Summary:
Dnmt3a-mutant stem cells gain a competitive advantage via upregulation of a Txnip–p53–p21 axis and protection from IFNγ induced exhaustion.
See related article by Zhang et al., p. 220 (5).
Eight years ago, it was first discovered that a significant fraction of elderly individuals harbor clones of hematopoietic cells which expand to above 4% of the total blood population, a fraction that cannot be explained by neutral genetic drift and that possesses mutations frequently observed in myeloid malignancies (1). This phenomenon, commonly referred to as clonal hematopoiesis of indeterminant potential (CHIP), has been the subject of intense studies aimed at understanding underlying biological mechanisms and functional consequences. CHIP is associated with increased risk of various maladies of old age, including leukemias, other cancers, cardiovascular disorders and overall mortality; however, we still lack a clear understanding of the biological mechanisms that lead to CHIP in the first place. Studies show that CHIP is associated with mutations that are known drivers of acute myeloid leukemia (AML) and originates in multipotent hematopoietic stem and progenitor cells (HSPCs), perhaps in hematopoietic stem cells (HSCs). Various animal models have been generated to investigate the mechanisms that lead to mutation-driven clonal fitness advantage and, recognizing that malignant mutations are stochastic and inevitable in a large population of cells, to search for interventions that can mitigate this fitness advantage to limit clonal expansion in the bone marrow. These studies have been complicated by the fact that most CHIP mutations only confer a small fitness benefit to the HSPCs in which they originate. Still, compounded over many years, this fitness differential results in significant colonization of the bone marrow and blood populations, a process that is hard to replicate in the limited life span of a mouse. Despite these challenges, some studies have shown that certain environmental stimuli like inflammation and cytotoxic therapies can promote the selective expansion of specific mutant clones (2, 3).
DNMT3A is the most commonly mutated gene in CHIP. Loss-of-function mutations include the dominant-negative R882H, the most frequent DNMT3A mutation in AML. Recent studies have demonstrated that inflammation, and in particular IFNγ signaling, is associated with DNMT3A-mutant CHIP in ulcerative colitis patients (4) and is sufficient to fuel the expansion of Dnmt3a−/− HSCs in bacterially infected mice (2). However, a mechanistic explanation for the advantage conferred by IFNγ was so far lacking. In this issue, Zhang and colleagues (5), building on their previous work, explore the mechanisms leading to the positive selection of Dnmt3a knock-out (KO) HSCs or those harboring the R878H mutation (analogous to the human R882H mutation). By using competitive transplant models, the authors show that Dnmt3a loss-of-function cells display higher engraftment capacity than wild-type (WT) cells. Importantly, IFNγ negatively impacts the engraftment capacity of WT HSCs but does not impair Dnmt3a-mutant HSC engraftment. Instead, retransplanted mutant HSCs gain an advantage in secondary recipients, with similar observations in DNMT3A KO human CD34+ cells. To further dissect the role of IFNγ signaling, the authors used Dnmt3a-mutant models lacking expression of the IFNγ receptor (IFNgr1), demonstrating that the ability of Dnmt3a-mutant HSCs to directly receive IFNγ signals is important for their expansion in unperturbed mice and for their enhanced potential to reconstitute the HSC and myeloid cell populations when transplanted into WT mice (expressing IFNγ). Furthermore, transplantation of Dnmt3a-mutant HSPCs into IFNγ KO mice similarly negates their fitness advantage over WT HSPCs. Altogether, these results suggest that a combination of the detrimental effect of IFNγ on WT HSCs and the positive effects on Dnmt3a-mutant HSC are key to generating the fitness differential that promotes Dnmt3a-mutant clonal expansion.
Compared to WT cells, Dnmt3a-mutated cells treated with IFNγ exhibit reduced apoptosis in culture and reduced proliferation and exit from quiescence in vitro and in vivo. Bulk and single-cell RNA-seq analyses show subtle transcriptional differences between IFNγ-treated Dnmt3a KO and WT cells with the largest transcriptional effects due to IFNγ. Among the few differentially expressed genes, Txnip and p53 target genes are upregulated in Dnmt3a KO cells, while E2F targets, Myc targets and cell-cycle genes are downregulated. While this pattern represents a counterintuitive impact on gene expression for a mutation involved in leukemia, HSC maintenance requires a sufficiently low level of cell-cycle activity. Notably, Txnip (thioredoxin interacting protein) is an inhibitor of thioredoxin, a regulator of antioxidant responses and is important for HSC engraftment potential. Furthermore, Txnip has previously been shown to interact with and stabilize p53 by reducing association with MDM2, and through this interaction with p53, to regulate a number of antioxidant response genes (6). Zhang and colleagues show that a genomic regulatory region upstream of the Txnip gene is hypomethylated in Dnmt3a KO cells. Whereas WT cells show modestly reduced expression of Txnip when treated with IFNγ, the expression of Txnip is higher in Dnmt3a KO cells and not affected by IFNγ. The expression of p53 protein is correlated to that of Txnip, being increased in Dnmt3a-mutant cells but not further increased in IFNγ-treated cells. In contrast, IFNγ results in increased expression of p21 in Dnmt3a KO cells, which is associated with increased p53/Txnip complex formation and p53 binding to the Cdkn1a (p21) promoter. In this context, the role of p21 might appear counterintuitive but is important to prevent excessive HSC division that is coupled to differentiation and the loss of stem cell potential. To demonstrate the biological importance of the Txnip–p53–p21 axis, the authors knock down p21 and Txnip. They show that each knockdown can prevent the IFNγ-dependent expansion of Dnmt3a-mutated cells in recipient mice, as well as reverting the cell cycle and survival effects observed in IFNγ-treated Dnmt3a-mutated cells (Fig. 1). In the same experiments, p53-silenced cells showed somewhat accelerated exit from quiescence but, at the same time, reduced apoptosis upon treatment with IFNγ, which is not surprising given the pleiotropic activities of p53 on the regulation of cell-cycle progression and apoptosis beyond Txnip-dependent regulation of p21.
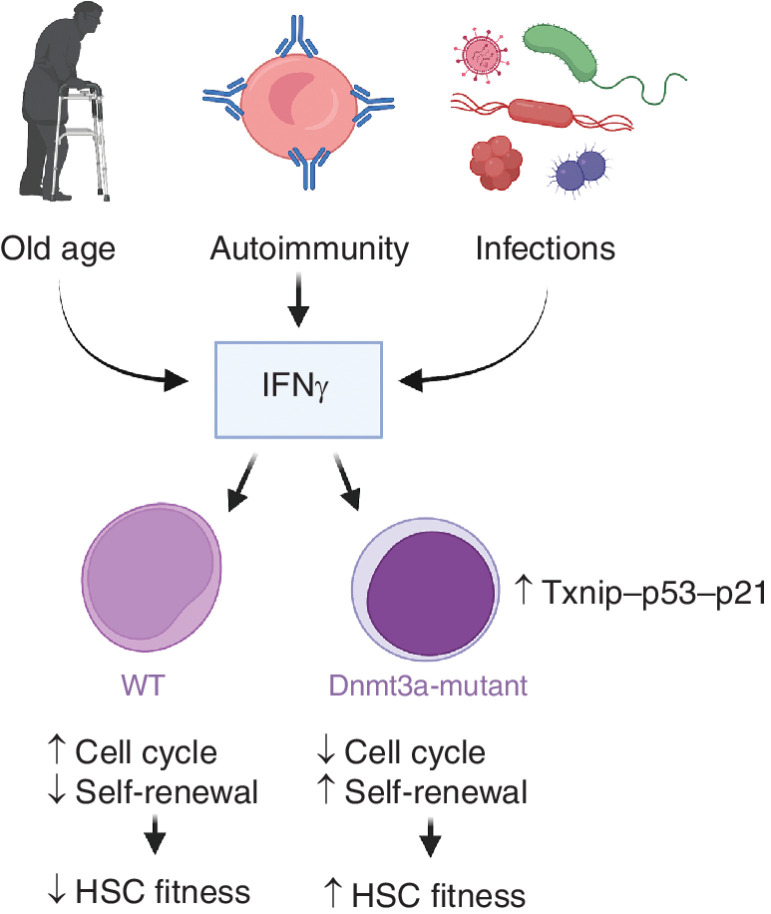
Figure 1.
Intrinsic and extrinsic changes to the microenvironment lead to increased IFNγ stimulation of HSCs. While IFNγ is detrimental to the self-renewal of WT HSCs, Dnmt3a-mutant HSCs are protected via Txnip–p53–p21 upregulation. Figure created using Biorender.
These results are striking for a number of reasons. First, they elucidate a molecular pathway that is responsible for the context-dependent increased fitness of Dnmt3a-mutated cells, a task that has been elusive despite the extensive research conducted by multiple groups; second, they provide a potential means to prevent the expansion of Dnmt3a-mutant HSC through modulation of IFNγ or the Txnip–p53–p21 axis; third, therapies aimed at reducing p21 expression might simultaneously reduce the fitness advantage of Dnmt3a-mutant HSCs and potentially increase competition by WT cells, although this needs to be tested experimentally. It is also peculiar that the basis for the advantage of Dnmt3a-mutated HSCs relies on activation of some p53 functions. Loss-of-function TP53 mutations are frequent in AML. Similarly, TP53 is one of the most commonly mutated genes in CHIP, and, not unexpectedly, TP53-mutant CHIP is associated with a high risk of developing leukemia (7). It is possible that DNMT3A and TP53 mutations might confer orthogonal ways for HSCs to gain an advantage over the WT bone marrow. Alternatively, they might be advantageous under different environmental stimuli (3). Finally, it is notable that Zhang and colleagues show that only a subset of p53 targets (such as p21) are altered by Dnmt3a loss, while proapoptotic targets like Bax and Puma are unaffected, which could spare these mutant cells from the deadly consequences of full p53 activation.
With regard to Txnip, it was previously shown that the Txnip KO exhibits phenotypes of precocious differentiation with enlarged but dysfunctional HSC populations, increased ROS production, myeloid skewing and reduced cell polarity (8), all features of HSCs in older individuals. Given that Txnip loss-of-function phenocopies aging in HSCs, it is intriguing to speculate that the increased expression of Txnip in Dnmt3a-mutated cells might promote a more youthful phenotype in mutated cells, leading to their clonal advantage (Fig. 1). In the work by Zhang and colleagues, Txnip operates primarily by inducing p21 expression. In contrast, Jung and colleagues showed that Txnip prevents the aging phenotype by suppressing p38 activity while p21 is increased in Txnip-KO HSCs (8), the opposite trend of what is seen in Dnmt3a-KO HSCs. Zhang and colleagues suggest that Txnip likely has many other functions besides the regulation of the p53–p21 axis, but it is also possible that the different molecular contexts between WT and Dnmt3a-mutated cells redirect the pathways regulated by Txnip.
It is important to note that the selective advantage of Dnmt3a loss-of-function cells is partially dependent on exposure to IFNγ. Interferon signaling can increase cell division and differentiation of WT HSCs leading to their exhaustion, and after chronic stimulation can lead to apoptosis (9). Zhang and colleagues show that Dnmt3a-mutant HSCs have an even higher interferon response than WT cells but are protected from its negative effects because of the cell-cycle control enacted by p21. It remains an open question how exactly p21 prevents excessive cell division associated with loss of stem cell self-renewal while simultaneously allowing sufficient proliferation of Dnmt3a-mutant cells necessary to competitively colonize the bone marrow niche. Another aspect that will require further investigation is the exact source of IFNγ and how it may promote expansion of clonal hematopoiesis with DNMT3A mutations in humans. A relevant study showed that in DNMT3A-R882H HSPCs, genes for endogenous retroviral elements are hypomethylated and more highly expressed, leading to double-stranded DNA efflux into the cytoplasm and induction of the interferon response (10). Mouse Dnmt3a- and Tet2-mutant hematopoietic cells (including macrophages and mast cells) exhibit enhanced production of multiple inflammatory cytokines, likely contributing to increased disease risks in individuals with CHIP (1). Thus, Dnmt3a-mutant hematopoietic cells may increase production of the “toxin” (IFN) that Dnmt3a-mutant HSPCs have the antidote for (through Txnip-p53-p21 upregulation), not unlike examples of “selfish” genomic elements that similarly ensure biased propagation through the germline (‘gene drive’). While this response is not detrimental to DNMT3A-mutant HSPCs under normal conditions, azacytidine treatment exacerbates the interferon response and leads to the depletion of the DNMT3A-mutant population, suggesting that while DNMT3A-mutant HSPCs competitively expand despite higher interferon signaling, there is a level after which such signaling becomes detrimental (10). In all, we can appreciate that Dnmt3a-mutant HSCs and their differentiated progeny are not simply gaining a competitive edge from increased IFN signaling, but they are actively responsible for shaping the inflammatory landscape in the bone marrow, a perspective that might be further elucidated in future studies.
Despite the fact that there are still many uncertainties for how Dnmt3a and other CHIP mutations gain their advantage and how this impacts the risk of future hematologic diseases and other negative health impacts, this work demonstrates the promise of identifying pathways that can potentially be manipulated to prevent hematologic malignancies at its roots. This knowledge, combined with research aimed at identifying which patients might benefit from therapies against CHIP, could prove invaluable in reducing mortality by preventing, rather than treating, debilitating diseases like AML.
Authors’ Disclosures
J. DeGregori reports grants from NIH/NIA during the conduct of the study. No disclosures were reported by the other author.
References
- 1. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science 2019;366:eaan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hormaechea-Agulla D, Matatall KA, Le DT, Kain B, Long X, Kus P, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 2021;28:1428–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020;52:1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang CRC, Nix D, Gregory M, Ciorba MA, Ostrander EL, Newberry RD, et al. Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp Hematol 2019;80:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang CR, Ostrander EL, Kukhar O, Mallaney C, Sun J, Haussler E, et al. Txnip enhances fitness of Dnmt3a-mutant hematopoietic stem cells via p21. Blood Cancer Discov 2022;3:220–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jung H, Kim MJ, Kim DO, Kim WS, Yoon SJ, Park YJ, et al. TXNIP maintains the hematopoietic cell pool by switching the function of p53 under oxidative stress. Cell Metab 2013;18:75–85. [DOI] [PubMed] [Google Scholar]
- 7. Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 2018;559:400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung H, Kim DO, Byun JE, Kim WS, Kim MJ, Song HY, et al. Thioredoxin-interacting protein regulates haematopoietic stem cell ageing and rejuvenation by inhibiting p38 kinase activity. Nat Commun 2016;7:13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demerdash Y, Kain B, Essers MAG, King KY. Yin and Yang: The dual effects of interferons on hematopoiesis. Exp Hematol 2021;96:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheller M, Ludwig AK, Göllner S, Rohde C, Krämer S, Stäble S, et al. Hotspot DNMT3A mutations in clonal hematopoiesis and acute myeloid leukemia sensitize cells to azacytidine via viral mimicry response. Nat Cancer 2021;2:527–44. [DOI] [PubMed] [Google Scholar]