Abstract
We present our institution's protocol for evaluating and transplanting thoracic organs from COVID-19 positive donors and report the outcomes to date. Hearts from donors testing positive for COVID-19 on any test were eligible for transplantation at our institution provided the donor exhibited no evidence of hypercoagulability or COVID-19 induced hyperinflammatory state during terminal hospitalization. Lungs were eligible if the donor first tested PCR positive on nasopharyngeal swab (NPS) for COVID-19 > 20 days prior to procurement and had a negative lower respiratory tract specimen. We performed 14 thoracic transplants in 13 recipients using organs from COVID-19 positive donors. None of the recipients or healthcare members acquired COVID-19. No recipients suffered unexpected acute rejection. Patient survival is 92% to date, with graft survival 93%. The use of hearts from COVID-19 positive donors may be safe and effective. Transplantation of lungs is unresolved but may be cautiously pursued under the restricted circumstances.
KEYWORDS: COVID-19, heart transplant, lung Transplant, organ donation, donor-derived infection
The COVID-19 pandemic has exacerbated the national shortage of thoracic organs. Understanding the safety of transplanting thoracic organs from COVID-19 positive donors is necessary to expand the donor pool for patients in critical need.1 We present our institution's protocol for evaluating and transplanting thoracic organs from COVID-19 positive donors and report our outcomes.
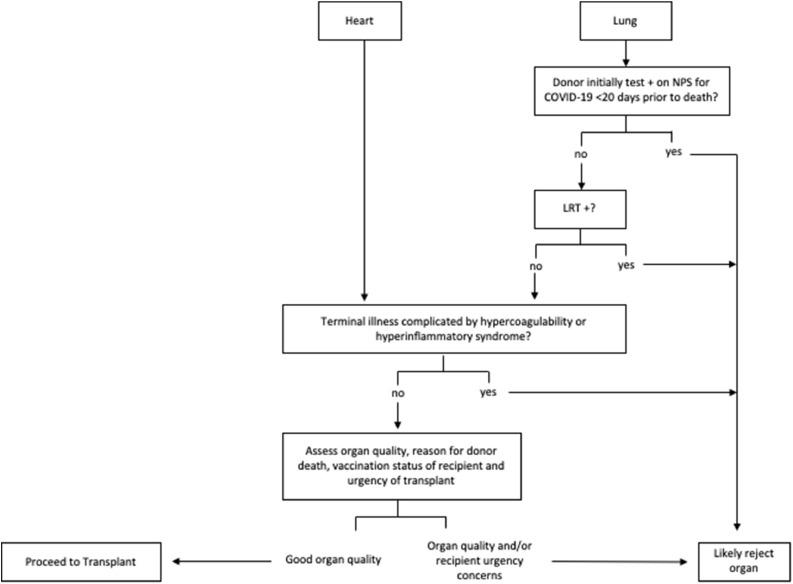
Hearts and lungs from donors testing positive by PCR on nasopharyngeal swab (NPS) for COVID-19 were eligible for transplantation provided the donor exhibited no evidence of hypercoagulability or COVID-19 induced hyperinflammatory state during terminal hospitalization (Figure 1 ). Lungs from donors whose first positive NPS COVID-19 test was <20 days prior to death were ineligible for transplantation. All lung donors underwent lower respiratory tract (LRT) testing for SARS-CoV-2. Lungs from any donor with SARS-CoV-2 detected on bronchoalveolar lavage (BAL) or tracheal aspirate were ineligible for transplant. Thoracic donor organs meeting the eligibility criteria were subsequently assessed for quality in accordance with our standard institutional protocol prior to acceptance. Repeat BAL with SARS-CoV-2 PCR was performed on donor lungs at the time of transplant for added caution. See Supplement for additional methods.
Figure 1.
Protocol for Donors Testing Positive for COVID-19: LRT, lower respiratory tract, NPS, nasopharyngeal swab. Hypercoagulability and hyperinflammatory syndrome as previously defined.8 For lung transplants, a period of <20 days was selected based on isolation guidance for immunosuppressed patients from the Centers for Disease Control and Prevention.9 A lower respiratory tract specimen consisted of either a bronchoalveolar lavage specimen or a tracheal aspirate.
Under our institution's protocol, 14 thoracic organs including 12 hearts and 2 sets of lungs from COVID-19 positive donors were transplanted in 13 recipients between January 1, 2021 and February 2, 2022 (Table 1 ). Dominant strains were alpha and delta (subjects 1-10) and omicron (subjects 11-13). No donors died from SARS-CoV-2 infection and no donors had moderate or severe COVID-19 symptoms. None of the recipients developed signs or symptoms of COVID-19 infection. Median duration of follow up is 215 days. Patient survival is 92% to date, and graft is survival 93%. No procurement or surgical team members developed COVID-19 because of this protocol.
Table 1.
Donor and Recipient Characteristics
| Donor |
Recipient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Organ | Donor with clinically moderate or severe COVID-19?aDonor vaccinated? | Donor information?Cause of death? | Donor COVID testb (days prior to procurement) | Donor heart EF at procurement>55%?c | Number of mRNA vaccine doses prior to transplant | Recipient indication for transplant?Waitlist allocation score? | Recipient develop COVID-19?Treatment? | Graft function?d | SARS-CoV-2 PCR detected on tissue biopsy? | Days since transplant and current recipient status |
| 1 | Heart | No; Not vaccinated |
23 years male, no medical history Motor vehicle accident |
D-3: tracheal aspirate PCR +, CT = 38.14. D-2: tracheal aspirate PCR + D-1: tracheal aspirate –; NPS – |
Yes | Unknown | 53 years male, HCM Status 2 |
No | 1R, pAMR 0 EF >55% |
N/A | D+351, discharged home |
| 2 | Heart | No; Unknown vaccination status |
32 years male, history of substance abuse; Suspected opioid overdose |
D-2: NPS+, CT = 34 | Yes | 2 | 62 years male, NICM Status 2 |
No | 2R, pAMR 0 EF >55% |
Negative | D+253, discharged home |
| 3 | Heart | No- asymptomatic; Unknown vaccination status |
24 years male; no medical history Drug overdose |
D-5: NPS +, D-2: BAL +, CT = 13.26; NPS +, CT = 16.1. |
Yes | 2 | 69 years male; NICM Status 2 |
No; received monoclonal antibody post exposure prophylaxis | 1R, pAMR 0 EF >55% |
Negative | D+237, discharged home |
| 4 | Heart & Liver | No- mild symptoms of headache; Not vaccinated |
20 years male; history of depression; Cerebral abscess |
D-7: NPS +, CT = 41.9; NPS antigen –; BAL – |
Yes | 2 | 36 years male; congenital heart disease with double inlet left ventricle, AV atresia, VSD) Status 4 |
No | Severe biventricular graft dysfunction | Negative | Transplant complicated by massive hemorrhage followed by hypercoagulability and coronary thrombus requiring redo heart transplant on D+6. |
| 4 | Heart | No-asymptomatic; Unknown vaccination status | 17 years male; no medical history; Suicide, gunshot wound |
D-17: NPS + D-5: NPS +, CT = 26 D-3 NPS +, CT = 20-21 and BAL +, CT for ORS gene = 32.7, CT for S gene = 39.0 |
Yes | 2 | Indication as above Status 1 |
No | 1R, pAMR 0 EF >55% |
N/A | D+88 deceased; primary cause of death: hemorrhagic shock from aortic anastomosis breakdown and Rhizopus mediastinitis |
| 5 | Heart | No; Unknown vaccination status | 26 years male; no medical history; Gunshot wound to head |
D-22: NPS + with CT= 31-34 D-1: NPS + with CT= 40; BAL – |
Yes | 2 | 48 years male with HCM; Status 3 |
No | 2R, pAMR 1-I EF >55% |
Negative | D+219, discharged home |
| 6 | Heart | No; Unknown vaccination status | 24 years male; history of anxiety, depression and substance abuse; Drug overdose |
D-2: NPS + with CT = 23 | Yes | 2 | 64 years male with ICM; Status 4 |
No | 1R, pAMR 1-I EF >55% |
N/A | D+216, discharged home |
| 7 | Heart | No; Unknown vaccination status | 28 years male with history of substance abuse; Gunshot wound to neck |
D-3: NPS +, CT=40.2, BAL – D-2: NPS – |
Yes | 2 | 66 years male with cardiac sarcoidosis; Status 4 |
No | 1R, pAMR 0 EF >55% |
N/A | D+215, discharged home |
| 8e | Heart | No-mild symptoms; Unknown vaccination status | 15 years female, no medical history; Pneumococcal meningitis |
D-3: NPS+ D-2: NPS+ D-1: NPS + |
Yes | 2 | 53 years female with NICM; Status 6 |
No | 1R, pAMR 0 EF >55% |
N/A | D+205, discharged home |
| 9 | Lung | No-asymptomatic; Not vaccinated | 18 years male with no medical history; Motor vehicle accident |
D-38: NPS + D-3: NPS + with CT = 38.5; BAL— D-2: NPS – D 0: BAL— |
N/A | 2 Recipient also with history of COVID-19 | 56 years male with pulmonary fibrosis due to inhalational lung injury and prior COVID-19 Lung Allocation Score: 63.1859 |
No | No rejection | N/A | D+219, discharged home |
| 10d | Heart | No- mild symptoms; Not vaccinated | 25 years male, history of substance abuse, hepatitis C, history of Lyme disease; Cause of death unknown |
D-12: NPS + D-4: NPS + D-2: NPS + D-1: BAL – |
Yes | 1; Recipient also with history of COVID-19 | 69 years male with NICM Status 2 |
No | 1R, pAMR 0 EF >55% |
N/A | D+92; index transplant hospitalization |
| 11 | Lung | No- asymptomatic; Unknown vaccination status |
21 years male, no medical history Suicide |
D-30: NPS + D-4: NPS +, with CT = 34.4 D-3: BAL – D-3: NPS – D 0: BAL – |
N/A | 3 | 54 years male, with ILD Lung Allocation Score: 43.4458 |
No | No rejection | N/A | D+110; index transplantation |
| 12 | Heart | No; Unknown vaccination status | 30y years male, no medical history; Drug overdose |
D-3: NPS +, with CT = 28.1 D-2: BAL + with CT = 27.97 |
Yes | 3 | 67 years male, history of ICM Status 2 |
No | 1R, pAMR 0 EF 50% |
N/A | D+90; index transplantation |
| 13 | Heart | No; Unknown vaccination status | 23 years male, no medical history Drug overdose |
D-4: NPS + with CT = 34.6 D-4: NPS (antigen) – D-3: NPS (antigen) – D-2: BAL – |
Yes | 2 | 51 years male, ICM Status 4 |
No | 1R, pAMR 0 EF 50% |
N/A | D+110; index transplantation |
NPS +, nasopharyngeal swab PCR positive for COVID-19; NPS -, nasopharyngeal swab PCR negative for COVID-19; CT, cycle threshold; D, number of days prior to organ procurement; BAL, bronchoalveolar lavage; EF, ejection; HCM, hypertrophic cardiomyopathy; fraction; NICM, nonischemic cardiomyopathy; ICM, ischemic cardiomyopathy; pAMR, pathologic antibody mediated rejection; AV, aortic valve; VSD, ventricular septal defect; N/A, not applicable.
Median duration of follow up of recipients is 215 days (Q1: 110 days, Q3: 219 days).
Dominant SARS-CoV-2 strains during this study period for subjects 1-10 were alpha and delta; for subjects 11-13 omicron.
Genomic targets and sensitivity of SARS-CoV-2 testing platforms vary. Organ procurement centers did not provide information on assays used for each donor, or did the centers provide sequencing information.
Heart function at procurement as assessed grossly and by echocardiogram was normal. All hearts had left ventricular EF >55%, normal right ventricular function and normal appearing valves.
Graft function: Patients underwent routine scheduled allograft biopsies according to schedule discussed in Supplement. The biopsy with the greatest level of rejection is included in this table.
Cycle threshold values not provided by Organ Procurement Centers.
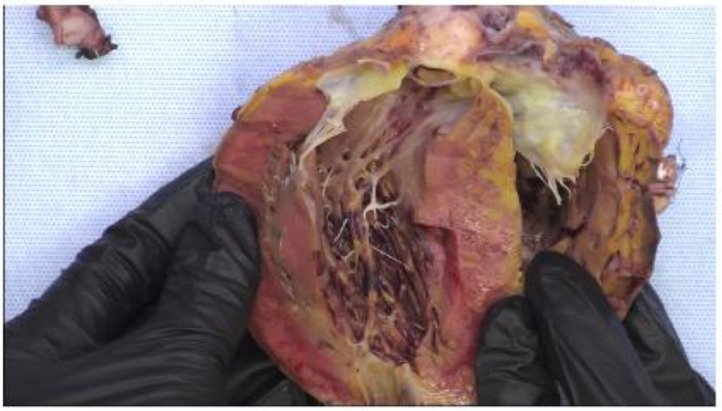
One heart-liver recipient (Subject #4) developed severe intraoperative coagulopathy with massive hemorrhage and thrombosis (Figure 2A , B ). Right coronary artery thrombosis and interventricular clot formed during implantation resulting in irreversible right ventricular ischemia requiring urgent re-do heart transplant utilizing a heart from another COVID-19 positive donor. After re-do heart transplant, the recipient did not develop recurrence of hypercoagulability. See Supplement for pathological finding.
Figure 2A.
Pathology of Explanted Donor Heart from Subject #4: Myocardium with mottled yellow-tan discoloration that is largely subendocardial and right ventricular and right septal. Some patchy yellow-tan discoloration was present in the left ventricular free wall. The yellow-tan discoloration also involved the trabecular and papillary muscles of the right ventricle. There was a mild amount of brown presumed clot material within the left ventricle adhered to the right ventricle.
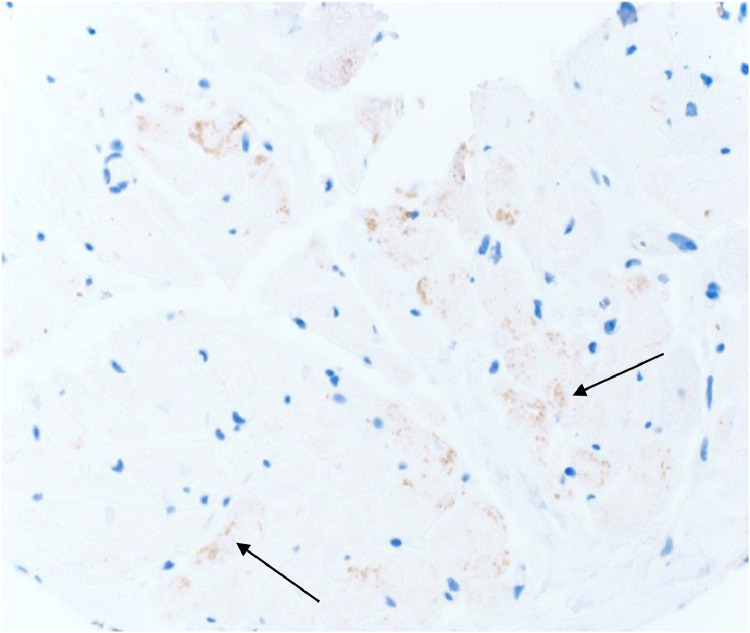
Figure 2B.
Nucleocapsid Immunohistochemistry Staining of the Right Ventricle Biopsy from Explanted Donor Heart (Subject #4)
We present the largest cohort of cardiac transplant recipients who received a heart from a COVID-19 positive donor to date. Our cohort, along with prior reports1, 2, 3 provide evidence that this practice may be safe and effective for patients with end stage heart disease, as none of the recipients developed donor-derived SARS-COV-2 infection, and to-date have expected graft function. While viral proteins have been identified in cardiac tissue on autopsy,4 no data indicates that viable, transmissible virus exists in organs outside of the respiratory tract. Further, no unexpected donor derived transmissions have occurred outside the lung. Whether virus in cardiac tissue represents an innocuous finding in the absence of moderate or severe symptoms, normal echocardiogram, and otherwise normal gross organ assessment, or is a harbinger of longer-term organ dysfunction remains unknown. Because evidence suggests that transmissible virus does not exist outside the respiratory tree, our protocol is deliberately agnostic to the LRT results for heart donors.
Subject #4 experienced significant complications after heart/liver transplantation as previously described. The explanted heart was examined for signs of direct myocardial injury due to COVID-19. A single right ventricular biopsy on the explanted heart IHC stained positive for nucleocapsid protein, but no other pathologic findings indicated direct myocyte damage due to COVID-19. There was no evidence of myocarditis, the SARS CoV-2 PCR from the heart tissue was negative, and the left ventricle was without gross or histologic abnormality. Additionally, the liver was clinically and histologically unaffected. While the significance of the IHC result is unclear, the PCR results and remaining pathological findings were reassuring and supportive that no viable virus exists in these extra-pulmonary tissues. The cause of hypercoagulability and graft failure was ultimately attributed to liver cirrhosis and underlying congenital heart disease, not COVID-19 related organ failure.
COVID-19 is primarily transmitted via respiratory secretions, as evidenced by 3 cases5 , 6 [personal communication] wherein donor-derived COVID-19 transmitted unexpectedly to a recipient via lung transplantation. In each instance, the donor tested positive for SARS-CoV-2 on BAL. In April 2021, UNOS mandated all donors undergo LRT testing for SARS-CoV-2 prior to lung procurement.7 Donors with a negative LRT test, but positive NPS likely represent a different entity wherein cautious evaluation of donor history and lungs may be useful to determine usability for transplant. In such cases, if the donor initially tested positive for COVID-19 on NPS >20 days prior, the donor may have a resolved infection with persistent, dead viral shedding. While viral culture would offer definitive evidence for active infection vs dead viral shedding, this takes several days to complete, rendering it impractical in real-time donor evaluation. Despite donors having positive NP swabs, our lung transplant recipients did not develop COVID-19 and recovered from transplant uneventfully. These cases provide incremental evidence that transplanting lungs from COVID-19 positive donors meeting the protocol's stringent criteria may provide a safe pathway to transplant for patients with end stage lung disease.
Seven donors had unknown vaccination status. A vaccinated donor may be less likely to develop hyperinflammatory/hypercoagulable complications, though unknown/negative donor vaccination did not preclude transplantation.
Our study has limitations. First, donor clinical information was limited to what OPOs collect and share, including COVID-19 assays used and donor vaccination. Second, sample size was small with only 14 recipients receiving thoracic organs from COVID-19 positive donors, including only 2 lung recipients. This reflects our abundant caution in evaluating lung donors with COVID-19, as prior transmission has occurred in this setting.5 , 6 Our results should be interpreted with caution and with the understanding that additional information, including impact of new variants, is needed to fully comprehend the safety of thoracic organ transplantation using COVID-19 positive donors. As we begin to explore the utility of thoracic organ transplantation from COVID-19 positive donors, we encourage all transplant candidates to receive the COVID-19 vaccination series. Additionally, any transplant candidates in whom COVID-19 positive donors are being considered should undergo informed consent prior to transplantation.
Immunohistochemistry was performed using a Sars-Cov-2 Nucleocapsid Protein Polyclonal Antibody (Novus biologicals NB100-56576). The antibody was validated for research, and not clinical purposes. There was equivocal staining in the cardiomyocytes (granular brown pigmented areas) with unclear significance from the right ventricular biopsy of the donor heart in the patient who underwent re-transplantation. However, the corresponding Sars-Cov-2 digital droplet PCR was negative for viral particles.
Author Contributions
All authors contributed to the conception, design, and drafting of the manuscript. All authors have approved of the final version to be published.
Disclosure statement
The authors of this manuscript have conflicts of interest to disclose. EP has participated in a physician advisory board for both Agilent and Diaceuticals for which she was compensated for her time. CW serves on the Data Safety Monitoring Board for COVID Therapeutics at Biogen and Atea Pharmaceuticals and performs consultancy for Regeneron, Enzychem Biopharma, and Adagio Therapeuticsp
Work contained in this manuscript was made possible by the following grant from the National Institute of Allergy and Infectious Disease (T32 -AI100851 [EME]).
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2022.06.018.
Appendix. Supplementary materials
References
- 1.Eichenberger EM, Kaul DR, Wolfe CR. The pandemic provides a pathway: What we know and what we need to know about using COVID positive donors. Transpl Infect Dis. 2021;23:e13727. doi: 10.1111/tid.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neidlinger NA, Smith JA, D'Alessandro AM, et al. Organ recovery from deceased donors with prior COVID-19: A case series. Transpl Infect Dis. 2021;23:e13503. doi: 10.1111/tid.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kute VB, Fleetwood VA, Meshram HS, Guenette A, Lentine KL. Use of organs from SARS-CoV-2 infected donors: Is It Safe? Contemporary Rev. Curr Transplant Rep. 2021;8:281–292. doi: 10.1007/s40472-021-00343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulfamante GP, Perrucci GL, Falleni M, et al. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. Biomedicines. 2020;8:626. doi: 10.3390/biomedicines8120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21:2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar D, Humar A, Keshavjee S, Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21:2623–2624. doi: 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organ Procurement and Transplantation Network. Lower respiratory SARS-CoV-2 testing for lung donors. Available at: 2022https://optn.transplant.hrsa.gov/media/4576/policy_notice_lunglowerrespiratorytesting_20210426.pdf. Accessed October 25, 21.
- 8.Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ. Coronavirus disease 2019-associated thrombosis and coagulopathy: Review of the pathophysiological characteristics and implications for antithrombotic management. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ending Isolation and Precautions for People with COVID-19: Interim Guidance. Available at: 2022 https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed January 27.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.