Abstract
While the coronavirus disease 2019 (COVID-19) pandemic placed a heavy burden on healthcare systems worldwide, it also induced urgent mobilisation of research teams to develop treatments preventing or curing the disease and its consequences. It has, therefore, challenged critical care research to rapidly focus on specific fields while forcing critical care physicians to make difficult ethical decisions. This narrative review aims to summarise critical care research —from organisation to research fields— in this pandemic setting and to highlight opportunities to improve research efficiency in the future, based on what is learned from COVID-19. This pressure on research revealed, i.e., (i) the need to harmonise regulatory processes between countries, allowing simplified organisation of international research networks to improve their efficiency in answering large-scale questions; (ii) the importance of developing translational research from which therapeutic innovations can emerge; (iii) the need for improved triage and predictive scores to rationalise admission to the intensive care unit. In this context, key areas for future critical care research and better pandemic preparedness are artificial intelligence applied to healthcare, characterisation of long-term symptoms, and ethical considerations. Such collaborative research efforts should involve groups from both high and low-to-middle income countries to propose worldwide solutions. As a conclusion, stress tests on healthcare organisations should be viewed as opportunities to design new research frameworks and strategies. Worldwide availability of research networks ready to operate is essential to be prepared for next pandemics. Importantly, researchers and physicians should prioritise realistic and ethical goals for both clinical care and research.
Keywords: COVID-19, Critical care, Research, Pandemic, Perspectives
Abbreviations: AI, artificial intelligence; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; eCASH, early Comfort using Analgesia, minimal Sedatives and maximal Humane care; EWS, early warning system; FAIR, Findability, Accessibility, Interoperability, and Reusability; HICs, high-income countries; HFNO, high-flow nasal oxygen; ICU, intensive care unit; ISARIC, International Severe Acute Respiratory and Emerging Infection Consortium; LMICs, low- and middle-income countries; NIH, National Institutes of Health; NIHR, National Institute of Health Research; PICU, post-intensive care syndrome; RRT, rapid response team; UK-CTAP, United Kingdom COVID-19 Therapeutics Advisory Panel; UPH, urgent public health
1. Introduction
As a worldwide emergent disease, the coronavirus disease 2019 (COVID-19) pandemic has placed extraordinary pressure on researchers for its prevention and treatment. Highlighted by mRNA vaccine development, which was a breakthrough based on decades of research, research teams and organisations have been challenged during the pandemic and have shown remarkable abilities to adapt. Compared to research previously done in other fields of sepsis, especially bacterial sepsis which has been the most studied [1], homogeneous groups of patients and site-specific alterations in COVID-19 critical patients were all favourable to achieving effective results faster than expected [2]. However, researchers also faced many obstacles. Several publications have addressed how this pandemic has impacted critical care organisations [3], [4], few of them – mainly surveys – discussed the effects on ongoing health research [5], [6]. But, to our knowledge, none of them has focused on the impact on critical care research topics and networks.
The objectives of this review are to summarise critical care research—from organisation to research fields—during the COVID-19 pandemic and provide insights on potential challenges for the future. Research is one of the main drivers of better health and patient care [7], and stress tests such as pandemics should be viewed as an opportunity to design new research frameworks and strategies [8]. Based on the issues experienced during this pandemic, we provide insights that will be transformative and impact how research is managed in intensive care, even after the pandemic is over.
2. Clinical research organizations & networks, a transnational harmonization to be reached
The COVID-19 pandemic clearly highlighted the importance of clinical research in the understanding of a novel disease and the rapid delivery of evidence-based interventions. The roles of clinical research organisations, networks, and learned societies were numerous, including the conception, design, and delivery of clinical research during a global pandemic, industry engagement for manufacturing and procurement, guidelines development and implementation to immediately show clinical impact, and governmental bodies to advise on policy and strategy.
One such example was the National Institute of Health Research (NIHR) in the UK, which developed an engagement with the office of the Chief Medical Officer that led to the urgent prioritisation of COVID-19 research [9]. This enabled resource allocation to urgent public health (UPH) research to facilitate the delivery of UPH study platforms such as Recovery, ReMAP-CAP, and PHOSP-COVID. This approach tapped leading funding bodies, research experts, and NHS/NIHR clinical research delivery platforms. It also enabled novel compounds to be rapidly assessed through the UK COVID-19 Therapeutics Advisory Panel (UK-CTAP) and UPH committee [10]. For its part, the World Health Organization has also carried out numerous actions on the epidemiological level, as well as on fundamental research and the therapeutic level. Hence, the Solidarity trial was conducted in 30 countries and included 11,330 patients to demonstrate that remdesivir, hydroxychloroquine, lopinavir, and interferon regimens had no effect on hospitalised patients with COVID-19 [11]. While national (e.g., Recovery) and international trials (e.g., Remap-CAP, Solidarity, and Discovery) have contributed to the immediate understanding of COVID-19, the results of international trials tend to be easier to generalise and require thinking about the development of large networks that go beyond national borders.
This new working knowledge and experience during the pandemic—namely, the challenges of global engagement—could substantially benefit medical research. Medicine transcends individual, organisational, and geopolitical boundaries. Henceforth, the learning gained from this global pandemic should be a steppingstone to further strengthen academic research networks. Exemplars of existing research and collaboration should be celebrated and examined in preparation for future health emergencies but also translated to other research areas. For instance, the European Commission announced that the Clinical Trials Regulation (No 536/2014) would be fully operational starting on the 31st of January 2022. Such scrutiny and harmonisation of regulatory processes to boost efficiency across geopolitical boundaries should be front and centre for future research in an increasingly interconnected world. Only the future can tell us whether such changes to legislation will serve as a catalyst for pan-European and global medical research.
3. Translational research, the new driver of innovation
Translational research has an important role in biomedical discovery, often serving as the pathway to clinical trials. “Forward” translation brings pre-clinical discoveries from the bench to the bedside while “reverse” translation brings clinical observations back to the laboratory. The COVID-19 pandemic has presented unique challenges to translational research that, by its design, requires use of research laboratory space, as well as access to patients. During the early phase of the COVID-19 pandemic, many laboratories and institutions across Europe and the USA were shut down partially, completely, or operated at limited capacity for weeks to months [12], [13]. As a result, collection, processing, and storage of clinical samples was not possible and mechanistic “reverse” translational studies had to be stopped. Furthermore, many institutions paused non-COVID-19 related clinical research, study visits became difficult to impossible, and elective procedures and certain treatments were often postponed [14], [15]. As a result, access to patients who serve as study populations was severely limited and study subjects could not be enrolled.
The response to these crippling restrictions was widely variable with some labs halting all translational studies and others pivoting to study patients with SARS-CoV-2 infection, something that was only possible for investigators with access to appropriate biocontainment facilities. Over time, many institutions have established centralised and coordinated research facilities and resources for translational studies of COVID-19 patients. These focused efforts have facilitated rapid enrolment of large numbers of COVID-19 patients and collection of biospecimens, with major support from the National Institutes of Health (NIH). As a result, there has been an explosion of new knowledge about SARS-CoV-2 and COVID-19 disease, much of which comes from translational studies, including clinical trials [16]. While the pandemic has highlighted the vulnerabilities of translational research to a global catastrophe, it also revealed the opportunities that arise with institutional investment, centralised resources and coordinated effort.
While a wide range of COVID-19 clinical presentations exists, approximately 30% of hospitalised patients require ICU care due to respiratory failure, of which 40% will eventually die [17], [18]. To maximise scarce healthcare resources, an important research priority has been to develop ways to identify early those COVID-19 patients at risk for ICU admission, mortality, and recovery [19]. Several attempts have been made to develop COVID-19-specific scoring systems to assist with triage and mortality prediction, with newly developed scoring systems performing better than adapted existing scoring systems [20]. Lymphocyte count and D-dimer testing were among the most informative parameters to predict mortality in COVID-19 patients [20]. Since then, several studies have leveraged high-content omics assays to identify biological signatures that differentiate patients with mild (non-hospitalised), moderate (hospitalised but non-ICU), and severe (hospitalised and ICU) COVID-19, such as virus-specific antibody levels [21], dysregulated pro-inflammatory immune signalling networks [22], dysregulated myeloid cells [23], [24], [25], and inflammation [26], [27]. As such, integration of high-content omics technologies to derive predictive models of COVID-19 severity is a promising strategy for the identification of actionable pathophysiological mechanisms to identify patients at risk for severe COVID-19 and the development of novel therapeutic strategies.
Importantly, reverse translational research during the COVID-19 pandemic has been stimulated, for instance, by the changing clinical practice of treating patients with severe COVID-19 pneumonia with high-flow nasal oxygen (HFNO). This mode of support has delayed—and in some patients avoided—the need for endotracheal intubation. As a result, the widespread use of HFNO for COVID-19 patients has prompted a re-evaluation of the Berlin Definition of the acute respiratory distress syndrome (ARDS), as discussed in a recent article, and paved the way to additional physiological and experimental research to define new ARDS endotypes, defined by specific pathophysiological mechanisms [28], [29]. In turn, this initiative has led to the formation of a global consensus conference to expand the Berlin Definition of ARDS.
In addition, several major clinical trials have been carried out by the NIH to test new therapies, including anticoagulation strategies and monoclonal antibody treatment [30], [31]. These trials have led to new biological studies on how plasma biomarkers can be used for pathogenesis and prognosis. These biomarkers include traditional measures of inflammation such as IL-6 and IL-8, endothelial injury such as angiopoietin-2, and alveolar epithelial injury such as RAGE. These studies also demonstrate the novel benefit of quantitative assays of the SARS-CoV-2 antigen (Quanterix assay), as well as measures for neutralising antibodies against SARS-CoV-2 at the time of hospitalisation to help guide the use of monoclonal antibody therapy [32].
However, the sheer volume and pace of COVID-19-related preprints and fast dissemination of peer-reviewed publications [33], [34], together with questionable clinical trial findings [35], [36], [37], has highlighted that reliable clinical and research practice is imperative, especially in pandemic times. Key to improving translational research is to have an efficient system for obtaining biological samples, which includes trained personnel and laboratory infrastructure [35]. In the context of COVID-19, this would be samples for protein measurements and RNA sequencing at the time of and during hospitalisation, and during out-patient follow-up, if possible, for up to 28 and 90 days, as is being done in the ACTIV-3 NIH trials on monoclonal antibodies. These samples need to be linked with detailed clinical data for each enrolled patient. In addition, this pandemic has highlighted that to safeguard the quality of translational research sufficiently statistically powered research should be conducted in well-defined patient categories, and preferably coordinated across multiple centres [35], [38], [39]. However, conducting large scale multi-centre research remains challenging in the current academic setting, but core protocols developed by for instance the World Health Organization could provide solution [38]. Lastly, notwithstanding unforeseen institutional shutdowns, researchers might want to start including alternative recruitment protocols to mitigate potential unforeseen events.
4. Developing key areas in ICU research
4.1. Data Science and Rapid Response Team to prevent ICU admission
Over the past two decades, data science has emerged as an active medical research field that has seen the development of innumerable artificial intelligence (AI) algorithms for predicting a variety of clinical outcomes at the individual level and assisting in medical decision-making in the ICU [40], [41].
Prior to the COVID-19 pandemic, predictive algorithms were mainly used in critical care to predict ICU complications or mortality [42], [43]. The COVID-19 pandemic has imposed an unprecedented strain on healthcare systems, with the most critical identified bottleneck being ICU admission. Since the beginning of the pandemic, many AI algorithms were developed to predict a variety of novel outcomes, including clinical deterioration of hospitalised patients that may require acute intervention (from rapid response teams, see below) and ultimately result in ICU transfer [44]. While preventing the future need for ICU admission is certainly helpful to proactively manage workflow and resources, using data and AI to help prevent ICU admission would be even more significant. In order to achieve this goal, AI algorithms would be required very early over the course of the infection to: (i) help diagnose the disease at its very early stage by predicting those with a high risk of being infected and should get tested; (ii) predict those likely to deteriorate and should thus be hospitalised and closely monitored for early signs of deteriorations; (iii) predict those likely to benefit from early treatment such as steroids or immunomodulatory therapies in order to avoid ICU admission.
Although data science and AI are associated with unprecedented opportunities in terms of improving healthcare workflow and ultimately patient care, there remain many roadblocks. First, to be able to predict and even more to prevent ICU admission, one needs to have access to a wealth of quality data prior to ICU admission. Although ICU data are becoming increasingly more structured, this is still not the case for pre-ICU biomedical data [45]. Second, data used to develop AI algorithms may differ from data that are collected during routine-care activities. Furthermore, data scientists usually pre-process data before using them to train an algorithm. This data-cleaning process, though useful for algorithm-training purposes, may be problematic when it comes to implementing algorithm indecision support systems for clinical practice. Thus, it is necessary to close the research-to-practice gap to create a virtuous cycle from basic data science to clinical practice and back again. Third, clinical AI algorithms have been shown to lack external validity [46], meaning that predictive performance is proven to decay when the algorithm is used in settings that are not exactly similar to the ones used for training. Finally, there is currently a lack of guidelines as to how AI algorithm performance should be monitored over time, as well as when they should be updated or recalibrated based on dynamic datasets that grow as the amount of data increases. These challenges are large yet critical to overcome for leveraging the power of data and AI to help clinicians in critical situations, such those encountered during the COVID-19 pandemic [47].
Another tool that has been largely used during the first waves of this pandemic to prevent decompensation in hospitalised patients are rapid response teams (RRTs), which are now prevalent in 85% of hospitals in Australia and New Zealand [48]. The perceived benefits of RRTs have varied, but it is accepted that they reduce out-of-ICU cardiac arrests [49] and reduce hospital mortality, as shown in a meta-analysis [50]. It has been recommended to move from intermittent vital-sign checks of hospitalised patients to continuous monitoring, but the lack of a highly sensitive and specific early warning system (EWS) may temper its benefit [51]. This lack of specificity is illustrated by the majority of RRT calls resulting in no intervention and only 30% requiring ICU transfer [52]. In addition to wasting staff resources, this high false-positive rate could contribute to alarm fatigue [53]. Application of a continuous EWS has demonstrated a reduction in mortality and length-of-stay for a subgroup of patients, although it resulted in increased RRT alerts and required increased RRT staffing [54]. The COVID-19 pandemic-induced staffing shortages may be inhibiting the expansion of current EWSs [55]. AI has shown the potential to improve the detection of deterioration while reducing RRT alerts when retrospectively applied to EWSs [56]. As we inevitably move towards continuously monitoring hospitalised patients using wearable devices, we will need better systems, including AI, to utilise more efficiently and accurately this vast amount of data [57], [58].
4.2. Cognitive dysfunction and long-term outcomes
Many COVID-19 patients have a good outcome, as they can recover to their status prior to SARS-CoV-2 infection, yet 70% complain of at least one remaining symptom [59]. Notably, one-third of patients develop dyspnoea at 12 months, and another 30% develop anxiety or depression. This so-called “long-COVID” [60] has many similarities with post-intensive care syndrome (PICU) [61], [62]. Moreover, many ICU patients who survived COVID-19 are dependent on chronic life support due to pulmonary fibrosis [63], [64], which leaves them in need of prolonged mechanical support, home ventilation, or long-term oxygen therapy [65]. It should be added that such patients are also at risk of developing long-term functional and psycho-cognitive impairments that impact their quality of life [60].
The pathophysiology of long COVID-19 is not fully understood. The initial severity of COVID-19 is a risk factor for long-term complications, yet some patients with mild symptoms will develop long-term complications [66], [67]. A protracted COVID-19-induced inflammatory state is one of its mechanisms [60], but direct viral toxicity has also been culpable, notably SARS-CoV-2-induced myositis [68]. Finally, post-COVID-19 syndromes are not specific to COVID-19 but rather are related to ICU management, such as ICU-acquired paresis from the use of neuromuscular blockers or corticosteroids, but also prolonged mechanical ventilation and muscular uploading. Long-term psycho-cognitive disorders certainly result from a protracted neuro-inflammatory process triggered by early systemic inflammatory response, with the latter also considered to be involved in acute central neurological dysfunction, including sickness behaviour, encephalopathy, and encephalitis.
Implementation of bundles of care such as the ABCDEF bundle [69], [70]—dedicated for ICU and after-ICU care [71] and composed of six evidence-based elements—has been shown to decrease the occurrence of delirium or psychological disorders in general ICU patients. Another dedicated quality-improving approach is the early Comfort using Analgesia, minimal Sedatives and maximal Humane care (e-CASH) concept aimed to provide comfort and patient-centred care without excessive sedation [72]. These bundles are likely to be useful for caring for COVID-19 patients in the ICU, even if they have not been specifically assessed or extensively applied [73]. With an increased use of midazolam and lack of family visitations, the set-back of care regarding sedation and delirium management in the ICU was associated with a dramatic increase in the rate of ICU delirium in COVID-19 patients [74]. Nevertheless, practices have rapidly changed during the pandemic, and the standards of care for ARDS patients before the COVID-19 pandemic are now reconsidered.
COVID-19 promoted innovative approaches such as telemedicine and virtual reality [75]. As an example, the out-patient clinics for long-COVID-19 or cognition improvement are promising [76], [77], as they provide multidisciplinary and personalised care [78], [79]. Long-term symptoms are being evaluated in multicentric studies aiming to recruit 10 000 patients (CO-FLOW and PHOSP-COVID) [80], [81]. A study on genetic polymorphisms through the Human Genetic Effort could also help identify susceptibility genes and introduce personalised therapeutics [82].
4.3. Ethics and humanization of care
The pandemic placed us in unusual situations of overwhelming demands. Considering triage appeared to be necessary if not essential and required complex thought processes to provide beyond-simplistic answers [83], [84]; they involve understanding quantitative and qualitative human and material resources, which are multi-level organisational issues with prioritisation matters that cannot be considered from an ICU perspective alone [85], [86]. They need to be continuously adjusted with flexible approaches while being aware of the importance of the political components of these decisions. Currently, an honest and unbiased analysis of the answers provided (including their efficiency and acceptability) should help define the best decision-making processes and strategies for the future, all while involving healthcare users and citizens in the elaboration of these prioritisation strategies, which need to be considered acceptable and legitimate by most people.
The COVID-19 pandemic reminded us of the significance of the human factor in the management of ICU patients, as the relationship between patients, families, and caregivers has been deeply affected [3], [87]. Among prevention measures, restrictions on family visits could have a significant impact on patient experiences and perceptions, with increased post-traumatic stress, anxiety, and depression among both patients and relatives [88]. Caregivers attempted to deal with these new constraints despite a significant rise in their own workload and a generally stressful situation [87]. Today, it seems essential to develop new relational strategies between family, relatives, and caregivers, such as ensuring that relatives are physically present in end-of-life situations; using hologram or android robot technology to visually or even physically substitute for the patient’s relatives [89]; proposing a psychologist’s support for families using distant communication tools and a post-ICU follow-up; offering a written report of the ICU stay such as a diary; and developing communication training for caregivers by regularly practicing during simulated sessions.
Ethical issues have been a central concern of all people involved. The pandemic improved the public’s awareness of ICU issues, as well as ICU healthcare professionals’ awareness of the citizens’ concerns. Healthcare user (patients, relatives, and citizens) empowerment must now be encouraged to legitimise the decision-making processes and to promote patient and relative-reported outcomes and experience measures [90]. This will also require, from both healthcare workers and users, to balance the perceived need to “do or prescribe something” (as prescribing no medications could lead to a perception of therapeutic helplessness) and the vital resistance against non-evidence-based treatments, even when insistently requested [91], [92].
The incredible development of research during this crisis must benefit the development of research in ethics: multidisciplinary research involving diverse experts and both quantitative and qualitative methodologies must be encouraged via dedicated funding to measure and improve the quality of care in an evidence-based manner [93].
5. Urgent challenges in the management of critical care research in resource-poor settings
Only a small portion of biomedical investigations originates in low- and middle-income countries (LMICs) [94], and this gap is likely even bigger in the area of critical care [95]. To date, infectious diseases that can lead to critical illness, such as malaria and melioidosis, have been the most successful areas of research in LMICs. There is a clear sense of urgency but also great ambiguity about what should be researched. Quality improvement in resource-limited ICUs has always been largely unexplored, and this is especially needed now in the context of the massive increase in patients that is putting hospitals and ICUs under great pressure and the multiple makeshift ICUs that are starting to cope with case surges. Next, specific research questions could be more prominent in LMICs than in high-income countries (HICs) but deciding whether equipoise exists for testing certain interventions in settings of high versus low resources is challenging. For example, there could be a much greater need for research on how to use oxygen most economically—given the severe oxygen shortages that many LMICs currently face [96]—than for the development of “emergency” ventilators, since a skilled workforce to operate them remains very limited [97]. And there are many more challenges [98]. Research staff in resource-limited hospitals often cannot spare even the short-term investment required to contribute to “simple” observational studies, and multi-centre trials are significantly more expensive to perform because “standard” clinical data are often not available at these sites. In addition, ethical considerations are complex, as all participants at resource-limited sites must be considered potentially “vulnerable” due to the at times extreme need and lack of healthcare—this also includes the relationships between researchers from LMICs and HICs, with unequal power dynamics and a realistic risk for abuse. Last, but not least, research always has two competing goals—developing local researchers lacking prior training and producing best-quality research.
However, there is important heterogeneity in both research needs and means among LMICs [99], and LMICs are relevant not only to answer "specific questions" but rather to contribute with crucial questions in the critical care field for the whole world. Examples of groups that created an infrastructure for global epidemiologic data before the COVID-19 pandemic include the “International Severe Acute Respiratory and Emerging Infection Consortium” (ISARIC) [100], the “Global Intensive Care Working Group” of the European Society of Intensive Care Medicine [98], the “Critical Care ASIA” of the Mahidol Oxford Research Unit in Bangkok, Thailand [101], the “Human Resources for Health” program in Rwanda [102], the “CERTAIN: Checklist for Early Recognition and Treatment of Acute IllNess” [103], and “Vital Signs Directed Therapy” [104]. There are also large funding bodies such as the Wellcome Trust that support several efforts, but clearly additional funds are needed. During the COVID-19 pandemic, major publications from LMICs were important to better understand the pandemic epidemiology or COVID-19 treatment options, and LMICs actively participated in many of the international platform trials. Since the worldwide outbreak of COVID-19, there have been multiple initiatives, such as the “LMIC taskforce” [105], the COVID-19 Clinical Research Coalition [106], the “Every Breath Counts coalition” [107], the Asian Critical Care Clinical Trial Group [59] or the Brazilian Research in Intensive Care and COVID-19 Coalition networks [108], [109].
Collaboration of researchers from established groups in HICs with researchers in LMICs has proven to be good model, at least if equal and reciprocal relations are guaranteed. The “Findability, Accessibility, Interoperability, and Reusability” (FAIR) guiding principles for data should be executed to enable the equitable use and reuse of data [110]. Hospitals and their healthcare professionals can easily become overloaded with their clinical duties, especially in the current wave of COVID-19 patients, leaving insufficient room for research. Finally, priority settings and governance regarding financial resources for research will remain extremely important, and local institutions and their institutional review boards should help create an environment for research benefiting the local population.
High-quality research takes time, but there are countless examples of it being possible even during a pandemic. Instead of spending precious time and resources on multiple investigations, it could be wise to consolidate the best efforts, supported by international collaborations, to focus on pragmatic research that would meet the priorities of LMICs and best serve to narrow the practice gap of evidence.
6. Conclusion
While the COVID-19 pandemic has challenged clinical and research organisations worldwide, multiple examples of projects or consortia, from governmental or non-governmental origins, also outlined promising ways of overcoming research-related issues under extreme strain. In many ways, the pandemic has taught us lessons, which should now be incorporated into better preparedness and long-term global solutions for future pandemic or mass disaster contexts.
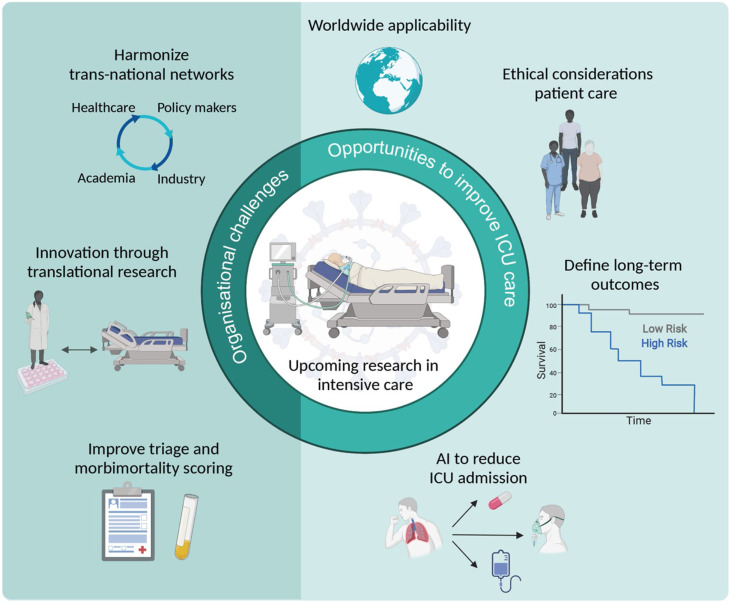
Among other aspects, the availability of transnational clinical and translational research organisations and networks that are ready to operate even under major strain is pivotal to help therapeutic innovations to emerge. In this context, key areas for future critical care research and better pandemic preparedness are artificial intelligence applied to healthcare, characterisation of long-term symptoms, and ethical considerations to improve evidence-based and patient-centred quality of care. Such collaborative research efforts should involve groups from both high and low-to-middle income countries to propose worldwide solutions (Fig. 1 ).
Fig. 1.
Organisational challenges in critical care research during the COVID-19 pandemic and opportunities to improve future intensive care unit (ICU) care and research. Created with BioRender.com.
Human and animal rights
Not applicable.
Informed consent and patient details
Not applicable.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Funding
This work was supported by the Department of Perioperative Medicine, CHU Clermont-Ferrand, Clermont-Ferrand, France.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Vincent J.-L., Abraham E. The last 100 years of sepsis. Am J Respir Crit Care Med. 2006;173:256–263. doi: 10.1164/rccm.200510-1604OE. [DOI] [PubMed] [Google Scholar]
- 2.Schinkel M., Virk H.S., Nanayakkara P.W.B., van der Poll T., Wiersinga W.J. What sepsis researchers can learn from COVID-19. Am J Respir Crit Care Med. 2021;203:125–127. doi: 10.1164/rccm.202010-4023LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi Y.M., Azoulay E., Al-Dorzi H.M., Phua J., Salluh J., Binnie A., et al. How the COVID-19 pandemic will change the future of critical care. Intensive Care Med. 2021;47:282–291. doi: 10.1007/s00134-021-06352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent J.-L., Wendon J., Martin G.S., Juffermans N.P., Creteur J., Cecconi M. COVID-19: what we’ve done well and what we could or should have done better-the 4 Ps. Crit Care. 2021;25:40. doi: 10.1186/s13054-021-03467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J., Yin Y., Myers K.R., Lakhani K.R., Wang D. Potentially long-lasting effects of the pandemic on scientists. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratan T., Aichinger H., Brkic N., Rueter J., Apfelbacher C., Boyer L., et al. Impact of the COVID-19 pandemic on ongoing health research: an ad hoc survey among investigators in Germany. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana L., Fasano A., Chong Y.S., Vineis P., Willett W.C. Transdisciplinary research and clinical priorities for better health. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SPH_VisionPaper_02062016.pdf n.d.
- 9.Urgent Public Health COVID-19 Studies n.d. https://www.nihr.ac.uk/covid-studies/ [Accessed 9 January 2022].
- 10.Outcomes of the UK COVID-19 Therapeutics Advisory Panel (UK-CTAP) n.d. https://www.ukri.org/about-us/policies-standards-and-data/data-collection/uk-covid-19-therapeutics-advisory-panel/ [Accessed 16 November 2021].
- 11.WHO Solidarity Trial Consortium, Pan H., Peto R., Henao-Restrepo A.-M., Preziosi M.-P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong K. Covid-19 and the investigator pipeline. N Engl J Med. 2021;385:7–9. doi: 10.1056/NEJMp2100086. [DOI] [PubMed] [Google Scholar]
- 13.Omary M.B., Eswaraka J., Kimball S.D., Moghe P.V., Panettieri R.A., Jr, Scotto K.W. The COVID-19 pandemic and research shutdown: staying safe and productive. J Clin Invest. 2020;130:2745–2748. doi: 10.1172/JCI138646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moletta L., Pierobon E.S., Capovilla G., Costantini M., Salvador R., Merigliano S., et al. International guidelines and recommendations for surgery during Covid-19 pandemic: a systematic review. Int J Surg. 2020;79:180–188. doi: 10.1016/j.ijsu.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finley C., Prashad A., Camuso N., Daly C., Aprikian A., Ball C.G., et al. Guidance for management of cancer surgery during the COVID-19 pandemic. Can J Surg. 2020;63:S2–4. doi: 10.1503/cjs.005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong R.A., Kane A.D., Kursumovic E., Oglesby F.C., Cook T.M. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia. 2021;76:537–548. doi: 10.1111/anae.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen N.T., Chinn J., Nahmias J., Yuen S., Kirby K.A., Hohmann S., et al. Outcomes and mortality among adults hospitalized with COVID-19 at US Medical Centers. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65:533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu K., Alharahsheh B., Garg N., Guha P. Evaluating risk stratification scoring systems to predict mortality in patients with COVID-19. BMJ Health Care Inform. 2021;28 doi: 10.1136/bmjhci-2021-100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Röltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feyaerts D., Hedou J., Gillard J., Chen H., Tsai E.S., Peterson L.S., et al. Integrated plasma proteomic and single-cell immune signaling network signatures demarcate mild, moderate, and severe COVID-19. BioRxiv. 2021 doi: 10.1101/2021.02.09.430269. Feb 10;2021.02.09.430269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B., et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440.e23. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvin A., Chapuis N., Dunsmore G., Goubet A.-G., Dubuisson A., Derosa L., et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418.e18. doi: 10.1016/j.cell.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevrier S., Zurbuchen Y., Cervia C., Adamo S., Raeber M.E., de Souza N., et al. A distinct innate immune signature marks progression from mild to severe COVID-19. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2020.100166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filbin M.R., Mehta A., Schneider A.M., Kays K.R., Guess J.R., Gentili M., et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L., et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183:1479–1495.e20. doi: 10.1016/j.cell.2020.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay M.A., Thompson B.T., Ware L.B. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;9:933–936. doi: 10.1016/S2213-2600(21)00105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos L.D.J., Artigas A., Constantin J.-M., Hagens L.A., Heijnen N., Laffey J.G., et al. Precision medicine in acute respiratory distress syndrome: workshop report and recommendations for future research. Eur Respir Rev. 2021;30 doi: 10.1183/16000617.0317-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie K.M., Li H., Bedinger D., Schendel S.L., Dennison S.M., Li K., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor P.C., Adams A.C., Hufford M.M., de la Torre I., Winthrop K., Gottlieb R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon D. How swamped preprint servers are blocking bad coronavirus research. Nature. 2020;581:130–131. doi: 10.1038/d41586-020-01394-6. [DOI] [PubMed] [Google Scholar]
- 34.Bauchner H., Fontanarosa P.B., Golub R.M. Editorial evaluation and peer review during a pandemic: how journals maintain standards. JAMA. 2020;324:453–454. doi: 10.1001/jama.2020.11764. [DOI] [PubMed] [Google Scholar]
- 35.Park J.J.H., Mogg R., Smith G.E., Nakimuli-Mpungu E., Jehan F., Rayner C.R., et al. How COVID-19 has fundamentally changed clinical research in global health. Lancet Glob Health. 2021;9:e711–20. doi: 10.1016/S2214-109X(20)30542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Menyar A., Mekkodathil A., Asim M., Consunji R., Rizoli S., Abdel-Aziz Bahey A., et al. Publications and retracted articles of COVID-19 pharmacotherapy-related research: a systematic review. Sci Prog. 2021;104 doi: 10.1177/00368504211016936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehra M.R., Ruschitzka F., Patel A.N. Retraction-hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimmel S.E., Califf R.M., Dean N.E., Goodman S.N., Ogburn E.L. COVID-19 clinical trials: a teachable moment for improving our research infrastructure and relevance. Ann Intern Med. 2020;173:652–653. doi: 10.7326/M20-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaemi M.S., Tarca A.L., Romero R., Stanley N., Fallahzadeh R., Tanada A., et al. Proteomic signatures predict preeclampsia in individual cohorts but not across cohorts — implications for clinical biomarker studies. J Matern Fetal Neonatal Med. 2021:1–8. doi: 10.1080/14767058.2021.1888915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beam A.L., Kohane I.S. Big data and machine learning in health care. JAMA. 2018;319:1317–1318. doi: 10.1001/jama.2017.18391. [DOI] [PubMed] [Google Scholar]
- 41.Pirracchio R., Cohen M.J., Malenica I., Cohen J., Chambaz A., Cannesson M., et al. Big data and targeted machine learning in action to assist medical decision in the ICU. Anaesth Crit Care Pain Med. 2019;38:377–384. doi: 10.1016/j.accpm.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 42.van de Sande D., van Genderen M.E., Huiskens J., Gommers D., van Bommel J. Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit. Intensive Care Med. 2021;47:750–760. doi: 10.1007/s00134-021-06446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez G. Artificial intelligence in the intensive care unit. Crit Care. 2020;24:101. doi: 10.1186/s13054-020-2785-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho A. AI systems aim to sniff out coronavirus outbreaks. Science. 2020;368:810–811. doi: 10.1126/science.368.6493.810. [DOI] [PubMed] [Google Scholar]
- 45.Weber G.M., Mandl K.D., Kohane I.S. Finding the missing link for big biomedical data. JAMA. 2014;311:2479–2480. doi: 10.1001/jama.2014.4228. [DOI] [PubMed] [Google Scholar]
- 46.Wong A., Otles E., Donnelly J.P., Krumm A., McCullough J., DeTroyer-Cooley O., et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181:1065–1070. doi: 10.1001/jamainternmed.2021.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verdonk C., Verdonk F., Dreyfus G. How machine learning could be used in clinical practice during an epidemic. Crit Care. 2020;24:265. doi: 10.1186/s13054-020-02962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sethi S.S., Chalwin R. Governance of rapid response teams in Australia and New Zealand. Anaesth Intensive Care. 2018;46:304–312. doi: 10.1177/0310057X1804600308. [DOI] [PubMed] [Google Scholar]
- 49.Chan P.S., Khalid A., Longmore L.S., Berg R.A., Kosiborod M., Spertus J.A. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513. doi: 10.1001/jama.2008.715. [DOI] [PubMed] [Google Scholar]
- 50.Maharaj R., Raffaele I., Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. doi: 10.1186/s13054-015-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeVita M.A., Smith G.B., Adam S.K., Adams-Pizarro I., Buist M., Bellomo R., et al. “Identifying the hospitalised patient in crisis” — a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81:375–382. doi: 10.1016/j.resuscitation.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Lyons P.G., Edelson D.P., Carey K.A., Twu N.M., Chan P.S., Peberdy M.A., et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the get with the guidelines resuscitation-medical emergency team registry. Crit Care Med. 2019;47:1283–1289. doi: 10.1097/CCM.0000000000003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Embi P.J., Leonard A.C. Evaluating alert fatigue over time to EHR-based clinical trial alerts: findings from a randomized controlled study. J Am Med Inform Assoc. 2012;19:e145–8. doi: 10.1136/amiajnl-2011-000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan C.K., Amspoker A.B., Howard C., Razjouyan J., Siddique M., D’Avignon S., et al. Continuous cloud-based early warning score surveillance to improve the safety of acutely ill hospitalized patients. J Healthc Qual. 2021;43:59–66. doi: 10.1097/JHQ.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 55.Wu H., Soe M.M., Konnor R., Dantes R., Haass K., Dudeck M.A., et al. Hospital capacities and shortages of healthcare resources among US hospitals during the coronavirus disease 2019 (COVID-19) pandemic, National Healthcare Safety Network (NHSN), March 27-July 14, 2020. Infect Control Hosp Epidemiol. 2021:1–4. doi: 10.1017/ice.2021.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho K.-J., Kwon O., Kwon J.-M., Lee Y., Park H., Jeon K.-H., et al. Detecting patient deterioration using artificial intelligence in a rapid response system. Crit Care Med. 2020;48:e285–e289. doi: 10.1097/CCM.0000000000004236. [DOI] [PubMed] [Google Scholar]
- 57.Weenk M., Bredie S.J., Koeneman M., Hesselink G., van Goor H., van de Belt T.H. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22 doi: 10.2196/15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verrillo S.C., Cvach M., Hudson K.W., Winters B.D. Using continuous vital sign monitoring to detect early deterioration in adult postoperative inpatients. J Nurs Care Qual. 2019;34:107–113. doi: 10.1097/NCQ.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 59.Phua J., Weng L., Ling L., Egi M., Lim C.-M., Divatia J.V., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marra A., Pandharipande P.P., Girard T.D., Patel M.B., Hughes C.G., Jackson J.C., et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martillo M.A., Dangayach N.S., Tabacof L., Spielman L.A., Dams-O’Connor K., Chan C.C., et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: cohort study from a New York City Critical Care Recovery Clinic. Crit Care Med. 2021;49:1427–1438. doi: 10.1097/CCM.0000000000005014. [DOI] [PubMed] [Google Scholar]
- 63.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lechowicz K., Drożdżal S., Machaj F., Rosik J., Szostak B., Zegan-Barańska M., et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med Res. 2020;9 doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gentile F., Aimo A., Forfori F., Catapano G., Clemente A., Cademartiri F., et al. COVID-19 and risk of pulmonary fibrosis: the importance of planning ahead. Eur J Prev Cardiol. 2020;27:1442–1446. doi: 10.1177/2047487320932695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 67.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manzano G.S., Woods J.K., Amato A.A. Covid-19-associated myopathy caused by type I interferonopathy. N Engl J Med. 2020;383:2389–2390. doi: 10.1056/NEJMc2031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devlin J.W., Skrobik Y., Gélinas C., Needham D.M., Slooter A.J.C., Pandharipande P.P., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 70.Pun B.T., Balas M.C., Barnes-Daly M.A., Thompson J.L., Aldrich J.M., Barr J., et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mart M.F., Pun B.T., Pandharipande P., Jackson J.C., Ely E.W. ICU survivorship — the relationship of delirium, sedation, dementia, and acquired weakness. Crit Care Med. 2021;49:1227–1240. doi: 10.1097/CCM.0000000000005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincent J.-L., Shehabi Y., Walsh T.S., Pandharipande P.P., Ball J.A., Spronk P., et al. Comfort and patient-centred care without excessive sedation: the eCASH concept. Intensive Care Med. 2016;42(6):962–971. doi: 10.1007/s00134-016-4297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu K., Nakamura K., Katsukawa H., Elhadi M., Nydahl P., Ely E.W., et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explor. 2021;3:e0353. doi: 10.1097/CCE.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pun B.T., Badenes R., Heras La Calle G., Orun O.M., Chen W., Raman R., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vlake J.H., Van Bommel J., Wils E.-J., Korevaar T.I.M., Hellemons M.E., Schut A.F.C., et al. Effect of intensive care unit-specific virtual reality (ICU-VR) to improve psychological well-being and quality of life in COVID-19 ICU survivors: a study protocol for a multicentre, randomized controlled trial. Trials. 2021;22:328. doi: 10.1186/s13063-021-05271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss B., Paul N., Balzer F., Noritomi D.T., Spies C.D. Telemedicine in the intensive care unit: a vehicle to improve quality of care? J Crit Care. 2021;61:241–246. doi: 10.1016/j.jcrc.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 77.Weiss E.F., Malik R., Santos T., Ceide M., Cohen J., Verghese J., et al. Telehealth for the cognitively impaired older adult and their caregivers: lessons from a coordinated approach. Neurodegener Dis Manag. 2021;11:83–89. doi: 10.2217/nmt-2020-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mayer K.P., Parry S.M., Kalema A.G., Joshi R.R., Soper M.K., Steele A.K., et al. Safety and feasibility of an interdisciplinary treatment approach to optimize recovery from critical coronavirus disease 2019. Crit Care Explor. 2021;3:e0516. doi: 10.1097/CCE.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Z., Yang M., Lai C.-L. Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021;9 doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bek L.M., Berentschot J.C., Hellemons M.E., Huijts S.M., Aerts J.G.J.V., van Bommel J., et al. CO-FLOW: COvid-19 Follow-up care paths and Long-term Outcomes Within the Dutch health care system: study protocol of a multicenter prospective cohort study following patients 2 years after hospital discharge. BMC Health Serv Res. 2021;21:847. doi: 10.1186/s12913-021-06813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.PHOSP-COVID Collaborative Group, Evans R.A., McAuley H., Harrison E.M., Shikotra A., Singapuri A., et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation — a multi-centre prospective cohort study. BioRxiv. 2021:1275–1287. doi: 10.1101/2021.03.22.21254057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.COVID HUMAN GENETIC EFFORT n.d. https://www.covidhge.com/ [Accessed 9 January 2022].
- 83.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 84.White D.B., Lo B. a framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323:1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 85.Rosenbaum L. Facing Covid-19 in Italy — ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 86.Azoulay É, Beloucif S., Guidet B., Pateron D., Vivien B., Le Dorze M. Admission decisions to intensive care units in the context of the major COVID-19 outbreak: local guidance from the COVID-19 Paris-region area. Crit Care. 2020;24:293. doi: 10.1186/s13054-020-03021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mistraletti G., Gristina G., Mascarin S., Iacobone E., Giubbilo I., Bonfanti S., et al. How to communicate with families living in complete isolation. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2020-002633. bmjspcare-2020-002633. [DOI] [PubMed] [Google Scholar]
- 88.Kentish-Barnes N., Cohen-Solal Z., Morin L., Souppart V., Pochard F., Azoulay E. Lived experiences of family members of patients with severe COVID-19 who died in Intensive Care Units in France. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi L., Li B., Kim C., Kellnhofer P., Matusik W. Towards real-time photorealistic 3D holography with deep neural networks. Nature. 2021;591:234–239. doi: 10.1038/s41586-020-03152-0. [DOI] [PubMed] [Google Scholar]
- 90.Bardes C.L. Defining “patient-centered medicine”. N Engl J Med. 2012;366:782–783. doi: 10.1056/NEJMp1200070. [DOI] [PubMed] [Google Scholar]
- 91.Reihani H., Ghassemi M., Mazer-Amirshahi M., Aljohani B., Pourmand A. Non-evidenced based treatment: an unintended cause of morbidity and mortality related to COVID-19. Am J Emerg Med. 2021;39:221–222. doi: 10.1016/j.ajem.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carley S., Horner D., Body R., Mackway-Jones K. Evidence-based medicine and COVID-19: what to believe and when to change. Emerg Med J. 2020;37:572–575. doi: 10.1136/emermed-2020-210098. [DOI] [PubMed] [Google Scholar]
- 93.Malterud K. The art and science of clinical knowledge: evidence beyond measures and numbers. Lancet. 2001;358:397–400. doi: 10.1016/S0140-6736(01)05548-9. [DOI] [PubMed] [Google Scholar]
- 94.Rahman M., Fukui T. Biomedical publication — global profile and trend. Public Health. 2003;117:274–280. doi: 10.1016/S0033-3506(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 95.Dondorp A.M., Iyer S.S., Schultz M.J. Critical care in resource-restricted settings. JAMA. 2016;315:753–754. doi: 10.1001/jama.2016.0976. [DOI] [PubMed] [Google Scholar]
- 96.Shrestha G.S., Lamsal R. Rational use of oxygen in COVID-19 pandemic — are we doing enough? JNMA J Nepal Med Assoc. 2021;59:429–431. doi: 10.31729/jnma.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schultz M.J., Neto A.S., Paulus F. Battling COVID-19-related mortality: from a fight for ventilators to a cry for oxygen. Lancet Respir Med. 2021;9:939–941. doi: 10.1016/S2213-2600(21)00267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schultz M.J., Dünser M.W., Dondorp A.M., Adhikari N.K.J., Iyer S., Kwizera A., et al. In: Sepsis management in resource-limited settings. Dondorp A.M., Dünser M.W., Schultz M.J., editors. Springer; Cham (CH): 2019. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. [PubMed] [Google Scholar]
- 99.Franzen S.R.P., Chandler C., Lang T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.International Severe Acute Respiratory and emerging Infection Consortium. ISARIC 2018. https://isaric.org/ [Accessed 16 January 2022].
- 101.CRIT CARE ASIA Establishing a critical care network in Asia to improve care for critically ill patients in low- and middle-income countries. Crit Care. 2020;24:608. doi: 10.1186/s13054-020-03321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Binagwaho A., Kyamanywa P., Farmer P.E., Nuthulaganti T., Umubyeyi B., Nyemazi J.P., et al. The human resources for health program in Rwanda — new partnership. N Engl J Med. 2013;369:2054–2059. doi: 10.1056/NEJMsr1302176. [DOI] [PubMed] [Google Scholar]
- 103.Vukoja M., Dong Y., Adhikari N.K.J., Schultz M.J., Arabi Y.M., Martin-Loeches I., et al. Checklist for early recognition and treatment of acute illness and injury: an exploratory multicenter international quality-improvement study in the ICUs with variable resources. Crit Care Med. 2021;49:e598–e612. doi: 10.1097/CCM.0000000000004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baker T., Schell C.O., Lugazia E., Blixt J., Mulungu M., Castegren M., et al. Vital signs directed therapy: improving care in an intensive care unit in a low-income country. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dondorp A.M., Papali A.C., Schultz M.J. Recommendations for the management of COVID-19 in low- and middle-income countries. Am J Trop Med Hyg. 2021;104(3_Suppl):1–2. doi: 10.4269/ajtmh.20-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swaminathan DS. Home 2020. https://covid19crc.org/ [Accessed 9 January 2022].
- 107.Home - Stop Pneumonia / Every Breath Counts 2016. https://stoppneumonia.org/ [Accessed 9 January 2022].
- 108.Tomazini B.M., Maia I.S., Cavalcanti A.B., Berwanger O., Rosa R.G., Veiga V.C., et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical Trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopes R.D., de Barros E Silva P.G.M., Furtado R.H.M., Macedo A.V.S., Bronhara B., Damiani L.P., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilkinson M.D., Dumontier M., Aalbersberg I.J.J., Appleton G., Axton M., Baak A., et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3 doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]