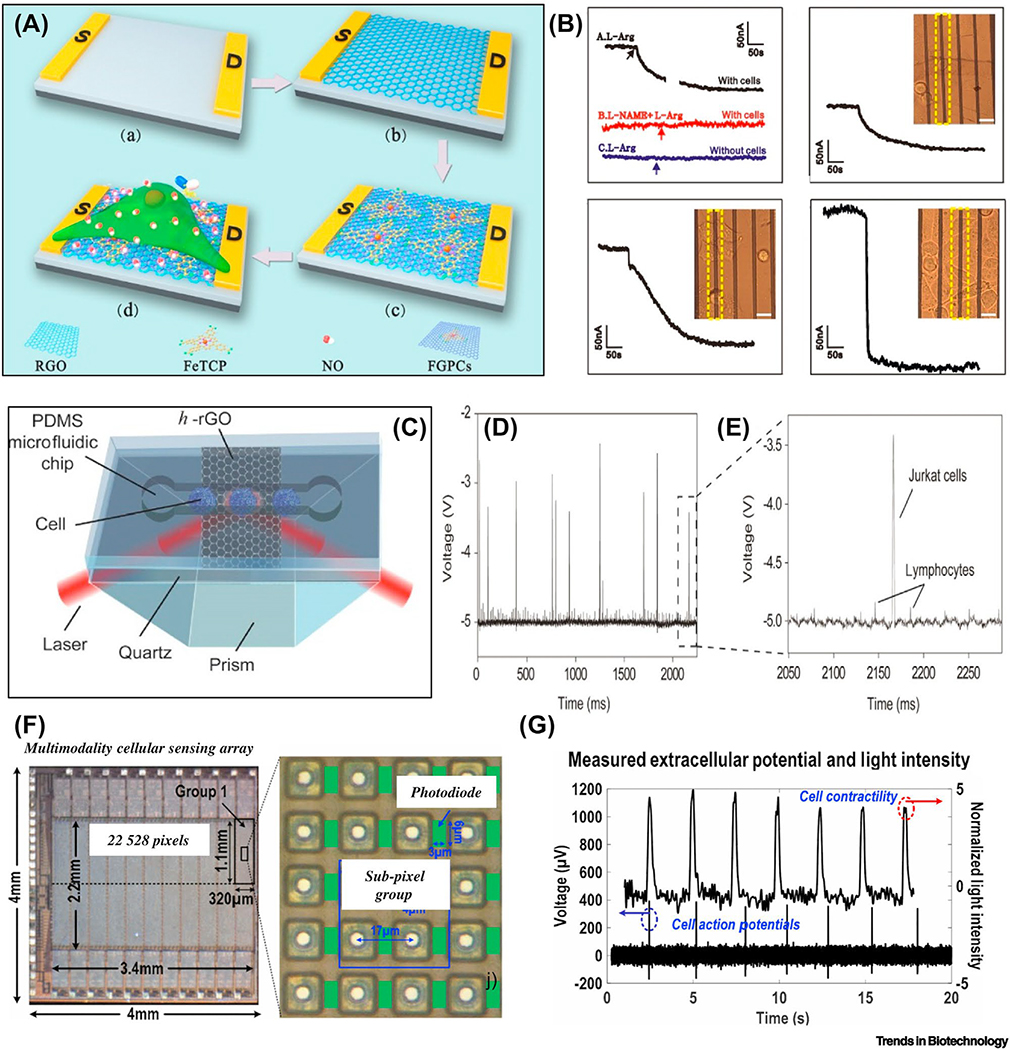
Figure 3: Single-cell sensors in wearable format.
(a) Diagram of rGO/FeTCPs composite FET biosensor for nitric oxide monitoring. (b) Signals of the FET under different conditions with detection range from single cell, three cells, and five cells. Reproduced with the permission from Ref. [38]. (c) Flow sensing device for single cells based on h-rGO layer between quartz and PDMS microfluidic chip. (d) Single cell detection using the flow sensing device. (e) Voltage difference between detected Jurkat (leukemia) cell and normal lymphocyte. Reproduced with the permission from Ref. [40]. (f) Multimodality sensing array for potential recording and optical detection. (g) Measurement of light intensity and action potentials in real-time. Reproduced with the permission from Ref. [41].
So far, wearable devices showing clear proof of single-cell sensing are limited. However, the integration of multiple sensors on a wearable device has shown the feasibility for sensing of single cells or a few cells, as evidenced in diagnosis of cancer cells, monitoring the metabolism of cardiomyocytes, and optogenetics [46]. Beside the transistor mentioned above, other techniques including microfluidic chips and multimodal sensors could also provide promising detection limits, specificity, and a breadth of information. For example, on-chip flow cytometry analysis has been frequently applied for ‘sensing’ cancer cells from normal cells [46]. Xing and co-workers developed an ultrasensitive high-temperature, reduced graphene oxide (h-rGO) optical sensor for the detection of cancerous cells at single-cell resolution based on a microfluidic chip (Figure 3c) [46]. A flow-sensing setup consisted of h-rGO sandwiched between a PDMS chip and a quartz layer on top of a prism. The variation of the refractive index of the flowing cells causes changes in the light reflected from the h-rGO layer. As a proof-of-concept application, Jurkat cells (a leukemia cell line) have been successfully distinguished from normal lymphocytes due to a voltage change on the sensor (Figures 3d, e) [46]. Miniaturizing these optical sensors on flexible and stretchable substrates is likely to enable implantable microdevices for real-time detection of hematopoietic malignancies in the body. A 22k-pixel sensing array was reported for both action potential recording and optical detection of individual cardiomyocytes, as shown in Figure 3f [40]. The pixels in the sensing array consist of transmission gate switches, a gold-plated electrode, a photodiode, and a buffer for the photodiode. Two sets of 2×2 groups of pixels were used to record the extracellular potential of the cells. The optical detection of cell activities (contraction and relaxation) and the recording of action potentials in each cardiomyocyte are simultaneously achieved on one device as shown in Figure 3g [40]. Besides, wearable devices for optogenetics also provide precise manipulation (i.e. activation/inhibition) and sensing of single neurons [51]. Park and co-workers developed a soft and stretchable microdevice for wireless optogenetic intervention [52]. The device is composed of two elastomeric substrates that sandwich a stretchable antenna, capacitors, Schottky diodes, a LED, and an inductor. They showed efficient cellular stimulation at the sciatic nerve and cause the rats to exhibit reactionary behavior when pain pathways were activated. These devices have shown the potential to realize multimodal sensors for simultaneous single-cell stimulation and action potential sensing. In practice, they have inspired wearable products for vision restoration with single-cell resolution based on optogenetics [53]. The single-cell sensors covered in this review have varied device architectures, operate with different sensing mechanisms, and interface with multiple cell types. Thus, the sensing platforms from single cells varies from application to application.
Wearable Devices for Single-cell Transfection
The use of skin and/or organ drug delivery devices is often limited to small molecules that can diffuse or be actively transported across the cell membrane, due to a great deal of challenges associated with macromolecules, including peptides and nucleic acids, to effectively penetrate the cell membrane to enter cells in both in vitro and in vivo settings. In the past few years, multiple advances have been made with the micro/nano devices that enable in vivo transfection of macromolecules at the single-cell level. Each one of them generally belongs to one of two types of designs that perform either electroporation-based transfection or micro/nanoneedle-based transfection via physical penetration.
In transdermal delivery or cell transfection, microneedles and electroporation have been applied for decades [3]. However, in vivo techniques still face challenges for delivering DNA plasmids among other macromolecular cargo with high efficiency and specificity. Single-cell electroporation has been studied for many years in in vitro settings [54, 55]. Recently, novel designs and fabrication techniques have been attempted to enable single-cell transfection in vivo. For instance, a nanochannel array-based, single-cell electroporation system was recently fabricated on a skin-patchable substrate [56]. A high efficiency of transfecting biomolecules into animal cells in the skin was achieved with minimum invasiveness. This method provides a sharp contrast to microneedle devices by physically penetration. To date, the nanochannel and micro-/nano-needle based single cell electroporation represents the closest technique for wearable single cell delivery, although further development is needed to bring integrated chips with drug reservoirs, microfluidic transportation and electronics.
Wearable electroporation for single-cell transfection
The intrinsic heterogeneity existed in virtually any given cell population is a well-known confounding factor with important biological meanings. To properly address this variable and take the cellular heterogeneity into account in research, many platforms that allow biological samples to be analyzed at the single-cell level have been developed and playing increasingly significant roles in the studies of genomics, transcriptomics, epigenomics and proteomics relevant to different fields [57–59]. The heterogeneity of cells in a pathological lesion (e.g. a tumor) is likely to determine the therapeutic outcome and long-term prognosis in patients. While having analysis at the single-cell level provides us with unprecedented capacity to understand biology and possibly disease progression in patients, it is also important to establish approaches that enable precise manipulation on specific cells to target rare-cell populations in organs or pathological lesions for therapeutic purposes [60]. Many technological advances have enabled genomic manipulation at single-cell resolution and with a high throughput [61–63]. The development and use of single-cell transfection techniques allows us to quantitatively deliver macromolecules, such as DNA or RNA, into individual cells [64, 65]. By temporarily cracking the cell membrane, cell permeability to macromolecules is increased [66]. This permeabilization can be achieved by several means including viral vectors, chemical vectors and physical methods [3, 67]. In in vitro conditions, multiple physical approaches, including cell squeezing [68], sonoporation [69], microinjection [70], and optical transfection [71] have been reported. Physical methods show less tendency than carrier-mediated methods to cause mutagenesis in cellular genomes, thus proving to be safer for in situ transfections [72]. For in vivo application, however, most reported physical methods have been limited to access the human organs.
Electroporation is a transfection process where molecules were delivered assisted by applying a voltage across the cell membrane. This produces a transmembrane potential difference (around 0.5 V) and induces nanoscale pore formation on the lipid bilayer of the membrane [73]. Bulk electroporation in vivo has been performed in muscle tissues by inserting a pair of electrode needles that create a few millimeter gap into the tissue to inject DNA plasmids prior to the electroporation [74, 75]. Despite its feasibility, the application of bulk electroporation in vivo remains limited, due to the fact that a large electric potential required to generate sufficient transmembrane potential leads to significant cell death and low efficiency [72]. In contrast, single-cell electroporation shows superior performance in terms of specificity, dosage control, cell viability and transfection efficiency [64, 76]. In single cell electroporation, a nanochannel connected to a microfluidic reservoir and filled with electrolytes deploys the electrical potential to a small patch of the cell membrane. The area of the membrane that is subjected to poration depends on the opening of the nanochannel which is normally less than 500 nm in diameter [64]. This nanochannel can be fabricated in an array [77], can be the opening of the apex of an atomic force microscopy (AFM) probe [64], or other features [78, 79]. These different forms of setup ensure porating a small patch of the cell membrane while electrophoretically forcing polarized macromolecules through the nanoscale pores into the single cell. The first in vivo single cell electroporation was performed on individual neurons using a micropipette constructed on a wearable patch [80] (Figure 4a).
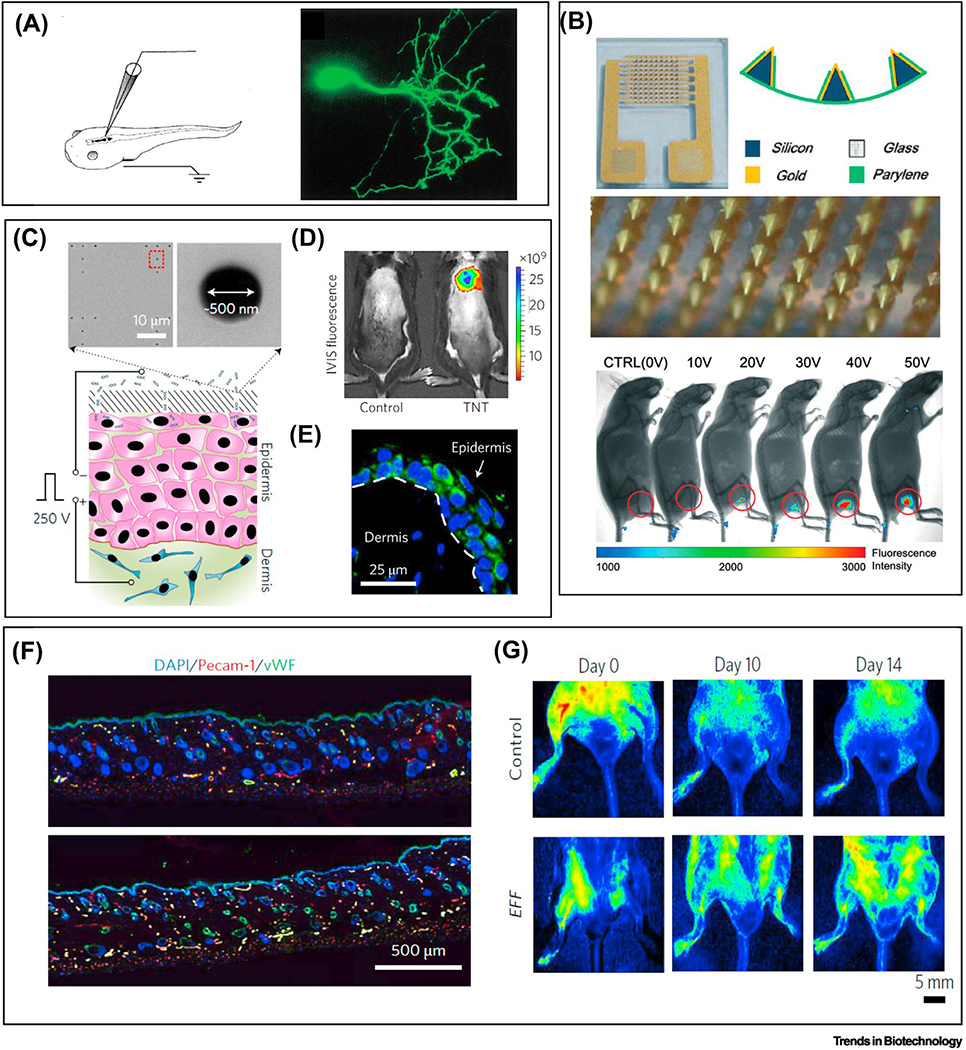
Figure 4: Single-cell transfection in vivo by electroporation.
(a) In vivo single cell transfection of neuron cells in xenopus laevis. Scale bars: 2 mm for left two and 1 mm for right. Reproduced with the permission from Ref. [80]. (b) In vivo transfection of skin cells by a patchable device using a flexible microneedle arrays with hollow tip for electroporation. Electroporation on the leg of mice induces dosage-depend expression of fluorescence markers. Reproduced with the permission from Ref. [82]. (c) A nanochannel based patch for transfection of epidermis cells in vivo with resolution at single cells. The reference electrode is inserted under the dermis of the mouse skin, and the silicon substrate with nanochannels (500 nm in diameter) is adhered onto the top of the skin. d) After single cell electroporation on mouse, the in vivo imaging system (IVIS) shows high levels of ABM expression. e) The cellular level transfection confirmation with confocal imaging of the dermis and epidermis layers. f) Increased angiogenesis of the skin tissue is observed with a one-time treatment of EFF transfection. g) Single cell electroporation based patch on a mouse limb rescues the limb from necrotizing ischemia as indicated by the increased blood flow for EFF treated limb compared with control. Reproduced with the permission from Ref. [56].
By miniaturization and integration of microneedle electrodes or microchannels, these devices are capable of patching the skin and performing single cell electroporation [81]. The first such example uses a silicon microneedle coated with a layer of gold (6 μm in thickness) and passivated with a layer of parylene on a parylene substrate (8 μm) (Figure 4b) [82]. The interdigitated arrangements of the microneedle arrays form opposing rows of cathodes and anodes to deliver the electrical potential. The parylene substrate with a designed size of 20 mm2 ensures the flexibility of the device to conform on mice skin. DNA transfection is achieved by an application of 20 V voltage or above. On-chip single cell electroporation was also demonstrated using a silicon-based nanochannel [56]. This paradigm-shift in how to deliver the electrical field also brings the convenient transformation from an in vitro testing to a wearable patch format. Figure 4c illustrates the setup of the study with the silicon wafer on top of the epidermis patterned with nanochannel arrays. The array of nanochannels is about 500 nm in diameter and about 10 μm in depth. To deliver the electrical potential, one reference electrode is inserted into the dermis and the other is placed above the silicon wafer. The electrical pulse of 250 V at 10 ms intervals delivers DNA plasmids into epidermal cells. Compared with bulk, this nanochannel offers a 50- to 250-fold increase in gene expression. Figure 4d and e show the mouse skin cells expressing the ABM (Ascl1/Brn2/Myt1l) factors in in vivo, and demonstrated the cellular reprogramming by delivery of 3-gene cocktails for direct on-skin reprogramming fibroblasts into induced endothelial cells. Figure 4f shows the increased vascularization after reprograming factor EFF (Etv2/Foxc2/Fli1) transfection, which leads to enhanced blood flow and whole limb rescue (Figure 4g). The animal studies demonstrates the potential with wearable single cell electroporation for therapeutic options for wound healing and regeneration [83]. Nanochannel based electroporation, the issue of accessibility remains. Studies are required to investigate the contact between the substrates with the skin to ensure a good seal is form between the channel and the cells on the epidermis; without this seal, electrical field strength will not be sufficient for delivery of large molecules.
Micro/nano needle for single cell gene transfection
Hypodermic needle injection has always been the most common method for drug or gene delivery in vivo [84]. Miniaturization of the conventional metal needles has the potential to eliminate a host of drawbacks inert to the method, such as pain and risk of infection [85]. This results in micro/nanoneedle designs tailored for delivering macromolecules for in vivo transfection. A host of these designs have indeed brought new developments in DNA vaccination and gene editing [85, 86]. By employing biocompatible materials and novel fabrication techniques, the new micro/nano devices improved delivery efficiency in a non-invasive and safe manner. Further, the use of thin and flexible polymer substrates that have the appropriate Young’s moduli offers ideal conformity with the skin. Considering the dimension of the functional units of needles, they can be categorized for use in local delivery or intracellular delivery. They also use distinct methods of delivering the cargo: as part of a multilayer coating on the needle [85], encapsulation within a dissolvable polymer needle [86], or diffusion through a hollow opening of the needles [82]. Conventional microneedle arrays fabricated using different materials have been studied for DNA vaccination applications [87]. Vaccine viruses or plasmid DNAs (pDNA) delivered into the vicinity of cells in the epidermis are subjected to cellular uptake. Therefore, delivery efficiency is highly dependent on capsizes of cargo molecules. Most of the microneedles fabricated out of polymer materials have a tip apex of a few tens of micrometers, which is unlikely to precisely pinpoint to the membrane of a single cell. Thus, delivery of plasmids or other molecules will most likely be local rather than intracellular; while needles with nanometer apex can perform intracellular deliver without cell damage.
With respect to single cell, nanoneedle arrays are capable of offering more precision due to a much smaller functional area. Their effectiveness as a vehicle for macromolecule delivery in in vivo transfection lies in the small size of the needle diameter (less than 100 nm). At this scale, direct penetration with the help of cell gravity is deemed possible [88], although endocytosis is also suspected to assist the transport of large molecules [89]. Cargo can be supplied by either a hollow nanofluidic channel or direct grafting. A number of nanoneedle arrays were fabricated from non-degradable materials, such as nanowires [90], carbon nanotubes [91] and nanofibers [92], or from degradable porous silicon. Recently, nanostraws with an inner diameter of less than 100 nm were fabricated on top of a compliant polycarbonate membrane (Figure 5a) [90]. Direct access to the cell cytosol was achieved through the nanofluidic channel within the nanostraw. This was demonstrated by expression of GFP marker proteins within 72 hours after plasmids delivery (Figure 5b). Since the arrays were fabricated on a mechanically flexible substrate, there should be no compatibility issue for a wearable format. In addition, the size of the nanostraw also presents a better alternative for penetration of individual cells, but the device may require a protective outer-shell to penetrate the outer layer of the skin [90]. Further, a recent study by the same group has demonstrated the ability of the nanostraw to perform intracellular sampling of proteins and mRNAs on live cells [93]. The same cells can be repeated sample for 5 days for longitudinal cell monitoring. This presents a platform where the dual functionality of sensing and delivery can be integrated on one device. Similar designs have also been reported with the hollow nanoneedle array on top of silicon membranes [94].
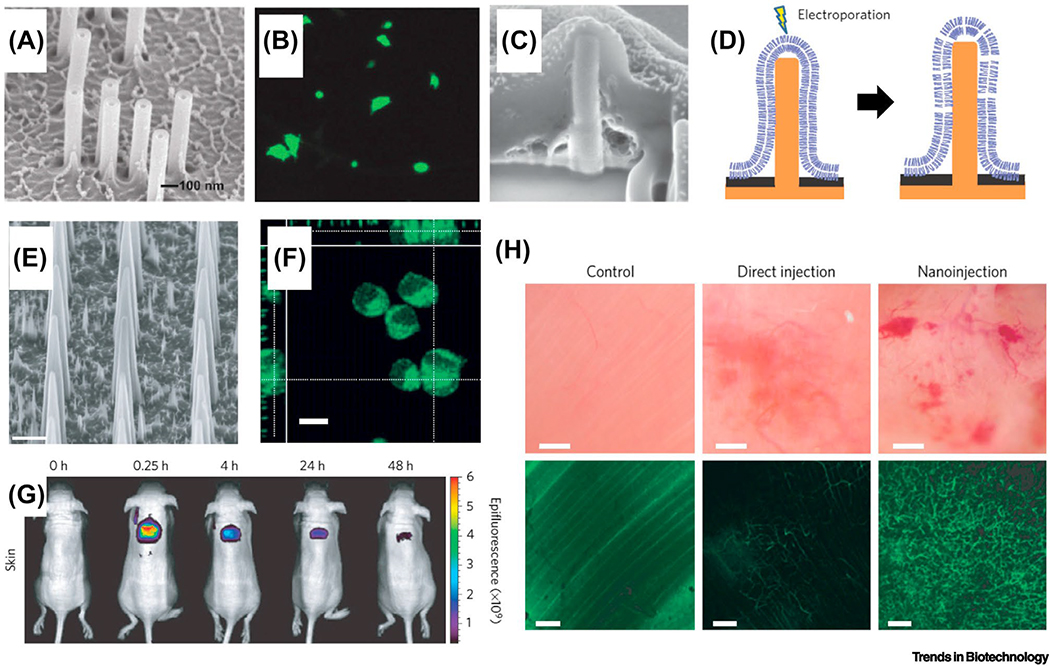
Figure 5: Micro/Nanoneedle based intracellular delivery and transfection.
(a) An array of nanostraws grow on a polycarbonate substrate with the outer diameters of 100 nm. Scale bar: 100 nm. Reproduced with the permission from Ref. [90]. (b) Single cell transfect was achieved with fluidic intracellular delivery of plasmids, and uniform expression levels of fluorescence markers are exhibited [90]. (c) Nanopillar arrays are used for the direct penetration of the cell membrane and for single cell electroporation. The nanopillar (150 nm in diameter, 1.5 μm in height) is fabricated out of platinum on a silicon nitride substrate. Scale bar: 200 nm. Reproduced with the permission from Ref. [78]. (d) The aspect ratio of the nanopillar enables either direct penetration. Moreover, the conductive Pt nanopillar also can apply a localized electrical field for single cell electroporation [78]. (e) Biodegradable silicon nanoneedles for deliver and transfection. An array of nanoneedles were fabricated out of porous silicon with tip apex of less than 100 nm. Scale bar: 2 μm. Reproduced with the permission from Ref. [95]. Similar design has also been reported in [96] for the delivery of nanoparticles. (f) Single cell transfection is demonstrated with delivery of fluorescence markers [95]. (g) The potential for a wearable patch format is shown by an in vivo experiment in mice [95]. (h) Significant neovascularization was achieved by a nanoneedle based single cell transfection compared with direct injection.
Nanoneedle featured electrodes can also be used in combination with in situ electroporation [78]. Due to the sharp apex of a nanopillar electrode (Figure 5c), a strong electrical field induces local permeability of the cell membrane and delivers target molecules into the cytosol (Figure 5d). Biodegradable nanoneedles were fabricated from porous silicon with a 50 nm apex width (Figure 5e) [95]. This sharp design facilitates the cell membrane penetration and cargo injection. Delivery of plasmids can be achieved by simply growing cells on top of the bed of nanoneedle arrays (Figure 5f). When tested on the skin and muscle tissues on mice, the localized nanoinjection method using nanoneedle arrays provides a more uniform delivery profile compared with direct injection (Figure 5g). Further, delivering vascular endothelial growth factor (VEGF) plasmids to the mouse muscle tissue induces neovascularization and increase vessel connectivity (Figure 5h). The success of the animal studies demonstrates the potential of nanoneedles for in vivo studies as a patchable single cell transfection platform.
Concluding Remarks and Future Perspectives
Advancements in nanotechnology have made it feasible to design nanodevices with applications for skin or organ sensing and therapy. Existing soft and wearable electronics provide a basis for transforming the currently rigid single-cell sensors into formats that are compliant with the curved interfaces of the human body. Greater effort is envisioned to establish precise and definitive diagnosis for wearable single-cell sensing, which may directly indicate the conditions of cells from patients. Various single-cell sensors for multiple cell types including endothelial, epithelial, cardiomyocytes, lymphoma, and nerve cells, have been discussed in this review, but these sensors have yet to be realized in wearable formats. Furthermore, wearable single-cell sensors still need to be developed for other cell types, such as pancreatic cells, osteocytes, chondrocytes, and other stem cells to better understand their behavior in pathophysiological or normal physiological states.
Currently, the majority of the single cell transfection platforms are still confined for in vitro experimentations. This is due to the difficulty in transforming these microfabricated devices into wearable format and the challenge in delivering sufficient voltage with compact wearable electronic units. Although some micro-/nano-needles have been fabricated on soft polymeric substrates, the wearable system goes beyond mechanical conformity, and requires the integration of drug reservoirs, microfluidic transports, electronics with the micro-/nano-needles. This is also true for nanochannel based electroporation. On the electronics aspect, single electroporation requires an input voltage ranging from a few tens Volts to a few hundred Volts, the wearable electronics unit will have to include amplification and power supply. Thus, to tailor these microfabricated device to completely wearable, research efforts needs to look into integrated microchips with all elements for transfection.
Targeted delivery with single cell resolution will increase accuracy and lessen the risk of disturbing cells that do not require any remedy in vivo. In the future, translational technologies will witness increasing nanodevices in wearable formats, e.g. nanoelectroporation and nanoneedles, with promises for precise drug or gene delivery in a wide variety of applications, ranging from on-skin gene therapy and wound healing to regenerative medicine and beyond.
ACKNOWLEDGEMENT
L.C. and Y. F. thank the funding from INSCA of Beijing Advanced Innovation Center for Biomedical Engineering and Beihang University, Start-up and PDS funding from UNT. R.Y. acknowledges the funding from the Nebraska Center for Integrated Biomolecular Communication (NCIBC) (NIH National Institutes of General Medical Sciences P20GM113126), from Nebraska Center for Nanomedicine (P30GM127200) and from the NSF (Award #1826135). Y.C.W. is supported by the UNTHSC Start-up Fund for Stem Cell Laboratory and Faculty Pilot Grant FY15 (RI6182). C.Y. would like to acknowledge the National Institutes of Health Grant (R21EB026175) and the Doctoral New Investigator grant from American Chemical Society Petroleum Research Fund (56840-DNI7).
REFERENCE
- 1.Windmiller JR and Wang J (2013) Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 25 (1), 29–46. [Google Scholar]
- 2.Liu Y et al. (2017) Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 11 (10), 9614–9635. [DOI] [PubMed] [Google Scholar]
- 3.Prausnitz MR et al. (2004) Current status and future potential of transdermal drug delivery. Nature Reviews Drug Discovery 3 (2), 115–124. [DOI] [PubMed] [Google Scholar]
- 4.Lee H et al. (2018) Device-assisted transdermal drug delivery. Advanced Drug Delivery Reviews 127, 35–45. [DOI] [PubMed] [Google Scholar]
- 5.Bai W et al. (2018) Patchable Micro/Nanodevices Interacting with Skin. Biosens Bioelectron. [DOI] [PubMed] [Google Scholar]
- 6.Kang WM et al. (2016) Micro- and Nanoscale Technologies for Delivery into Adherent Cells. Trends Biotechnol 34 (8), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong GS et al. (2018) A method for single-neuron chronic recording from the retina in awake mice. Science 360 (6396), 1447–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu TM et al. (2016) Stable long-term chronic brain mapping at the single-neuron level. Nature Methods 13 (10), 875–+. [DOI] [PubMed] [Google Scholar]
- 9.Wang SQ et al. (2016) Flexible Substrate-Based Devices for Point-of-Care Diagnostics. Trends in Biotechnology 34 (11), 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandodkar AJ and Wang J (2014) Non-invasive wearable electrochemical sensors: a review. Trends in Biotechnology 32 (7), 363–371. [DOI] [PubMed] [Google Scholar]
- 11.Bettinger CJ (2015) Materials Advances for Next-Generation Ingestible Electronic Medical Devices. Trends in Biotechnology 33 (10), 575–585. [DOI] [PubMed] [Google Scholar]
- 12.Jang H et al. (2016) Graphene-Based Flexible and Stretchable Electronics. Advanced Materials 28 (22), 4184–4202. [DOI] [PubMed] [Google Scholar]
- 13.Baik S et al. (2019) Bioinspired Adhesive Architectures: From Skin Patch to Integrated Bioelectronics. Advanced Materials, 1803309. [DOI] [PubMed] [Google Scholar]
- 14.Wei D et al. (2009) Electrochemical biosensors at the nanoscale. Lab on a Chip 9 (15), 2123–2131. [DOI] [PubMed] [Google Scholar]
- 15.Amatore C et al. (2008) Electrochemical Monitoring of Single Cell Secretion: Vesicular Exocytosis and Oxidative Stress. Chem Rev 108 (7), 2585–2621. [DOI] [PubMed] [Google Scholar]
- 16.Koh A et al. (2016) A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Transl Med 8 (366), 366ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JW et al. (2016) Soft, thin skin-mounted power management systems and their use in wireless thermography. Proceedings of the National Academy of Sciences 113 (22), 6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagdeviren C et al. (2015) Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nature Materials 14, 728. [DOI] [PubMed] [Google Scholar]
- 19.Gao W et al. (2016) Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton JJS et al. (2015) Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S et al. (2016) Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Advanced Materials 28 (22), 4203–4218. [DOI] [PubMed] [Google Scholar]
- 22.Nyein HYY et al. (2016) A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 10 (7), 7216–7224. [DOI] [PubMed] [Google Scholar]
- 23.Yuk H et al. (2016) Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nature Communications 7, 12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H et al. (2017) Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Science Advances 3 (3), e1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X et al. (2016) A Low-Operating-Power and Flexible Active-Matrix Organic-Transistor Temperature-Sensor Array. Advanced Materials 28 (24), 4832–4838. [DOI] [PubMed] [Google Scholar]
- 26.Chortos A et al. (2016) Pursuing prosthetic electronic skin. Nature Materials 15, 937. [DOI] [PubMed] [Google Scholar]
- 27.Yokota T et al. (2016) Ultraflexible organic photonic skin. Science Advances 2 (4), e1501856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X et al. (2017) Flexible Sensing Electronics for Wearable/Attachable Health Monitoring. Small 13 (25), 1602790. [DOI] [PubMed] [Google Scholar]
- 29.Yao S et al. (2017) A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Advanced Healthcare Materials 6 (6), 1601159. [DOI] [PubMed] [Google Scholar]
- 30.Lee S et al. (2016) A transparent bending-insensitive pressure sensor. Nature Nanotechnology 11, 472. [DOI] [PubMed] [Google Scholar]
- 31.Kim J et al. (2016) Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Science Advances 2 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu BX et al. (2016) An Epidermal Stimulation and Sensing Platform for Sensorimotor Prosthetic Control, Management of Lower Back Exertion, and Electrical Muscle Activation. Advanced Materials 28 (22), 4462–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hattori Y et al. (2014) Multifunctional Skin-Like Electronics for Quantitative, Clinical Monitoring of Cutaneous Wound Healing. Advanced Healthcare Materials 3 (10), 1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willand MP (2015) Electrical stimulation enhances reinnervation after nerve injury. European journal of translational myology 25 (4), 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu B et al. (2015) An Epidermal Stimulation and Sensing Platform for Sensorimotor Prosthetic Control, Management of Lower Back Exertion, and Electrical Muscle Activation. Adv Mater 28 (22), 4462–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SM et al. (2014) Self-adhesive epidermal carbon nanotube electronics for tether-free long-term continuous recording of biosignals. Sci Rep 4, 6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattori Y et al. (2014) Multifunctional Skin-Like Electronics for Quantitative, Clinical Monitoring of Cutaneous Wound Healing. Advanced Healthcare Materials 3 (10), 1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thakral G et al. (2013) Electrical stimulation to accelerate wound healing. Diabetic Foot & Ankle 4 (1), 22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moshayedi P et al. (2014) The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 35 (13), 3919–3925. [DOI] [PubMed] [Google Scholar]
- 40.Park J et al. , A CMOS 22k-pixel single-cell resolution multi-modality real-time cellular sensing array, 2017 IEEE Custom Integrated Circuits Conference (CICC), 2017, pp. 1–4. [Google Scholar]
- 41.Susloparova A et al. (2015) Electrical cell-substrate impedance sensing with field-effect transistors is able to unravel cellular adhesion and detachment processes on a single cell level. Lab on a Chip 15 (3), 668–679. [DOI] [PubMed] [Google Scholar]
- 42.Li X et al. (2018) Label-Free Optofluidic Nanobiosensor Enables Real-Time Analysis of Single-Cell Cytokine Secretion. Small 14 (26), 1800698. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y et al. (2015) Development of Au Disk Nanoelectrode Down to 3 nm in Radius for Detection of Dopamine Release from a Single Cell. Analytical Chemistry 87 (11), 5531–5538. [DOI] [PubMed] [Google Scholar]
- 44.Li X et al. (2015) Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes. Angewandte Chemie International Edition 54 (41), 11978–11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie H et al. (2016) Real-Time Monitoring of Nitric Oxide at Single-Cell Level with Porphyrin-Functionalized Graphene Field-Effect Transistor Biosensor. Anal Chem 88 (22), 11115–11122. [DOI] [PubMed] [Google Scholar]
- 46.Xing F et al. (2014) Ultrasensitive Flow Sensing of a Single Cell Using Graphene-Based Optical Sensors. Nano Lett 14 (6), 3563–3569. [DOI] [PubMed] [Google Scholar]
- 47.Sahoo PK et al. (2016) Nanowire Arrays as Cell Force Sensors To Investigate Adhesin-Enhanced Holdfast of Single Cell Bacteria and Biofilm Stability. Nano Letters 16 (7), 4656–4664. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y et al. (2016) Spearhead Nanometric Field-Effect Transistor Sensors for Single-Cell Analysis. ACS Nano 10 (3), 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y-L et al. (2016) Stretchable Electrochemical Sensor for Real-Time Monitoring of Cells and Tissues. Angewandte Chemie International Edition 55 (14), 4537–4541. [DOI] [PubMed] [Google Scholar]
- 50.Cai B et al. (2014) Ultrasensitive Label-Free Detection of PNA–DNA Hybridization by Reduced Graphene Oxide Field-Effect Transistor Biosensor. ACS Nano 8 (3), 2632–2638. [DOI] [PubMed] [Google Scholar]
- 51.Shemesh OA et al. (2017) Temporally precise single-cell-resolution optogenetics. Nat Neurosci 20 (12), 1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SI et al. (2015) Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol 33, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galluppi F et al. , Live Demonstration: A Wearable Device for Optogenetic Vision Restoration, 2018 IEEE International Symposium on Circuits and Systems (ISCAS), 2018, pp. 1–1. [Google Scholar]
- 54.Kar S et al. (2018) Single-cell electroporation: current trends, applications and future prospects. Journal of Micromechanics and Microengineering 28 (12). [Google Scholar]
- 55.Khine M et al. (2005) A single cell electroporation chip. Lab on a Chip 5 (1), 38–43. [DOI] [PubMed] [Google Scholar]
- 56.Gallego-Perez D et al. (2017) Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol 12 (10), 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallego-Perez D et al. (2016) On-Chip Clonal Analysis of Glioma-Stem-Cell Motility and Therapy Resistance. Nano Letters 16 (9), 5326–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang FC et al. (2011) Development and applications of single-cell transcriptome analysis. Nature Methods 8 (4), S6–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leslie M (2011) The Power Of One. Science 331 (6013), 24–26. [DOI] [PubMed] [Google Scholar]
- 60.Lecault V et al. (2012) Microfluidic single cell analysis: from promise to practice. Current Opinion in Chemical Biology 16 (3–4), 381–390. [DOI] [PubMed] [Google Scholar]
- 61.Kalisky T and Quake SR (2011) Single-cell genomics. Nature methods 8 (4), 311. [DOI] [PubMed] [Google Scholar]
- 62.Rotem A et al. (2015) Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nature Biotechnology 33 (11), 1165–U91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang DJ and Bodovitz S (2010) Single cell analysis: the new frontier in ‘omics’. Trends in Biotechnology 28 (6), 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang W et al. (2013) Nanofountain probe electroporation (NFP-E) of single cells. Nano Lett 13 (6), 2448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang R et al. (2018) Monoclonal Cell Line Generation and CRISPR/Cas9 Manipulation via Single-Cell Electroporation. Small 14 (12), e1702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephens DJ and Pepperkok R (2001) The many ways to cross the plasma membrane. Proceedings of the National Academy of Sciences 98 (8), 4295–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin L et al. (2014) Current Progress in Gene Delivery Technology Based on Chemical Methods and Nano-carriers. Theranostics 4 (3), 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharei A et al. (2013) A vector-free microfluidic platform for intracellular delivery. Proc Natl Acad Sci U S A 110 (6), 2082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helfield B et al. (2016) Biophysical insight into mechanisms of sonoporation. Proc Natl Acad Sci U S A 113 (36), 9983–9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y and Yu LC (2008) Single-cell microinjection technology in cell biology. Bioessays 30 (6), 606–10. [DOI] [PubMed] [Google Scholar]
- 71.Stevenson DJ et al. (2010) Single cell optical transfection. Journal of the Royal Society Interface 7 (47), 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang M et al. (2010) Single-cell electroporation. Anal Bioanal Chem 397 (8), 3235–48. [DOI] [PubMed] [Google Scholar]
- 73.Tsong T (1991) Electroporation of Cell Membrane. [DOI] [PMC free article] [PubMed]
- 74.Aihara H and Miyazaki J (1998) Gene transfer into muscle by electroporation in vivo. Nature Biotechnology 16 (9), 867–870. [DOI] [PubMed] [Google Scholar]
- 75.Aung W et al. (2009) Visualization of in vivo electroporation-mediated transgene expression in experimental tumors by optical and magnetic resonance imaging. Gene Ther 16 (7), 830–9. [DOI] [PubMed] [Google Scholar]
- 76.Chang LQ et al. (2016) Controllable Large-Scale Transfection of Primary Mammalian Cardiomyocytes on a Nanochannel Array Platform. Small 12 (43), 5971–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boukany PE et al. (2011) Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat Nanotechnol 6 (11), 747–54. [DOI] [PubMed] [Google Scholar]
- 78.Xie C et al. (2012) Intracellular recording of action potentials by nanopillar electroporation. Nat Nanotechnol 7 (3), 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie X et al. (2013) Nanostraw-Electroporation System for Highly Efficient Intracellular Delivery and Transfection. Acs Nano 7 (5), 4351–4358. [DOI] [PubMed] [Google Scholar]
- 80.Haas K et al. (2001) Single-cell electroporation for gene transfer in vivo. Neuron 29 (3), 583–91. [DOI] [PubMed] [Google Scholar]
- 81.Judkewitz B et al. (2009) Targeted single-cell electroporation of mammalian neurons in vivo. Nat Protoc 4 (6), 862–869. [DOI] [PubMed] [Google Scholar]
- 82.Wei ZW et al. (2014) A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery. Lab on a Chip 14 (20), 4093–4102. [DOI] [PubMed] [Google Scholar]
- 83.Kurita M et al. (2018) In vivo reprogramming of wound-resident cells generates skin epithelial tissue. Nature 561 (7722), 243–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim Y-C et al. (2012) Microneedles for drug and vaccine delivery. Advanced drug delivery reviews 64 (14), 1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeMuth PC et al. (2013) Polymer multilayer tattooing for enhanced DNA vaccination. Nature materials 12 (4), 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan SP et al. (2010) Dissolving polymer microneedle patches for influenza vaccination. Nat Med 16 (8), 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ita K (2016) Transdermal delivery of vaccines - Recent progress and critical issues. Biomedicine & Pharmacotherapy 83, 1080–1088. [DOI] [PubMed] [Google Scholar]
- 88.Xu AM et al. (2014) Quantification of nanowire penetration into living cells. Nature Communications 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shalek AK et al. (2010) Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci U S A 107 (5), 1870–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.VanDersarl JJ et al. (2011) Nanostraws for direct fluidic intracellular access. Nano Lett 12 (8), 3881–3886. [DOI] [PubMed] [Google Scholar]
- 91.Park S et al. (2009) Carbon nanosyringe array as a platform for intracellular delivery. Nano Lett 9 (4), 1325–1329. [DOI] [PubMed] [Google Scholar]
- 92.Kamble P et al. (2017) Nanofiber based drug delivery systems for skin: A promising therapeutic approach. Journal of Drug Delivery Science and Technology 41, 124–133. [Google Scholar]
- 93.Cao Y et al. (2017) Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc Natl Acad Sci U S A 114 (10), E1866–E1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peer E et al. (2012) Hollow nanoneedle array and its utilization for repeated administration of biomolecules to the same cells. ACS Nano 6 (6), 4940–6. [DOI] [PubMed] [Google Scholar]
- 95.Chiappini C et al. (2015) Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nature materials 14 (5), 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiappini C et al. (2015) Biodegradable nanoneedles for localized delivery of nanoparticles in vivo: exploring the biointerface. ACS Nano 9 (5), 5500–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]