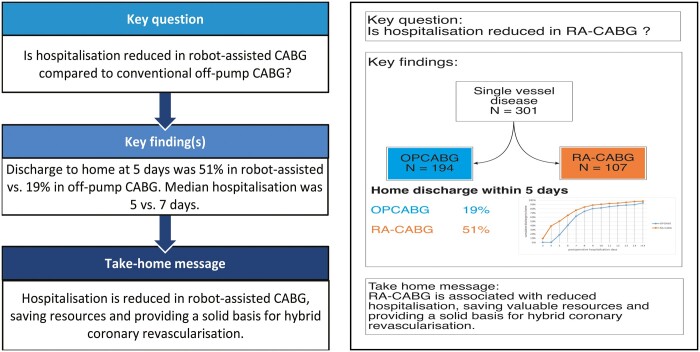
Abstract
OBJECTIVES
Robot-assisted coronary artery bypass grafting (CABG) has been developed as a less invasive alternative for conventional CABG to enhance postoperative recovery, patient satisfaction and early discharge to home. Furthermore, it may provide a basis for hybrid coronary revascularization. To determine the feasibility of this procedure, we compared robot-assisted with conventional off-pump CABG.
METHODS
All consecutive patients undergoing a robot-assisted left internal mammary artery-to-left anterior descending coronary artery procedure were compared to consecutive patients undergoing conventional off-pump CABG for single-vessel disease from October 2016 to July 2019. The primary outcome was discharge to home within 5 days after the operation. Secondary outcomes were total hospital stay, reoperations within 48 h, transfusions, atrial fibrillation, 30-day mortality and quality of life 1 month postoperatively. A propensity matched cohort was assembled to correct for possible confounders.
RESULTS
A total of 107 patients who had robot-assisted CABG were compared to 194 patients who had conventional off-pump CABG. The primary outcome was reached in 51% of the robot-assisted group versus 19% of the conventional off-pump group (P < 0.01). The median postoperative hospital stay was 5 days for the robot-assisted group versus 7 days in the conventional off-pump group (P < 0.01). Other secondary outcomes did not differ significantly between the groups, and the quality of life 1 month after the operation was equal. The results after propensity matching were similar.
CONCLUSIONS
Early discharge to home is more frequent for patients who have robot-assisted CABG than in those who have conventional off-pump CABG, with no difference in health-related quality of life. Therefore, this approach may reduce healthcare resources and provide a solid basis for hybrid coronary revascularization.
Keywords: Off-pump CABG, Robotic surgery, Minimally invasive surgery, Hospitalization, Health-related quality of life
Coronary artery bypass grafting (CABG) is a proven, safe and feasible treatment of coronary artery disease [1, 2].
INTRODUCTION
Coronary artery bypass grafting (CABG) is a proven, safe and feasible treatment of coronary artery disease [1, 2]. The left internal mammary artery (LIMA) on the left anterior descending artery (LAD) undisputedly provides the most significant benefit of CABG. It is associated with a significantly reduced risk of death, myocardial infarction and recurrent angina. Furthermore, long- term patency of the LIMA is high [3, 4]. The LIMA resists atherosclerosis and thrombosis and protects the native coronary tree from disease progression [5]. With the development of minimally invasive surgery, the LIMA can be harvested through a robot-assisted approach combined with a minithoracotomy to anastomose the LIMA to the LAD, avoiding sternotomy or the use of cardiopulmonary bypass. Moreover, surgical trauma is minimized, with reduced pain, shorter hospital stay, faster return to normal activities and improved cosmetics [6]. It has the potential benefits of lower transfusion rate and reduced systemic inflammatory response [7, 8].
So far, there are only a few publications on robot-assisted bypass surgery from European centres and none from the Netherlands [9–11]. Furthermore, there are only a few controlled studies, none of them directly comparing LIMA-LAD robot-assisted CABG (RA-CABG) to conventional off-pump CABG (OPCABG) [11–13]. We evaluated the effect of RA-CABG on hospital stay, discharge destination and health-related quality of life (HRQoL) compared to OPCABG. Safety parameters were also studied.
PATIENTS AND METHODS
Ethics statement
The ethical committee of Isala Zwolle, The Netherlands, approved the protocol and waived the need for formal evaluation according to the Dutch Law on Scientific Medical Research with Humans (reference: 21.0114). Data were anonymized before analysis.
Study design
All consecutive patients undergoing RA-CABG from January 2016 to October 2019 were included. Patients were selected for RA-CABG if their anatomy was suitable for a robotic approach (significant proximal LAD disease, not intramyocardial or of poor quality) and if logistic criteria were met (availability of the da Vinci robot and team). Exclusion criteria were a significant lesion of the diagonal branch, history of subclavian artery or LIMA stenosis, previous left-sided thoracic surgery, lung adhesions, severe chronic obstructive pulmonary disease, severe pulmonary hypertension, morbid obesity (body mass index > 40), and general contraindications for off-pump surgery like haemodynamic instability and the need for additional valve surgery. In case of conversion to full sternotomy, patients were analysed as RA-CABG (intention-to-treat).
We constructed a control group from all consecutive patients with single-vessel disease who underwent OPCABG, in whom only the LIMA was used with a maximum of 2 distal and no proximal anastomoses, no Y-grafts, from 1 January 2016 until December 2019. We excluded patients who had an emergency operation, patients with severely reduced left ventricular function and those with off-pump procedures due to a porcelain aorta. All data were collected from our prospective managed registry of cardiac surgery procedures.
Outcome parameters
Primary outcome was discharge to home within 5 days postoperatively. Secondary outcomes were total hospitalization time, reoperations within 48 h, transfusion of red blood cell concentrate, atrial fibrillation, 30-day mortality and HRQoL. Furthermore, data on the conversion rate to sternotomy (in the RA-CABG group), neurological complications and postoperative infarction were collected.
HRQoL was measured using the Medical Outcomes Study Questionnaire Short Form 36 (SF-36), a well-documented and validated self-reporting questionnaire. Patients completed the forms 1 month after surgery.
Definitions
Time in the hospital included a possible stay in referring hospitals.
Patients were discharged to home when they met identical criteria for all cardiac surgery procedures: good clinical condition with no need of opioids for pain management, free from drains, intravenous medication and additional oxygen supply, no signs of infection, able to walk stairs, able to wash or take a shower independently.
Myocardial infarction was defined as CK-MB >90 U/l and wall movement abnormalities by echocardiography within 48 h postoperatively. The feasibility outcome was defined by the percentage of patients who needed to be converted from robot-assisted CABG to full sternotomy off-pump CABG.
Operative procedure for robot-assisted coronary artery bypass grafting
Patients were anaesthetized, intubated with a single-lumen tube with a bronchial blocker (VivaSight-SL, Ambu A/S, Ballerup, Denmark) and mechanically ventilated. Patients were positioned supine with a slight elevation of the left side of the chest. Using an endobronchial blocker, the left lung was deflated, and the first port placement was made in the fifth intercostal space just lateral to the midclavicular line, medial to the nipple. Carbon dioxide was inflated via this port at a target pressure between 7 and 10 mmHg throughout the procedure. The da Vinci robot (Intuitive Surgical, Sunnyvale, CA, USA), positioned on the patient's right side (Fig. 1), was docked, and the robotic cautery spatula and forceps instruments were inserted. Pericardial fat was dissected, and the pericardium was opened at the site where the LAD was suspected. Judgement was made about the suitability and accessibility of the LAD for grafting. In case of dense adhesions or a not visible or deep intramural course of the LAD, the robotic procedure was converted to an open procedure. The LIMA was harvested and skeletonized, with a narrow pedicle near its origin to its bifurcation at the xiphoid. The patient was heparinized to a target activated clotting time > 300 s. Two clips were applied to the distal end, and the LIMA was divided between the clips. The robot was undocked and removed from the operative field.
Figure 1:
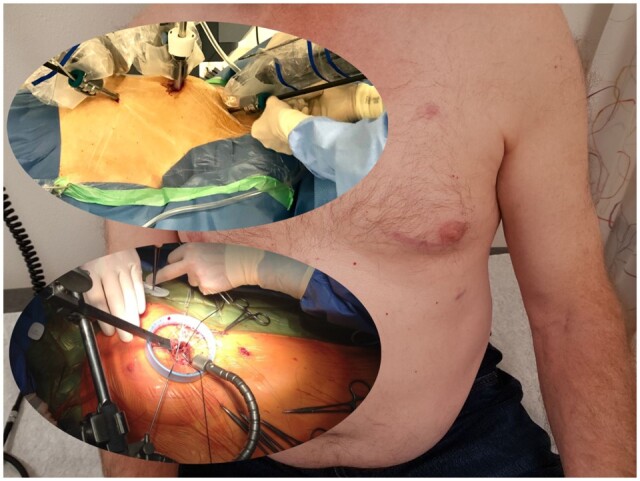
Port placement and exposure during left internal mammary artery anastomosis and cosmetic result of robot-assisted coronary artery bypass grafting.
The camera port, just medial to the nipple in the fifth intercostal space, was extended 4 cm medially. A soft tissue retractor (Alexis Retractor, Applied Medical California, Rancho Santa Margarita, CA) was inserted, providing maximum exposure without rib spreading. An Octopus Nuvo tissue stabilizer (Medtronic, Minneapolis, MN, USA) was inserted and used to stabilize the LAD through the port site in the seventh intercostal space. The LAD was opened at the selected anastomosis site after it was surrounded proximally and distally with vessel loops. A LIMA-to-LAD end to side anastomosis was performed using a standard off-pump technique. Finally, an intercostal nerve block was applied, and all surgical incisions were closed in a standard manner, leaving a chest tube through the port site in the seventh intercostal space.
Statistics
Statistical analyses were performed with SPSS software (version 25 for macOS; SPSS Inc., Chicago, IL, USA). Graphical analyses were performed with Microsoft Excel. Quantitative variables were expressed as the mean with standard deviation when normally distributed and categorical variables as frequencies with percentages. The median with the interquartile range (IQR) was used for non-normally distributed continuous variables. Normality of continuous data was tested with the Shapiro-Wilk test. Continuous data were compared using the two-sided Student t-test or the Mann-Whitney test for non-normally distributed variables. A χ2 test was used to compare the differences between categorical variables.
Health-related quality of life: The raw data from the questionnaire were edited to calculate the scores on the 8 different component scales: physical functioning, role limitations due to physical problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems and mental health. These scores were further processed to compute a mental component score (MCS) and a physical component score (PCS), such that a score of 50 represents the average of an age-adjusted Dutch population.
Missing data: patients with missing data on the primary outcome parameter were excluded from that analysis. Missing data on HRQoL were replaced with medians only if a completed test was present.
A propensity score was calculated from age, renal function, comorbidity and anticoagulant use. A 1:1 propensity score matched cohort was constructed, based on nearest neighbour matching, with a tolerance of 0.1.
RESULTS
A total of 107 patients underwent RA-CABG and 194 patients had OPCABG for single-vessel disease. Baseline characteristics of the 2 groups are represented in Table 1. The median age in the RA-CABG group was lower (65 years vs 69 years, P = 0.02) than in the OPCABG group, and renal function (expressed as eGFR) was better (76.2 vs 70.3 ml/kg/m2) (P = 0.05). More patients with neurological dysfunction (4.7% vs 0.5%, P = 0.01) were in the RA-CABG group. Fewer patients were taking clopidogrel in the RA-CABG group (27.1% vs 44%, P < 0.01). All other differences between the groups were not statistically significant. In the propensity score matched cohort of 102 patients with RA-CABG and 102 with ROBO-CABG, there were no significant differences between the groups.
Table 1:
Patient characteristics
| Original data |
Propensity matched |
|||||||
|---|---|---|---|---|---|---|---|---|
| OPCABG | RA-CABG | P-value | SMD | OPCABG | RA-CABG | P-value | SMD | |
| (N = 194) | (N = 107) | (N = 102) | (N = 102) | |||||
| Age, median (IQR) | 67.7 (10.2) | 65.3 (8.6) | 0.04 | 0.255 | 66.2 (10.4) | 65.3 (8.7) | 0.47 | 0.102 |
| Logistic EuroSCORE I, median (IQR) | 3.3 (2.8) | 2.94 (3.4) | 0.32 | 0.118 | 2.9 (2.7) | 2.7 (2.3) | 0.45 | 0.106 |
| eGFR, median (IQR) | 70.3 (20.1) | 75.4 (17.8) | 0.03 | 0.273 | 73.4 (19.2) | 75.1 (17.8) | 0.52 | 0.089 |
| Female gender, N (%) | 42 (21.6) | 30 (28.0) | 0.27 | 0.148 | 24 (23.5) | 27 (26.5) | 0.75 | 0.068 |
| Diabetes, N (%) | 0.06 | 0.295 | 0.92 | 0.058 | ||||
| Type 1 | 3 (1.5) | 4 (3.7) | 3 (2.9) | 4 (3.9) | ||||
| Type 2 | 50 (25.8) | 16 (15.0) | 17 (16.7) | 16 (15.7) | ||||
| No diabetes | 141 (72.7) | 87 (81.3) | 82 (80.4) | 82 (80.4) | ||||
| Hypertension, N (%) | 117 (60.3) | 56 (52.3) | 0.22 | 0.161 | 67 (65.7) | 54 (52.9) | 0.09 | 0.262 |
| Recent myocardial infarction, N (%) | 55 (28.4) | 20 (18.7) | 0.09 | 0.229 | 18 (17.6) | 19 (18.6) | 1.00 | 0.025 |
| Atrial fibrillation, N (%) | 8 (4.1%) | 3 (2.8%) | 0.79 | 0.072 | 3 (2.9%) | 2 (2.0%) | 1.00 | 0.063 |
| Chronic pulmonary disease, N (%) | 14 (7.2) | 15 (14.0) | 0.09 | 0.222 | 12 (11.8) | 12 (11.8) | 1.00 | <0.001 |
| Extracardiac arteriopathy, N (%) | 11 (5.7) | 8 (7.5) | 0.71 | 0.073 | 4 (3.9) | 7 (6.9) | 0.54 | 0.130 |
| Neurological dysfunction, N (%) | 1 (0.5) | 5 (4.7) | 0.04 | 0.264 | 1 (1.0) | 3 (2.9) | 0.61 | 0.142 |
| LVEF = 30-50%, N (%) | 41 (21.1) | 25 (23.4) | 0.76 | 0.054 | 21 (20.6) | 23 (22.5) | 0.87 | 0.048 |
| Aspirin, N (%) | 159 (82.0) | 85 (79.4) | 0.70 | 0.064 | 87 (85.3) | 81 (79.4) | 0.36 | 0.155 |
| Clopidogrel, N (%) | 86 (44.3) | 29 (27.1) | 0.01 | 0.365 | 25 (24.5) | 28 (27.5) | 0.75 | 0.067 |
| Ticagrelor, N (%) | 5 (2.6) | 4 (3.7) | 0.83 | 0.066 | 2 (2.0) | 4 (3.9) | 0.68 | 0.116 |
| Coumadin, N (%) | 11 (5.7) | 6 (5.6) | 1.00 | 0.003 | 3 (2.9) | 5 (4.9) | 0.72 | 0.101 |
CABG: coronary artery bypass grafting; eGFR: estimated glomerular filtration rate; IQR: interquartile range; LVEF: left ventricular ejection fraction; OPCABG: conventional off-pump CABG; RA-CABG: robot-assisted CABG; SMD: standardized mean difference.
Outcome parameters
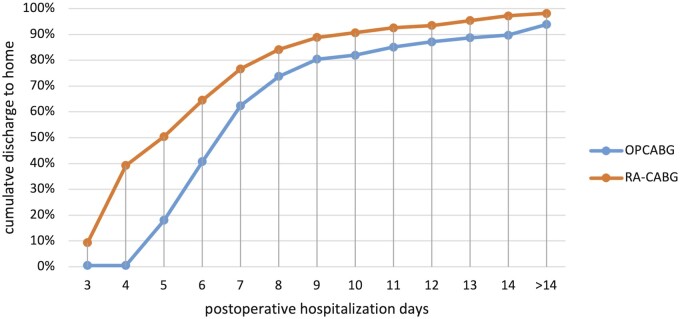
Outcome parameters are represented in Table 2. Figure 2 is a graphical representation of the postoperative hospitalization time in both groups. Data on total postoperative hospitalization days were missing for 14 patients (4.6%): 12 in the OPCABG group (6.2%) and 2 in the RA-CABG group (1.9%). Whereas some of these patients stayed more than 5 postoperative days in Isala, data on the primary outcome were missing for 7 patients (2.3%), all in the OPCABG group. The primary outcome, discharge to home within 5 days, was reached in 51% of the patients who had RA-CABG compared to 19% of the patients who had OPCABG (P < 0.01). In the propensity score matched cohort, data on total postoperative hospitalization were missing for 6 patients: 4 in the OPCABG group and 2 in RA-CABG group; data on the primary outcome were missing in 3 patients, all in the OPCABG group. The primary outcome was reached in 20% in the OPCABG group versus 51% in the RA-CABG group (P < 0.01).
Table 2:
Primary and secondary outcomes in both study groups
| Original data | Propensity matched | |||||
|---|---|---|---|---|---|---|
| OPCABG | RA-CABG | P-value | OPCABG | RA-CABG | P-value | |
| (N = 194) | (N = 107) | (N = 102) | (N = 102) | |||
| Home discharge within 5 days, N (%) | 35 (19%) | 54 (51%) | <0.01 | 20 (20%) | 52 (51%) | <0.01 |
| Total postoperative hospitalization, median (IQR) in days | 7 (6-8) | 5 (4-7) | <0.01 | 7 (6-8) | 5 (4-7) | <0.01 |
| Resternotomy within 48 h, N (%) | 5 (2.6) | 2 (1.9) | 0.70 | 2 (2.0) | 2 (2.0) | 1.0 |
| Atrial fibrillation, N (%) | 64 (33.0) | 21 (19.6) | 0.01 | 28 (27.5) | 20 (19.6) | 0.19 |
| Transfusion RBC, N (%) | 17 (8.8%) | 10 (9.3%) | 0.87 | 7 (6.9%) | 9 (8.8%) | 0.60 |
| 30-Day mortality, N (%) | 0 (0) | 0 (0) | NA | 0 | 0 | NA |
| SF-36 PF | 70 (55-80) | 72.5 (55-85) | 0.41 | 70 (52.5-80) | 72.5 (55-85) | 0.43 |
| SF-36 RP | 31.3 (18.8-50) | 31.3 (18.8-50) | 0.37 | 31.3 (18.8-56.3) | 31.3 (18.8-50) | 0.65 |
| SF-36 BP | 52 (41-74) | 62 (41-74) | 0.55 | 52 (41-74) | 62 (41-74) | 0.23 |
| SF-36 GH | 72 (55-82) | 65 (55-77) | 0.06 | 68.5 (52-82) | 65 (55-77) | 0.22 |
| SF-36 VT | 55 (45-65) | 55 (45-60) | 0.11 | 55 (40-65) | 55 (45-60) | 0.40 |
| SF-36 SF | 62.5 (37.5-75) | 62.5 (37.5-75) | 0.54 | 62.5 (37.5-81.3) | 62.5 (50-75) | 0.82 |
| SF-36 RE | 66.7 (41.7-100) | 66.7 (50-100) | 0.50 | 75 (37.5-100) | 66.7 (50-100) | 0.88 |
| SF-36 MH | 72 (60-80) | 72 (60-80) | 0.50 | 72 (54-80) | 72 (56-80) | 0.83 |
| SF-36 MCS | 48.7 (43.7-53.0) | 48.8 (43.3-52.7) | 0.54 | 48.0 (42.3-53.1) | 48.9 (43.4-53.1) | 0.88 |
| SF-36 PCS | 46.6 (39.8-51.1) | 45.8 (39.0-50.0) | 0.45 | 46.8 (37.4-51.8) | 45.8 (38.8-50.0) | 0.59 |
BP: bodily pain; CABG: coronary artery bypass grafting; GH: general health; IQR: interquartile range; MCS: mental component score; MH: mental health; OPCABG: conventional off-pump CABG; PCS: physical component score; PF: physical function; RA-CABG: robot-assisted CABG; RBC: red blood cell concentrate; RE: role limitations due to emotional problems; RP: role limitations due to physical problems; SF: social functioning; SF-36: Medical Outcomes Short Form-36 questionnaire; VT: vitality.
Figure 2:
Home discharge after surgery, in cumulative percentages from group total, by surgery type, in the first 2 weeks after surgery. OPCABG: traditional off-pump coronary artery bypass surgery; RA-CABG: robot-assisted coronary artery bypass surgery.
The median postoperative hospital stay was significantly shorter in the RA-CABG group: 5 days (IQR 4–7) in the RA-CABG group versus 7 days (IQR 6–8) in the OPCABG group (P < 0.01). In addition, in the OPCABG group, 6 (3.1%) patients were transferred to a nursing home or rehabilitation centre versus 1 in the RA-CABG group (0.9%) (P = 0.23). These numbers were similar in the propensity score matched cohort; median postoperative hospitalization was 7 (IQR 6–8) days in the OPCABG group and 5 (IQR 4–7) in the RA-CABG group (P < 0.01). In this cohort, 2 patients who had OPCABG and 1 patient who had RA-CABG were transferred to a nursing home.
In the RA-CABG group, 2 patients had reinterventions within 48 h. In the OPCABG group, 5 (2.6%) patients required resternotomy within 48 h.
No deaths had occurred in either group 30 days postoperatively.
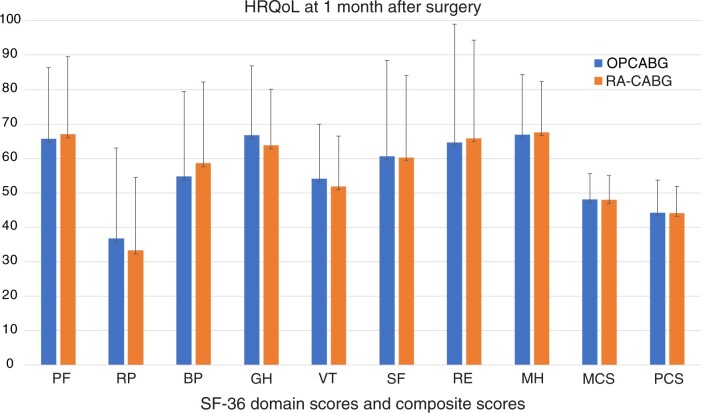
The mean observed scores from the SF-36 questionnaire in both groups, one month after surgery, are displayed in (Fig. 3). The percentage of complete SF-36 scales was comparable between the 2 groups: in the propensity matched group, 82% (84/102) in the control group and 88% (90/102) in the RA-CABG group. In addition, the scores on the 8 components were not significantly different: Both groups had comparable composite HRQoL scores (MCS and PCS) 1 month after surgery.
Figure 3:
Health-related quality of life in the propensity score matched cohort from the Medical Outcomes Short Form-36 questionnaire 1 month after surgery. Bars are mean scores with standard deviation, for each of the 8 domains, combined with a mental component score and a physical component score. BP: bodily pain; CABG: coronary artery bypass surgery; GH: general health; HRQoL: health-related quality of life; MCS: mental component score; MH: mental health; OPCABG: traditional off-pump CABG; PCS: physical component score; PF: physical function; RA-CABG: robot-assisted CABG; RE: role limitations due to emotional problems; RP: role limitations due to physical problems; SF: social functioning; VT: vitality.
Safety outcome parameters are represented in Table 3. Conversion to sternotomy was 6.5% (n = 7) of the 107 included patients who had RA-CABG. Reasons were poorly identifiable LAD because of pericardial adhesions, possibly caused by preoperative pericarditis (n = 2), severe bleeding of the subclavian vein during preparation of the LIMA (n = 1), no adequate blood flow through the LIMA after takedown (n = 1), a strongly calcified LAD (n = 1) and blood in the pericardium of unknown origin before harvesting the LIMA (n = 2). Of the latter 2, in 1 case no explanation for the blood was found, and in 1 patient, the preoperative placement of the central venous line was responsible for the blood in the pericardium.
Table 3:
Additional safety outcome parameters after both conventional off-pump coronary artery bypass grafting and robot-assisted coronary artery bypass grafting
| OPCABG | RA-CABG | P-value | |
|---|---|---|---|
| N = 194 | N = 107 | ||
| Count(%) | Count(%) | ||
| Conversion to sternotomy, N (%) | NA | 7 (6.7) | NA |
| Myocardial infarction, N (%) | 0 (0) | 2 (1.9) | 0.06 |
| Neurological complication, N (%) | 0 (0) | 0 (0) | NA |
CABG: coronary artery bypass grafting; OPCABG: off-pump CABG; RA-CABG: robot-assisted CABG.
There were no neurological complications in either group. There were no peri- or postoperative myocardial infarctions in the OPCABG group. In the RA-CABG group, myocardial infarction was observed in 2 patients (1.9%).
DISCUSSION
Robot-assisted CABG is associated with early discharge to home and reduced hospitalization compared to conventional OPCABG. The HRQoL 1 month after surgery was equal. The significant reduction in total postoperative hospital stay for patients undergoing RA-CABG is in line with the data reported in the literature [6, 10–20]. It should be noted that no additional attempt was made for early discharge in the RA-CABG group. A timelier discharge policy may further reduce the hospitalization days of the RA-CABG group.
In 107 RA-CABG cases in this study, the short-term results are comparable to those of conventional off-pump CABG concerning safety parameters. The percentage of reinterventions for bleeding in the RA-CABG group is in line with the numbers reported in the literature [12, 20]. There was no significant difference in reinterventions for bleeding between the groups. There were no neurological complications, and at 30 days, no deaths occurred in either group.
The conversion rate to sternotomy is in line with the data in the literature, although reported numbers vary widely [6, 13, 16, 20, 21]. An initial learning curve may contribute to this range. As we continued to perform these procedures, the conversion rate declined. Other studies confirm a decrease in the number of conversions with increased experience [20]. Furthermore, conversion should not primarily be seen as a failure [6]. We have a low threshold for conversion because patient safety is paramount, and sternotomy is a safe alternative.
Most of the patients in our study were treated for a single LAD lesion [3, 4, 22]. Su et al. compared multivessel RA-CABG with percutaneous cardiac intervention and observed that the numbers of in-hospital and long-term deaths, myocardial infarctions and strokes were similar [23]. Nevertheless, the incidence of target vessel revascularization was lower in the RA-CABG group. Therefore, for patients with multivessel disease, a hybrid approach of robot-assisted LIMA-LAD grafting combined with stenting of non-LAD lesions could be a prime revascularization strategy [24, 25]. Initial results are promising; however, the benefits of such a hybrid revascularization approach for multivessel disease have to be validated in larger multicentre prospective clinical trials [25–27].
The SF-36 response rate of over 80% in both groups after 1 month is high. We observed a similar quality of life in both groups 1 month after surgery. Our data are comparable to findings in patients from the SYNTAX study who had CABG [28]. In a recent meta-analysis of patients who had CABG, their quality of life improved compared to baseline at 1 year [29]. However, we wanted to compare early quality of life between the groups. Therefore, we did not include baseline and 1-year measurements. A recent study comparing robot-assisted to on-pump CABG found superior quality of life values (SF-36) for patients who had robot-assisted surgical procedures, although initial pain scores were higher [30]. Our observed scales are in between the reported scales in this study. Unfortunately, the balanced PCS and MCS are not reported and also, the moment the SF-36 was taken is lacking, making comparisons with our findings more complicated.
The fact that we could not detect any difference between the groups may have several explanations. First, until now, our postoperative rehabilitation has been focused on patients who had sternotomies. Second, although initial healing is faster with the minimally invasive approach, residual pain or discomfort may remain longer, limiting full activity. Finally, patients without sternotomy are encouraged to return to normal activity faster but may feel more impaired by their operation. To discriminate among these possibilities, a diary with activities, in combination with a registry of the use of pain medication and pain scores, would be required.
This study had several limitations. First, there was a selection bias: patients who had RA-CABG were selected based on their coronary anatomy and absence of significant comorbidities. We did not include a severity score for LAD lesions (SYNTAX score). However, the control group in the propensity matched cohort did not differ significantly in age or comorbidities.
Second, all procedures were performed at a single centre, and a single surgeon performed the robot-assisted procedures. This situation is understandable, because this is a relatively new technique with a specific learning curve. Therefore, more long-term follow-up is needed.
Third, the numbers in this study are relatively small, and the incidence of adverse outcomes is low, increasing the possibility of a type II error. Larger multicentre studies are required to study the safety of this approach reliably.
Finally, although the initial costs are higher, this strategy may have a financial benefit in light of shorter hospital stays and faster recovery leading to earlier participation in society. An attempt is underway to gather nationwide data for robot-assisted revascularization.
CONCLUSION
RA-CABG is a safe procedure, leading to early home discharge and equal quality of life compared to routine OPCABG, thus saving valuable resources and providing a solid basis for hybrid coronary revascularization. However, further cost-effectiveness analysis is necessary.
Funding
There was no external funding for this study.
Conflict of interest: All of the authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) in the matter discussed in this article.
Data availability
The data used for this manuscript can be shared upon request with the corresponding author.
Author contributions
Alexander Spanjersberg: Formal analysis; Writing–original draft; Writing–review & editing; Leendert Hoek: Writing–original draft; Writing–review & editing; Formal analysis; Project administration; Jan Paul Ottervanger: Supervised and validated the analysis; Writing–review & editing; Thi-Yen Nguyen: Data curation and visualization; Emel Kaplan: Conceptualization; Investigation; Roland Laurens: Investigation; Writing–review & editing; Sandeep Singh: Conceptualization; Investigation; Writing–review & editing.
Glossary
Abbreviations
- CABG
Coronary artery bypass grafting
- HRQoL
Health-related quality of life
- LAD
Left anterior descending coronary artery
- LIMA
Left internal mammary artery
- MCS
Mental component score
- OPCABG
Off-pump CABG
- PCS
Physical component score
- RA-CABG
Robot-assisted coronary artery bypass grafting
- RE
Role limitations due to emotional problems
- SF-36
Medical Outcomes Short Form 36
Contributor Information
Alexander Spanjersberg, Division Cardiothoracic Anesthesiology: Department of Anesthesiology and Intensive Care, Isala Heart Centre, Isala Zwolle, Netherlands.
Leendert Hoek, ICON, Early development services, Groningen, Netherlands.
Jan Paul Ottervanger, Department of Cardiology, Isala Heart Centre, Isala Zwolle, Netherlands.
Thi-Yen Nguyen, Division Cardiothoracic Anesthesiology: Department of Anesthesiology and Intensive Care, Isala Heart Centre, Isala Zwolle, Netherlands.
Emel Kaplan, Universitätsspital Basel, Switzerland.
Roland Laurens, Department of Cardiothoracic Surgery, Isala Heart Centre, Isala Zwolle, Netherlands.
Sandeep Singh, Department of Cardiothoracic Surgery, Isala Heart Centre, Isala Zwolle, Netherlands.
REFERENCES
- 1. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, ESC Scientific Document Group et al. 2018 ESC/EACTS Guidelines on myocardial revascularisation. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 2. Members WC, Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM. et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;79:e21–129. [DOI] [PubMed] [Google Scholar]
- 3. Yusuf S, Zucker D, Passamani E, Peduzzi P, Takaro T, Fisher LD. et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563–70. [DOI] [PubMed] [Google Scholar]
- 4. Boylan MJ, Lytle BW, Loop FD, Taylor PC, Borsh JA, Goormastic M. et al. Surgical treatment of isolated left anterior descending coronary stenosis: comparison of left internal mammary artery and venous autograft at 18 to 20 years of follow-up. J Thorac Cardiovasc Surg 1994;107:657–62. [PubMed] [Google Scholar]
- 5. Otsuka F, Yahagi K, Sakakura K, Virmani R.. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg 2013;2:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonatti J, Wallner S, Crailsheim I, Grabenwöger M, Winkler B.. Minimally invasive and robotic coronary artery bypass grafting—a 25-year review. J Thorac Dis 2021;13:1922–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie ME, Romsa J, Fox SA, Vezina WC, Akincioglu C, Warrington JC et al Long-Term Angiographic Follow-Up of Robotic-Assisted Coronary Artery Revascularization. Ann Thorac Surg 2012;93:1426–31. [DOI] [PubMed] [Google Scholar]
- 8. Lee JD, Bonaros N, Hong PT, Kofler M, Srivastava M, Herr DL. et al. Factors Influencing Hospital Length of Stay After Robotic Totally Endoscopic Coronary Artery Bypass Grafting. Ann Thorac Surg 2013;95:813–8. [DOI] [PubMed] [Google Scholar]
- 9. Caynak B, Sagbas E, Onan B, Onan IS, Sanisoglu I, Akpinar B.. Robotically enhanced coronary artery bypass grafting: the feasibility and clinical outcome of 196 procedures. Int J Med Robot 2009;5:170–7. [DOI] [PubMed] [Google Scholar]
- 10. Reichenspurner H, Boehm DH, Gulbins H, Detter C, Damiano R, Mack M. et al. Robotically assisted endoscopic coronary artery bypass procedures without cardiopulmonary bypass. J Thorac Cardiovasc Surg 1999;118:960–1. [DOI] [PubMed] [Google Scholar]
- 11. Sabashnikov A, Patil NP, Weymann A, Mohite PN, Zych B, Sáez DG. et al. Outcomes after different non-sternotomy approaches to left single-vessel revascularisation: a comparative study with up to 10-year follow-up. Eur J Cardio-Thorac 2014;46:e48–55–e55. [DOI] [PubMed] [Google Scholar]
- 12. Leyvi G, Forest SJ, Srinivas VS, Greenberg M, Wang N, Mais A. et al. Robotic Coronary Artery Bypass Grafting Decreases 30-Day Complication Rate, Length of Stay, and Acute Care Facility Discharge Rate Compared with Conventional Surgery. Innovations�(Phila) 2014;9:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gong W, Cai J, Wang Z, Chen A, Ye X, Li H. et al. Robot-assisted coronary artery bypass grafting improves short-term outcomes compared with minimally invasive direct coronary artery bypass grafting. J Thorac Dis 2016;8:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subramanian VA, Patel NU, Patel NC, Loulmet DF.. Robotic Assisted Multivessel Minimally Invasive Direct Coronary Artery Bypass With Port-Access Stabilization and Cardiac Positioning: paving the Way for Outpatient Coronary Surgery? Ann Thorac Surg 2005;79:1590–6. [DOI] [PubMed] [Google Scholar]
- 15. DeRose JJ, Balaram SK, Ro C, Swistel DG, Singh V, Wilentz JR. et al. Mid-term results and patient perceptions of robotically-assisted coronary artery bypass grafting. Interact CardioVasc Thorac Surg 2005;4:406–11. [DOI] [PubMed] [Google Scholar]
- 16. Turner WF, Sloan JH.. Robotic-Assisted Coronary Artery Bypass on a Beating Heart: initial Experience and Implications for the Future. Ann Thorac Surg 2006;82:790–4. [DOI] [PubMed] [Google Scholar]
- 17. Srivastava S, Gadasalli S, Agusala M, Kolluru R, Naidu J, Shroff M. et al. Use of Bilateral Internal Thoracic Arteries in CABG Through Lateral Thoracotomy With Robotic Assistance in 150 Patients. Ann Thorac Surg 2006;81:800–6. [DOI] [PubMed] [Google Scholar]
- 18. BACHINSKY WB, ABDELSALAM M, BOGA G, KILJANEK L, MUMTAZ M, MCCARTY C.. Comparative Study of Same Sitting Hybrid Coronary Artery Revascularization versus Off‐Pump Coronary Artery Bypass in Multivessel Coronary Artery Disease. J Interv Cardiol 2012;25:460–8. [DOI] [PubMed] [Google Scholar]
- 19. Poston RS, Tran R, Collins M, Reynolds M, Connerney I, Reicher B. et al. Comparison of Economic and Patient Outcomes With Minimally Invasive Versus Traditional Off-Pump Coronary Artery Bypass Grafting Techniques. Transactions Meet Am Surg Assoc 2008;126:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giambruno V, Chu MW, Fox S, Swinamer SA, Rayman R, Markova Z. et al. Robotic‐assisted coronary artery bypass surgery: an 18‐year single‐centre experience. Int J Med Robot 2018;14:e1891. [DOI] [PubMed] [Google Scholar]
- 21. Yang M, Wu Y, Wang G, Xiao C, Zhang H, Gao C.. Robotic Total Arterial Off-Pump Coronary Artery Bypass Grafting: seven-Year Single-Center Experience and Long-Term Follow-Up of Graft Patency. Ann Thorac Surg 2015;100:1367–73. [DOI] [PubMed] [Google Scholar]
- 22. Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW. et al. Influence of the Internal-Mammary-Artery Graft on 10-Year Survival and Other Cardiac Events. N Engl J Med 1986;314:1–6. [DOI] [PubMed] [Google Scholar]
- 23. Su C-S, Shen C-H, Chang K-H, Lai C-H, Liu T-J, Chen K-J. et al. Clinical outcomes of patients with multivessel coronary artery disease treated with robot-assisted coronary artery bypass graft surgery versus one-stage percutaneous coronary intervention using drug-eluting stents. Medicine (Baltimore) 2019;98:e17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gąsior M, Zembala MO, Tajstra M, Filipiak K, Gierlotka M, Hrapkowicz T, POL-MIDES (HYBRID) Study Investigators et al. Hybrid Revascularisation for Multivessel Coronary Artery Disease. Jacc Cardiovasc Interventions 2014;7:1277–83. [DOI] [PubMed] [Google Scholar]
- 25. Hannan EL, WU Y-F, Cozzens K, Tamis-Holland J, Ling FSK, Jacobs AK et al Hybrid coronary revascularisation vs. percutaneous coronary interventions for multivessel coronary artery disease. J Geriatric Cardiol Jgc 2021;18:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tajstra M, Hrapkowicz T, Hawranek M, Filipiak K, Gierlotka M, Zembala M, POL-MIDES Study Investigators et al. Hybrid Coronary Revascularization in Selected Patients With Multivessel Disease 5-Year Clinical Outcomes of the Prospective Randomized Pilot Study. Jacc Cardiovasc Interventions 2018;11:847–52. [DOI] [PubMed] [Google Scholar]
- 27. Praet KMV, Kofler M, Shafti TZN, Al AAE, Kampen A van, Amabile A. et al. Minimally Invasive Coronary Revascularisation Surgery: a Focused Review of the Available Literature. Interventional Cardiol Rev 2021;16:e08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen DJ, Hout BV, Serruys PW, Mohr FW, Macaya C, den HP, Synergy between PCI with Taxus and Cardiac Surgery Investigators et al. Quality of Life after PCI with Drug-Eluting Stents or Coronary-Artery Bypass Surgery. New Engl J Medicine 2011;364:1016–26. [DOI] [PubMed] [Google Scholar]
- 29. Creber RM, Dimagli A, Spadaccio C, Myers A, Moscarelli M, Demetres M. et al. Effect of coronary artery bypass grafting on quality of life: a meta-analysis of randomised trials. European Hear J - Qual Care Clin Outcomes 2021; gcab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ezelsoy M, Oral K, Caynak B, Bayramoglu Z, Akpinar B.. Pain and the Quality of Life Following Robotic Assisted Minimally Invasive Surgery. Hsf 2016;19:165–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this manuscript can be shared upon request with the corresponding author.