Abstract
Serum bactericidal assay (SBA) is the method to investigate in vitro complement-mediated bactericidal activity of sera raised upon vaccination. The assay is based on incubating the target bacteria and exogenous complement with sera at different dilutions and the result of the assay is represented by the sera dilution being able to kill 50% of bacteria present in the inoculum. The traditional readout of the assay is based on measurement of colony-forming units (CFU) obtained after plating different reaction mixes on agar. This readout is at low throughput and time consuming, even when automated counting is used. We previously described a novel assay with a luminescence readout (L-SBA) based on measurement of ATP released by live bacteria, which allowed to substantially increase the throughput as well as to reduce the time necessary to perform the assay when compared to traditional methods. Here we present a further improvement of the assay by moving from a 96-well to a 384-well format, which allowed us to further increase the throughput and substantially reduce costs while maintaining the high performance of the previously described L-SBA method. The method has been successfully applied to a variety of different pathogens.
Keywords: serum bactericidal assay, vaccine, functional assay, high throughput, luminescent SBA
1. Introduction
Serum bactericidal assay (SBA) represents a method to determine in vitro the ability of antibodies present in serum to kill bacteria through complement activation. The assay has been established as an in vitro correlate of protection for bacterial vaccines against cholera [1] and meningococcal disease [2], and is widely used to evaluate functionality of sera raised against pathogens for which a functional assay has not been yet defined as a correlate of protection [3].
In the SBA, bacteria are mixed with dilutions of heat-inactivated serum in the presence of exogenous complement. The number of live bacteria is determined at each serum dilution after a certain amount of time. The dilution of serum resulting in killing 50% of bacteria in the reaction represents the bactericidal antibody titer [4,5].
The traditional SBA methods had some bottlenecks, mainly represented by the need for manually plating onto agar plates and counting the colony-forming units (CFU) both at the beginning and at the end of incubation at each serial dilution. Thus, the assay is considered time consuming and labor intensive for screening large datasets, even when automated colony-counting systems are in place.
In order to overcome these issues, several groups have worked in increasing throughput [6,7]. We developed a luminescence-based high-throughput SBA (L-SBA) in 96-well format. Indeed, in our assay, the reaction mix is directly mixed with BacTiter-Glo Reagent (Promega, Madison, WI, USA), containing a thermostable luciferase and its substrate luciferin that is oxidized and thus emits light in the presence of bacterial ATP. Thus, the level of metabolic ATP released by bacteria surviving the complement-mediated killing can be detected by measuring the level of luminescent signal, which is directly proportional to the number of living bacteria in the assay wells and inversely proportional to the level of functional antibodies that are present in the serum. Hence, in the L-SBA setup, the bactericidal titer can be calculated directly at the end of the bactericidal reaction by reading the microplate in a luminometer, without the need to plate and count CFU. We demonstrated the performance of this method and the equivalence of results compared to the traditional CFU-based method against several pathogens, including Citrobacter freundii, Salmonella serovars Typhimurium and Enteritidis, Shigella flexneri serotypes 2a and 3a, Shigella sonnei, Neisseria meningitidis [8] and S. Paratyphi A [9]. We have also characterized the assay in an intralaboratory manner in terms of specificity, linearity and precision by using human sera raised against an S. sonnei GMMA-based vaccine (1790GAHB) as model, demonstrating high performance of L-SBA and further optimizing the analysis method [10]. The L-SBA method has already been extensively applied to evaluate functionality of both preclinical [11,12,13] and clinical sera from our [14] and other groups’ studies [15].
Here, we present a further improvement of the L-SBA method in terms of throughput by moving from a 96-well to a 384-well format. We demonstrated consistent results and high correlation between serum titers obtained using the two L-SBA formats against different bacteria: S. sonnei, S. flexneri 1b, S. flexneri 2a, S. flexneri 3a, S. Typhimurium, S. Enteritidis, S. Paratyphi A and C. freundii. All of those pathogens represent the etiological agent of large, and often underestimated, disease burdens in low- and middle-income countries, especially in children under the age of five. Shigella and Salmonella cause significant diarrheal disease resulting in illness and death mostly in low-income countries [16]. Shigellosis is the second-leading cause of diarrheal-related mortality, with >200,000 deaths per year, globally [17]; invasive non-typhoidal Salmonella (iNTS) disease is a leading cause of morbidity and mortality among infants and HIV-positive adults in sub-Saharan Africa, with an up to 30% mortality rate [18]; enteric fever caused by S. enterica serovar Typhi and Paratyphi A is a bacteremic disease with clinical features different from those of other Gram-negative bacteremias [19]. Typhoid fever is most prevalent among children living in areas of Asia and Africa especially, where access to clean water and adequate sanitation is limited, but it is also an important travel-associated disease [20]. Based on clinical severity, disease burden and emergence of antimicrobial resistance, Shigella and Salmonella are prime targets for vaccine development [21,22,23]. The improvement of SBA in terms of throughput results is considered to be very important for the development of vaccines against both Shigella and Salmonella enteric diseases pathogens [24,25,26].
2. Materials and Methods
2.1. Bacterial Strains and Reagents
Bacterial strains used in this work are listed in Table 1. They were stored in glycerol stocks at −80 °C until use.
Table 1.
Bacterial strains used in this study.
| Species and Serovar | Strain | Characteristics | Reference(s) |
|---|---|---|---|
| Shigella flexneri serotype 1b | 140 | Clinical isolate | Public Health England (PHE) |
| Shigella flexneri serotype 2a | 142 | Clinical isolate | Public Health England (PHE) |
| Shigella flexneri serotype 3a | 144 | Clinical isolate | Public Health England (PHE) |
| Shigella sonnei | 71 | S. sonnei 53G ΔvirG::cat | [26] |
| Citrobacter freundii | NVGH328 | Clinical isolate from Novartis Master Culture | [27] |
| Salmonella enterica serovar Typhimurium | D23580 | Clinical isolate from blood culture, Malawi | [28,29] |
| Salmonella enterica serovar Enteritidis | CMCC4314 | (corresponding to ATCC4931) obtained from the Novartis Master Culture Collection (NMCC) | [30] |
| Salmonella Paratyphi A | NVGH308 | Invasive isolates, Nepal | [31] |
An overnight culture (16 h at 37 °C, shaking at 180 rpm) was started from a loop of material from frozen stocks in Luria Bertani (LB) medium (Sigma-Aldrich, Saint Louis, MO, USA), supplemented with 20 µg/mL of chloramphenicol (Sigma-Aldrich, Saint Louis, MO, USA) only in the case of the S. sonnei strain. The bacterial suspension was then diluted in fresh LB to start a bacterial culture from an optical density (OD600) of 0.05 at 600 nm and incubated at 37 °C with 180 rpm agitation, until it reached 0.20–0.25 OD600.
2.2. Serum Samples
The serum samples used were polyclonal sera raised in mice or rabbits immunized with glycoconjugates (Vi-CRM197 [32,33] and O:2-CRM197 [34]), or with GMMA-based vaccines obtained from S. flexneri 1b, 2a, 3a, S. sonnei, S. Typhimurium and S. Enteritidis GMMA-producing strains [11,24,25,26,31,35].
All sera tested in L-SBA were heat-inactivated (HI) at 56 °C for 30 min to remove endogenous complement activity prior to performing the L-SBA.
2.3. Luminescent-Based SBA (L-SBA) in 96- and 384-Well Plates
L-SBA was conducted in a 96-well plate (100 μL volume reaction mix containing 25,000 bacteria) format under the same assay conditions and reagent proportions as previously described [8,9] with an optimized method for raw data fitting [10]. Baby rabbit complement (BRC) from Cedarlane (Euroclone) was used as an exogenous complement source (20% BRC in the case of S. sonnei, 15% in the case of S. flexneri 1b and 3a, 7.5% in the case of S. flexneri 2a, 50% in the case of S. Typhimurium and Enteritidis, 20% in the case of S. Paratyphi A and 5% in the case of C. freundii). Phosphate-buffered saline (PBS) and LB medium (only in the case of S. flexneri 1b strain) were used for serum and bacteria dilutions for preparation of the reaction mix.
Initially, L-SBA using 384-well plates was performed in the same experimental conditions established for 96-well plates. After the initial bridging, the L-SBA in 384-well format was performed using the same proportion of reagents as for 96-well plates, but in 50 μL final volume.
Up to eight independent replicates of heat-inactivated test sera were serially diluted 11 times, 3-fold apart in 96-well Corning plate.
An additional well containing buffer only was also added as negative control and was used for fitting purposes [10]. Furthermore, ratio of luminescence detected at T180 in wells containing buffer and BRC only and luminescence at T0 is used to evaluate the optimal growth in the assay and as quality control to validate the assay [10].
Heat-inactivated sera, exogenous BRC and diluted bacteria were mixed and incubated for 180 min at 37 °C. At the end of the incubation, the plate containing the assay reaction was centrifuged at 25 °C (room temperature, RT) for 10 min at 4000× g. The supernatant was discarded to remove bacterial debris, dead bacteria and the other SBA reagents (for this step, direct aspiration using an automated liquid handler or a plate washer was implemented); the bacterial pellet was resuspended in PBS, transferred to white round-bottom 96- or 384-well plates (Greiner Bio-One, Kremsmünster, Austria) and mixed 1:1 (v:v) with BacTiter-Glo Reagent (Promega, Madison, WI, USA). After 5 min of incubation at RT on an orbital shaker, the luminescence signal was measured by a luminometer (Synergy Biotek, Winooski, VT, USA).
2.4. Calculations
For data analysis, a 4-parameter non-linear regression was applied to raw luminescence data obtained at different dilutions tested for each serum sample.
Fitting was performed by weighting the data for the inverse of luminescence^2, as previously described [10].
GraphPad Prism (GraphPad Software, La Jolla, CA, USA) was used for fitting and IC50 determination. IC50 corresponds to the reciprocal serum dilution necessary to obtain 50% bacterial growth inhibition (SBA titer).
3. Results
3.1. Moving from 96- to 384-Well Plates
After having verified the feasibility of performing L-SBA in 384-well plates by using the same experimental conditions (bacteria dilution, final volume reaction, BRC percentage, sera dilution volume and method for dispensing buffers or removing supernatants) established with 96-well-plates L-SBA (data not shown), we optimized the 384-well format using half of the reaction volume used for the 96-well format (50 µL rather than 100 µL, maintaining same proportion of reagents).
3.2. Comparison between L-SBA in 96- and 384-Well Plates
The relative performance of 384-well-plate L-SBA was evaluated by comparing results obtained with this method to the results of 96-well-plate L-SBA for sera raised against multiple bacteria, such as S. flexneri serotypes 1b, 2a and 3a, S. sonnei and S. serovars Typhimurium, Enteritidis and Paratyphi A.
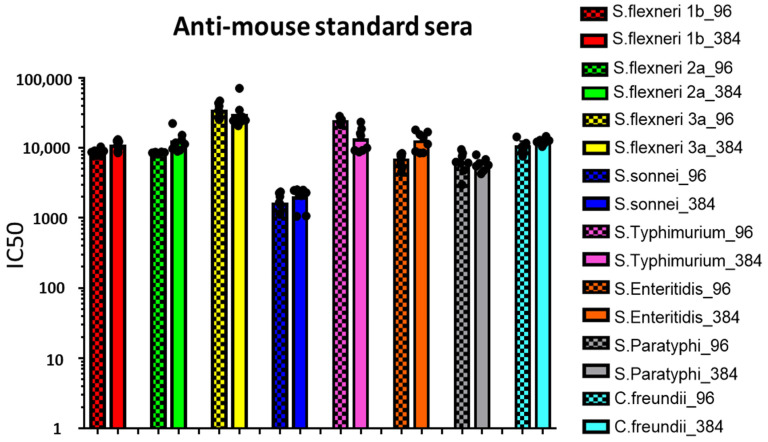
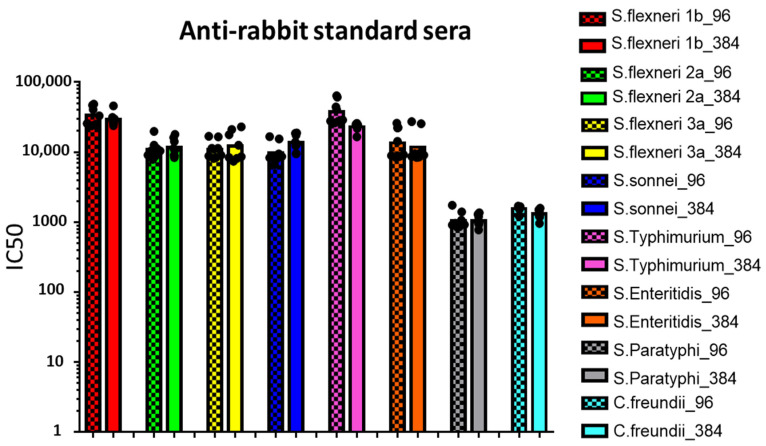
Mouse (Figure 1) and rabbit (Figure 2) reference sera were tested against the homologous strains in seven or eight independent replicates with each bactericidal reaction simultaneously assayed by both 96-well and 384-well L-SBA.
Figure 1.
L-SBA titers (IC50) against S. flexneri serotypes 1b, 2a and 3a, S. sonnei, S. serovars Typhimurium, Enteritidis and Paratyphi A and C. freundii strains measured in mouse reference sera. Dots represent IC50 values corresponding to each replicate, while bars represent the related geometric means. Checkered bars represent data deriving from 96-wells-plate L-SBA, while solid bars represent data deriving from 384-wells-plate L-SBA.
Figure 2.
L-SBA titers (IC50) against S. flexneri serotypes 1b, 2a and 3a, S. sonnei, S. serovars Typhimurium, Enteritidis and Paratyphi A and C. freundii strains measured in rabbit reference sera. Dots represent IC50 values corresponding to each replicate while bars represent the related geometric means. Checkered bars represent data deriving from 96-wells-plate L-SBA, while solid bars represent data deriving from 384-wells-plate L-SBA.
Similar SBA titers (IC50) were obtained by testing the same reference sera multiple times with both 96- and 384-well L-SBA format (Figure 1 and Figure 2). Moreover, very low variability in the measured IC50 was observed between the two different L-SBA formats, with standard error (SE) among replicates being less than 20% and in the majority of test samples around 10% (Table 2).
Table 2.
L-SBA titers (IC50) against homologous bacteria tested as determined by L-SBA in 96- and 384-well plates in multiple independent replicates (Rep.).
| L-SBA Titers (IC50) | L-SBA Titers (IC50) | |||||||
|---|---|---|---|---|---|---|---|---|
| 96-Wells-Plate SBA | 384-Wells-Plate SBA | 96-Wells-Plate SBA | 384-Wells-Plate SBA | |||||
| S. flexneri 1b Strain | Mouse Standard Antiserum | Rep. 1 | 8126 | 8490 | Rabbit Standard Antiserum | Rep. 1 | 24,732 | 29,776 |
| Rep. 2 | 7738 | 12,255 | Rep. 2 | 25,699 | 31,448 | |||
| Rep. 3 | 8257 | 10,670 | Rep. 3 | 33,031 | 26,493 | |||
| Rep. 4 | 9092 | 8694 | Rep. 4 | 46,997 | 28,192 | |||
| Rep. 5 | 8697 | 9869 | Rep. 5 | 48,571 | 24,008 | |||
| Rep. 6 | 10,322 | 8784 | Rep. 6 | 40,635 | 25,847 | |||
| Rep. 7 | 8965 | 13,089 | Rep. 7 | 23,901 | 45,903 | |||
| Rep. 8 | 8533 | 12,554 | Rep. 8 | 28,454 | ||||
| GeoMean | 8687 | 10,406 | GeoMean | 33,418 | 29,456 | |||
| SE | 260 | 621 | SE | 3481 | 2258 | |||
| SE% | 3 | 6 | SE% | 10 | 8 | |||
| S. flexneri 2a strain | Mouse standard antiserum | Rep. 1 | 8119 | 8905 | Rabbit Standard antiserum | Rep. 1 | 8770 | 8380 |
| Rep. 2 | 8473 | 9682 | Rep. 2 | 11,246 | 9054 | |||
| Rep. 3 | 8477 | 22,253 | Rep. 3 | 12,632 | 18,019 | |||
| Rep. 4 | 8450 | 15,172 | Rep. 4 | 19,843 | 14,242 | |||
| Rep. 5 | 8657 | 9233 | Rep. 5 | 10,420 | 8821 | |||
| Rep. 6 | 8741 | 11,761 | Rep. 6 | 10,428 | 16,238 | |||
| Rep. 7 | 8591 | 11,323 | Rep. 7 | 9104 | 10,757 | |||
| Rep. 8 | 8376 | 12,974 | Rep. 8 | 7926 | 11,323 | |||
| GeoMean | 8484 | 12,116 | GeoMean | 10,863 | 11,652 | |||
| SE | 63 | 1456 | SE | 1243 | 1206 | |||
| SE% | 1 | 12 | SE% | 11 | 10 | |||
| S. flexneri 3a Strain | mouse Standard Antiserum | Rep. 1 | 46,819 | 24,862 | Rabbit standard antiserum | Rep. 1 | 8811 | 12,624 |
| Rep. 2 | 25,205 | 70,660 | Rep. 2 | 11,374 | 21,178 | |||
| Rep. 3 | 43,233 | 56,883 | Rep. 3 | 11,343 | 23,076 | |||
| Rep. 4 | 27,388 | 40,699 | Rep. 4 | 16,600 | 17,792 | |||
| Rep. 5 | 39,309 | 25,032 | Rep. 5 | 16,928 | 7450 | |||
| Rep. 6 | 34,009 | 24,001 | Rep. 6 | 9086 | 8619 | |||
| Rep. 7 | 32,125 | 24,675 | Rep. 7 | 8417 | 8149 | |||
| Rep. 8 | 26,146 | 23,684 | Rep. 8 | 8141 | 8387 | |||
| GeoMean | 33,454 | 33,063 | GeoMean | 10,897 | 12,171 | |||
| SE | 2691 | 6030 | SE | 1180 | 2110 | |||
| SE% | 8 | 18 | SE% | 11 | 17 | |||
| S. sonnei Strain | Mouse Standard Antiserum | Rep. 1 | 1658 | 2517 | Rabbit standard antiserum | Rep. 1 | 6630 | 17,512 |
| Rep. 2 | 1150 | 2290 | Rep. 2 | 8097 | 9570 | |||
| Rep. 3 | 2357 | 2081 | Rep. 3 | 8673 | 12,372 | |||
| Rep. 4 | 1529 | 2460 | Rep. 4 | 9433 | 18,587 | |||
| Rep. 5 | 1139 | 2489 | Rep. 5 | 16,669 | 18,851 | |||
| Rep. 6 | 1121 | 1068 | Rep. 6 | 15,471 | 9528 | |||
| Rep. 7 | 2014 | 2160 | Rep. 7 | 8789 | 12,956 | |||
| Rep. 8 | 2258 | 1054 | Rep. 8 | 8342 | 14,005 | |||
| GeoMean | 1585 | 1912 | GeoMean | 9770 | 13,721 | |||
| SE | 168 | 201 | SE | 1219 | 1250 | |||
| SE% | 11 | 11 | SE% | 12 | 9 | |||
| S. Typhimurium strain | Mouse standard antiserum | Rep. 1 | 28,133 | 16,038 | Anti-rabbit standard serum | Rep. 1 | 61,185 | 25,018 |
| Rep. 2 | 21,515 | 23,282 | Rep. 2 | 40,788 | 24,404 | |||
| Rep. 3 | 21,259 | 9986 | Rep. 3 | 27,883 | 25,895 | |||
| Rep. 4 | 22,669 | 14,926 | Rep. 4 | 28,813 | 23,882 | |||
| Rep. 5 | 25,324 | 18,654 | Rep. 5 | 44,251 | 23,305 | |||
| Rep. 6 | 23,509 | 9197 | Rep. 6 | 27,725 | 21,824 | |||
| Rep. 7 | 23,182 | 9208 | Rep. 7 | 26,312 | 16,567 | |||
| Rep. 8 | 8749 | Rep. 8 | 63,920 | 23,675 | ||||
| GeoMean | 23,557 | 12,905 | GeoMean | 37,765 | 22,888 | |||
| SE | 783 | 1777 | SE | 5077 | 956 | |||
| SE% | 3 | 14 | SE% | 13 | 4 | |||
| S. Enteritidis strain | Mouse standard antiserum | Rep. 1 | 7984 | 18,043 | Rabbit standard antiserum | Rep. 1 | 14,317 | 8527 |
| Rep. 2 | 8327 | 11,270 | Rep. 2 | 8448 | 9505 | |||
| Rep. 3 | 6037 | 16,781 | Rep. 3 | 8931 | 8681 | |||
| Rep. 4 | 7377 | 13,950 | Rep. 4 | 22,548 | 27,381 | |||
| Rep. 5 | 5105 | 15,416 | Rep. 5 | 25,966 | 25,482 | |||
| Rep. 6 | 4482 | 8436 | Rep. 6 | 22,255 | 9927 | |||
| Rep. 7 | 7463 | 8867 | Rep. 7 | 9179 | 9091 | |||
| Rep. 8 | 7867 | 8457 | Rep. 8 | 8701 | 8442 | |||
| GeoMean | 6683 | 12,116 | GeoMean | 13,532 | 11,793 | |||
| SE | 475 | 1293 | SE | 2448 | 2675 | |||
| SE% | 7 | 11 | SE% | 18 | 23 | |||
| S. Paratyphi strain | Mouse standard antiserum | Rep. 1 | 6012 | 6008 | Rabbit standard antiserum | Rep. 1 | 1400 | 941 |
| Rep. 2 | 2962 | 4244 | Rep. 2 | 851 | 1296 | |||
| Rep. 3 | 6090 | 6353 | Rep. 3 | 865 | 974 | |||
| Rep. 4 | 7579 | 6832 | Rep. 4 | 968 | 998 | |||
| Rep. 5 | 9471 | 5474 | Rep. 5 | 1748 | 1361 | |||
| Rep. 6 | 8381 | 5657 | Rep. 6 | 1065 | 775 | |||
| Rep. 7 | 4740 | 4694 | Rep. 7 | 924 | 1201 | |||
| Rep. 8 | 6259 | 7945 | Rep. 8 | 912 | 1026 | |||
| GeoMean | 6107 | 5798 | GeoMean | 1058 | 1056 | |||
| SE | 680 | 389 | SE | 105 | 65 | |||
| SE% | 11 | 7 | SE% | 10 | 6 | |||
| C. freundii strain | Mouse standard antiserum | Rep. 1 | 11,600 | 12,664 | Rabbit standard antiserum | Rep. 1 | 1216 | 959 |
| Rep. 2 | 9214 | 10,450 | Rep. 2 | 1579 | 1317 | |||
| Rep. 3 | 14,273 | 11,422 | Rep. 3 | 1693 | 1503 | |||
| Rep. 4 | 9719 | 12,792 | Rep. 4 | 1665 | 1490 | |||
| Rep. 5 | 10,345 | 14,563 | Rep. 5 | 1634 | 1588 | |||
| Rep. 6 | 8914 | 12,121 | Rep. 6 | 1629 | 1222 | |||
| Rep. 7 | 7585 | 11,946 | Rep. 7 | 1539 | 1300 | |||
| Rep. 8 | 12,104 | Rep. 8 | 1195 | |||||
| GeoMean | 10,052 | 12,209 | GeoMean | 1557 | 1307 | |||
| SE | 710 | 393 | SE | 53 | 67 | |||
| SE% | 7 | 3 | SE% | 3 | 5 | |||
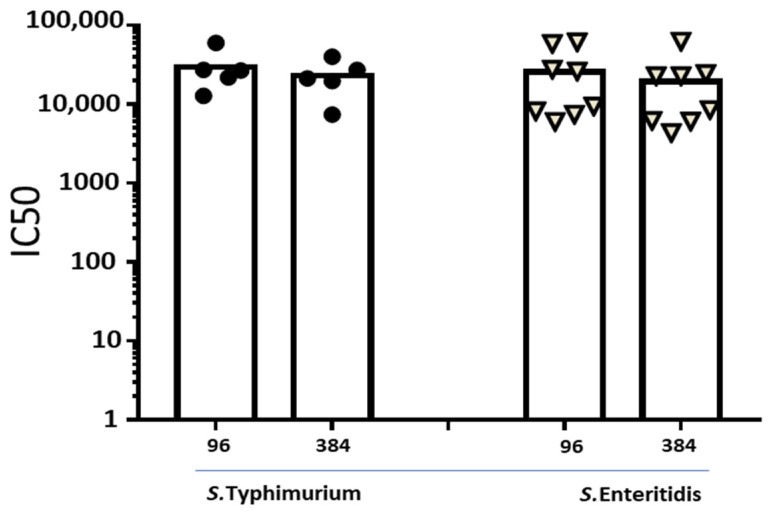
Finally, to demonstrate equivalence of results obtained by 96- and 384-wells-plate L-SBA in the presence of the intrinsic biological variability of animal response against the same immunogen, individual rabbit sera raised against S. Typhimurium or S. Enteritidis GMMA (Figure 3) were directly compared using the two methods. By applying paired non-parametric Wilcoxon test, we did not show statistical difference between the two L-SBA methods (p = 0.1875 and p = 0.1094 for S. Typhimurium and S. Enteritidis, respectively).
Figure 3.
L-SBA titers (IC50) against S. Typhimurium and S. Enteritidis strains calculated on single sera obtained after immunization of New Zealand White rabbits with a mix of individually formulated S. Typhimurium and S. Enteritidis GMMA vaccine obtained on day 14, 28, 42 and 71. Dots represent serum samples from individual rabbits.
4. Discussion
Serum bactericidal assay (SBA) is the method of choice to investigate in vitro complement-mediated bactericidal activity of antibodies present in sera, especially induced upon immunization [4,36,37]. The traditional SBA method is CFU-based, and thus depends on the laborious practice of plating bacteria on solid media at the end of the assay reaction, requiring an overnight incubation and afterwards CFU counting, so it is time consuming and at low throughput.
To overcome those bottlenecks, several groups have worked in increasing throughput by developing both conventional CFU-based assays implementing automated CFU counting [6] or non-conventional SBA by measuring cellular respiration as a survival readout [38]. We have developed a luminescence-based high-throughput SBA method based on luminescence readout (L-SBA) and direct measurement of ATP released by live bacteria on the 96-well format. This method is highly reproducible and has a strong correlation between SBA titers (IC50) determined with traditional CFU counting method [8]. L-SBA has been applied to determine the functionality of sera raised against a broad range of bacterial targets, both at preclinical [11,12,13] and clinical levels [14,15]. The sensitivity of the L-SBA has been evaluated as part of an in depth characterization of the assay (data not shown) performed for each serotype: L-SBA was able to efficiently, specifically and sensibly discriminate between positive and negative samples under the same assay conditions used here.
In this work, we have shown a further optimization of the L-SBA method by adapting the 96-well L-SBA to the 384-wells-plate format.
We demonstrated a good concordance of results obtained using 96-well and 384-well L-SBA formats in all the cases analyzed: (1) against multiple clinically relevant enteric bacterial strains (S. sonnei, S. flexneri 1b, S. flexneri 2a, S. flexneri 3a, S. Typhimurium, S. Enteritidis, S. Paratyphi A and C. freundii); (2) using sera raised in two animal species (mice and rabbits); (3) testing independent replicates of the same reference sera or directly comparing the functionality of multiple individual single sera raised against the same vaccine.
A direct comparison of the three methods (traditional CFU-based with manual counting, 96-wells-plate luminescence-based and 384-wells-plate luminescence-based SBA) in terms of performances is shown in Table 3.
Table 3.
Estimation of throughput comparing traditional CFU-based method with 96- and 384-well L-SBA.
| Traditional CFU-Based SBA | 96-Wells-Plate L-SBA | 384-Wells-Plate L-SBA | |
|---|---|---|---|
| Final Volume Reaction | 100 µL/well | 100 µL/well | 50 µL/well |
| Assay Time | 1.5 working day | 6 h | 6 h |
| Plate Reading | 2–3 h/SBA plate | 2 min/SBA plate | 5 min/SBA plate |
| Reproducibility | Lower operator independence (for manual CFU counting) than 96- and 384-wells L-SBA | High operator independence | Higher operator independence than 96-well L-SBA |
| Throughput | Plates/day: 2 | Plates/day: 8 | Plates/day: 4 (equivalent to sixteen 96-wells plates) |
| 1 operator/1.5 day: 22 individual sera in single | 1 operator/day: 88 individual sera in single | 1 operator/day: 188 individual sera in single | |
| Reagent Costs | 4 EUR/serum | 12 EUR/serum | 8 EUR/serum |
In the case of 384-well L-SBA, the assay time remained the same as the 96-well L-SBA with an apparent increase of individual plate reading. However, it needs to be considered that a 384-well plate can accommodate four 96-well-plate layouts; therefore, with the new format, the time for reading a single layout remained basically equivalent.
The main achievements of 384-well L-SBA format are represented by the increase of throughput per day per operator, which goes from 88 to 188 individual sera. This increase is paralleled by a relevant cost reduction (decreasing from about twelve to around eight EUR for each serum assayed) due mostly to the reduction of the reaction volume. During the optimization, we also gained an increase of operator independence, due to the implementation of the automatic liquid handler/plate washer to discard reaction mix prior to the reading, applicable both to 96- and 384-wells L-SBA format.
Overall, the assay costs of reagents were higher for L-SBA compared to traditional SBA; however, the increased throughput of L-SBA method allows one to substantially reduce the labor costs, making the use of the L-SBA method, especially in the 384-well format, attractive and competitive in terms of costs, overall very similar to the ones for the traditional method.
Thus, 384-well-plate L-SBA represents a promising assay particularly for very large-scale studies, as this allows significant savings in terms of costs, time and human resources while maintaining the high performances of the previously developed and well-established 96-well-format L-SBA method. This increase in throughput is particularly important to analyze sera from clinical trials, and it opens the opportunity to analyze a larger number of sera, also against more than one strain, as the assay requires less sera volume. Therefore, this assay will be critical to support the development of vaccines against multiple bacterial targets.
Acknowledgments
We thank Laura B. Martin for critical revision of the manuscript.
Author Contributions
Conceived and designed the experiments: M.G.A., M.C., O.R., F.M. and F.N. Performed the experiments: M.G.A. and M.C. Analyzed the data: M.G.A. and O.R. Writing—original draft preparation: M.G.A. and O.R.; writing—review and editing: M.G.A., O.R., M.C., F.M. and F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was undertaken at the request of and sponsored by GlaxoSmithKline Biologicals SA. The GSK Vaccines Institute for Global Health (Srl) (GVGH) is an affiliate of GlaxoSmithKline Biologicals SA.
Institutional Review Board Statement
All animal sera used in this study were derived from mouse or rabbits immunization experiments performed at the GSK Vaccines Animal Facility (Siena, Italy), or at the Toscana Life Sciences Facility (Siena, Italy) or at Covance Laboratories Limited (Harrogate, UK). All experiments have been conducted in compliance with the relevant guidelines of Italy (Italian Legislative Decree n. 116/1992) and EU policies on animal experimentation, as well as with the institutional policies of GSK Vaccines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
All authors were employees of the GSK Vaccines Institute for Global Health at the time in which the study was conducted. GSK Vaccines Institute for Global Health Srl is an affiliate of GlaxoSmithKline Biologicals SA. Some strains used in this study are GSK antigen-producing strains, for which a negotiation with GSK will be necessary in case of material sharing. Other than this, all authors adhere to Journal policies on data and material sharing.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Son M.S., Taylor R.K. Vibriocidal assays to determine the antibody titer of patient sera samples. Curr. Protoc. Microbiol. 2011;23:6A.3.1–6A.3.9. doi: 10.1002/9780471729259.mc06a03s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow R., Carlone G.M., Rosenstein N., Blake M., Feavers I., Martin D., Zollinger W., Robbins J., Aaberge I., Granoff D.M., et al. Neisseria meningitidis group B correlates of protection and assay standardization—International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 3.Giersing B.K., Porter C.K., Kotloff K., Neels P., Cravioto A., MacLennan C.A. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine. 2019;37:4778–4783. doi: 10.1016/j.vaccine.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Boyd M.A., Tennant S.M., Saague V.A., Simon R., Muhsen K., Ramachandran G., Cross A.S., Galen J.E., Pasetti M.F., Levine M.M. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin. Vaccine Immunol. 2014;21:712–721. doi: 10.1128/CVI.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang M.S., Sahastrabuddhe S., Yun C.H., Han S.H., Yang J.S. Serum bactericidal assay for the evaluation of typhoid vaccine using a semi-automated colony-counting method. Microb. Pathog. 2016;97:19–26. doi: 10.1016/j.micpath.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahm M.H., Yu J., Weerts H.P., Wenzel H., Tamilselvi C.S., Chandrasekaran L., Pasetti M.F., Mani S., Kaminski R.W. Development, Interlaboratory Evaluations, and Application of a Simple, High-Throughput Shigella Serum Bactericidal Assay. Msphere. 2018;3:e00146-18. doi: 10.1128/mSphere.00146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H.W., Kim K.H., Kim J., Nahm M.H. A high throughput serum bactericidal assay for antibodies to Haemophilus influenzae type b. BMC Infect. Dis. 2016;16:473. doi: 10.1186/s12879-016-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Necchi F., Saul A., Rondini S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS ONE. 2017;12:e0172163. doi: 10.1371/journal.pone.0172163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Necchi F., Saul A., Rondini S. Setup of luminescence-based serum bactericidal assay against Salmonella Paratyphi A. J. Immunol. Methods. 2018;461:117–121. doi: 10.1016/j.jim.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Rossi O., Molesti E., Saul A., Giannelli C., Micoli F., Necchi F. Intra-Laboratory Evaluation of Luminescence Based High-Throughput Serum Bactericidal Assay (L-SBA) to Determine Bactericidal Activity of Human Sera against Shigella. High Throughput. 2020;9:14. doi: 10.3390/ht9020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mancini F., Gasperini G., Rossi O., Aruta M.G., Raso M.M., Alfini R., Biagini M., Necchi F., Micoli F. Dissecting the contribution of O-Antigen and proteins to the immunogenicity of Shigella sonnei generalized modules for membrane antigens (GMMA) Sci. Rep. 2021;11:906. doi: 10.1038/s41598-020-80421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmieri E., Arato V., Oldrini D., Ricchetti B., Aruta M.G., Pansegrau W., Marchi S., Giusti F., Ferlenghi I., Rossi O., et al. Stability of Outer Membrane Vesicles-Based Vaccines, Identifying the Most Appropriate Methods to Detect Changes in Vaccine Potency. Vaccines. 2021;9:229. doi: 10.3390/vaccines9030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson N.I., Ravenscroft N., Arato V., Oldrini D., Micoli F., Kuttel M.M. Conformational and Immunogenicity Studies of the Shigella flexneri Serogroup 6 O-Antigen: The Effect of O-Acetylation. Vaccines. 2021;9:432. doi: 10.3390/vaccines9050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micoli F., Rossi O., Conti V., Launay O., Scire A.S., Aruta M.G., Nakakana U.N., Marchetti E., Rappuoli R., Saul A., et al. Antibodies Elicited by the Shigella sonnei GMMA Vaccine in Adults Trigger Complement-Mediated Serum Bactericidal Activity: Results from a Phase 1 Dose Escalation Trial Followed by a Booster Extension. Front. Immunol. 2021;12:671325. doi: 10.3389/fimmu.2021.671325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juel H.B., Thomaides-Brears H.B., Darton T.C., Jones C., Jones E., Shrestha S., Sie R., Eustace A., Galal U., Kurupati P., et al. Salmonella Typhi Bactericidal Antibodies Reduce Disease Severity but Do Not Protect against Typhoid Fever in a Controlled Human Infection Model. Front. Immunol. 2017;8:1916. doi: 10.3389/fimmu.2017.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdelalim A., Abdollahpour I., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil I.A., Troeger C., Blacker B.F., Rao P.C., Brown A., Atherly D.E., Brewer T.G., Engmann C.M., Houpt E.R., Kang G., et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990-2016. Lancet Infect. Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feasey N.A., Dougan G., Kingsley R.A., Heyderman R.S., Gordon M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong H.K., Parry C.M., van der Poll T., Wiersinga W.J. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012;8:e1002933. doi: 10.1371/journal.ppat.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor B.A., Schwartz E. Typhoid and paratyphoid fever in travellers. Lancet Infect. Dis. 2005;5:623–628. doi: 10.1016/S1473-3099(05)70239-5. [DOI] [PubMed] [Google Scholar]
- 21.von Seidlein L., Kim D.R., Ali M., Lee H., Wang X., Thiem V.D., Canh D.G., Chaicumpa W., Agtini M.D., Hossain A., et al. A multicentre study of Shigella diarrhoea in six Asian countries: Disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine M.M., Kotloff K.L., Barry E.M., Pasetti M.F., Sztein M.B. Clinical trials of Shigella vaccines: Two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan C.A., Martin L.B., Micoli F. Vaccines against invasive Salmonella disease: Current status and future directions. Hum. Vaccin Immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micoli F., Rondini S., Alfini R., Lanzilao L., Necchi F., Negrea A., Rossi O., Brandt C., Clare S., Mastroeni P., et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA. 2018;115:10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Benedetto G., Alfini R., Cescutti P., Caboni M., Lanzilao L., Necchi F., Saul A., MacLennan C.A., Rondini S., Micoli F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35:419–426. doi: 10.1016/j.vaccine.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 26.Gerke C., Colucci A.M., Giannelli C., Sanzone S., Vitali C.G., Sollai L., Rossi O., Martin L.B., Auerbach J., Di Cioccio V., et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE. 2015;10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rondini S., Micoli F., Lanzilao L., Pisoni I., Di Cioccio V., Saul A.J., Martin L.B. Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM(197) glycoconjugate vaccine against Salmonella Typhi. J. Infect. Dev. Ctries. 2012;6:763–773. doi: 10.3855/jidc.2495. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan C.A., Gondwe E.N., Msefula C.L., Kingsley R.A., Thomson N.R., White S.A., Goodall M., Pickard D.J., Graham S.M., Dougan G., et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Investig. 2008;118:1553–1562. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingsley R.A., Msefula C.L., Thomson N.R., Kariuki S., Holt K.E., Gordon M.A., Harris D., Clarke L., Whitehead S., Sangal V., et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onsare R.S., Micoli F., Lanzilao L., Alfini R., Okoro C.K., Muigai A.W., Revathi G., Saul A., Kariuki S., MacLennan C.A., et al. Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya. PLoS Negl. Trop. Dis. 2015;9:e0003573. doi: 10.1371/journal.pntd.0003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobinson H.C., Gibani M.M., Jones C., Thomaides-Brears H.B., Voysey M., Darton T.C., Waddington C.S., Campbell D., Milligan I., Zhou L., et al. Evaluation of the Clinical and Microbiological Response to Salmonella Paratyphi A Infection in the First Paratyphoid Human Challenge Model. Clin. Infect. Dis. 2017;64:1066–1073. doi: 10.1093/cid/cix042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micoli F., Bjarnarson S.P., Arcuri M., Aradottir Pind A.A., Magnusdottir G.J., Necchi F., Di Benedetto R., Carducci M., Schiavo F., Giannelli C., et al. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc. Natl. Acad. Sci. USA. 2020;117:24443–24449. doi: 10.1073/pnas.2005857117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcuri M., Di Benedetto R., Cunningham A.F., Saul A., MacLennan C.A., Micoli F. The influence of conjugation variables on the design and immunogenicity of a glycoconjugate vaccine against Salmonella Typhi. PLoS ONE. 2017;12:e0189100. doi: 10.1371/journal.pone.0189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micoli F., Rondini S., Gavini M., Lanzilao L., Medaglini D., Saul A., Martin L.B. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS ONE. 2012;7:e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasperini G., Raso M.M., Arato V., Aruta M.G., Cescutti P., Necchi F., Micoli F. Effect of O-Antigen Chain Length Regulation on the Immunogenicity of Shigella and Salmonella Generalized Modules for Membrane Antigens (GMMA) Int. J. Mol. Sci. 2021;22:1309. doi: 10.3390/ijms22031309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell H., Borrow R., Salisbury D., Miller E. Meningococcal C conjugate vaccine: The experience in England and Wales. Vaccine. 2009;27((Suppl. 2)):B20–B29. doi: 10.1016/j.vaccine.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 37.Gill C.J., Baxter R., Anemona A., Ciavarro G., Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo(R)) or Menactra(R) among healthy adolescents. Hum. Vaccines. 2010;6:881–887. doi: 10.4161/hv.6.11.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak P.A., Santos G.F., Masterman K.A., Janes J., Wacknov B., Vienken K., Giuliani M., Herman A.E., Cooke M., Mbow M.L., et al. Development of an automated, high-throughput bactericidal assay that measures cellular respiration as a survival readout for Neisseria meningitidis. Clin. Vaccine Immunol. 2011;18:1252–1260. doi: 10.1128/CVI.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.