Abstract
Profiles of dissolved O2 and methane with increasing depth were generated for Lake Washington sediment, which suggested the zone of methane oxidation is limited to the top 0.8 cm of the sediment. Methane oxidation potentials were measured for 0.5-cm layers down to 1.5 cm and found to be relatively constant at 270 to 350 μmol/liter of sediment/h. Approximately 65% of the methane was oxidized to cell material or metabolites, a signature suggestive of type I methanotrophs. Eleven methanotroph strains were isolated from the lake sediment and analyzed. Five of these strains classed as type I, while six were classed as type II strains by 16S rRNA gene sequence analysis. Southern hybridization analysis with oligonucleotide probes detected, on average, one to two copies of pmoA and one to three copies of 16S rRNA genes. Only one restriction length polymorphism pattern was shown for pmoA genes in each isolate, and in cases where, sequencing was done, the pmoA copies were found to be almost identical. PCR primers were developed for mmoX which amplified 1.2-kb regions from all six strains that tested positive for cytoplasmic soluble methane mono-oxygenase (sMMO) activity. Phylogenetic analysis of the translated PCR products with published mmoX sequences showed that MmoX falls into two distinct clusters, one containing the orthologs from type I strains and another containing the orthologs from type II strains. The presence of sMMO-containing Methylomonas strains in a pristine freshwater lake environment suggests that these methanotrophs are more widespread than has been previously thought.
Methane is an important greenhouse gas estimated to contribute approximately 20% to global warming (4). The bacteria that consume methane, methanotrophs, are predicted to play a major role in global methane consumption, and they are thought to play an important role in carbon, oxygen, and nitrogen cycling in both aquatic and terrestrial environments (15). In addition to their role in nutrient cycling, methanotrophs have also gained interest in recent years because of their ability to degrade chlorinated solvents such as trichloroethylene (TCE) (11).
Freshwater sediments, including wetlands, rice paddies, and lakes, are thought to contribute 40 to 50% of the annual atmospheric methane flux (4). Characteristics of methane oxidation in Lake Washington, a freshwater lake in Seattle, Wash., have been reported previously (17, 18). The zone of methane oxidation was shown to be restricted to the top 0.7 cm of sediment, where the concentration of methane appeared to be the limiting factor in the biological oxidation (17). About half of the methane from the lake depths is oxidized to CO2 within this 0.7-cm zone, consuming about 7 to 10% of the total oxygen flux into the sediment (17). Previous work in Lake Washington has also shown that little methane oxidation occurs in the water column and that the oxic-anoxic interface where the methanotrophs are found is apparently seasonably stable (18).
Different groups of methanotrophs have distinct physiological characteristics that can be predicted to affect the role of methanotrophs in nutrient cycling in situ. Methanotrophs can be divided into two major phylogenetic groups: the type I methanotrophs, which are γ-proteobacteria, and the type II methanotrophs, which are α-proteobacteria (11). The type I methanotrophs use the ribulose monophosphate (RuMP) pathway for assimilation, while the type II methanotrophs use the serine cycle (1). The RuMP pathway is more efficient than the serine cycle, with conversion efficiencies of about 65 to 80% and 40 to 60% for methylotrophs containing each of the respective pathways (1).
Groups of methanotrophs also differ from each other in the types of methane mono-oxygenase (MMO) they produce. All methanotrophs contain the membrane-bound or particulate MMO (pMMO) (11). In addition to the production of pMMO, some methanotrophs are also capable of producing a distinct cytoplasmic soluble MMO (sMMO) (11). The type of MMO expressed is important environmentally, since sMMO shows substantially higher rates for the cometabolism of halogenated solvents such as TCE than does pMMO (6, 24).
Some recent environmental studies based on molecular approaches provide information on the breadth of in situ methanotroph diversity at the genus level (5, 12, 14, 20, 22, 35). However, little work has been done to directly measure the relative abundance of key physiological subgroups, to determine the extent of in situ diversity of sMMO-containing strains, or to ascertain which sMMO-containing strains are environmentally important. The existing information on methane oxidation in Lake Washington (18, 19) makes this environment a good study site for such an investigation.
Initial characterization of the in situ methanotrophic population in Lake Washington has been carried out (5). This work includes the analysis of environmental clone banks of both partial 16S rRNA genes and pmoA genes (encoding a conserved subunit of pMMO) from total community DNA extracted from Lake Washington sediment. These sequences were compared to the 16S rRNA gene and pmoA sequences from a number of methanotroph strains isolated from the same environment (5). This work revealed a broad diversity of methanotrophs, including both type I and type II methanotrophs, that are present in Lake Washington sediment. We report here a more detailed analysis of these and other isolates, including partial sequences for mmoX, encoding the conserved α-subunit of the hydroxylase component of sMMO. This study lays the groundwork for understanding the composition of the in situ population and its current and potential role in carbon and nitrogen cycling.
MATERIALS AND METHODS
Collection of samples.
Sediment samples were collected on 21 June 1996, 26 September 1997, and 9 September 1998 from a 62-m deep station off Madison Park in Lake Washington in Seattle, Wash. (17), using a spade box core sampler. Subcores were taken from these boxcores using 3 in.- and 4 in.-diameter Plexiglas cylinders.
Generation of gas profiles in sediment.
The 3-in. subcores were used immediately for generating pore water oxygen and methane profiles with increasing depth using a “squeezer” apparatus previously described (2). Dissolved oxygen was measured from the squeezer samples using a water-cooled Plexiglas cell (616XL; Cameron Instrument Company, Port Aransas, Tex.) in which an oxygen electrode (E101; Cameron Instrument Company) was fitted. The oxygen electrode was calibrated just prior to the sampling trip using flasks of water with nitrogen or air bubbled through as 0 and 100% oxygen, respectively. A needle was attached to the outlet tube from the oxygen electrode, and the pore water from each half turn was collected for methane determinations in 8.7-ml evacuated serum vials, each containing 0.7 ml of 7 N NaOH, capped with gray butyl rubber-covered stoppers (Wheaton, Millville, N.J.) and aluminum crimp seals. The evacuated vials were stored inverted until they could be analyzed (ca. 2 to 3 days). For methane analysis, these vials were equilibrated to atmospheric pressure, and 0.2-ml samples of head space gas were injected into an HP 5890 Series II Gas Chromatograph (Hewlett-Packard, San Fernando, Calif.) equipped with a microseal inlet septum (Merlin Instrument Company, Half Moon Bay, Calif.), a DB-5 column, and a flame ionization detector. The injector, oven, and detector temperatures were 110, 150, and 110°C, respectively. The peak areas were determined using Hewlett-Packard ChemStation integration software. The concentrations of the samples were calculated by comparison of the peak areas to a standard curve of methane concentrations.
Measurement of methane oxidation rates.
The 4-in. subcores were stored on ice for 1 to 3 h and then were divided into 0.5-cm sections using a 60-ml syringe to remove sediment for each layer successively from the top. Methane oxidation rates in the top three 0.5-cm sediment layers were determined by incubation with 14CH4 and measurement of labeled products in different subfractions by scintillation counting (17, 18). Sediment from each of the layers was diluted 1:1 or 1:3 with bottom water from Lake Washington filtered with a 2-μm (pore-size) filter. Aliquots (2 ml) of diluted sediment were dispensed into sterile 21-ml serum vials (Pierce, Rockford, Ill.) that were closed with Teflon-coated gray butyl rubber stoppers and aluminum crimp seals. Killed controls (zero time points) were run for each time course, in which 7 N NaOH was added to a final concentration of 1 N prior to gas addition. Then, 17.15 μmol of unlabeled methane was added through the stoppers with syringes. Next, 1.6 μCi of synthetic 14CH4 (DuPont-NEN, Boston, Mass.; specific activity, 58.1 mCi/mmol) was added using Pressure-Lok gas-tight syringes (Precision Sampling Corp., Baton Rouge, La.). Vials were made in duplicate and incubated with shaking at room temperature at 250 rpm for 1, 2, 4, or 15 h. At each time point, 7 N NaOH was added to a pair of vials to a final concentration of 1 N to stop the methane oxidation and to allow absorption of gas-phase CO2 and 14CO2 as bicarbonate ions. After 1 h to allow for the absorption of the CO2, the vials were flushed with air for 3 to 5 min to remove any unincorporated methane. Next, 0.5-ml aliquots were placed in 4.5 ml of scintillation fluid (Opti-Fluor; Packard Instrument Company, Meridien, Conn.) in 7-ml scintillation vials and counted using a liquid scintillation counter. This value is a measure of total methane oxidation, and includes all base-stable products (17, 18). In order to estimate the fraction of methane oxidized to CO2, stoppers with suspended pieces of β-phenethylamine-soaked filter paper were then inserted into the vials, and the vials were sealed with aluminum crimp seals. Next, 8 N HCl was added to the liquid via syringe to a final concentration of 1 N to release dissolved CO2 and 14CO2. After overnight incubation at room temperature, the β-phenethylamine-soaked filters with adsorbed CO2 and 14CO2 were counted in 4.5 ml of liquid scintillation fluid in 7-ml scintillation vials. Aliquots (0.5 ml) of the remaining fluid, representing the amount of methane incorporated into acid-stable compounds, were also counted in 20 ml of liquid scintillation fluid in 20-ml scintillation vials. Rates were determined by plotting the amount of methane oxidized versus time. The methane utilization rates leveled off by 4 h so the Vmax values were determined using the time points of 0, 1, and 2 h. In general, about 80% of the total methane oxidized to base-stable compounds was recovered in the two subfractions. Controls using 14C-labeled methanol and CO2 suggested that the main loss term was in the CO2. Therefore, for the carbon conversion calculations, it was assumed that the missing oxidation products were in the form of CO2. Previous work has shown that this experimental system is not limited by mass transfer of methane (32) and that the final methane concentrations used provide estimates of Vmax (17, 18, 32).
Enrichment and isolation of Lake Washington strains.
Strains from Lake Washington were isolated from sediment collected on 21 June 1996 and 26 September 1997. For the former samples, 1-ml samples of the upper 1 cm of sediment were inoculated into 37-ml amber serum vials each containing 10 ml of either nitrate mineral salts medium (NMS) (34) or nitrate-free mineral salts medium with no added copper. The slurries were incubated with methane/air headspace ratios of either 50:50, 80:20, or 5:95 (vol/vol). The vials were incubated with shaking at 200 rpm at room temperature. After 2 days, 2-ml aliquots from each vial were inoculated into 37-ml amber vials each containing 10 ml of NMS with 10 μM CuSO4 · 5H2O and were all incubated with methane/air headspace ratios of 50:50 (vol/vol). After two more days of shaking at 200 rpm at room temperature, serial dilutions were plated onto NMS with 10 μM CuSO4 · 5H2O and incubated at 30°C with a methane/air headspace ratio of 50:50 (vol/vol). Five methanotroph strains were isolated and purified. For the 26 September 1997 samples, different enrichment conditions were used in an attempt to broaden the diversity of isolates obtained. Three enrichments were set up using sediment from the top 0.5-cm layer, this time containing added copper. Sediment was mixed 1:1 (vol/vol) with filter-sterilized lake water and then inoculated into NMS containing a vitamin solution (8) and 10, 20, or 40 μM CuSO4 · 5H2O. The final dilution of the sediment was 1:10, with a total volume of 10 ml. A fourth enrichment was also set up by diluting 1 ml of unfiltered pore water into 9 ml of NMS with vitamins and 10 μM CuSO4 · 5H2O. The dilutions were put into 125-ml flasks which were closed with rubber stoppers and incubated at 25°C with shaking at 200 rpm under a methane-air atmosphere of 50:50 (vol/vol). The gases in the flasks were replaced approximately every 3 days. After 10 days, each enrichment was serially diluted and plated onto NMS agar with vitamins and 10 μM CuSO4. In addition, each enrichment was streaked out directly onto the same medium. These plates were incubated at 30°C under a methane-air atmosphere of 50:50 (vol/vol). From these plates, six methanotroph strains were isolated and purified. All 11 strains were characterized microscopically, using a phase-contrast microscope (Zeiss). Strains were tested for their ability to grow at 37°C. Strains were also tested for sMMO activity using a colorimetric plate assay as previously described (10). For this assay, strains were grown on NMS plates with 1 mM sodium formate and 200 μM FeCl3, as described elsewhere (33).
Isolation of chromosomal DNA.
Chromosomal DNA was isolated using a method similar to one previously described (26). Chromosomal DNA was also isolated from cells grown on agar plates using a method previously described (33).
Southern hybridization.
EcoRI- and PstI-digested aliquots of chromosomal DNA from each isolate and from Methylococcus capsulatus Bath were subjected to agarose gel electrophoresis. The gels were then treated, and the DNA was transferred to nylon membranes (Hybond; Amersham, Piscataway, N.J.) as described previously (27). The membranes were then probed with the following oligonucleotides: type1b (5′-GTCAGCGCCCGAAGGCCT-3′), which targets the coding strand in 16S rRNA genes from type I methanotrophs (equivalent to the region of nucleotides 74 to 98 in Escherichia coli 16S rRNA [A. M. Costello and M. E. Lidstrom, unpublished data]); type2 (5′-GCTCTTTCGCYAGGGACGA-3′), which targets the complement of the coding strand in 16S rRNA genes from type II methanotrophs (equivalent to the region of nucleotides 457 to 475 in E. coli 16S rRNA [Costello and Lidstrom, unpublished]); LWpmoA (5′-AACTTCTGGGGHTGGAC-3′), which targets the reverse complement of nucleotides 319 to 335 in pmoA (numbered from ATG in M. capsulatus Bath pmoA); and mmoXD (5′-CCGATCCAGATDCCRCCCCA-3′), which targets nucleotides 937 to 956 in mmoX (numbered from ATG in M. capsulatus Bath mmoX). These probes were designed based on alignments of existing sequences. Searches of sequence databases showed that the type II and mmoX probes are specific, but the type I and pmoA probes may also hybridize to Ectothiorhodospira and Halothiobacillus 16S sequences and human sequences, respectively. The oligonucleotide probes were labeled with [γ32P]ATP (Dupont-NEN) by phosphorylation at the 5′ end using polynucleotide kinase (Boehringer Mannheim) according to the supplier's protocol. The reactions were stopped by adding 80 μl of 50 mM Tris–50 mM EDTA. Prior to use, the labeled probes were denatured by incubation at 100°C for 5 min. Then, 10 μl of probe was added to the prehybridization buffer, and the membranes were allowed to hybridize overnight. The prehybridization-hybridization temperatures for LWpmoA, mmoXD, type1b, and type2 were 42, 48, 55, and 50°C, respectively. After hybridization, the membranes were rinsed three times for 10 to 15 min each time in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at the hybridization temperature. The membranes were then blotted dry and exposed to X-ray film at −80°C for 3 to 5 days before development. The use of oligonucleotides that did not contain either PstI or EcoRI sites as probes ensures that each can bind to only one DNA fragment per gene copy target.
RFLP analysis.
Fragments of pmoA of about 510 bp were amplified from chromosomal DNA samples from the LW strains using the primers A189gc (13) and mb661 (5). The reactions were carried out in an MJ Research PTC-200 thermocycler, with an initial denaturation step of 30 s at 94°C, followed by 30 cycles of 92°C for 1 min, 60°C for 1 min, and 72°C for 1 min, with a final extension step of 72°C for 5 min. The PCR reactions contained final concentrations of 1× PCR buffer (Gibco-BRL, Rockville, Md.), 1.5 mM MgCl2 (Gibco-BRL), 333 nM A189gc, 333 nM mb661, 0.167 mM deoxynucleoside triphosphates (Boehringer Mannheim), and 2.5 U of Taq polymerase (Gibco-BRL) in a total volume of 30 μl. For each LW strain, the pmoA fragments were then cloned into pCR2.1 using the Topo-TA Cloning Kit (Invitrogen, San Diego, Calif.), and 8 to 10 separate transformants with inserts were analyzed for each pmoA. pmoA fragments were reamplified as noted above, except the initial denaturation step was extended to 5 min, and these PCR products were digested with HhaI (Gibco-BRL) and a combination of MspI (Gibco-BRL) and HaeIII (Gibco-BRL) in a total of 5 μl for each digestion. The digests were then subjected to agarose gel electrophoresis using 3.0% (wt/vol) Nu-Sieve GTG agarose (FMC Bioproducts, Rockland, Maine) gels. DNA from transformants showing unique restriction fragment length polymorphism (RFLP) patterns was sequenced.
PCR amplification of genes from LW strains.
16S rRNA and pmoA genes were amplified from LW strain DNA as described previously (5). New primers for mmoX were designed using existing GenBank sequences of mmoX. These primers were mmoXA (5′-ACCAAGGARCARTTCAAG-3′) and mmoXB (5′-TGGCACTCRTARCGCTC-3′), which amplify an approximately 1,230-bp fragment from mmoX corresponding to nucleotides 166 to 1401 in the M. capsulatus Bath mmoX. The PCR conditions used were the same as those described for the amplification of pmoA from LW strain chromosomal DNA.
DNA sequencing and analysis.
DNA sequencing of the 16S rRNA genes and the pmoA and mmoX gene fragments was carried out on both strands using the ABI Prism BigDye terminators sequencing kit (PE Applied Biosystems, Foster City, Calif.). The sequencing reactions and analysis were performed by the University of Washington Center for AIDS Research DNA Sequencing Facility and the Department of Biochemistry Sequencing Facility using an Applied Biosystems Automated Sequencer. Analyses and translation of DNA sequences were performed using the Genetics Computer Group programs. MmoX sequences were aligned with translated mmoX sequences obtained from the GenBank database using SeqPup (Indiana University) and GeneDoc (www.psc.edu/biomed/genedoc). Dendograms were constructed using the programs PROTDIST, PROTPARS, NEIGHBOR, SEQBOOT, and CONSENSE from PHYLIP v3.5c (7), and tree files were analyzed using Tree View (25).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the pmoA gene sequences from LW4, LW8 (copy A), LW8 (copy B), LW12, and LW14 are AY007282 to AY007286, respectively. The accession numbers for the mmoX gene sequences from LW3, LW4, LW8, LW13, LW15, and PW1 are AY007287 to AY007292, respectively. The accession numbers for the 16S rRNA gene sequences from LW4, LW8, LW12, and LW14 are AY007293 to AY007296, respectively.
RESULTS
Characterization of Lake Washington sediment. (i) Generation of methane and oxygen profiles.
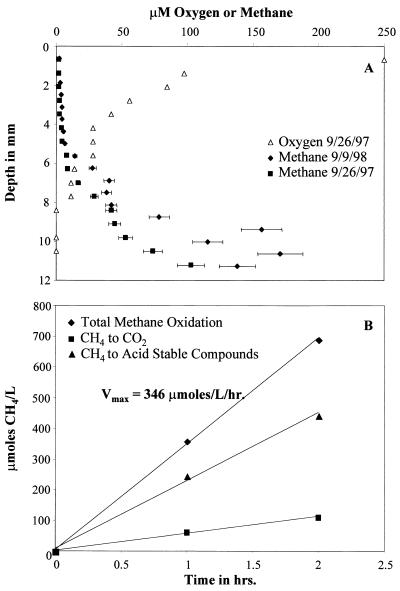
In order to define the zone of maximum methane consumption, the upper layers of sediment were characterized with regard to methane, oxygen, and methane oxidation potential. Methane profiles were determined for the subcores obtained on 26 September 1997 and 9 September 1998, and an oxygen profile was also determined for the 26 September 1997 subcore. The two methane profiles were very similar. The methane concentration increased from approximately 2 to 170 μM within the top 11 mm of sediment, while the oxygen concentration decreased from approximately 250 μM to nondetectable levels within the top 8 mm (Fig. 1A). These data suggest that the area of peak methanotrophic activity should be within the top 0.8 cm of sediment, where both oxygen and methane are present in sufficient concentrations to support methanotrophic metabolism. These profiles are almost superimposable over profiles generated for this site at various seasons from 1980 to 1984 (17), supporting the previous suggestion that the sediment at this 62-m deep station in Lake Washington is quite stable (17, 18).
FIG. 1.
Characterization of methane oxidation in Lake Washington sediment. (A) Profiles of methane and/or O2 with increasing sediment depth for subcores obtained 26 September 1997 and 9 September 1998. The y axis represents sediment depth with 0 mm representing the sediment-water interface. (B) Example of the partitioning of methane to cells and metabolites versus CO2 from the top 0.5-cm layer.
(ii) Methane oxidation rate. In order to characterize the area of peak methane oxidation in the sediment, methane oxidation potentials (Vmax values) were determined for the top three 0.5-cm depths of sediment from the 9 September 1998 sampling trip. Vmax values were determined to be 346, 308, and 269 μmol/liter/h for the 0- to 0.5-cm, 0.5- to 1.0-cm, and 1.0- to 1.5-cm depths, respectively. By Michaelis-Menten kinetics, the Vmax determined for a sample is proportional to the total catalytic potential within the sample. Since the methane oxidation rate per cell is fairly constant over a broad range of growth conditions and methanotrophic species, the Vmax is roughly proportional to the cell number (32). Because the Vmax values for all three depths were similar, this suggests that the numbers of potentially active methanotrophs present at each depth did not differ significantly. In addition, the percentage of methane incorporated into acid stable compounds (cells and metabolites) versus oxidized to CO2 was determined (Fig. 1B). These data provide an estimate of the carbon-conversion efficiency for the methanotrophic population. At all three depths approximately 65% of the oxidized methane was converted to acid stable products while the remainder was converted to CO2.
Isolation and characterization of methanotrophic strains.
In order to begin to characterize methanotrophic populations in Lake Washington sediments, we isolated strains from different enrichment conditions, designed to favor either type I or type II strains (Table 1). These strains can then serve as a baseline for comparison to results obtained by direct techniques not involving culturing (5). The first enrichments (using 21 June 1996 samples) contained no copper and in one case contained no fixed nitrogen, conditions expected to favor growth of sMMO- and nitrogenase-containing type II methanotrophs. Different methane/air headspace ratios were used in an attempt to increase diversity of the isolates. Five strains were isolated that were classed as type II strains, either as Methylocystis (two strains) or as Methylosinus (three strains), by 16S rRNA gene sequence analysis (Table 1). Methylosinus-like strains were obtained in enrichments from all conditions tested, while Methylocystis-like strains were not isolated in the enrichment with no added nitrogen. From the 26 September 1997 sediment, enrichments were carried out with added Cu2+ at various concentrations, conditions expected to favor type I methanotrophs. In this case, five strains were isolated, which 16S rRNA gene sequences indicated could be classed with type I strains, either with Methylomonas (three strains) or Methylobacter (two strains) (Table 1). There was no significant difference in colony types among the plates from the enrichments containing various copper concentrations, suggesting that varying the initial copper concentrations did not strongly influence the final strains. In addition, an enrichment was carried out with pore water to avoid nutrients contained in the sediment, and a type II strain (classing as a Methylosinus; 5) was isolated. All of the strains were short fat rods except the Methylosinus-like strains, which were curved rods characteristic of this genus (11). The Methylomonas-like strains were all pink pigmented, as is characteristic of this genus (11). The strains were also assayed for sMMO activity by a plate assay (see Materials and Methods), using Methylosinus trichosporium OB3b and Methylomicrobium album BG8 as positive and negative controls, respectively. All the Methylosinus-like strains and two of the three Methylomonas-like strains were shown to oxidize naphthalene, the positive test for sMMO (Table 1).
TABLE 1.
Enrichment and characterization of Lake Washington methanotrophsa
| Strain | Classification by 16S rRNA genesb | Enrichment conditionc
|
Pigmentation | sMMO activityd | mmoX type | ||
|---|---|---|---|---|---|---|---|
| % CH4 | % Air | Cu2+ (μM) | |||||
| LW2 | Methylocystis* | 50 | 50 | NA | Cream | − | None |
| LW3 | Methylosinus* | 50 | 50 | NA | Cream | + | Type II |
| LW4 | Methylosinus | 80 | 20 | NAe | Cream | + | Type II |
| LW5 | Methylocystis* | 5 | 95 | NA | Cream | − | None |
| LW8 | Methylosinus | 5 | 95 | NA | Cream | + | Type II |
| LW12 | Methylobacter | 50 | 50 | 10 | White | − | None |
| LW13 | Methylomonas* | 50 | 50 | 20 | Pink | + | Type I |
| LW14 | Methylobacter | 50 | 50 | 20 | White | − | None |
| LW15 | Methylomonas* | 50 | 50 | 20 | Pink | + | Type I |
| LW21 | Methylomonas* | 50 | 50 | 40 | Pink | − | None |
| PW1 | Methylosinus* | 50 | 50 | 10 | Cream | + | Type II |
Strains LW2, LW3, LW4, LW5, and LW8 were isolated from sediment collected 21 June 1996. Strains LW12, LW13, LW14, LW15, and LW21 were isolated from sediment collected 26 September 1997. Strain PW1 was isolated from pore water collected 26 September 1997. Phase-contrast microscopy showed all strains to be short fat rods except the Methylosinus strains, which were curved rods. 16S rRNA gene sequences were determined for strains LW4, LW8, LW12, and LW14 as described in Materials and Methods.
Genera indicated by an asterisk were from sequences published previously (5).
In NMS. NA, none added.
By naphthalene plate assay (10).
No fixed N added.
Genetic characterization of strains.
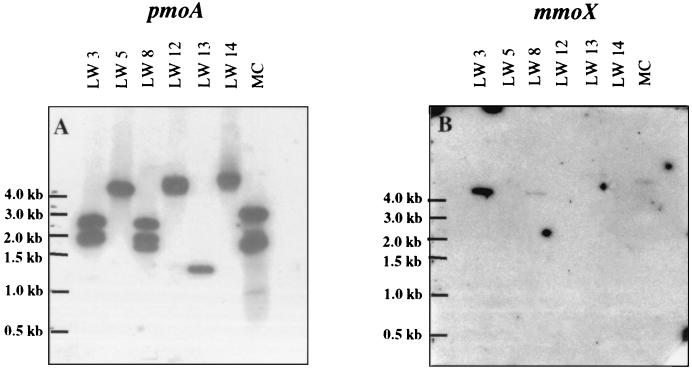
In order to interpret probing experiments for environmental DNA samples, it is important to determine the number of gene copies that are detected with probes for both 16S ribosomal DNA and pmoA in environmental isolates. Therefore, the 11 LW strains were further characterized by Southern hybridization analysis. Blots of digested chromosomal DNA from each strain were probed with four oligonucleotides for type I 16S rRNA genes, type II 16S rRNA genes, pmoA (encoding the catalytic subunit of pMMO), and mmoX (encoding a conserved catalytic subunit of sMMO), respectively. Probing with the two 16S rRNA gene oligonucleotides gave the same results as the 16S rRNA gene sequences: strains LW12, LW13, LW14, LW15, and LW21 were classified as type I methanotrophs, while strains LW2, LW3, LW4, LW5, LW8, and PW1 were classified as type II methanotrophs. In addition, in the type I strains one to two copies were detected, while in the type II strains one to three copies were detected. These results were confirmed with two different restriction digests. An oligonucleotide specific for pmoA detected multiple copies of this gene in several of the strains (see Fig. 2A for examples). Three copies were detectable in LW8, two copies were detectable in LW2, LW3, LW4, and PW1 (all type II strains), and one copy was detectable in LW5, LW12, LW13, LW14, LW15, and LW21 (all type I strains except LW5). Probing with an oligonucleotide specific for mmoX confirmed the capacity to generate sMMO (see Fig. 2B for examples) in strains LW3, LW4, LW8, LW13, LW15, and PW1, and mmoX was not detected in any of the strains that were negative for sMMO.
FIG. 2.
Examples of Southern blot analyses for pmoA (A) and mmoX (B) with representative Lake Washington strains. These chromosomal DNA samples were digested with PstI. Chromosomal DNA samples were also digested with EcoRI. MC, M. capsulatus Bath.
RFLP analyses of pmoA copies.
The presence of multiple copies of pmoA within a single strain has been shown previously for laboratory strains (29, 33), and for both M. capsulatus Bath and M. trichosporium OB3b, the two full copies of the pmoCAB operon are almost identical (9, 33). However, if in situ methanotrophs have more divergent pmo gene copies, then interpretation of denaturing gradient gel electrophoresis (DGGE) and probing analyses of environmental DNA will become more complicated. Therefore, we analyzed the pmo genes of the LW isolates in more detail. The hybridization pattern shown in Fig. 2 suggests that strain LW8 has three copies of pmoA. Although this is the first reported instance of three copies of pmoA in a methanotroph, nitrifiers containing three copies of amoA, encoding a subunit of a related enzyme ammonia monooxygenase, have been found previously (23, 28).
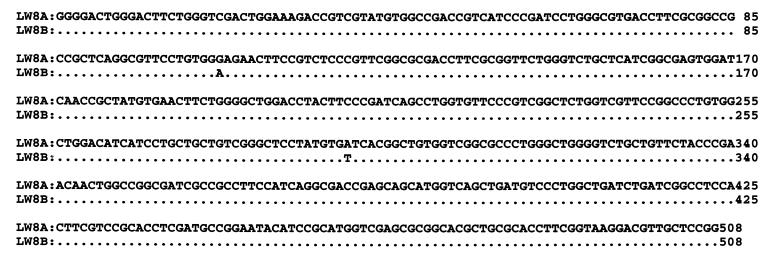
In order to investigate whether the multiple pmoA copies in single strains differed significantly, RFLP analysis was performed for multiple PCR clones from each strain that showed multiple pmoA hybridization bands. All of the isolates each showed only one distinct RFLP pattern for 8 to 10 different transformants. A partial pmoA sequence had been previously reported for strains LW2, LW3, LW5, LW13, LW15, LW21, and PW1 (5). The same region of pmoA sequence was determined for three of the other strains (LW4, LW12, and LW14). LW8 and PW1, two strains shown by Southern analysis to contain three and two pmoA copies, respectively, were investigated further by sequencing pmoA fragments from approximately 8 to 10 separate clones PCR-generated from each strain. For LW8, in eight clones that were sequenced, two highly related sequences have been found; five of one type and three of the other. These types have only two nucleotide differences over the 510-bp PCR product (Fig. 3). This is similar to the case for M. capsulatus Bath and M. trichosporium OB3b pmoA copy sequences. For PW1, all 10 sequences were identical. For all of the pmoA sequences in these isolates, the sequences grouped together in the same genus-level classes as the 16S rRNA gene sequences.
FIG. 3.
Alignment of multiple DNA sequences of pmoA PCR products from LW8. Eight pmoA PCR products from this strain were cloned and sequenced. Five sequences were identical to LW8A, while three sequences differed in two nucleotide positions and were identical to LW8B. The nucleotide changes (positions 106 and 292 in the PCR products) resulted in amino acid differences (E versus K and I versus F, respectively).
PCR amplification and analysis of mmoX.
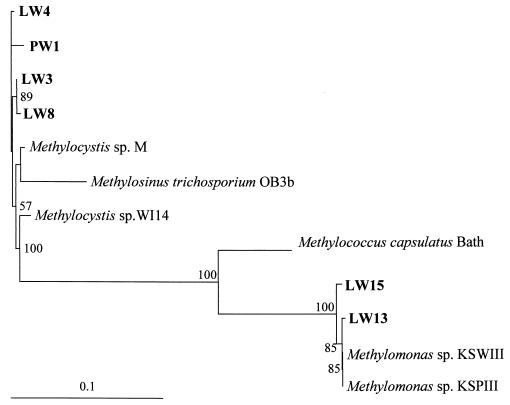
mmoX encodes the α-subunit of the hydroxylase component of sMMO and has been used as a marker for sMMO (19, 21, 31). In order to extend the limited database of mmoX sequences, mmoX was studied in the LW strains. Existing mmoX primers (19, 21) were tested for hybridization to DNA from the Methylomonas strains, but binding was not observed. However, comparison of these primers to two recently published Methylomonas mmoX sequences showed a number of mismatches. Therefore, new primers were designed to amplify an approximately 1,230-bp region of the 1,585-bp mmoX gene, based on the six known full mmoX sequences, including the two from Methylomonas strains (31). PCR amplification using these primers was attempted for the 11 LW strains. PCR products were obtained only for the six strains already shown to exhibit sMMO activity. These PCR products were sequenced and translated, and the amino acid alignments show the sequences to be highly similar, with 75% identity overall (Fig. 4). The products were shown to fall into two major categories by amino acid sequence similarity, type II MmoX (from strains LW3, LW4, LW8, and PW1) and type I MmoX (from strains LW13 and LW15). Phylogenetic analysis of these sequences with previously published sequences shows two distinct groups of MmoX sequences that correspond to the type I-type II delineation (Fig. 5).
FIG. 4.
Alignment of deduced amino acid sequences of the 1.2-kb partial mmoX genes from LW13, LW15, Methylomonas sp. strain KSWIII (accession no. AB025022), Methylomonas sp. strain KSPIII (AB025021), M. capulatus Bath (M90050), M. trichosporium OB3b (X55394), Methylocystis sp. strain WI 14 (AF153282), Methylocystis sp. strain M (U81594), LW3, LW4, LW8, and PW1. Identical residues are in black boxes, and similar residues are in gray boxes.
FIG. 5.
Phylogenetic analysis of the derived amino acid sequences of mmoX genes. BOOTSTRAP values of >50% are shown near the clades. The bar represents 10% sequence divergence, as determined by the lengths of the horizontal lines connecting any two species.
DISCUSSION
This study has provided new information regarding the physiological diversity of methanotrophs in a specific methanotrophic niche, a freshwater lake sediment with a history of stable methane profiles (17). The high carbon conversion efficiency from methane to cells and metabolites of about 65% (Fig. 1B) indicates the dominance of methanotrophs containing the ribulose monophosphate cycle in this environment. This conclusion is supported by the dominance of type I sequences in 16S rRNA gene and pmoA clone banks generated by amplification of total community DNA extracted from Lake Washington (5). However, a variety of both type I and type II methanotrophs were isolated from this niche using different enrichment conditions, suggesting that the underlying physiological potential in the methanotrophic community is greater than for type I strains alone. Significant methanotroph diversity has also been indicated in other studies involving landfills, soils, and peat bogs (12, 14, 20, 22, 35).
The strains described here were tested for the number of detectable copies of key target genes for environmental probing: 16S rRNA genes, pmoA genes, and mmoX genes. Our results and those from other studies of laboratory strains (9, 29) suggest that these probes will detect one to three copies of the first two sets of genes, and one copy of mmoX. In general, type II strains had more detectable copies of pmoA than type I strains, a factor that should be taken into account for estimates of total methanotrophic populations based on direct probing of environmental DNA with pmoA probes. In addition, our results and results from other published studies (9, 29) suggest that in those methanotrophs containing multiple copies of pmoA (as detected using standard pmoA probes), the individual copies are sufficiently similar such that probes and PCR primers will detect these copies and will not distinguish between them. It is also unlikely that DGGE analysis would separate out PCR products of the multiple copies if they differed by one to two nucleotides (12), as in the case of LW8. It has been shown with laboratory strains that pmoA sequence similarity groups fall into the same classes as the 16S rRNA gene sequence genus-level groups (5, 22, 35). This correlation has held up with the new isolates reported in this study, providing further support for the idea that environmental pmoA sequences may be useful as indicators of the phylogenetic position (22).
A surprising result from this study is the presence of Methylomonas-like strains containing sMMO. So far, sMMO-containing Methylomonas strains have only been described from sites contaminated with oil or TCE (16, 30, 31). It has been generally assumed that in natural populations, type II methanotrophs will be the dominant sMMO-containing population, and most in situ bioremediation protocols involving methanotrophs focus on type II strains (3). Our work, however, suggests that type I methanotrophs may contribute significantly to the sMMO-containing population in Lake Washington, a pristine freshwater lake. 16S ribosomal DNA sequences that were classified as Methylomonas were common in an environmental clone bank generated from Lake Washington sediment, including some that were identical to that of LW15 (5), one of the strains that contains sMMO. These results suggest that Methylomonas strains are important components of the in situ population at this site and that LW15 itself may be environmentally significant. The presence of sMMO in two of the four Methylomonas isolates suggests that sMMO may be more prevalent in type I populations than was previously thought. Since type I strains have higher carbon conversion efficiencies than type II strains (1), these methanotrophs may be more attractive than type II strains for bioremediation protocols.
The small number of available mmoX sequences has made the development of robust probes and PCR primers for sMMO difficult. A recently published primer set based on the six available mmoX sequences targets a 396-bp region of mmoX (21, 31). However, one of these primers has six mismatches for the mmoX gene from LW15, a Methylomonas strain, suggesting that this primer set may not be appropriate for environments containing Methylomonas strains. In the present study, new degenerate PCR primers were developed that take into account the more divergent Methylomonas mmoX sequences. These primers target a 1.2-kb region of the approximately 1.6-kb mmoX gene with no mismatches with any of the known mmoX sequences, including the six new sequences reported here, and no matches to any other sequences in the public databases. In addition, the mmoX probe we describe here has no mismatches with any of the known mmoX sequences but shows no identity to any other sequences in public databases, suggesting that the specificity is sufficient for use as an environmental probe.
Alignment of the translated 1.2-kb regions of the mmoX genes from previously published sequences and from the LW strains shows that these sequences are highly conserved. Phylogenetic analysis of these translated sequences shows that MmoX sequences fall into two clusters, one corresponding to type I methanotrophs and one corresponding to type II methanotrophs. This finding, if confirmed by additional mmoX sequences, could form the basis for distinguishing between type I and type II methanotrophs containing sMMO in environmental communities.
ACKNOWLEDGMENTS
This work was supported by grants from the DOE (as a subcontract to grant DE-AC05-960R22464 with Oak Ridge National Laboratory, managed by Lockheed-Martin Energy Research Corp.) and the NSF (DEB-9707383).
We thank Al Devol (University of Washington) for his assistance during this study.
REFERENCES
- 1.Anthony C. The biochemistry of methylotrophs. London, England: Academic Press, Inc.; 1982. [Google Scholar]
- 2.Bender M, Martin W, Hess J, Sayles F, Ball L, Lambert C. A whole-core squeezer for interfacial pore-water sampling. Limnol Oceanogr. 1987;32:1214–1225. [Google Scholar]
- 3.Bowman J P, Jimenez L, Rosario I, Hazen T C, Sayler G S. Characterization of the methanotrophic bacterial community present in a trichloroethylene-contaminated subsurface groundwater site. Appl Environ Microbiol. 1993;59:2380–2387. doi: 10.1128/aem.59.8.2380-2387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicerone R J, Oremland R S. Biogeochemical aspects of atmospheric methane. Global Biogeochem Cycles. 1988;1:61–86. [Google Scholar]
- 5.Costello A M, Lidstrom M E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol. 1999;65:5066–5074. doi: 10.1128/aem.65.11.5066-5074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dispirito A A, Gulledge J, Murrell J C, Shiemke A K, Lidstrom M E, Krema C L. Trichloroethylene oxidation by the membrane associated methane monooxygenase in type I, type II and type X methanotrophs. Biodegradation. 1992;2:151–164. [Google Scholar]
- 7.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 8.Fulton G F, Nunn D N, Lidstrom M E. Molecular cloning of a malyl CoA lyase gene (Mcl) from Pseudomonas AM1, a facultative methylotroph. J Bacteriol. 1984;160:718–723. doi: 10.1128/jb.160.2.718-723.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert B, McDonald I R, Finch R, Stafford G P, Nielsen A K, Murrell J C. Molecular analysis of the pmo (particulate methane monooxygenase) operons from two type II methanotrophs. Appl Environ Microbiol. 2000;66:966–975. doi: 10.1128/aem.66.3.966-975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham D W, Korich D G, LeBlanc R P, Sinclair N A, Arnold R G. Applications of a colorimetric plate assay for soluble methane monooxygenase activity. Appl Environ Microbiol. 1992;58:2231–2236. doi: 10.1128/aem.58.7.2231-2236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henckel T, Friedrich M, Conrad R. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl Environ Microbiol. 1999;65:1980–1990. doi: 10.1128/aem.65.5.1980-1990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes A J, Costello A, Lidstrom M E, Murrell J C. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett. 1995;132:203–208. doi: 10.1016/0378-1097(95)00311-r. [DOI] [PubMed] [Google Scholar]
- 14.Holmes A J, Roslev P, McDonald I R, Iversen N, Henriksen K, Murrell J C. Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol. 1999;65:3312–3318. doi: 10.1128/aem.65.8.3312-3318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King G M. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. In: Marshall K C, editor. Advances in microbial ecology. New York, N.Y: Plenum Press; 1992. pp. 431–474. [Google Scholar]
- 16.Koh S-C, Bowman J P, Sayler G S. Soluble methane monooxygenase production and trichloroethylene degradation by a type I methanotroph, Methylomonas methanica 68-1. Appl Environ Microbiol. 1993;59:960–967. doi: 10.1128/aem.59.4.960-967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuivila K M, Murray J W, Devol A H, Lidstrom M E, Reimers C E. Methane cycling in the sediments of Lake Washington. Limnol Oceanogr. 1988;33:571–581. [Google Scholar]
- 18.Lidstrom M E, Somers L. Seasonal study of methane consumption in Lake Washington. Appl Environ Microbiol. 1984;47:1255–1260. doi: 10.1128/aem.47.6.1255-1260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald I R, Kenna E M, Murrell J C. Detection of methanotrophic bacteria in environmental samples with the PCR. Appl Environ Microbiol. 1995;61:116–121. doi: 10.1128/aem.61.1.116-121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald I R, Upton M, Hall G, Pickup R W, Edwards C, Saunders J R, Ritchie D A, Murrell J C. Molecular ecological analysis of methanogens and methanotrophs in blanket bog peat. Microb Ecol. 1999;38:225–233. doi: 10.1007/s002489900172. [DOI] [PubMed] [Google Scholar]
- 21.Miguez C B, Bourque D, Sealy J A, Greer C W, Groleau D. Detection and isolation of methanotrophic bacteria possessing soluble methane monooxygenase (sMMO) genes using the polymerase chain reaction (PCR) Microb Ecol. 1997;33:21–31. doi: 10.1007/s002489900004. [DOI] [PubMed] [Google Scholar]
- 22.Murrell J C, McDonald I R, Bourne D G. Molecular methods for the study of methanotroph ecology. FEMS Micriobiol Ecol. 1998;27:103–114. [Google Scholar]
- 23.Norton J M, Jackie M L, Klotz M G. The gene encoding ammonia monooxygenase subunit A exists in three nearly identical copies in Nitrosospira sp. NpAV. FEMS Microbiol Lett. 1996;139:181–188. doi: 10.1111/j.1574-6968.1996.tb08200.x. [DOI] [PubMed] [Google Scholar]
- 24.Oldenhuis R, Oedzes J Y, Van der Waarde J J, Janssen D B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991;57:7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page R D M. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 26.Saito H, Miura K-I. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Sayavedra-Soto L A, Hommes N G, Alzerreca J J, Arp D J, Norton J M, Klotz M G. Transcription of the amoC, amoA and amoB genes in Nitrosomonas europaea and Nitrosospira sp. NpAV. FEMS Microbiol Lett. 1998;167:81–88. doi: 10.1111/j.1574-6968.1998.tb13211.x. [DOI] [PubMed] [Google Scholar]
- 29.Semrau J D, Chistoserdov A, Lebron J, Costello A, Davagnino J, Kenna E, Holmes A J, Finch R, Murrell J C, Lidstrom M E. Particulate methane monooxygenase genes in methanotrophs. J Bacteriol. 1995;177:3071–3079. doi: 10.1128/jb.177.11.3071-3079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen R-N, Yu C-L, Ma Q-Q, Li S-B. Direct evidence for a soluble methane monooxygenase from type I methanotrophic bacteria: purification and properties of a soluble methane monooxygenase from Methylomonas sp. GYJ3. Arch Biochem Biophys. 1997;345:223–229. doi: 10.1006/abbi.1997.0239. [DOI] [PubMed] [Google Scholar]
- 31.Shigematsu T, Hanada S, Eguchi M, Kamagata Y, Kanagawa T, Kurane R. Soluble methane monooxygenase gene clusters from trichloroethylene-degrading Methylomonas sp. strains and detection of methanotrophs during in situ bioremediation. Appl Environ Microbiol. 1999;65:5198–5206. doi: 10.1128/aem.65.12.5198-5206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith K S, Costello A M, Lidstrom M E. Methane and trichloroethylene oxidation by an estuarine methanotroph, Methylobacter sp. strain BB5.1. Appl Environ Microbiol. 1997;63:4617–4620. doi: 10.1128/aem.63.11.4617-4620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stolyar S, Costello A M, Peeples T L, Lidstrom M E. Role of multiple gene copies in particulate methane monooxygenase activity in the methane-oxidizing bacterium Methylococcus capsulatus Bath. Microbiology. 1999;145:1235–1244. doi: 10.1099/13500872-145-5-1235. [DOI] [PubMed] [Google Scholar]
- 34.Whittenbury R, Philips K, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 35.Wise M G, McArthur J V, Shimkets L J. Methanotroph diversity in landfill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl Environ Microbiol. 1999;65:4887–4897. doi: 10.1128/aem.65.11.4887-4897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]