Abstract
OBJECTIVES
Preoperative selection bias led to the inability to generalize the proposed benefit of subxiphoid uniportal video-assisted thoracoscopic surgery (SVATS) as having less postoperative pain than uniportal intercostal VATS. So, we conducted this prospective, single-blinded, randomized controlled trial to investigate the hypothesis that SVATS may have less early postoperative pain than UVATS in patients who undergo major lung resection for early-stage lung cancer.
METHODSA total of
262 patients were randomly allocated between 2 groups (each with 131 patients), the first being the UVATS group and the second being the SVATS group. The values indicated on the numerical rating scale (NRS) of pain were collected at 24 h and 48 h during rest and during coughing. In addition, different perioperative variables were analysed and compared between the 2 groups.
RESULTS
Multiple linear regression analysis showed that the type of surgical approach was a significant predictor of the postoperative NRS values. The postoperative NRS pain values were significantly lower in the SVATS group after 24 h during rest and coughing and after 48 h during coughing. Postoperatively, patients in the SVATS group got out of bed significantly earlier [16.37 (2.54) vs 18.05 (3.29) h, p < 0.001]. The SVATS group showed a significantly higher rate of intraoperative arrhythmia [20 (15.3%) vs 3 (2.3%) patients, p = 0.03].
CONCLUSIONS
SVATS major pulmonary resection in early-stage lung cancer is associated with less early postoperative pain than the UVATS approach. Operating on patients with cardiac problems using the SVATS approach is still a limiting factor for randomization due to the potential compression on the heart with resulting arrhythmia.
Clinical trial registration
The trial was registered under clinical trials.gov Identifier: NCT03331588. https://clinicaltrials.gov/ct2/show/NCT03331588.
Keywords: Subxiphoid, Lung resection, VATS, Pain
Subxiphoid uniportal video-assisted thoracoscopic surgery (SVATS) is known as a less painful alternative for uniportal intercostal video-assisted thoracoscopic surgery (UVATS).
INTRODUCTION
Subxiphoid uniportal video-assisted thoracoscopic surgery (SVATS) is known as a less painful alternative for uniportal intercostal video-assisted thoracoscopic surgery (UVATS). However, due to technical considerations, specific patient selection criteria were followed in SVATS to ease the procedure especially at the beginning of the learning curve. For subxiphoid surgery, it was advised to avoid obese patients with a BMI > 30 kg/m2; patients with central masses or enlarged lymph nodes with confirmed N1 or N2 disease; patients with cardiomyopathy or impaired cardiac function; or patients with posterior lesions [1, 2]. Those exclusion criteria added a selection bias that prevents the generalization of the proposed benefit of SVATS to all populations. So, we performed this prospective, single-blinded, randomized controlled trial to investigate the hypothesis that SVATS may cause less early postoperative pain than UVATS in patients who undergo major lung resection for early-stage lung cancer.
METHODS
Study design and participants
This prospective randomized controlled study was performed at the thoracic surgery department, Shanghai Pulmonary Hospital, Shanghai, China. Patients who were expected to undergo pulmonary segmentectomy or lobectomy for stage I or II non-small-cell lung cancer from January 2018 to February 2020 were eligible for this trial. Patients with cardiomyopathy (any disorder that affected the cardiac muscle) or impaired cardiac function (with ejection fraction < 41%) and those who had previous pulmonary resection or had more than 1 pulmonary lesion that needed concomitant pulmonary resection in another ipsilateral or contralateral lobe were excluded from the trial. This trial was in compliance with the updated Consolidated Standards of Reporting Trials (CONSORT) criteria [3, 4].
Ethics statement
The trial was approved by the hospital’s institutional review board (K17-160, 17/10/2017). Written informed consent was provided by all patients after they received a detailed explanation of the trial methodology.
Randomization and masking
After application of the aforementioned criteria, 262 patients were subjected to simple randomization using computer-generated tables (SPSS program version 23.0, IBM-SPSS Inc., Armonk, NY, USA) in a parallel 1:1 ratio between 2 groups according to the surgical approach without prior specific patient selection. The first group comprised 131 patients who underwent UVATS segmentectomy or lobectomy and the second group comprised 131 patients who underwent SVATS segmentectomy or lobectomy.
The calculation of the sample size was based on the previously calculated difference in the mean pain score between the UVATS and the SVATS groups [5]. Using the G*power version 3.0.10 (https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower) with α error =0.05, power = 90.0%, effect size =0.42 (common standard deviation =0.986), the sample size was 121, and 5% was added for possible dropouts; then the total sample size was at least 128 in each group.
After the randomized allocation and until the day of surgery, neither the operating surgeon nor the patient knew the approach of the upcoming procedure. The surgeon performed the procedure via the approach that was written on a specific card in the patient's file at the time of the operation. After the operation, regardless of the approach, for both groups, a dressing covering the entire operated hemithorax and one-third of the anterior non-operated hemithorax was applied to mask the type of approach. The identical dressing was left in place until discharge to render patients, nurses and doctors doing ward rounds blinded about the approach. If the dressing needed to be changed, either the project nurse or a nurse from another ward would assist.
Surgical technique
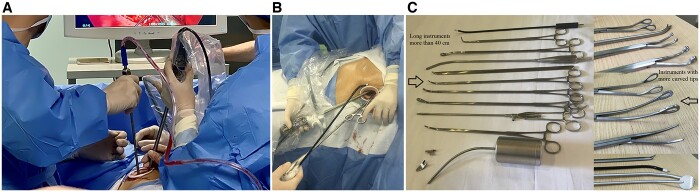
Patients who had the UVATS procedure were operated on in the dead lateral decubitus position with direct access to the thoracic cavity through a 3- to 4-cm incision between the mid-axillary and anterior axillary lines in the 4th or 5th intercostal space according to the location of the resection (Fig. 1A). Patients who had the SVATS procedure were placed in the lateral decubitus position with 30° backward inclination and operated on via a 4-cm longitudinal incision extending from the xipho-sternal junction to 1 cm below the xiphoid process (Fig. 1B). In SVATS, the rectus abdominis muscle was dissected longitudinally, then the xiphoid process was resected to provide a widened operative access to the thoracic cavity. Detailed explanations of lobectomy and segmentectomy procedures through both UVATS and SVATS approaches have been described previously [6–10]. Usual VATS instruments were used in the UVATS cases. Specially designed longer instruments with more angled ends were used for SVATS approaches (Shanghai Medical Instruments Group Ltd) (Fig. 1C).
Figure 1:
(A) Intercostal incision between the mid-axillary and anterior axillary lines in the 4th or 5th intercostal space; (B): subxiphoid incision extending from the xiphi-sternal junction to 1 cm below the xiphoid process; (C): specially designed subxiphoid instruments (Shanghai Medical Instruments Group Ltd, )
Anaesthetic and pain control management
All patients were subjected to the same anaesthetic and pain control protocol. There was no specific preoperative preparation of pre-emptive analgesia. Anaesthesia was achieved via a mixture of midazolam, propofol and an initial dose of 0.5–1 μg/kg sufentanil injected intravenously, followed by an intravenous injection of rocuronium 0.6–0.8 mg/kg and atropine 0.5 mg. Then, an additional dose of sufentanil 0.2–0.4 μg/kg was given when the mean arterial blood pressure (MAP) or heart rate (HR) was 20% higher than the preoperative baseline. MAP and HR were recorded at the time of incision, 1 h after starting surgery and at the end of the operation. Postoperatively, for the initial 48 h, patients received a patient-control intravenous analgesia (PCIA) pump with intravenous infusions of sufentanil 1 mcg/ml and flurbiprofen axetil 1 mg/ml, running at a continuous infusion of 2 ml/h. A PCIA bolus administration of 0.5 ml of the same mixture was available to patients if they could not stand the pain, with a lockout interval of 15 min. Following the initial 24 h, a combination of ibuprofen 400 mg and codeine phosphate 25 mg sustained release tablets was prescribed according to the patient's needs. The values on the 11-point numerical rating scale (NRS) of pain were collected at 24 h and 48 h during rest and during coughing by an attending nurse who was unaware of the ongoing study. The patient selected a number ranging from zero (no pain) to 10 (the worst pain) that described pain on a horizontal bar.
Patients were usually discharged 1 day after drain removal and were seen 2 weeks, 1 month and 3 months postoperatively at a special outpatient clinic moderated by the project nurse. Patients who had pain that incapacitated their life until the third month postoperatively were considered to have chronic pain.
Statistical analysis and data interpretation
Data were analysed using the IBM SPSS software package version 23 (IBM-SPSS Inc., Armonk, NY, USA). Data were described using number and percent. Quantitative data were described using the median (interquartile range 25th–75th percentiles) for non-normally distributed data and the mean (standard deviation) for normally distributed data after testing normality using normality plots (P-P plots and Q-Q). The χ2, Monte Carlo and Fisher exact tests were used for comparing 2 or more groups of categorical variables. The Student t-test and the Mann-Whitney U test were used to compare 2 independent groups of continuous variables. Multiple linear regression analysis was applied to detect independent variables that affected postoperative pain. A P value < 0.05 was considered statistically significant.
RESULTS
Preoperative patient demographic data showed no significant differences between the 2 groups. The study included 5 patients with a BMI > 30 kg/m2: 2 patients in the UVATS group and 3 patients in SVATS group. However, the BMI showed no significant difference between the 2 groups (Table 1).
Table 1:
Demographic and studied group characteristics
| Intercostal | Subxiphoid | P-value | |
|---|---|---|---|
| Age (years), mean (SD) | 58.17 (6.95) | 58.31 (7.84) | 0.881 |
| Gender, count (%) | 0.451 | ||
| Male | 57 (43.5%) | 51 (38.9%) | |
| Female | 74 (56.5%) | 80 (61.1%) | |
| BMI (kg/m2), mean (SD) | 23.52(2.81) | 23.29(3.06) | 0.541 |
| Procedure, count (%) | |||
| Lobectomy | 69 (52.7) | 75 (57.3) | 0.456 |
| Segmentectomy | 62 (47.3) | 56 (42.7) | |
| Educational level, count (%) | 0.948 | ||
| High school | 93 (71.0%) | 93 (71.0%) | |
| College | 32 (24.4%) | 33 (25.2%) | |
| Postgraduate | 6 (4.6%) | 5 (3.8%) | |
BMI: body mass index; SD: standard deviation.
Operative and postoperative MAP values were significantly lower in the SVATS group. Also, HR values were significantly lower in the SVATS group during skin incision, at the end of the operation and during the first 24 h postoperatively (Table 2). Consequently, extra doses of needed intraoperative sufentanil were significantly lower in SVATS group (Table 2).
Table 2:
Perioperative mean arterial pressure and heart rate and operative sufentanil dose
| Intercostal N = 131 | Subxiphoid N = 131 | P-value | |
|---|---|---|---|
| MAP, mean (SD) | |||
| Preoperative MAP | 82.54 (3.64) | 82.23 (4.04) | 0.511 |
| Operative MAP | |||
| Incision | 91.02 (4.46) | 87.27 (4.25) | < 0.001a |
| 1 h | 78.39 (3.53) | 76.67 (4.58) | 0.001a |
| Finish | 86.49 (4.52) | 83.24 (4.33) | < 0.001a |
| Postoperative MAP | |||
| 24 h | 88.08 (4.48) | 81.64 (5.36) | < 0.001a |
| 48 h | 84.99 (4.47) | 79.83 (5.60) | < 0.001a |
| HR, mean (SD) | |||
| Preoperative HR, bpm | 68.98 (3.67) | 69.24 (4.31) | 0.601 |
| Operative HR, bpm | |||
| Incision | 76.14 (3.52) | 73.51 (3.98) | < 0.001a |
| 1 h | 64.09 (2.71) | 64.42 (3.93) | 0.432 |
| Finish | 73.17 (3.73) | 72.07 (3.68) | 0.017a |
| Postoperative HR, bpm | |||
| 24 h | 72.18 (3.54) | 70.90 (4.29) | 0.009a |
| 48 h | 69.73 (3.99) | 69.73 (4.15) | 1.0 |
| Sufentanil dose, median (IQR) | |||
| Sufentanil dose (ml) | 65 (60-75) | 55 (50-60) | < 0.001a |
BPM: beats per minute; HR: heart rate; IQR: interquartile range; MAP: mean arterial pressure.
Statistically significant.
The SVATS group showed significantly higher rates of intraoperative arrhythmia in the form of premature ventricular contraction and premature beats (Table 3); however, there was no reported haemodynamic instability secondary to those arrhythmias. There was no reported conversion to open thoracotomy in either group. Also, there was no reported conversion to UVATS in the SVATS group.
Table 3:
Operative time, operative blood loss and operative arrhythmia
| Intercostal N = 131 | Subxiphoid N = 131 | P-value | |
|---|---|---|---|
| Operative time (min), mean (SD) | |||
| Incision | 4.70 (0.72) | 5.18 (1.39) | 0.001a |
| Operation | 51.34 (7.96) | 51.38 (8.04) | 0.963 |
| Specimen extraction | 4.64 (0.69) | 4.39 (0.72) | 0.005a |
| Closure | 4.76 (0.53) | 4.93 (0.70) | 0.023a |
| Amount of blood loss (ml), median (IQR) | 50 (20-50) | 50 (30-50) | 0.084 |
| Intraoperative arrythmia, count (%) | |||
| Premature vent contraction | 2 (1.5) | 11 (8.4) | 0.01a |
| Premature beats | 1 (0.8) | 7 (5.3) | 0.03a |
| Atrial fibrillation | 0 (0.0) | 2 (1.5) | 0.498 |
IQR: interquartile range; SD: standard deviation.
Statistically significant.
The operative time needed to reach the thoracic cavity and to close the incision in layers was significantly longer in the SVATS group. On the other hand, the time needed to extract the specimen from the chest was significantly shorter in the SVATS group. However, there was no significant difference between the 2 groups regarding the time needed to do the definitive lobectomy or segmentectomy procedure (Table 3).
Resection of the left S1+S2 was the most commonly performed segmentectomy in this series (21 cases, 8%), whereas a right upper lobectomy was the most commonly performed lobectomy in the series (52 cases, 19.8%) (see Supplemental Table 1, which demonstrates the detailed surgical procedures).
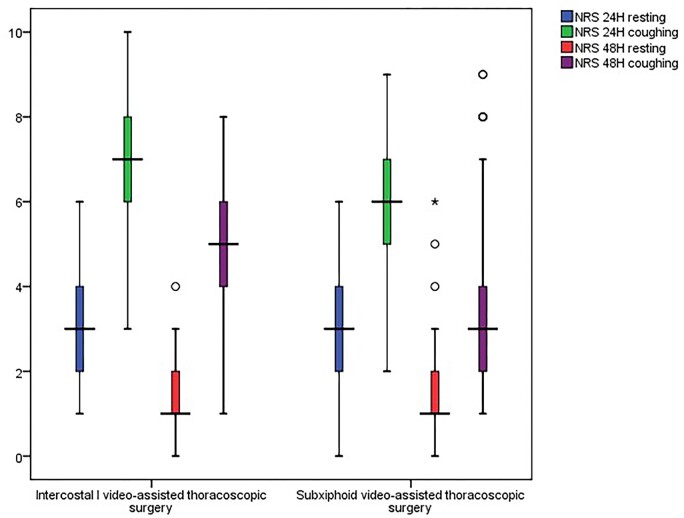
The NRS of pain was significantly lower in the SVATS group after 24 h and after 48 h during coughing (Table 4; Fig. 2). Despite the higher frequency of postoperative PCIA and oral analgesia needed in the UVATS group, there was no significant difference between the 2 groups regarding postoperative doses of analgesia (Table 4). Postoperatively, patients in the SVATS group got out of bed significantly earlier than patients in UVATS group and even sustained significantly longer periods of mobilization during their first activity (Table 5).
Table 4:
Postoperative pain score (numerical rating score) and frequency of postoperative analgesics
| Intercostal N = 131 | Subxiphoid N = 131 | P-value | |
|---|---|---|---|
| NRS, median (IQR) | |||
| 24 h resting | 3 (2-4) | 3 (2-4) | 0.04a |
| 24 h coughing | 7 (6-8) | 6 (5-7) | < 0.001a |
| 48 h resting | 1 (1-2) | 1 (1-2) | 0.289 |
| 48 h coughing | 5 (4-6) | 3 (2-4) | < 0.001a |
| Frequency of oral analgesic, count (%) | |||
| No times | 104 (79.4) | 115 (87.8) | 0.182 |
| 1 Time | 23 (17.6) | 14 (10.7) | |
| 2 Times | 4 (3.1) | 2 (1.5) | |
| Frequency of PCIA, count (%) | |||
| No times | 105 (80.2) | 114 (87.0) | 0.379 |
| 1 Time | 12 (9.2) | 9 (6.9) | |
| 2 Times | 8 (6.1) | 6 (4.6) | |
| 3 Times | 6 (4.6) | 2(1.5) | |
IQR: interquartile range; NRS: numerical rating scale; PCIA: patient controlled intravenous analgesia.
Statistically significant.
Figure 2:
Box-and-whisker plot showing the median NRS of pain between the 2 studied groups (circles denote outliers that are more than 1.5 interquartile ranges and less than 3 interquartile ranges (stars denote outliers that are more than 3 interquartile ranges).
Table 5:
Postoperative data and complications
| Intercostal N = 131 | Subxiphoid N = 131 | P value | |
|---|---|---|---|
| Duration of staying in bed before first activity (h), mean (SD) | 18.05 (3.29) | 16.37 (2.54) | < 0.001 a |
| Duration of first activity time (min), median (IQR) | 3 (2-5) | 5 (2-5) | < 0.001 a |
| Duration of chest tube (h), mean (SD) | 49.66 (11.17) | 45.88 (8.46) | 0.002 a |
| Hospital stay (days), mean (SD) | 7.73 (2.78) | 7.05 (1.91) | 0.02 a |
| Postoperative complications, count (%) | |||
| Atelectasis | 9 (6.9) | 2 (1.5) | 0.03 a |
| Chest infection | 4 (3.1) | 1 (0.8) | 0.370 |
| Pulmonary oedema | 4 (3.1) | 2 (1.5) | 0.684 |
| Prolonged air leak (more than 7 days) | 6 (4.6) | 7 (5.3) | 0.776 |
| Postoperative bleeding | 3 (2.3) | 2 (1.5) | 1.0 |
| Pulmonary embolism | 3 (2.3) | 1 (0.8) | 0.622 |
| Wound infection | 1 (0.8) | 2 (1.5) | 1.0 |
| Arrhythmia | 2 (1.5) | 6 (4.6) | 0.151 |
| Chronic pain (after 3 months) | 9 (6.9) | 4( 3.1) | 0.155 |
IQR: interquartile range; SD: standard deviation.
Statistically significant.
Patients in the SVATS groups showed significantly lower rates of postoperative atelectasis, earlier removal of chest drains and shorter hospital stays. However, there was no significant difference between the 2 groups regarding other postoperative complications. During follow-up at the outpatient clinic after 3 months, there were no significant differences between the 2 groups regarding complaints of chronic pain (Table 5). We did not report any case of abdominal herniation in the SVATS group.
Multiple linear regression analysis showed that the type of the surgical approach was a significant predictor for postoperative NRS pain after 24 h during rest and coughing and after 48 h during coughing with no relation to other mentioned variables (Table 6).
Table 6:
Multiple linear regression analysis showing predictors of postoperative numerical rating scale
| NRS24 resting |
NRS24 coughing |
NRS48 resting |
NRS48 Coughing |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | β | P-value | |
| Constant | 0.760 | <0.001 | 1.151 | <0.001 | 0.337 | 0.062 | 1.162 | <0.001 |
| Age | −0.002 | 0.167 | −0.001 | 0.163 | −0.002 | 0.395 | −0.004 | 0.031 |
| Gender | 0.007 | 0.729 | −0.013 | 0.352 | 0.029 | 0.302 | 0.000 | 0.981 |
| Operation | −0.047 | 0.027 | −0.082 | <0.001 | −0.017 | 0.531 | −0.158 | <0.001 |
| BMI | −0.004 | 0.212 | −0.003 | 0.148 | −0.005 | 0.297 | −0.001 | 0.771 |
| Hospital stay | 0.002 | 0.194 | 0.001 | 0.414 | 0.000 | 0.822 | 0.000 | 0.963 |
| Lobectomy/segmentectomy | −0.017 | 0.414 | −0.014 | 0.335 | 0.006 | 0.835 | −0.012 | 0.646 |
| R Square | 0.043 | 0.144 | 0.02 | 0.148 | ||||
BMI: body mass index; NRS: numerical rating score.
DISCUSSION
A shortage of randomized trials showing the effect of SVATS in decreasing postoperative pain after major lung resection was considered one of the main limitations of the many previous studies [1, 11, 12]. The need for randomization has emerged from the difficulty to extrapolate the SVATS values for decreasing postoperative pain to the general population due to the specific patient selection criteria that had been followed preoperatively [13, 14]. The incentive behind the specific patient selection criteria was primarily to ease the difficulty of the SVATS approach, with its different orientation at the beginning of its learning curve [1]. However, as the learning curve has progressed and the surgeons' expertise and instruments have been upgraded, many SVATS limitations have been overcome with the reporting of operating on posteriorly located lesions and lymph nodes and patients with a BMI > 30 kg/m2 [10,12]. The progress in the learning curve with non-adherence to the previously followed exclusion criteria has prepared us to conduct this prospective randomized controlled trial to assess the early postoperative pain after an SVATS major lung resection in comparison to the UVATS approach.
All patients who were eligible for this trial were operated on, regardless of their BMI and of the site, size or laterality of the lesion. Only patients with cardiac problems, patients who had previous pulmonary resection and patients who needed concomitant pulmonary resection in another lobe were excluded from the trial. Operating on a previously operated on patient or on a patient with more than 1 pulmonary lesion who needs additive resection is itself an exhausting job that may add extra effort and time to the procedure, whether it is through the intercostal or the subxiphoid approach. That may lead to some sort of confounding bias that may affect the results in another way.
Despite a reported decreased rate of intraoperative arrhythmias during SVATS as a result of progress along the learning curve [12] and the development of appropriate instruments [15], intraoperative induced cardiac arrhythmia in SVATS procedures is still a predicted critical event due to potential compression on the heart. In this trial, there was a significantly higher incidence of intraoperative arrhythmia in the SVATS group than in the UVATS group. For that reason, we decided preoperatively to decrease the operative risk by excluding patients with cardiomyopathy or impaired cardiac function from both groups, which led to non-reported haemodynamic instability or mortality secondary to those arrhythmias. At the same time, we maintained randomization by operating on any lesion, regardless of its side or location. These exclusion criteria were applied to both groups. As a result, we postulated that selection bias was decreased in this study as much as possible.
Operating on lung malignancy through the SVATS approach without interrupting the intercostal nerves could be a fundamental factor in lessening postoperative pain [1, 5, 13]. In this randomized trial, NRS pain scoring data were collected at 24 h and 48 h postoperatively during rest and during coughing after patient education by an attending nurse who was unaware of the ongoing study to ensure unbiased registration of scores and to avoid any leading questions for patients. At the same time, patients in both groups had no significant difference regarding their level of education. As a result, we assumed uniform understanding of score expression by patients in both groups.
Multiple linear regression analysis showed that the type of surgical approach was a significant predictor for postoperative NRS pain scores after 24 h during rest and coughing and after 48 h during coughing. Patients in the SVATS group had significantly lower NRS pain scores after 24 h during rest and coughing. After 48 h, the pain threshold mostly increased, with decreased pain scores under rest conditions. However, pain may be elicited during movement or muscle contraction, like that which occurs during coughing [16]. That fact may explain the significantly lower NRS pain scores in the SVATS group after 48 h during coughing only, because during rest, pain scores decreased as early as postoperative day (POD) 2. So, there was no difference in NRS pain scores between the 2 groups during rest. However, during coughing with irritation of chest wall nerves, the benefit of not interrupting intercostal nerves in the SVATS had emerged in the form of less pain sensation and lower NRS pain scores. That reasoning may be supported by the findings conveyed by Tong et al., who showed gradual decreasing NRS pain scores during PODs 1, 2 and 3 (2.60 ± 1.31, 2.06 ± 1.11 and 1.34 ± 0.88), respectively [17]. Also, Wang et al. suggested lower postoperative NRS pain scores after VATS during rest (3.91 ± 2.12) than during mobilization (5.73 ± 2.26) on POD 1 [18].
We previously reported lower pain scores on PODs 0, 1 and 2 after SVATS segmentectomy than after UVATS segmentectomy [11]. Also, Cai and his colleagues emphasized the same findings by reporting lower early postoperative pain scores on POD 1 and before discharge after SVATS for bilateral lung resections than after UVATS [5]. Moreover, lower pain scores for patients who had an SVATS lobectomy were reported by Chen et al. [13] after 1, 3 and 6 months postoperatively than for patients who had a UVATS lobectomy. In a meta-analysis comparing SVATS versus UVATS for lung resection, which included 11 studies involving 934 patients, significantly lower postoperative pain scores on PODs 1, 2 and 3 were shown after SVATS resection [19]. However, all aforementioned studies were non-randomized and increased the importance of conducting a randomized trial to support their findings. In a randomized trial comparing postoperative pain after SVATS versus UVATS for spontaneous pneumothorax, Li and his colleagues reported significantly lower pain scores for the SVATS approach on PODs zero, 1, 2 and 3 [20]. To our knowledge and until submission of this manuscript, this study is considered to be the first controlled randomized trial to compare early postoperative pain after SVATS and UVATS approaches for major lung resection for early-stage lung cancer.
In our study, the decreased postoperative pain in the SVATS group probably led to significantly earlier ambulation and longer duration of physical exercise during the initial postoperative activity in the patients in the SVATS group compared with those in the UVATS group. In turn, that might explain the significantly lower incidence of atelectasis, earlier removal of chest drains and shorter hospital stay in the SVATS group [21].
Intense postoperative pain results in increased plasma catecholamine levels with consequent increased systemic vascular resistance and cardiac workload [22]. This fact may explain the resultant significantly higher postoperative MAP and HR in the UVATS group than in the SVATS group due to the associated higher pain scores. But the questionable findings in this trial are the significantly lower intraoperative MAP values in the SVATS group along with the significantly lower HR values in the SVATS group during the skin incision and at the end of the operation, even though the patients were completely anaesthetized. An explanation of these findings might be that the intraoperative use of an intravenous opioid (sufentanil) as an analgesic works mainly by reducing the central perception of pain by acting upon central specific opioid receptors, but it cannot abolish completely the sympathetic effect of pain on changing HR and MAP (23). However, we cannot refer these intraoperative lower levels of MAP and HR in the SVATS group mainly to the fact that the type of operative approach is subxiphoid rather than intercostal because other factors can influence the level of MAP and HR; these include depth of anaesthesia, amount of bleeding and infused fluid. These findings need further study to explain.
We followed up on our patients at an outpatient clinic after 2 weeks, 1 month and 3 months postoperatively. Actually, we could not apply NRS scoring during those visits because most complaints regarding pain were in the form of intermittent discomfort, vague chest pain and paresthesia or, on the other hand, severe pain that incapacitated normal activity. So, we decided to consider the “severe pain that incapacitated normal activity” after 3 months postoperatively (24) as an indicator to assess “chronic chest pain” in the current trial. We have not found a statistically significant difference between the 2 groups regarding complaints of chronic pain after 3 months. In their meta-analysis, Mei and his colleagues found less paresthesia after SVATS lung resection at the first, third and sixth months postoperatively than after UVATS lung resection (19).
Limitations of the study
We consider the short-term follow-up of postoperative pain for just 3 months postoperatively as one of the limitations of this study. Actually, the main scope of this clinical trial was to show the effect of SVATS on early postoperative pain specifically. However, longer follow-up of the patients included in this study or in randomized trials by other centres could be considered in future publications to show the long-term effect of SVATS on chronic postoperative pain and on the quality of life. Secondly, cardiac arrhythmia in patients who have the SVATS approach is still a predicted incident that warrants exclusion of patients with cardiac problems to avoid intraoperative hazards.
CONCLUSION
The SVATS approach for major pulmonary resection in early-stage lung cancer is associated with less early postoperative pain than the UVATS approach. Operating on patients with cardiac problems using the SVATS approach is still a limiting factor for randomization due to potential compression on the heart with resulting arrhythmia. Further long-term randomized trials are recommended to investigate the prolonged effect of the SVATS approach on postoperative chronic pain.
Supplementary Material
ACKNOWLEDGEMENT
None.
Funding
This trial is supported by the Fundamental Research Funds for the Central Universities (22120180019) and Health and Family Planning Commission of Shanghai Municipality (20184Y0090).
Conflict of interest: None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
Zhigang Chen: Conception and design; provision of study materials or patients; collection and assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript; Lei Jiang: conception and design; administrative support; provision of study materials or patients; manuscript writing; final approval of manuscript; Hua Zheng: provision of study materials or patients; manuscript writing; final approval of manuscript; Wentian Zhang: collection and assembly of data; final approval of manuscript; Xin Lv: administrative support; manuscript writing; final approval of manuscript; and Amr Abdellateef: conception and design; administrative support; collection and assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript.
Contributor Information
Zhigang Chen, Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China.
Lei Jiang, Thoracic Surgery Department, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China.
Hua Zheng, Thoracic Surgery Department, Second People's Hospital Jingdezhen, JiangXi 333000, China.
Wentian Zhang, Thoracic Surgery Department, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China.
Xin Lv, Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China.
Amr Abdellateef, Thoracic Surgery Department, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China; Cardiothoracic Surgery Department, Mansoura University Hospital, Mansoura University School of Medicine, Mansoura, 35111, Egypt.
REFERENCES
- 1. Hernandez-Arenas LA, Lin L, Yang Y, Liu M, Guido W, Gonzalez-Rivas D. et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060–6. [DOI] [PubMed] [Google Scholar]
- 2. Abdellateef A, Yang C, Chen J, Qu J, Huang J, Jiang L.. Subxiphoid uniportal video assisted thoracoscopic surgery lobectomy, evolution of the technique and progress of learning curve. Shanghai Chest 2018;2:88. [Google Scholar]
- 3. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. Published 2010 23. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ. et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. Bmj 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai H, Xie D, Al Sawalhi S, Jiang L, Zhu Y, Jiang G. et al. Subxiphoid versus intercostal uniportal video-assisted thoracoscopic surgery for bilateral lung resections: a single-institution experience. Eur J Cardiothorac Surg 2020;57:343–9. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Rivas D, Fieira E, Delgado M, Mendez L, Fernandez R, de la Torre M.. Uniportal video-assisted thoracoscopic lobectomy. J Thorac Dis 2013;5 Suppl 3(Suppl 3):S234–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez-Rivas D, Mendez L, Delgado M, Fieira E, Fernandez R, de la Torre M.. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5:S226–S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aresu G, Wu L, Lin L, Jiang G, Jiang L.. The Shanghai Pulmonary Hospital subxiphoid approach for lobectomies. J Vis Surg 2016;2:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aresu G, Weaver H, Wu L, Lin L, Jiang G, Jiang L.. The Shanghai Pulmonary Hospital uniportal subxiphoid approach for lung segmentectomies. J Vis Surg 2016;2:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali J, Haiyang F, Aresu G, Chenlu Y, Gening J, Gonzalez-Rivas D. et al. Uniportal subxiphoid video-assisted thoracoscopic anatomical segmentectomy: technique and results. Ann Thorac Surg 2018;106:1519–24. [DOI] [PubMed] [Google Scholar]
- 11. Abdellateef A, Ma X, Chen Z, Wu L, Cai J, Jiang L.. Subxiphoid uniportal thoracoscopic pulmonary segmentectomy for stage I non-small cell lung cancer: feasibility, quality of life and financial worthiness. Thorac Cancer 2020;11:1414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdellateef A, Ma X, Qiao W, Chen Z, Wu L, Cai J. et al. Subxiphoid uniportal video-assisted thoracoscopic pulmonary segmentectomy: effect of learning curve and future perspectives. Eur J Cardiothorac Surg 2020;58:i50–i57. [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Volpi S, Ali JM, Aresu G, Wu L, Chen Z. et al. Comparison of post-operative pain and quality of life between uniportal subxiphoid and intercostal video-assisted thoracoscopic lobectomy. J Thorac Dis 2020;12:3582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aresu G, Jiang L, Bertolaccini L.. Subxiphoid video-assisted major lung resections: the Believers’ speech. J Thorac Dis 2017;9:E387–E389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdellateef A, Ali JM, Jiang G, Aresu G, Jiang L.. Tips and tricks for success in subxiphoid video-assisted thoracic surgery. J Thorac Dis 2019;11:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pennefather SH, McKevith J, Pain management after thoracic surgery. In: Slinger P, editor. Principles and Practice of Anesthesia for Thoracic Surgery, vol. 675. Berlin: Springer;2011. 10.1007/978-1-4419-0184-2_46. [DOI] [Google Scholar]
- 17. Tong Y, Wei P, Wang S, Sun Q, Cui Y, Ning N. et al. Characteristics of Postoperative Pain After VATS and Pain-Related Factors: the Experience in National Cancer Center of China. J Pain Res 2020;13:1861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Li S, Liang N, Liu W, Liu H, Liu H.. Postoperative pain experiences in Chinese adult patients after thoracotomy and video-assisted thoracic surgery. J Clin Nurs 2017;26:2744–54. [DOI] [PubMed] [Google Scholar]
- 19. Mei LX, Wang YY, Chen Y, Dai L, Chen MW.. Subxiphoid versus intercostal video-assisted thoracic surgery for lung resection: a meta-analysis. Minim Invasive Ther Allied Technol 2020. 15;1–11. [TQ3] [DOI] [PubMed] [Google Scholar]
- 20. Li L, Tian H, Yue W, Li S, Gao C, Si L.. Subxiphoid vs intercostal single-incision video-assisted thoracoscopic surgery for spontaneous pneumothorax: a randomized controlled trial. Int J Surg 2016;30:99–103. [DOI] [PubMed] [Google Scholar]
- 21. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M. et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91–115. [DOI] [PubMed] [Google Scholar]
- 22. Gerner P. Post-thoracotomy pain management problems. Anesthesiol Clin 2008;26:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan ZZ, Tershner SA, Fields HL.. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 1997;389:382–405. [DOI] [PubMed] [Google Scholar]
- 24. Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S.. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on quality of life. PLoS One 2014;9:e90014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.