Abstract
Background:
Coronavirus disease 2019 (COVID-19) has an established impact on multiple organ systems, including the vascular and urogenital systems. Vascular effects may include venous thromboembolic disease, which could theoretically be a precursor to priapism—a urological emergency defined as an abnormal condition of prolonged penile erection lasting >4 hours. To better explore this association, we critically appraised all the published COVID-19 cases associated with priapism.
Materials and methods:
After PROSPERO registration (CRD42021245257), a systematic search of Google Scholar, Scopus, Embase, Web of Science, PubMed, Cumulative Index to Nursing and Allied Health Literature, Global Index Medicus, and Cochrane Database of Systematic Reviews was performed using specific search terms. The following study metadata were extracted: age, requirement for respiratory support, cavernous blood gas findings, management of priapism, and patient outcomes.
Results:
Fifteen single-patient case reports were included in this review. Of these, all of the patients presented with ischemic priapism, 9 patients (60.0%) were >60 years of age, 4 (26.7%) reported more than a single episode of priapism, 11 (73.3%) presented with pneumonia, 8 (53.3%) required mechanical ventilation, D-dimer was elevated in 5 of the 6 (83.3%) patients in whom this was reported, and among the 13 patients in whom mortality was reported, 4 (30.8%) died.
Conclusions:
Early reports suggest a prognostic relationship between COVID-19 and coexisting priapism. However, owing to commonalities in their pathophysiology and the small dataset reported in the literature, the probable association between COVID-19 and priapism is still theoretical. Further research is needed to confirm this association.
Keywords: Coronavirus, COVID-19, Priapism, SARS-CoV-2, Severe COVID-19, Thromboembolic disease, Thromboembolisms
1. Introduction
Coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China, in December 2019, and spread rapidly worldwide.[1] Typical presentations of COVID-19 include dry cough, sore throat, loss of smell, and fever. In severe cases, patients may develop dyspnea requiring hospitalization. Up to 50% of patients with dyspnea require intensive care unit admission, of which 46%–65% develop respiratory failure and die. Evidence suggests that COVID-19 also affects other organ systems, including the haematological (venous thromboembolism), cardiovascular (myocarditis), and central nervous (encephalitis, Guillaine-Barre syndrome) systems.[2]
The pathophysiology of COVID-19 includes the activation of the inflammatory and coagulation pathways. Studies have shown that patients with fatal COVID-19 have lower platelet counts and higher D-dimer levels than survivors.[3,4] Guidelines recommend that D-dimer and other coagulopathy markers should be monitored in hospitalized patients with COVID-19 associated coagulopathy.[5] Priapism has recently been reported as a possible thromboembolic complication of COVID-19.[6–20]
Priapism is a urological emergency defined as an abnormal condition of prolonged or constant penile erection lasting >4 hours in duration. It can be categorized as either ischemic (low-flow or veno-occlusive) or non-ischemic (high-flow or arterial). Priapism is sometimes characterized by pain and may or may not be associated with sexual stimulation. The causes of priapism can be idiopathic or secondary to recreational drugs (eg, cocaine), medications (eg, antipsychotics, antidepressants), central nervous system pathology (eg, spinal cord trauma, brain tumors), sickle cell disease, neoplasms (eg, leukemia), metabolic diseases (gout, diabetes mellitus), spider bites, and scorpion bites.[16,21]
Priapism is most commonly observed in adults, with an estimated incidence of 1.5 per 100,000 patients. Approximately 95% of presentations are of the ischemic type.[22] If treatment is not initiated timeously, permanent damage to the corpora cavernosa may occur, leading to ischemia, necrosis, and erectile dysfunction. Emergent treatments for priapism include ice pack application, cavernosal aspiration, and intracavernous injections of sympathomimetic agents.[23] Apart from the emergent therapy required in its management, priapism carries a medical liability risk in cases of delay, omission, or neglect.[24]
A limited number of cases of COVID-19 with coexisting priapism have been reported.[6–19] These cases must be reviewed to identify whether there is a potential association between these two conditions. Owing to the incidence and adverse events associated with priapism, coupled with the theoretical correlation between priapism and COVID-19 pathophysiology, we explored the potential relationship between the two by conducting this systematic review.
2. Methodology
2.1. Search strategy
Prior to commencement of the search, this systematic review was registered with the Prospective Register of Systematic Reviews (PROSPERO) (CRD42021245257). The following electronic databases were searched (February 9, 2022): Google Scholar, Scopus, Embase, Web of Science, PubMed, Cumulative Index to Nursing and Allied Health Literature, Global Index Medicus, and the Cochrane Database of Systematic Reviews. The following search terms were used: “COVID-19,” OR “coronavirus,” OR “SARS-CoV-2,” AND “priapism.” The search was restricted to human studies. No language or date restrictions were applied.
2.2. Study selection
Studies included in the review met the following criteria: (i) studies were clinical publications, (ii) studies were limited to human subjects, and (iii) publication text was available. All publications relating to the topic, including correspondence articles, letters to the editor, and conference proceedings, were eligible for inclusion. “Case report” inclusion was pivotal in this review, since this early association had not been previously explored.
2.3. PICO (participants/intervention/control)
Participants/population: COVID-19-positive male patients with priapism.
Intervention/exposure: Male patients were exposed and diagnosed with COVID-19 with concurrent priapism.
Comparator/control: Male patients without a confirmed diagnosis of COVID-19 with the presence of priapism were excluded.
2.4. Review study definition of priapism
For the purpose of this systematic review, priapism was defined as a urological emergency characterized by prolonged or constant penile erection lasting >4 hours in duration, with all subtypes considered for inclusion.[21]
2.5. Data extraction and methodology evaluation
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were used to guide the electronic search.[25] Two independent reviewers (DM and JM) screened the articles using eligibility criteria. Using the inclusion criteria, a descriptive narrative for each article was compiled by the reviewers. All differences, disagreements, and conflicting entries were resolved by a third reviewer (AA). All points of interest were tabulated (country of origin, patient age, diagnosis, category of respiratory support required, number of episodes and duration of priapism, cavernosal blood gas, D-dimer level, management of priapism, patient outcomes, and authors’ conclusions).
2.6. Data synthesis
The outcomes reported were summary data pertaining to the clinical presentation of priapism, severity of COVID-19 infection, relevant laboratory findings, patient outcomes, and management. Since all included studies were case reports, summative statistics and a narrative synthesis approach were primarily used to describe the findings.
2.7. Assessment of methodological quality of included articles
As all studies meeting the inclusion criteria were case reports, the tool proposed by Murad et al. was used to assess the methodological quality of the included manuscripts.[26] The tool comprises four domains, with eight questions in total. Since two questions related to drug reactions were not relevant to our study, they were omitted. The overall methodological quality of each of the included articles was described as low, intermediate, or high quality. High quality was defined as a “yes” answer to four or more of the included questions; intermediate quality was defined as a “yes” answer to three of the included questions; and low quality was defined as a “yes” answer to less than three of the included questions (Table 1).
Table 1.
Methodological quality assessment of included reports.
| Selection | Ascertainment | Causality | Reporting | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Author | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Question 6 | Overall quality∗ |
| Lamamri et al. [6] | Yes | Yes | Yes | Yes | Unclear | Yes | High |
| Lam et al.[7] | Yes | Yes | Yes | Yes | No | Yes | High |
| Silverman et al. [8] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Addar et al. [9] | Yes | Yes | Yes | No | Yes | Yes | High |
| Larrarte-Arenas et al. [10] | Yes | Yes | Yes | No | No | Yes | High |
| Grimberg et al. [11] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Giuliano et al. [12] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Carreño et al. [13] | Yes | Yes | Yes | Yes | No | Yes | High |
| Anderson et al. [14] | Yes | Yes | Yes | Yes | No | Yes | High |
| Ahmed et al. [15] | Yes | Yes | No | No | Unclear | Yes | Intermediate |
| Laaribi et al. [16] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Cruz et al. [17] | Yes | Yes | No | Yes | Yes | Yes | High |
| Brönimann et al. [18] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Ameyaw et al. [19] | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Alsaedi et al. [20] | Yes | Yes | Yes | Yes | Yes | Yes | High |
Questions 1–6 comprise the tools for assessing the methodological quality of each of the included articles.
1. Does the patient(s) represent(s) the entire experience of the investigator or is the selection method unclear to the extent that other patients with similar presentations may not have been reported?
2. Was the condition ascertained adequately?
3. Was the outcome ascertained adequately?
4. Were alternative causes that may explain the observation ruled out?
5. Was the follow-up long enough for outcomes to occur?
6. Is the case(s) described with sufficient detail to allow other investigators to replicate the research or allow practitioners to make inferences related to their own practice?
High quality: “yes” answer to four or more questions; intermediate quality: “yes” answer to three questions; low quality: “yes” answer to less than three questions.
3. Results
3.1. Search
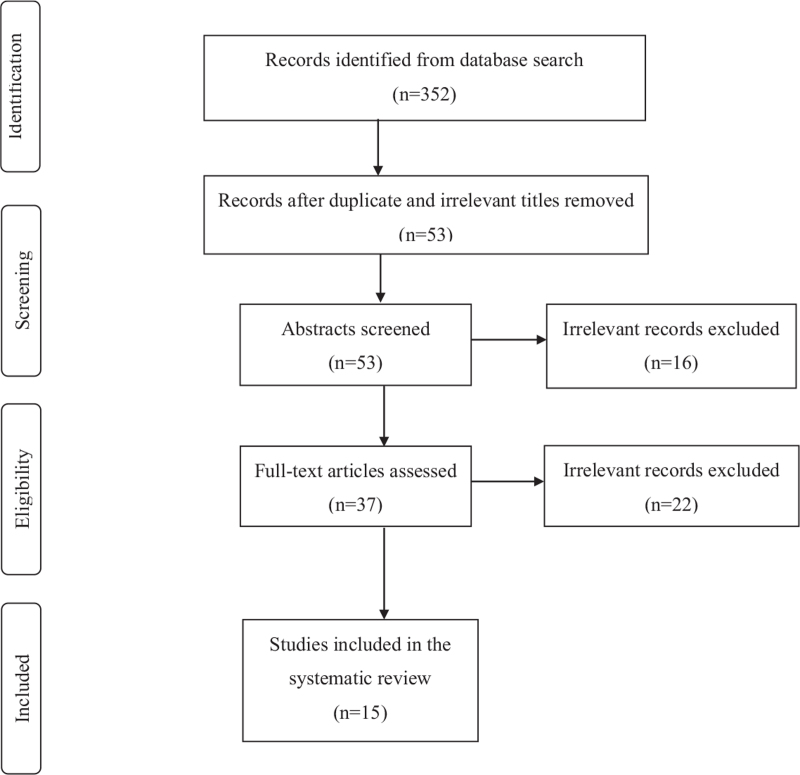
The electronic database search yielded 352 records as follows: Google Scholar (n = 254), Scopus (n = 41), Embase (n = 26), Web of Science (n = 13), PubMed (n = 12), Cumulative Index to Nursing and Allied Health Literature (n = 5), Global Index Medicus (n = 1), and Cochrane Database of Systematic Reviews (n = 0). Of these, 299 titles were excluded (83 duplicates and 216 irrelevant to the topic). A further 16 records were excluded after abstract review. Of the remaining 37 records that were eligible for full-text assessment, 17 articles were not relevant to the topic, while 5 were review-type articles. Hence, 15 records were eligible for inclusion in this study. The details of this process are shown in Figure 1. Table 2 summarizes the findings of the manuscript included in the text.
Figure 1.
Study flow diagram.
Table 2.
Summary of the literature included in the review.
| Cavernosal blood gas | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| First author, study design and date of publication | Country of origin | Age | Diagnosis | Respiratory support | No. of episodes and duration of priapism | pH | PCO2 (mm Hg) | PO2 (mm Hg) | D-dimer (ng/mL) | Management of priapism | Patient outcomes | Author's conclusion |
| Lamamri et al., CR, June 2020[6] | France | 62 | COVID-19 pneumonia with priapism | MV | 1 episode, >4 hours | 6.98 | 121 | 68 | 2210 | Ice packs, cavernosal aspiration, intracavernosal ethylephrine injection, enoxaparin | Survived to hospital discharge | Strongly suggests priapism related to SARS-CoV-2 infection. |
| Lam et al., CR, July 2020[7] | United Kingdom | 67 | COVID-19 pneumonia with priapism | CPAP and HFNC | 1 episode, duration not stated | NR | NR | NR | 428 | Conservative management due to rapid clinical deterioration of COVID-19 pneumonia | Deceased | Coagulopathy and possible microemboli formation secondary to COVID-19 infection may have led to obstruction of draining venules, resulting in low-flow priapism. |
| Silverman et al., CR, January 2021[8] | USA | 69 | COVID-19 pneumonia with priapism | MV | 1 episode, <1 day | 6.93 | >98.3 | <30.1 | NR | Ice packs, cavernosal aspiration, intracavernosal phenylephrine injection, heparin | Deceased | The evidence has suggested an association between COVID-19 and hypercoagulability. |
| Addar et al., CR, April 2021[9] | Saudi Arabia | 62 | COVID-19 pneumonia with priapism | HFNC | 1 episode, 10 days | 6.86 | 33.3 | 26.9 | NR | Cavernosal aspiration, intracavernosal phenylephrine injection, enoxaparin | Survived to hospital discharge | NR |
| Larrarte-Arenas et al., CR, May 2021[10] | Columbia | 65 | Mild COVID-19 with priapism, renal dialysis for the past 2 years | No oxygen required due to mild COVID-19 symptoms | 1 episode, 30 hours | 6.88 | 93.2 | 35 | NR | Ice packs, cavernosal aspiration, intracavernosal epinephrine injection | Survived to hospital discharge | Haemodialysis and kidney disease may have predisposed the patient to priapism. Role of COVID-19 is unclear. |
| Grimberg et al., CR, June 2021[11] | USA | 45 | COVID-19 pneumonia with priapism | MV | 2 episodes 8 hours apart, duration of first episode was >4 hours | 7.05 | 21 | 86 | NR | Cavernosal aspiration, intracavernosal phenylephrine, enoxaparin | Survived to hospital discharge | Priapism secondary to COVID-19 can occur while receiving prophylactic doses of heparin. Priapism can be stuttering in nature. Corporal aspiration and injection are feasible and effective, even in prone patients. |
| Giuliano et al., CR, July 2021[12] | Italy | 34 | Priapism post mild COVID-19 | No oxygen required due to mild COVID-19 symptoms | 1 episode, 36 hours | 7.47 | 33 | 65 | 5855 | Cavernosal aspiration, intracavernosal phenylephrine and spongio-cavernosal shunt all failed. Bilateral T-shunt relieved tumescence | Survived to hospital discharge | Priapism could occur due to mild COVID-19 infection. |
| Carreño et al., CR, September 2021[13] | Columbia | 39 | COVID-19 pneumonia with priapism | MV | 1 episode, >72 hours | 7.32 | 46.6 | 66.8 | 865 | Intracavernous injection of adrenaline, LMWH | Deceased | Potential association between ischemic priapism and COVID-19 |
| Anderson et al., CR, October 2021[14] | USA | 68 | COVID-19 pneumonia with priapism | MV | 4 episodes, duration of first episode was >4 hours | <6.8 | 19 | 86 | NR | Cavernosal aspiration, intracavernosal phenylephrine | Deceased | Priapism may be a rare thromboembolic complication of severe COVID-19. |
| Ahmed et al., CR, October 2021[15] | United Kingdom | 58 | COVID-19 pneumonia with priapism | MV | NR | NR | NR | NR | NR | Cavernosal aspiration | NR | We highlight the importance of vigilance for extra-pulmonary manifestations of COVID-19 |
| Laaribi et al., CR, December 2021[16] | Morocco | 67 | COVID-19 pneumonia with priapism | MV | 1 episode, prolonged duration with refractory priapism | NR | NR | NR | NR | Cavernosal aspiration, intracavernosal phenylephrine, caverno-cancellous shunt and posthectomy heparin | Survived to hospital discharge | Priapism is a rare thromboembolic complication of COVID-19 and requires rapid treatment in order to limit the often-irreversible sequelae. |
| Cruz et al., CR, December 2021[17] | Argentina | 62 | COVID-19 pneumonia with priapism | MV | 1 episode, 5 hours | 7.2 | 60 | 80 | 4885 | Ice packs, cavernosal aspiration, intracavernosal adrenaline, enoxaparin | NR | Failure to establish timely and effective treatment may lead to permanent sequela. |
| Brönimann et al., CR, January 2022[18] | Austria | 12 | Priapism post mild COVID-19 | No oxygen required due to mild COVID-19 symptoms | 3 episodes in 4 days | 6.95 | 3.1 | 85.1 | NR | Ice packs, cavernosal aspiration, intracavernosal phenylephrine, etilefrine, enoxaparin | Survived to hospital discharge | The underlying SARS-CoV-2 induced pathophysiological mechanisms need to be confirmed by future studies. |
| Ameyaw et al., CR, February 2022[19] | Ghana | 9 | Mild COVID-19 with priapism | No oxygen required due to mild COVID-19 symptoms | 1 episode, 6 hours | NR | NR | NR | NR | Ice packs, conservative management with spontaneous resolution after an hour of hospital presentation | Survived to hospital discharge | Priapism may be a rare clinical feature of COVID-19 among children and should be looked for following COVID-19 infection. |
| Alsaedi et al., CR, February 2022[20] | Saudi Arabia | 66 | COVID-19 pneumonia with priapism | HFNC | 1 episode, 3 days | NR | NR | NR | 200 | Cavernosal aspiration, partial penectomy as the patient had clinical evidence of penile gangrene on presentation | Survived to hospital discharge | Strongly suggest the need for the diagnosis and prevention of thrombotic diseases in at-risk patients with COVID-19 infection. |
CPAP = continuous positive airway pressure; CR = case report; HFNC = high-flow nasal cannula oxygen; LMWH = low-molecular-weight heparin; MV = mechanical ventilation; NR = not reported; PCO2 = partial pressure of carbon dioxide; PO2 = partial pressure of oxygen.
3.2. Country of origin
Three cases (20.0%) originated in the United States[8,11,14]; two (13.3%) each from Columbia,[10,13] the United Kingdom,[7,15] and Saudi Arabia[9,20]; and one (6.7%) each from France,[6] Italy,[12] Morocco,[16] Argentina,[17] Austria,[18] and Ghana.[19]
3.3. The age range of study subjects
Nine (60.0%) patients were aged between 60 and 70 years.[6–10,14,16,17,20] There were 2 (13.3%) pediatric patients aged 12[18] and 9[19] years. The ages of the remaining 4 (26.7%) cases ranged from 34 to 58 years.[11–13,15]
3.4. Subtype of priapism and likely aetiology
Low-flow (ischemic) priapism was diagnosed in all 15 cases.[6–20] In 14 (93.3%) of these cases, the authors indicated that thromboembolic complications of COVID-19 was the most likely cause of priapism.[6–9,11–20] In one case of mild COVID-19 infection, the authors were unsure whether the onset of priapism was precipitated by COVID-19 infection or whether it was a complication of the hemodialysis that the patient received 2 years prior.[10]
3.5. Number of episodes and duration of priapism
Eleven (73.3%) publications reported a single episode of priapism,[6–14,16,17,19,20] while one (6.7%) publication reported 2,[11] 3,[18] and 4[14] episodes of priapism. The reported duration of priapism ranged from 4 hours to 10 days.
3.6. Severity of COVID-19
COVID-19 pneumonia was diagnosed in 11 cases (73.3%),[6–9,11,13–17,20] of whom 8 (53.3%) required mechanical ventilation,[6,8,11,13–17] 1 (6.7%) required a combination of continuous positive airway pressure non-invasive ventilation and high-flow nasal canal oxygen,[7] and 2 (13.3%) required high-flow nasal canal oxygen only.[9,20] Of the 4 (26.7%) remaining cases, all were reported to have mild COVID-19 infection and did not require supplemental oxygen.[10,12,18,19]
3.7. Cavernous blood gas
Among the 10 cases in which cavernous blood gas was recorded,[6,8–14,17,18] the pH was <7.25 in 8 (80.0%) cases,[6,8–11,14,17,18] PaCO2 was ≥60 mm Hg in 4 (40.0%),[6,8,10,17] and PaO2 was <30.1 mm Hg in 2 (20.0%) cases.[8,9]
3.8. D-dimer
Of the 6 cases in which D-dimer levels were reported,[6,7,12,13,17,20] only 1 had a D-dimer level of <500 ng/mL.[7] The highest recorded D-dimer level was 5855 ng/mL,[12] while the lowest recorded level was 428 ng/mL.[7]
3.9. Design of the included publications
All 15 included publications were single patient case reports.[6–20]
3.10. Follow-up of survivors
Of the 9 (60.0%) cases in whom survival to hospital discharge was reported,[6,9–12,16,18,19] follow-up findings were recorded in 5 cases. Of these, the first publication reported that the patient was having night-time erections at 2-week follow-up[9]; the second publication reported that the patient had complete erectile dysfunction at 3-month follow-up[12]; the third publication only mentioned that the patient had no further episodes of priapism at 30-day follow-up[11]; the fourth publication reported that the patient required a glandulectomy, resection of non-viable corpora cavernosa, and a ventral penile urethrostomy due to extensive glandular necrosis[16]; and the fifth publication reported that the patient did not complain about priapism recurrence while confirming spontaneous physiologic erections, at 8-week follow-up.[18]
3.11. Treatment
Priapism was managed conservatively in 2 (13.3%) cases: one was a 67-year-old man who developed rapid clinical deterioration of COVID-19 pneumonia and died shortly after admission,[7] and the other was a 9-year old boy who achieved spontaneous resolution of priapism an hour after hospital presentation.[19] Except for a single case that required a bilateral T-shunt (corpora-glandular) procedure,[12] a case requiring a caverno-cancellous shunt and posthectomy,[16] and another case that presented 3 days after the onset of priapism with established penile gangrene and required a partial penectomy,[20] the remaining 10 (66.7%) cases responded to standard therapy that included a combination of ice pack application, cavernosal aspiration/irrigation, and intracavernosal sympathomimetic injections.[6,8–11,13–15,17,18] With regard to the treatment of concomitant COVID-19, 4 cases required mechanical ventilation,[6–8,13] while anticoagulation therapy was administered to 8 (53.3%).[6,8,9,11,13,16–18]
3.12. Patient outcomes
Of the 13 publications that reported on mortality outcomes, 4 patients (30.8%) died from COVID-19 related complications prior to hospital discharge.[7,8,13,14]
4. Discussion
Despite 2 years of the COVID-19 pandemic, the precise pathophysiology of the disease is not yet fully understood. COVID-19 enters the host cells via the S spike protein, which binds to angiotensin-converting enzyme 2. In addition to the direct injury caused by the virus itself, activation of the inflammatory and coagulation pathways, suppression of immune function, and downregulation of angiotensin-converting enzyme 2 also contribute to the pathophysiology of multi-organ pathology associated with COVID-19 infection.[27,28]
Microangiopathic thrombosis is commonly observed in patients with COVID-19. A meta-analysis of 42 studies comprising 8271 patients found that 21% of patients infected with COVID-19 also developed venous thromboembolism, which was associated with a significantly higher likelihood of mortality (odds ratio = 1.74; 95%CI = 1.01–2.98; p = 0.04).[29]
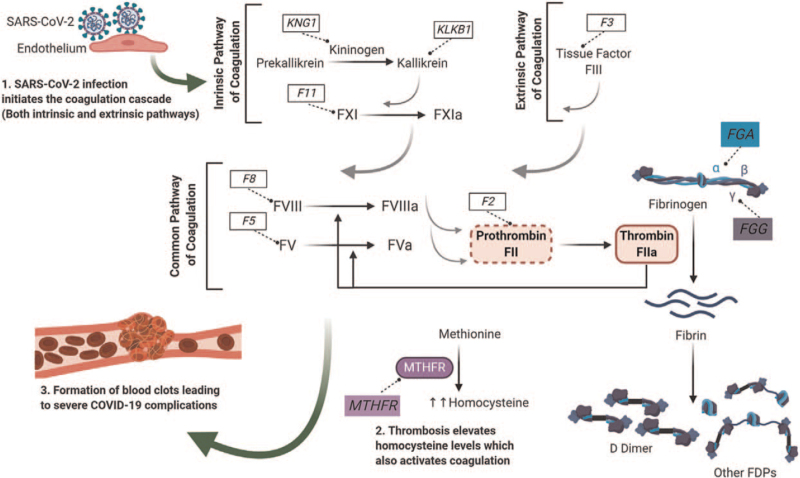
Activation of the coagulation cascade secondary to COVID-19 infection is a complex sequence of events that is precipitated by viral-induced endothelial cell injury (Fig. 2), resulting in activation of the intrinsic, extrinsic, and common pathways, thereby leading to the formation of blood clots. Additionally, SARS-CoV-2 induced thrombosis also leads to elevated levels of homocysteine, which further enhances the coagulation cascade.[30]
Figure 2.
Illustration of the SARS-CoV-2 (COVID-19) infection process in the human body From “Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A focused Analysis on D-dimer, Homocysteine and Thromboembolism” by Abu-Farha M, Al-Sabah S, Hammad MM, et al. Front Pharmacol 2020;11:e587451. Reused with permission.
Ischemic priapism is the most common form of priapism and results from occlusion of the venous outflow of the corpora cavernosa, leading to tissue ischemia. When aspirated, penile blood appears dark red, implying the presence of tissue hypoxia.[31] Penile cavernous blood gas analysis in the presence of ischemic priapism is commonly associated with a pH of <7.25, PCO2 >60 mm Hg, and PO2 <30 mm Hg.[32] In keeping with this, the penile cavernous blood pH was <7.25 in 8 of the 10 included cases cavernous blood gas was recorded. Of the 2 cases in which the pH was >7.25, it was still below the normal range of 7.36–7.44 in one case,[13] but within the normal range in the other article.[12] Notably, the latter patient was a 34-year-old adult who presented with mild COVID-19 infection, a very high D-dimer level (5855 ng/mL), and refractory priapism that necessitated a bilateral T-shunt procedure.
Elevated D-dimer levels (>500 ng/mL) are a prominent feature of COVID-19 induced hypercoagulability.[33] Consistent with this, D-dimer levels were elevated in 5 of the 6 cases in which D-dimer levels were recorded.[6,12,13,17] This finding suggests that priapism could be listed as a thromboembolic complication of COVID-19. Thromboembolic complications of COVID-19 were postulated to be the most likely cause of priapism by the authors in 14 of the 15 included publications.[6–13,15–19] In the remaining publication, the authors indicated that priapism may have been secondary to hemodialysis rather than a complication of COVID-19.[10] Haemodialysis has previously been described as a rare cause of priapism.[34]
As COVID-19 has been associated with significantly higher mortality in the elderly,[35] age may also play a role. Although the majority of patients in this study were aged between 60 and 70 years, 4 patients were below the age of 40 years,[12,13,18,19] 3 of whom had mild COVID-19 infection,[12,18,19] and 2 were in the pediatric age group.[18,19] This suggests that, although older patients with COVID-19 are more prone to developing priapism, it may also occur in younger patients.
Approximately three-quarters of the patients (73.3%) were diagnosed with COVID-19 pneumonia, more than half (53.3%) required mechanical ventilation, and one-third (30.8%) died prior to hospital discharge. This finding suggests that priapism may be an indicator of severe COVID-19. However, since COVID-19 infection was regarded as mild in 26.7% of patients who did not require supplemental oxygen, the relationship between priapism and severe COVID-19 is inconclusive.
An association between COVID-19 and other adverse effects in the male genital tract has also been reported.[36] Recently, Kresch et al. demonstrated the presence of COVID-19 virus particles in the penile tissue of two individuals with persistent severe dysfunction following recovery from COVID-19 respiratory symptoms.[37] Another study reported that sperm concentration was significantly lower in men previously infected with COVID-19.[38] Hence, the findings of our systematic review shed further light on the impact of COVID-19 on sexual health in men.
The overall impact of COVID-19 has interrupted other urological surgical interventions during the pandemic, and an emphasis on giving priority to COVID-19 patients has been advocated. Since priapism is an established medical emergency, its management cannot be deferred.[39,40]
An obvious limitation of this systematic review was that only 15 publications were eligible for inclusion, all of which were single-patient case reports. This is understandable, as the possible association between priapism and COVID-19 has only recently been described. Another limitation is that most studies did not report on laboratory tests pertaining to inflammatory biomarker levels or coagulation profiles; hence, we were unable to determine whether there was an association between these tests and the risk of developing priapism.
5. Conclusions
Priapism was more common in cases of severe COVID-19 infection than mild infection, suggesting a possible prognostic relationship between the two conditions. However, priapism has also been described in mild COVID-19 cases. Despite the described theoretical association between the two conditions, there is still a scarcity of data in international literature. Regardless of insufficient evidence outlining a significant association between COVID-19 and priapism, the management of priapism still constitutes a medical emergency that requires urgent conventional intervention. Further research is required to confirm the probable association between COVID-19 infection and priapism.
Acknowledgments
None.
Statement of ethics
Not applicable.
Conflict of interest statement
AA is an Editorial Board member of Current Urology.
Funding source
None.
Author contributions
DMM: Formal analysis, investigation, methodology, literature review, writing-original draft AEL, JM & AA: Conceptualization; supervision; writing-review and editing.
References
- [1].Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19: Studies needed. N Engl J Med 2020;382(13):1194–1196. [DOI] [PubMed] [Google Scholar]
- [2].Li Y, Bai W, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020;92(6):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].American Society of Haematology—COVID-19 and coagulopathy: Frequently Asked Questions [Internet]. 2021 [cited January 10, 2022]. Available at: https://www.hematology.org/covid-19/covid-19-and-coagulopathy. [Google Scholar]
- [6].Lamamri M, Chebbi A, Mamane J, et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am J Emerg Med 2021;39:251.e5–251.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lam G, McCarthy R, Haider R. A peculiar case of priapism: The hypercoagulable state in patients with severe COVID-19 infection. Eur J Case Rep Intern Med 2020;7(8):e001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silverman ML, VanDerVeer SJ, Donnelly TJ. Priapism in COVID-19: A thromboembolic complication. Am J Emerg Med 2021;45:686.e5–686.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Addar A, Al Fraidi O, Nazer A, Althonayan N, Ghazwani Y. Priapism for 10 days in a patient with SARS-CoV-2 pneumonia: A case report. J Surg Case Rep 2021;2021(4):rjab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Larrarte-Arenas C, Vargas-Ángel DC. Priapism in a patient on hemodialysis and with COVID-19. Case report. Rev la Fac Med 2021;69(1):e90632. [Google Scholar]
- [11].Grimberg DC, Tejwani R, Allkanjari A, Forrester MT, Kraft BD, Kaye DR. Ischemic priapism due to coagulopathy of severe COVID-19 infection. J Clin Urol 2022;published online ahead of print. [Google Scholar]
- [12].Giuliano AFM, Vulpi M, Passerini F, et al. SARS-CoV-2 infection as a determining factor to the precipitation of ischemic priapism in a young patient with asymptomatic COVID-19. Case Rep Urol 2021;2021:e9936891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carreño BDV, Perez CP, Vasquez D, Oyola JA, Suarez O, Bedoya C. Veno-occlusive priapism in COVID-19 disease. Urol Int 2021;105(9–10):916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andersen K, Dargin J, Childs B. A case of priapism in a man admitted to the ICU for COVID-19 ARDS. Chest 2021;160(4):A638. [Google Scholar]
- [15].Ahmed S, Alabai M, Datta S. Low flow priapism as a complication of Covid-19 infection. Br J Surg 2021;108(Supplement_6):1254. doi: 10.1093/bjs/znab259.265. [Google Scholar]
- [16].Laaribi I, Monchi M. COVID-19 infection complicated by priapism. World J Adv Res Rev 2021;12(3):072–074. [Google Scholar]
- [17].Cruz AJ, Leandro L, Claudio K, Jorge L, Paul C, Roberto VB. Priapism in a patient with COVID-19: A case report. Ann Clin Case Rep 2021;6:2070. [Google Scholar]
- [18].Brönimann S, Talhammer F, Springer A, Tonnhofer U, Shariat SF, D’Andrea D. Ischemic priapism in a 12-year old patient associated with coronavirus disease 2019 (COVID-19): A case report. Urology 2022;published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ameyaw E, Okyere I, Ameyaw R, et al. Priapism in a 9-year-old Ghanaian boy with COVID-19: A case report. Transl Res Urol 2022;4(1):4–8. [Google Scholar]
- [20].Alsaedi SM, Alsarwani RM, Ali AI, Aladhrai SA. Ischemic priapism progressing to penile gangrene in a patient with COVID-19 infection: A case report with literature review. Case Rep Med 2022;2022:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song PH, Moon KH. Priapism: Current updates in clinical management. Korean J Urol 2013;54(12):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Al-marhoon MS, Al-masruri MS. Demography of priapism: Comparison between sickle cell and non-sickle cell disease patients. ARC J Urol 2020;5(1):3–9. [Google Scholar]
- [23].Montague DK, Jarow J, Broderick GA, et al. American urological association guideline on the management of priapism. J Urol 2003;170:1318–1324. [DOI] [PubMed] [Google Scholar]
- [24].Matz A, Ambinder D, Spencer E, Phillips J, Wong NC. Review of priapism litigation in the United States. Urology 2021;156:169–172. [DOI] [PubMed] [Google Scholar]
- [25].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. Evid Based Med 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Azer SA. COVID-19: Pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect 2020;37:e100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020;24(1):e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Awortwe C, Cascorbi I. Meta-analysis on outcome-worsening comorbidities of COVID-19 and related potential drug-drug interactions. Pharmacol Res 2020;161:e105250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abu-Farha M, Al-Sabah S, Hammad MM, et al. Prognostic genetic markers for thrombosis in COVID-19 patients: A focused analysis on D-Dimer, homocysteine and thromboembolism. Front Pharmacol 2020;11:e587451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hudnall M, Reed-Maldonado AB, Lue TF. Advances in the understanding of priapism. Transl Androl Urol 2017;6(2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Levey HR, Segal RL, Bivalacqua TJ. Management of priapism: An update for clinicians. Ther Adv Urol 2014;6(6):230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Voicu S, Ketfi C, Stépanian A, et al. Pathophysiological processes underlying the high prevalence of deep vein thrombosis in critically Ill COVID-19 patients. Front Physiol 2021;11:e608788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shih WV, Wong C. Priapism and hemodialysis: Case report and literature review. Clin Nephrol 2018;90(1):64–70. [DOI] [PubMed] [Google Scholar]
- [35].Koh HK, Geller AC, VanderWeele TJ. Deaths from COVID-19. JAMA 2020;325(2):133–134. [DOI] [PubMed] [Google Scholar]
- [36].Abbas AM, Fathy SK, Khamees AA, Salem AS, Ahmed L. A focused review on the genital and sexual affection of COVID-19 patients. J Gynecol Obstet Hum Reprod 2020;49(8):e101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kresch E, Achua J, Saltzman R, et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: Histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health 2021;39(3):466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Donders GGG, Bosmans E, Reumers J, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: A prospective, observational study and validation of the SpermCOVID test. Fertil Steril 2022;117(2):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Quarto G, Grimaldi G, Castaldo L, et al. Avoiding disruption of timely surgical management of genitourinary cancers during the early phase of the COVID-19 pandemic. BJU Int 2020;126(4):425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Branche B, Carbonara U, Crocerossa F, Autorino R, Hampton LJ. Robotic urological surgery in the time of COVID-19: Challenges and solutions. Urol Pract 2020;7(6):547–553. [DOI] [PubMed] [Google Scholar]