Summary:
Here we review the pathophysiology and management of cytokine release syndrome (CRS) secondary to immunotherapy, and potential options for CRS refractory to IL6 inhibition and glucocorticoids, for which there are no proven treatments. To illustrate, we describe a patient with B-cell acute lymphoblastic leukemia who developed refractory grade 4 CRS following CD19-directed chimeric antigen receptor T-cell therapy, treated with tocilizumab, methylprednisolone, siltuximab, and the IFNγ inhibitor emapalumab, with complete remission from leukemia for 12 months.
Introduction
Chimeric antigen receptor (CAR)–modified T cells have emerged as highly effective therapies in the treatment of hematologic malignancies. High rates of remission and durable responses in the treatment of relapsed and refractory pediatric and young adult B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma, and mantle cell lymphoma have resulted in the FDA approval of tisagenlecleucel (Kymriah; Novartis), axicabtagene ciloleucel (Yescarta; Kite Pharma), lisocabtagene maraleucel (Breyanzi; Bristol Myers Squibb), and brexucabtagene autoleucel (Tecartus; Kite Pharma), and there are innumerable CAR T-cell and immune effector cell therapies being studied in clinical and preclinical trials. Tisagenlecleucel resulted in complete remission rates of >80% in children and young adults with relapsed/refractory B-ALL and 40% in adults with relapsed/refractory DLBCL (1, 2). Axicabtagene ciloleucel and lisocabtagene maraleucel have demonstrated similarly impressive responses in DLBCL (3, 4). The successes of these transformative immunotherapies are leading to widespread use but are accompanied by the challenge of managing their major acute toxicities: cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).
CRS is a systemic inflammatory response mediated by immune cell activation and release of high levels of cytokines. Incidence of CRS varies depending on the underlying disease and immunotherapy used, but is reported following CAR T-cell therapies in up to 93% of patients and is grade 3 and above in up to 47% of patients (1, 3). The pathophysiology of CRS involves T-cell proliferation promoting macrophage activation and cytokine release in addition to endothelial dysfunction (5, 6). This cascade results in the onset, at a median of 3 to 7 days after CAR T-cell infusion, of clinical manifestations that range from fever and flu-like symptoms to life-threatening toxicities such as hypotension, vascular leak, coagulopathy, and multi-organ failure (3, 5, 6). In its severe form, CRS symptomatology shares features of hemophagocytic lymphohistiocytosis (HLH), which appears to be on the spectrum of CRS. Indeed, in pediatric patients with ALL treated with tisagenlecleucel (formerly known as CTL019), we found that all patients with severe CRS met HLH-2004 criteria for HLH (5), and the association of CRS with HLH manifestations was recently reported with a CD22-directed CAR T-cell product (7). Common laboratory abnormalities in severe CRS include hyperferritinemia, elevated C-reactive protein (CRP), transaminitis, and coagulopathy marked by hypofibrinogenemia. Abnormal cytokine levels are seen, including elevations in IL1, IL6, IL8, IL10, IFNγ, TNFα, granulocyte macrophage-colony stimulating factor (GM-CSF), IP10 (CXCL10), MCP1, MIG (CXCL9), and MIP1β—a pattern shared with HLH (5, 6, 8). It is notable that both high-grade CRS and HLH manifest substantial elevations in IFNγ (5, 7). In HLH, IFNγ is considered a key driver of inflammation, and interestingly, elevated IFNγ following CAR T-cell infusion and prior to the onset of CRS has been shown to predict grade 4 or 5 CRS (5, 7, 9). In addition, IFNγ is one of the several serum cytokines that are differentially elevated in patients with neurologic toxicity after CAR T-cell therapy (10). However, IFNγ blockade has not been explored in the treatment of CRS, with no blocking agent available until recently.
Consensus recommendations for the management of high-grade CRS include the use of supportive care and CRS-directed therapies, such as cytokine blockade using the IL6 receptor antagonist tocilizumab and glucocorticoids (11). For CRS refractory to these therapies, no standardized treatment regimen exists, but blockade of alternative cytokine pathways (anakinra, IL1 receptor antagonist; siltuximab, IL6 mAb; etanercept, TNFα inhibitor), tyrosine kinase inhibitors (including dasatinib and ruxolitinib), cyclophosphamide, and extracorporeal cytokine adsorption devices have been proposed (11–14). In ICANS, glucocorticoids and supportive care are recommended, but tocilizumab has not been shown to be beneficial (11).
Emapalumab is a fully human anti-IFNγ mAb that was FDA approved for the treatment of adult and pediatric primary HLH in 2018 after demonstrating significant improvements in parameters of HLH including thrombocytopenia, neutropenia, hypofibrinogenemia, and hyperferritinemia in patients that had recurrent or refractory HLH following standard-of-care chemotherapy with dexamethasone and etoposide (9). In a phase II/III study, 10 of 12 patients with neurologic manifestations of HLH showed normalization or improvement in neurologic symptoms of HLH after being treated with emapalumab (9). As IFNγ is thought to be a critical cytokine in driving HLH (9), and elevated IFNγ is associated with and predictive of severe CRS (5) and associated with ICANS (10), it is intriguing to consider as a target for inhibition in the management of refractory and life-threatening CRS or ICANS (12). While theoretical concerns exist that IFNγ blockade could negatively affect T-cell cytolytic activity, preclinical data have suggested equivalent cytotoxicity against hematologic malignancies in vitro and in vivo with IFNγ inhibition or knockout in CAR T cells when compared with IFNγ-producing CAR T cells (15). To our knowledge, the clinical use of emapalumab has not been described in the treatment of CAR T-cell–mediated toxicities. Here, we describe a patient with refractory B-ALL treated with tisagenlecleucel who developed grade 4 CRS refractory to tocilizumab and glucocorticoids, and received siltuximab and emapalumab with subsequent resolution of fever and hypotension and improvements in hyperferritinemia, CRP, and coagulopathy. Leukemia remission and B-cell aplasia were achieved and sustained for 12 months after tisagenlecleucel infusion, suggesting CD19-directed CAR T-cell persistence.
Case Report
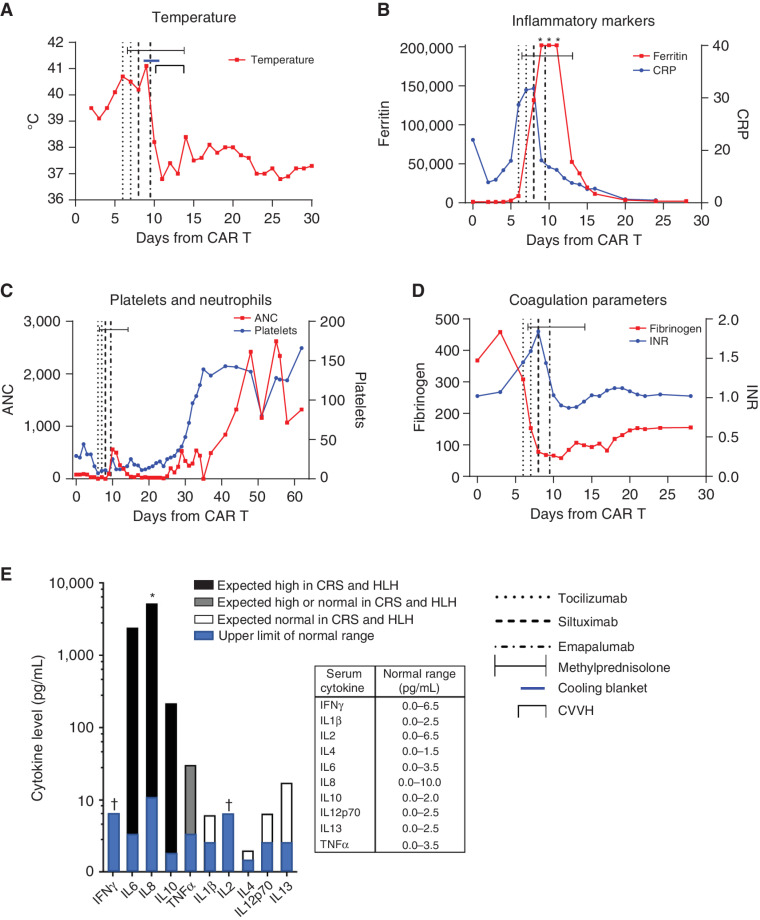
A 19-year-old male presented with his first isolated medullary relapse of B-ALL 4 years after his initial diagnosis. After reinduction, bone marrow aspirate and biopsy (BMBA) showed minimal residual disease (MRD) of 9.7%. He then received a 28-day cycle of blinatumomab, after which his MRD increased to 18% with continued CD19 expression. Due to refractory disease, he was referred to our center for CD19-directed CAR T-cell therapy. The patient underwent leukapheresis and subsequently received bridging chemotherapy with cyclophosphamide, etoposide, and intrathecal methotrexate. He returned to our center for lymphodepleting chemotherapy with cyclophosphamide (500 mg/m2 on days -7 to -6) and fludarabine (30 mg/m2 on days -7 to -4). Prior to tisagenlecleucel infusion, a BMBA demonstrated lymphoblasts accounting for >90% of the cellularity. Outpatient infusion of tisagenlecleucel on day 0 was uncomplicated. On day +2, the patient was admitted with fever to 38.8°C and tachycardia but was normotensive with normal respiratory exam, classified as grade 1 CRS on the American Society for Transplantation and Cellular Therapy (ASTCT) grading scale (11). Blood cultures were obtained, and he was started on broad-spectrum antibiotics. On day +6, he remained febrile and developed hypotension (grade 2 CRS). The patient was given 1 L of normal saline with minimal response. A second 1 L of normal saline was given with improvement in blood pressure. Additional fluid resuscitation included transfusion of platelets and packed red blood cells for ongoing cytopenias. He began showing signs of ICANS with word-finding difficulties and confusion. While still febrile up to 40.5°C, his encephalopathy worsened with agitation. Hypotension worsened, requiring the initiation of norepinephrine and epinephrine infusions (grade 4 CRS), and the patient became hypoxic, requiring supplemental oxygen at 5 L/minute via nasal cannula. Eight mg/kg of tocilizumab was given, and the patient's temperature initially improved to 38°C (Fig. 1A), but he remained on norepinephrine and epinephrine infusions without the ability to wean. On day +7, methylprednisolone 60 mg every 12 hours was started. That same day, the patient's temperature climbed back to 39.7°C, and his hypotension worsened, prompting the addition of a vasopressin infusion and increasing vasopressor infusion rates. He was then given hydrocortisone 100 mg and intubated for worsening circulatory failure. A second 8 mg/kg dose of tocilizumab was given 24 hours after his first dose without a notable change in fever, but vasopressor requirements decreased. On day +8, with ongoing fevers to 40.3°C, stalled vasopressor wean, continued increase in CRP to 21.8 mg/dL, and an increase in ferritin to 113,945 ng/mL (Fig. 1B), the patient was given 11 mg/kg of siltuximab. An echocardiogram was performed demonstrating mildly diminished cardiac function with left ventricular shortening fraction (LVSF) of 26%, left ventricular ejection fraction (LVEF) of 57%, and a small pericardial effusion. He developed profound hypofibrinogenemia and pancytopenia requiring frequent transfusions with cryoprecipitate, platelets, and red blood cells (Fig. 1C and D). A serum cytokine panel sent on day +8 after two doses of tocilizumab and corticosteroids showed elevated IL6, IL8, and IL10, while IFNγ was not elevated (Fig. 1E). On day +9, high fevers continued, and vasopressor requirement increased with worsening renal function and profound coagulopathy. An echocardiogram was repeated and showed moderately to severely diminished cardiac function with an LVSF of 24% and LVEF of 49%. The patient's ferritin climbed above detectable range at >202,000 ng/mL, with a CRP that had downtrended to 8.1 mg/dL, consistent with blockade of IL6 signaling (16). With refractory grade 4 CRS 12 hours after siltuximab and accompanying features of HLH, a decision was made to administer emapalumab on day +9 prior to receiving results of the cytokine panel. In concert, a vecuronium infusion was initiated to allow for therapeutic cooling with a cooling blanket, and he received 1 mg/kg of emapalumab. Following these interventions, his temperature normalized and vasopressors rapidly weaned. By day +10, vasopressin and norepinephrine infusions were discontinued, and the patient remained afebrile. Epinephrine infusion continued to allow diuresis with continuous furosemide infusion. Vecuronium and therapeutic cooling were discontinued. Echocardiogram showed a worsened LVSF of 16% and LVEF (biplane) of 41%, with an elevated brain natriuretic peptide (BNP) of 1,012.7 pg/mL. On day +11, worsening renal function (creatinine 5.0 mg/dL), decreased urine output despite initiation of a continuous furosemide infusion, a weight increase of 9%, and an elevated phosphorous of 6.6 mg/dL prompted initiation of continuous venovenous hemodiafiltration (CVVH). On day +12, milrinone infusion was initiated, and bedside echocardiogram noted qualitatively improved function. On day +14, methylprednisolone was stopped after a 3-day wean, the patient was extubated, and CVVH was discontinued. Following extubation and removal of sedative infusions, his level of alertness improved, but he had ongoing aphasia. Epinephrine and milrinone infusions were weaned and then discontinued on day +15. On day +17, echocardiogram showed a significantly improved and normal LVSF of 36% and LVEF (biplane) of 64%. On day +18, the patient's weight returned to baseline, and his creatinine and fibrinogen normalized. On day +21, he was transferred from the intensive care unit to the stem cell transplant unit. His mental status was back to baseline by that time, but he continued with dysarthria for another week before it too returned to baseline.
Figure 1.
Clinical and laboratory features surrounding the use of emapalumab for refractory CAR T-cell–induced CRS. A, Temperature in degrees Celsius (°C). B, Ferritin in ng/mL, and CRP in mg/dL. C, Absolute neutrophil count (ANC) in cells/μL and platelets ×1,000 cells/μL. D, Fibrinogen in mg/dL and international normalized ratio (INR). E, Serum cytokine levels in pg/mL on a logarithmic scale. Expected cytokine-level elevations from previously published reports (5, 8). Asterisk (*) indicates above limit of quantification. Dagger (†) indicates below limit of quantification.
On day +29, a BMBA and cerebrospinal fluid (CSF) analysis demonstrated MRD-negative remission via flow cytometry (<0.01% of mononuclear cells) and high-throughput sequencing (<1 × 10−5) techniques, and the patient's CSF showed no lymphoblasts. He had an absolute CD19 count of 0 cells per microliter in the peripheral blood. At months 3 and 6 post-tisagenlecleucel, BMBA showed MRD-negative remission with ongoing B-cell aplasia. Clinical remission and B-cell aplasia were sustained for 12 months after infusion.
Discussion
We describe a patient with a high burden of CD19+ B-ALL treated with tisagenlecleucel who developed life-threatening CRS with features of HLH, including persistent fever, hyperferritinemia (>202,000 ng/mL), cytopenias, splenomegaly, and hypofibrinogenemia, that was initially refractory to tocilizumab, and was treated with corticosteroids, a second dose of tocilizumab, siltuximab, and emapalumab with subsequent clinical improvement. As there were numerous interventions including IL6 blockade, corticosteroids, and therapeutic cooling surrounding the use of emapalumab, it is not possible to attribute definitive causality to any particular intervention. However, persistent fever, hypotension, and persistently elevated CRP suggested IL6 blockade was insufficient after two doses of tocilizumab. Furthermore, fever and hypotension persisted, and vasopressor requirements increased after siltuximab, despite a decreasing CRP suggesting effective IL6 blockade. While it is unknown if delayed action of these agents would have led to clinical improvement, the initial refractory nature led to the consideration of additional cytokine blockade using an agent that is indicated in HLH and that blocks IFNγ activity, which is known to be hyperactivated in CRS (5, 6, 8, 12). The timing of emapalumab on day +9 preceded improvements in markers of HLH that had been worsening, as was seen with emapalumab use in primary HLH (9). Fever, ferritin, and CRP measurably improved within a day of emapalumab, and hypofibrinogenemia, acute heart failure, and renal failure, which are typically late findings in CRS, resolved within the following week. However, on day +8 prior to emapalumab, IL6 and IL8 levels were notably elevated, but IFNγ was normal. It is therefore unclear whether IFNγ elevation was associated with CRS severity in this case, although correlative data from clinical trials of tisagenlecleucel would suggest IFNγ substantially increases early in the course of severe CRS (5). CXCL9, induced by IFNγ and thought to be a more sensitive measure of tissue IFNγ (17), was not available. We hypothesize that CXCL9 would have been a more accurate measure of IFNγ pathway activity and blockade in this case; however, limited cytokine data and lack of rapid testing options precluded the use of cytokine levels in clinical decision-making.
This case illustrates the challenges of identifying and studying effective therapies for refractory CRS. In the setting of life-threatening CRS refractory to initial cytokine blockade with the now-standard management of tocilizumab, often multiple interventions are utilized in quick succession, making it difficult to tease out individual contributions. Moreover, additional cytokine blockade may be more effective at an earlier time point. Real-time cytokine measurements may point to potential targets and optimal timing; however, the limited availability of cytokine assays to provide serial measurements with sufficient rapidity challenges their widespread use in clinical practice. Correlative studies and preclinical modeling can assist in identifying potential targets. As elevated IFNγ is associated with and predictive of severe CRS (5) and preclinical studies suggest blockade reduces macrophage activation and cytokine production (15), IFNγ may be a potential target for the treatment of severe CRS. Several other therapies garnered support through preclinical or anecdotal data. Anakinra, an IL1R antagonist, is currently being studied in clinical trials (NCT04432506, NCT04150913, NCT04148430) for the prevention or treatment of CAR-associated CRS and ICANS based on preclinical evidence for the role of IL1 in these toxicities (6, 8). The tyrosine kinase inhibitor dasatinib has been shown to reversibly inhibit cytokine production and CAR T-cell cytotoxicity in preclinical models, suggesting its potential utility in life-threatening CRS (14). While prospective clinical trial data are lacking, these therapies warrant further study.
Ideal management of CRS or ICANS would reduce hyperinflammation and end-organ damage without significantly limiting the cytotoxicity or persistence of CAR T cells. As emapalumab neutralizes IFNγ that CAR T cells and genetically unmodified lymphocytes secrete, there is potential for reduction of CAR T-cell cytotoxicity with IFNγ blockade. Indeed, IFNγ signaling has been shown to be indispensable for CAR T-cell killing in solid tumor models (18). However, there is preclinical evidence that IFNγ blockade or genetic knockout may not impair CAR T-cell cytotoxicity against hematologic malignancies (15). Our patient's ongoing remission and B-cell aplasia 12 months after tisagenlecleucel suggest that use of emapalumab in the setting of refractory grade 4 CRS did not meaningfully interfere with the efficacy or persistence of CAR T cells; however, it is not known whether IFNγ blockade could interfere with CAR T-cell expansion or function in other situations, particularly if utilized prior to peak expansion. Prospective clinical trials of IFNγ blockade are needed to determine the impact on both toxicity and efficacy.
Treatment options for life-threatening CRS and ICANS refractory to first-line treatment with tocilizumab and corticosteroids are needed, as there is no well-studied or standard approach. Suggested alternatives and agents used in the treatment of primary HLH (cyclophosphamide, tyrosine kinase inhibitors, T-cell lytic agents, etoposide) are likely to be toxic to T cells and carry additional toxicity risks. Therefore, targeted cytokine blockade directed at the pathophysiology of HLH and the triggering process is preferable. As the use of T-cell–engaging therapies expands, further study of IFNγ blockade in human subjects with CRS after CAR T-cell and immune effector cell therapy is warranted.
Acknowledgments
The authors thank Michele Paessler, Hamid Bassiri, and Chakkapong Burudpakdee for development and validation of the serum cytokine panel. D.T. Teachey is supported by grants from the NIH (R01CA263837, R01CA193776, R03CA256550), Leukemia & Lymphoma Society, Alex's Lemonade Stand Foundation, and Children's Oncology Group. S.L. Maude is supported by grants from the St. Baldrick's Foundation–Stand Up To Cancer (SU2C-AACR-DT-27-17), V Foundation, and Children's Oncology Group. Stand Up To Cancer is a division of the Entertainment Industry Foundation. The indicated SU2C grant is administered by the American Association for Cancer Research, the scientific partner of SU2C.
Authors' Disclosures
D.T. Teachey reports personal fees from Sobi during the conduct of the study; nonfinancial support from Janssen, grants and personal fees from BEAM Therapeutics, and grants from NeoImmune Tech outside the submitted work; and a patent for US2018252727A1 pending. S.A. Grupp reports research and/or clinical trial support from Novartis, Servier, Vertex, and Kite, and has participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, Allogene, CBMG, Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex/CRISPR, and Roche. S.L. Maude reports grants, personal fees, and nonfinancial support from Novartis; personal fees from Wugen; and personal fees and nonfinancial support from Kite outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt Het al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JPet al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 3. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CAet al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason Jet al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet North Am Ed 2020;396:839–52. [DOI] [PubMed] [Google Scholar]
- 5. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey Net al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lichtenstein DA, Schischlik F, Shao L, Steinberg SM, Yates B, Wang HWet al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T-cells. Blood 2021;138:2469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua Met al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24:739–48. [DOI] [PubMed] [Google Scholar]
- 9. Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao Aet al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med 2020;382:1811–22. [DOI] [PubMed] [Google Scholar]
- 10. Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi Jet al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017;35:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila MLet al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer 2020;8:e001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FLet al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frimmel S, Schipper J, Henschel J, Yu TT, Mitzner SR, Koball S. First description of single-pass albumin dialysis combined with cytokine adsorption in fulminant liver failure and hemophagocytic syndrome resulting from generalized herpes simplex virus 1 infection. Liver Transpl 2014;20:1523–4. [DOI] [PubMed] [Google Scholar]
- 14. Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv 2019;3:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bailey SR, Vatsa S, Larson RC, Bouffard AA, Scarfò I, Kann MCet al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov 2022;3:136–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szalai AJ, van Ginkel FW, Dalrymple SA, Murray R, McGhee JR, Volanakis JE. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 1998;160:5294–9. [PubMed] [Google Scholar]
- 17. Fulkerson PC, Rothenberg ME. Chemokines, CXC | CXCL9 (MIG). In:Laurent GJ, Shapiro SJ, editors. Encyclopedia of respiratory medicine. Amsterdam (the Netherlands): Elsevier; 2006. p.398–402. [Google Scholar]
- 18. Dong E, Yue XZ, Shui L, Liu BR, Li QQ, Yang Yet al. IFN-γ surmounts PD-L1/PD1 inhibition to CAR-T cell therapy by upregulating ICAM-1 on tumor cells. Signal Transduct Target Ther 2021;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]