Abstract
Background: SARS-CoV-2 vaccination in cancer patients is crucial to prevent severe COVID-19 disease course. Methods: This study assessed immunogenicity of cancer patients on active treatment receiving mRNA-based SARS-CoV-2 vaccine by detection of anti-SARS-CoV-2 S1 IgG antibodies in serum, before, after the first and second doses and 3 months after a complete primary course of vaccination. Results were compared with healthy controls. Results: Of 112 patients, the seroconversion rate was 96%. A significant reduction in antibody levels was observed 3 months after vaccination in patients receiving immune checkpoint inhibitors versus control participants (p < 0.001). Adverse events were mostly mild. Conclusion: Immunogenicity after mRNA-based vaccine in cancer patients is adequate but influenced by the type of anticancer therapy. Antibody levels decline after 3 months, and thus a third vaccination is warranted.
Keywords: : anticancer treatment, immunogenicity, mRNA-based vaccination, seroconversion, solid cancer
Plain language summary
Because cancer patients are especially endangered by SARS-CoV-2 infection and have worse disease course and outcomes, it is crucial to protect them from this infection. This study was aimed at assessing protective antibodies after patients received mRNA-based SARS-CoV-2 vaccines. Protective antibodies (e.g., anti-SARS-CoV-2 S1 IgG antibodies) were assessed in patients’ blood before vaccination, after the first and second doses and 3 months after a complete primary course vaccination. Patients’ oncological treatment was unaffected by the vaccination received. The results of protective antibodies were also compared with healthy control subjects who were vaccinated in the same manner. More than 110 cancer patients participated and agreed to have their blood samples analyzed. The rate of antibody production was 96% after a complete primary course of vaccination and was similar with that of healthy control subjects. However, there were some differences noted regarding the oncological treatment that the patients were receiving, with patients who were treated with targeted therapy achieving the highest levels of protective antibodies. Adverse events after vaccination were mostly mild and did not interfere with patients’ general performance. The rate of antibody production for cancer patients after SARS-CoV-2 vaccination is high and similar to that in healthy control subjects but varies with regard to the oncological treatment that patients are receiving. However, antibodies decline substantially after 3 months, and thus a third vaccination is desirable. There were no new safety concerns after vaccination, and most adverse events were mild and short-lived.
The COVID-19 pandemic had an enormous medical and socioeconomic impact on the global population health, with more than 250 million cases and 5 million deaths reported at the time of this writing [1]. Moreover, the COVID-19 pandemic has had devastating consequences in the cancer community, with a delayed cancer diagnosis, deferred treatment decisions and mortality rates among cancer patients reaching as high as 30% [2–7]. Additional safety measures have been incorporated during the pandemic, such as telemedicine, and many patients’ treatment was delayed due to the circumstances created by the pandemic [8,9]. Because COVID-19 disease adversely affects cancer patients, prophylactic strategies are crucial.
An unprecedented global effort has been made to develop effective and safe vaccines that could end the pandemic. Nevertheless, data on the safety and efficacy of current anti-SARS-CoV-2 vaccines is scarce for cancer patients who are receiving active treatment. None of the more than 117,000 volunteers included in the phase III SARS-CoV-2 vaccination trials were recently diagnosed cancer patients or patients on active cancer treatment or any sort of immunomodulatory treatment [10–13]. Because vaccine efficacy is not always optimal in cancer patients and is prone to many variables, as seen in real-world evidence on the influenza vaccine, more data on the efficacy and safety of cancer patients vaccinated against SARS-CoV-2 is urgently needed [14,15].
Many oncological organizations were quick to respond and suggested cancer patients be among the first to be vaccinated and thus protected, due to the nature of their disease and treatment as well as their frequent exposure to the healthcare system – all of which leaves them vulnerable, immunocompromised and at higher risk of contracting the virus. Initiatives were also taken to prospectively collect these data to provide additional safe options for cancer patients [16,17]. Thus, a prospective observational study (PRO-ONCO-COVID-19) of cancer patients with solid tumors treated in two oncology centers in Slovenia was initiated; these patients were voluntarily vaccinated with one of the available SARS-CoV-2 vaccines. This study aimed to assess the development of anti-SARS-CoV-2 S1 IgG antibodies (immunogenicity) and the safety of the mRNA-based vaccines in cancer patients treated with chemotherapy, immunotherapy with immune checkpoint inhibitors (ICIs) or targeted therapy.
Materials & methods
Study design and participants
This was a prospective observational study of patients with solid cancers who were currently receiving or had received systemic cancer therapy in the past year and had also been vaccinated for COVID-19. Between 1 March and 21 July 2021, patients with known solid malignancy treated at two academic institutions in Slovenia (University Clinic Golnik and University Medical Centre Maribor) who were also fully vaccinated were included. All patients gave written Informed consent before study inclusion. The study was approved by the National Ethics Committee (no. 0120-39/2021/6).
Procedures
Cancer patients included in this study were receiving systemic oncological treatment without interruption by the vaccination procedures. Data collected included patient age, sex, time of cancer diagnosis, stage and histological type of cancer, concomitant immunomodulatory therapy and systemic cancer therapy that they were receiving – chemotherapy, immunotherapy with ICIs, a combination of the latter two or targeted therapy (either tyrosine kinase inhibitors or endocrine therapy).
Blood samples from patients were obtained at four prespecified time points – time point 1: before the vaccination (-7 days); time point 2: 2–3 weeks after the first vaccination dose; time point 3: 2–3 weeks after the second vaccination dose; time point 4: 3 months after the complete primary course of vaccination (± 14 days). Blood was then centrifuged and stored if not analyzed shortly after centrifuge for the presence of anti-SARS-CoV-2 S1 IgG antibodies. The antibody levels produced by cancer patients were then compared with healthy control subjects who were previously tested at similar time points after vaccination. All laboratory analyses were conducted at the Laboratory for Clinical Immunology and Molecular Genetics at the University Clinic Golnik.
Vaccination was carried out according to the manufacturing instructions: BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) were received 3 and 4 weeks apart, respectively. Because the adenoviral AZD1222 (AstraZeneca) vaccine boost dose is recommended 4–12 weeks apart and soon only mRNA-based vaccines were recommended for cancer patients, only patients vaccinated with mRNA-based vaccines were included in this analysis. Vaccination of patients adhered to the National Vaccination Strategy, which was regularly updated by the Slovenian COVID-19 Vaccination Advisory Group.
Telephone consultations were carried out 24–48 h after each vaccination dose to assess possible safety concerns and adverse events (AEs), which were then noted on prepared forms. AEs were graded with grades (G) 0–4 (G0 = no AEs; G1 = mild AEs; G2 = moderate AEs that does not interfere with daily activities; G3 = severe AEs that interferes with daily activities; G4 = life-threatening AEs, hospitalization required). The AEs evaluated were local (pain, redness or swelling on injection site) or systemic (fever, chills, fatigue, myalgia, arthralgia, headache, vomiting, diarrhea) in line with trials of mRNA vaccines [10,11]. All AEs were also reported by the national pharmacovigilance system to the National Institute for Public Health, as appropriate.
Detection of anti-SARS-CoV-2 S1 IgG antibodies in serum
The immunogenicity of mRNA-based vaccines was assessed by S1-protein-based commercial ELISA assay IDK anti-SARS-CoV-2 IgG by Immundiagnostik AG (Bensheim, Germany) according to the manufacturer’s instructions. The assay is designed to detect IgG antibodies directed against the receptor-binding domain of the S1 subunit of the spike protein of SARS-CoV-2. The test has no cross-reactivity to plasma probes for adenovirus, Epstein–Barr virus, influenza A/B, HCoV-229E, HCoV-HKU1, HCoV-NL63 or HCoV-OC4. The results of quantitative anti-SARS-CoV-2 IgG ELISA show excellent correlation with the WHO International Standard for anti-SARS-CoV-2 immunoglobulin measurement (R2 = 0.9909; National Institute for Biological Standards and Control code: 20/136) and strong correlation with SARS-CoV-2 neutralization [18]. Results were interpreted according to the manufacturer’s recommendation: samples with ≥175 ng/ml were considered positive; measurements >2020 ng/ml (upper limit of the assay) were truncated at 2020 ng/ml. To obtain the concentration in binding antibody units (BAU)/ml, the results in ng/ml were divided by a factor of 20 (20 ng/ml ≙1 BAU/ml). Subjects with values ≥880 ng/ml (≥44 BAU/ml) were considered to have a high probability of immune protection against SARS-CoV-2 and were classified as adequate responders.
Outcomes
The primary outcome was defined as the development of anti-SARS-Cov-2 S1 IgG antibodies after vaccination of cancer patients with mRNA-based vaccines against SARS-CoV-2 measured 2–3 weeks after the first and second vaccine dose. Antibodies were also monitored later at 3 months to assess the longevity of antibody protection.
Secondary end points included safety analysis of AEs and reactogenicity following the first and second vaccination doses. Another secondary end point was the proportion of patients with SARS-CoV-2 infection after the complete primary course of vaccination and with worse COVID-19 disease course after vaccination.
Statistical analysis
Data distribution was evaluated using the D’Agostino and Pearson test. Wilcoxon signed-rank test and Mann–Whitney U test were used as appropriate to calculate differences of anti-SARS-Cov-2 S1 IgG antibody levels between different time points and groups. The frequency distribution of patients with anti-SARS-Cov-2 S1 IgG antibodies between different time points and groups was compared with Fisher’s exact test based on contingency tables. Statistical analysis was performed using GraphPad PRISM software (version 9.2 for Windows; GraphPad Software, CA, USA). A p-value < 0.05 was considered statistically significant.
Results
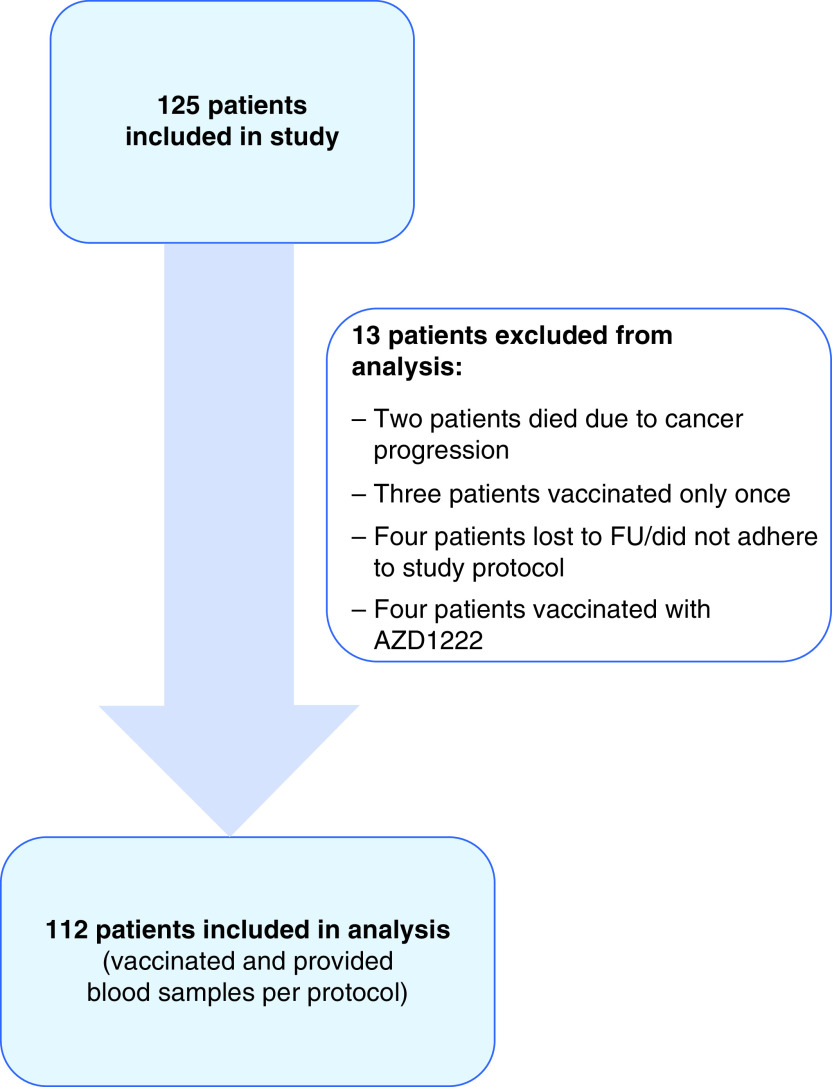
From March to the end of July 2021, 125 patients were recruited in the study and signed the informed consent form. Thirteen patients were excluded from analysis; two died due to disease progression, three were vaccinated only once and refused second vaccination dose, four were either lost to follow-up or did not adhere to the study protocol and four were vaccinated with adenoviral vaccine and were excluded due to differing dosing schedule and difficulty interpreting the result. Hence, 112 patients were included in the final analysis. A CONSORT diagram of patient flow is shown in Figure 1.
Figure 1. CONSORT diagram showing the included and analysis population.
The median age was 62 years (range 24–81 years), 58% were females and 88% were currently receiving anticancer therapy. Most of the patients were metastatic (70%) and treated for non-small-cell lung cancer (NSCLC; 71%). Types of cancer therapy being applied currently or within the past year are shown in Table 1. Four patients (4%) were on continuous steroid therapy that was not used as part of routine oncological schemes, and one patient was on immunomodulatory therapy with the anti-IL-23 drug due to psoriasis. Detailed demographic and clinical data are presented in Table 1.
Table 1. Demographic and clinical characteristics of included patients.
| n = 112 | |
|---|---|
| Age (years), median (range) | 62 (24–81) |
|
Sex, n (%) – Male – Female |
47 (42%) 65 (58%) |
|
Cancer type, n (%) – NSCLC – Breast cancer – Genitourinary cancer – Malignant mesothelioma – SCLC – Ovarian cancer – Gastrointestinal cancer |
80 (71%) 13 (12%) 5 (4%) 5 (4%) 4 (4%) 3 (3%) 2 (2%) |
|
Stage, n (%) – Limited – Locoregionaly advanced – Metastatic |
22 (20%) 11 (10%) 79 (70%) |
|
Currently receiving anticancer therapy, n (%) – Yes – No |
98 (88%) 14 (12%) |
|
Anticancer therapy, n (%) Chemotherapy alone – Age in y, median (range) – Male – Currently receiving therapy |
26 (23%) 61 (36–76) 12 (46%) 15 (58%) |
| Immune checkpoint inhibitors – Age, years, median (range) – Male – Currently receiving therapy |
44 (39%) 63 (24–73) 26 (59%) 42 (95%) |
| Targeted therapy (tyrosine kinase inhibitors or endocrine therapy) – Age, years, median (range) – Male – Currently receiving therapy |
42 (38%) 61 (33–81) 9 (21%) 41 (98%) |
|
Currently receiving steroids, n (%)‡ – No – Yes |
108 (96%) 4 (4%) |
|
Other immunomodulatory therapy, n (%)§ – No – Yes |
111 (99%) 1 (1%) |
|
Type of vaccination received, n (%)† – mRNA-based BNT162b2 (Pfizer/BioNTech) – mRNA-based mRNA-1273 (Moderna) |
109 (97%) 3 (3%) |
|
History of SARS-CoV-2 infection, n (%)# – No – Yes |
99 (88%) 13 (12%) |
|
Positive SARS-CoV-2 IgG antibodies before vaccination, n (%)†† – No – Yes |
90 (80%) 22 (20%) |
All patients received two doses.
Receiving methylprednisolone, dexamethasone or prednisolone not received within cancer treatment regimens.
One patient receiving anti-IL-23 therapy for psoriasis.
SARS-CoV-2 status was positive at baseline if the patient had clinical or virological evidence of COVID-19 illness either by positive patient history and positive reverse transcriptase-PCR or positive reverse transcriptase-PCR test alone.
Patients were considered to have positive SARS-Cov-2 IgG antibodies if the level of anti-SARS-CoV-2 S1 IgG was above the threshold of 175 ng/ml.
NSCLC: Non-small-cell lung cancer; SCLC: Small-cell lung cancer.
Most patients (97%) received the mRNA-based BNT162b2 (Pfizer/BioNTech) vaccine. There were 13 patients (12%) with a history of COVID-19 infection before the first vaccination. An additional nine patients (8%) tested positive for anti-SARS-CoV-2 S1 IgG before vaccination but had an asymptomatic disease course. Hence, 22 patients were classified as patients with prior COVID-19 infection. For comparison analysis, 64 healthy control subjects with a median age of 58 years (range 22–77 years) were included; 75% were females. All controls received two doses of the mRNA-based BNT162b2 vaccine. Twenty-two controls were classified as individuals with prior COVID-19 infection.
Primary outcomes
All but four patients in our cohort developed anti-SARS-CoV-2 S1 IgG antibody concentration of >175 ng/ml (≥8.8 BAU/ml) resulting in 96% of seropositive samples at time point 3, which was 2–3 weeks after the complete primary course of vaccination with mRNA-based vaccines. Subjects with values ≥880 ng/ml (≥44 BAU/ml) were considered to have a high probability of immune protection against SARS-CoV-2, and in our cohort, and all but seven cancer patients achieved that level and were classified as adequate responders.
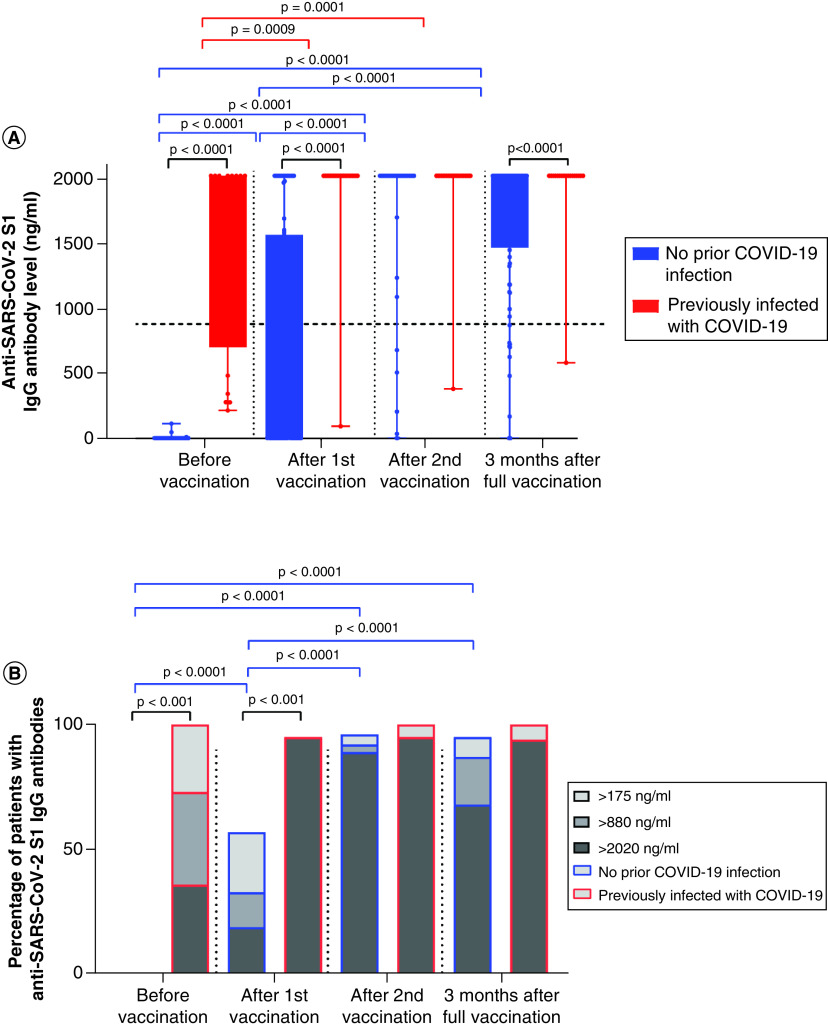
Twenty-two patients were considered previously infected, having positive anti-SARS-CoV-2 S1 IgG levels of ≥175 ng/ml before the first vaccination dose, thus leaving 90 patients who were COVID-19 naive (Figure 2). At time point 2, 52 of 90 (58%) non-previously infected patients achieved seroconversion with anti-SARS-CoV-2 S1 IgG levels ≥175 ng/ml, and one-third of patients (31/90 [35%]) achieved anti-SARS-CoV-2 S1 IgG levels ≥880 ng/ml (≥44 BAU/ml) and thus were considered adequate responders. In contrast, all but one patient in the previously infected group were adequate responders after the first vaccination. Differences were significant comparing the non-previously infected group before and after their first vaccination, p < 0.001. Median anti-SARS-CoV-2 S1 IgG levels after first vaccination for the non-previously infected and infected groups were 255 ng/ml (0–2020) and 2020 ng/ml (196–2020 ng/ml), p < 0.001, respectively. At time point 3 (after the second vaccination), 86 of 90 (96%) non-previously infected patients achieved seroconversion with median SARS-CoV-2 S1 IgG titers of 2020 ng/ml (0–2020), which was statistically significant compared with the levels before and after the first vaccination – both p < 0.001. At time point 4 (3 months after the complete primary course of vaccination), anti-SARS-CoV-2 S1 IgG levels were still significantly higher compared with before and after the first vaccination dose for non-previously infected patients, p < 0.001, and comparable to the antibody levels after the second dose of vaccine. In the non-previously infected group, 92 and 87% of patients were adequate responders after the complete primary course of vaccination and 3 months after vaccination, respectively. The difference between the non-previously infected and previously infected groups was statistically significant 3 months after the completing the primary course of vaccination with p = 0.033.
Figure 2. Anti-SARS-CoV-2 S1 IgG antibody levels in previously infected and non-previously infected cancer patients.
(A) Differences in antibody titers against S1 protein of SARS-CoV-2 in cancer patients at different time points in patients without previous COVID-19 infection (blue) and those with prior COVID-19 infection (red); Wilcoxon signed-rank test. (B) Proportion of cancer patients with antibodies against S1 protein of SARS-CoV-2 at different time points in patients without previous COVID-19 infection (blue borders) and in patients with prior COVID-19 infection (red borders); chi-square test. Patients with IgG values ≥880 ng/ml (≥44 BAU/ml) were considered to have a high probability of immune protection against SARS-CoV-2.
Seroconversion according to anticancer therapy
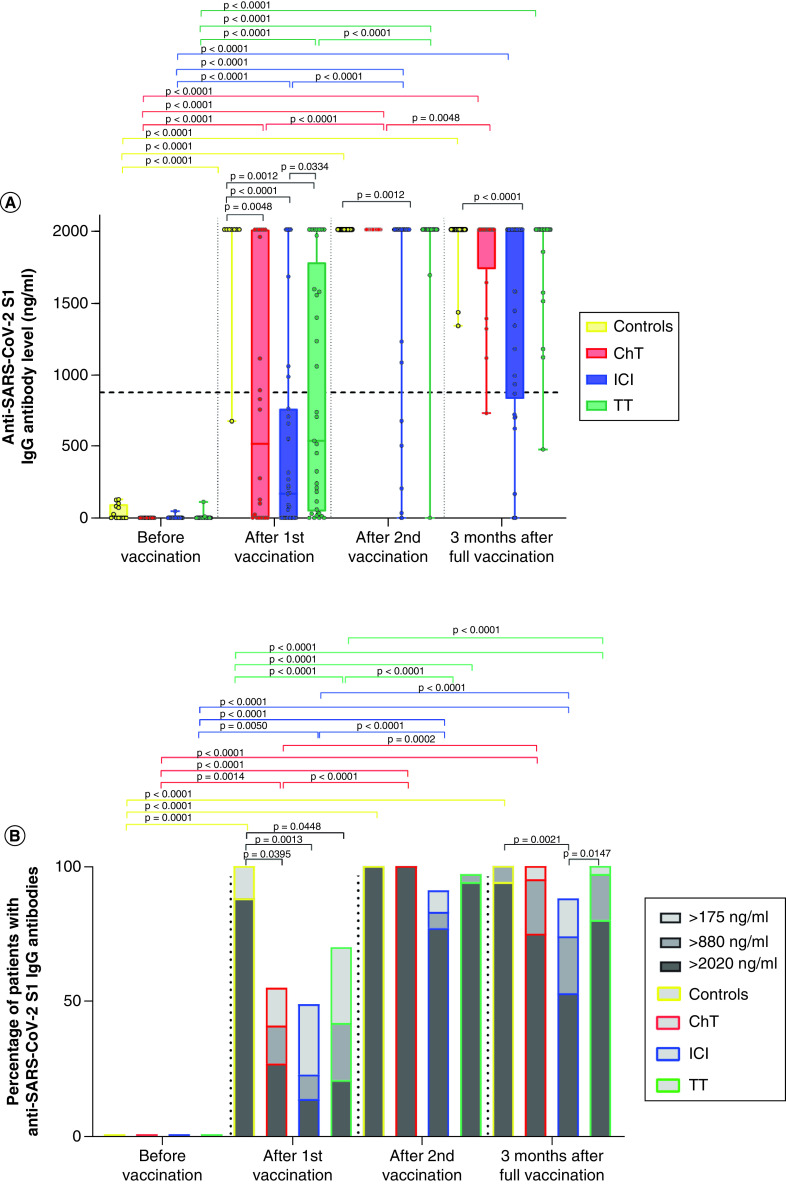
A different antibody response was observed when comparing non-previously infected patients according to different anticancer therapies, especially after the first vaccine course (Figure 3). Compared with healthy controls, who had seroconversion rates of 100% after the first vaccination dose and median anti-SARS-CoV-2 S1 IgG levels of 2020 ng/ml (678–2020), patients on chemotherapy, ICI or targeted therapy had seroconversion rates of 55, 49 and 70%, respectively. Similarly, 88% of controls were adequate responders, whereas this rate was much lower in patients on chemotherapy, ICI or targeted therapy, reaching 41% (p = 0.040), 23% (p = 0.001) and 42% (p = 0.045), respectively. Compared with healthy controls, median anti-SARS-CoV-2 S1 IgG levels after only one vaccine dose for patients receiving chemotherapy were significantly lower: 519 ng/ml (0–2020), p = 0.005. This was also the case for patients on ICI: median levels 169 ng/ml (0–2020), p < 0.001, and targeted therapy: median levels 541 ng/ml (0–2020), p = 0.001. Patients on ICI had the lowest anti-SARS-CoV-2 S1 IgG levels compared with patients on targeted therapy (p = 0.033) and combined patients on chemotherapy and targeted therapy (p = 0.045).
Figure 3. Anti-SARS-CoV-2 S1 IgG antibody levels in non-previously infected patients according to anticancer therapies.
(A) Differences in antibody titers against S1 protein of SARS-CoV-2 in cancer patients at different time points according to anticancer therapy received and in control subjects; Wilcoxon signed-rank test and Mann–Whitney U test. No subjects had previous COVID-19 infection. (B) Proportion of cancer patients with antibodies against S1 protein of SARS-CoV-2 at different time points according to anticancer therapy received and in control subjects; chi-square test. None had previous COVID-19 infection. Patients with IgG values ≥880 ng/ml (≥44 BAU/ml) were considered to have a high probability of immune protection against SARS-CoV-2.
ChT: Chemotherapy; Controls: Healthy control; ICI: Immune checkpoint inhibitor; TT: Targeted therapy.
After the complete primary course of vaccination, similar seroconversion rates were observed, with 100, 100, and 97% for healthy controls and patients receiving chemotherapy or targeted therapy, respectively. Conversely, patients receiving ICI achieved significantly lower seroconversion (91%) and anti-SARS-COV-2 S1 IgG compared to healthy controls (p = 0.001). Equally, rates of adequate response were similar in healthy controls (100%) and patients on chemotherapy (100%) and targeted therapy (97%), but only 83% of patients treated with ICI.
Patients and healthy controls previously infected with COVID-19 both achieved high levels of anti-SARS-CoV-2 S1 IgG even after the first dose of vaccination, with no significant differences in antibody levels after the first and second vaccinations (Figure 4).
Figure 4. Anti-SARS-CoV-2 S1 IgG antibody levels in previously infected patients according to anticancer therapies.
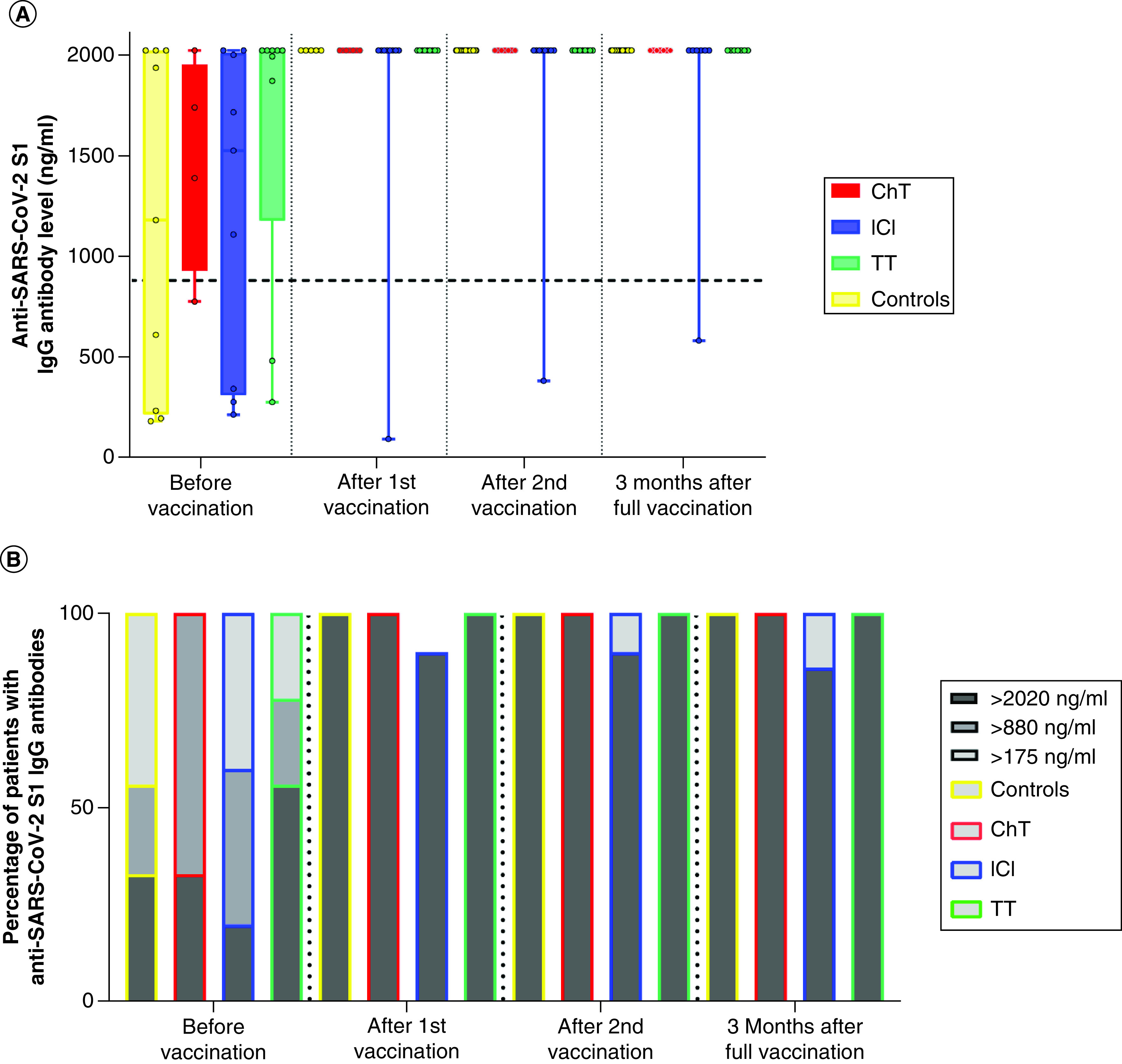
(A) Differences in antibody titers against S1 protein of SARS-CoV-2 in cancer patients at different time points according to anticancer therapy received and in control subjects; Wilcoxon signed-rank test and Mann–Whitney U test. All had previous COVID-19 infection. (B) Proportion of cancer patients with antibody against S1 protein of SARS-CoV-2 at different time points according to anticancer therapy received and in control subjects; chi-square test. All had prior COVID-19 infection. Patients with IgG values ≥880 ng/ml (≥44 BAU/ml) were considered to have a high probability of immune protection against SARS-CoV-2.
ChT: Chemotherapy; Controls: Healthy control; ICI: Immune checkpoint inhibitor; TT: Targeted therapy.
Long-term outcomes (after 3 months)
Three months after the complete primary course of vaccination data for 102 patients were available; of those, 84 had not been previously infected with COVID-19. Results show consistent positive anti-SARS-CoV-2 S1 IgG levels in cancer patients (95%), although a significant decline was observed. Patients receiving ICI had significantly lower levels of anti-SARS-CoV-2 S1 IgG compared with healthy controls 3 months after vaccination (p < 0.0001). At this time point, one patient in the chemotherapy group, four in the ICI group and three in the targeted therapy group exhibited anti-SARS-CoV-2 S1 IgG levels below 880 ng/ml (<44 BAU/ml) – the level considered to offer protection against SARS-CoV-2 with high probability. Results are shown in Figures 3 & 4.
Despite receiving a complete primary course of vaccination, four patients did not develop detectable antibodies against the S1 protein of SARS-CoV-2. One patient was treated for metastatic NSCLC with ICI but was also receiving glucocorticoids due to a severe AE of these treatments, the second was treated for metastatic NSCLC with a combination of chemo-immunotherapy but also had an underlying chronic lymphocytic leukemia in remission that did not require treatment at that time. The third patient was treated for metastatic renal cell carcinoma with an mTOR inhibitor, and the fourth patient was treated for metastatic NSCLC with a combination of chemo-immunotherapy; the latter two patients had no other significant history. An additional three non-previously infected patients had serum anti-SARS-CoV-2 S1 IgG values <880 ng/ml after the complete primary course of vaccination, which is considered suboptimal. Eleven patients altogether had serum anti-SARS-CoV-2 S1 IgG values <880 ng/ml after 3 months of the complete primary course of vaccination, owing to a rapid drop of antibody levels.
Safety
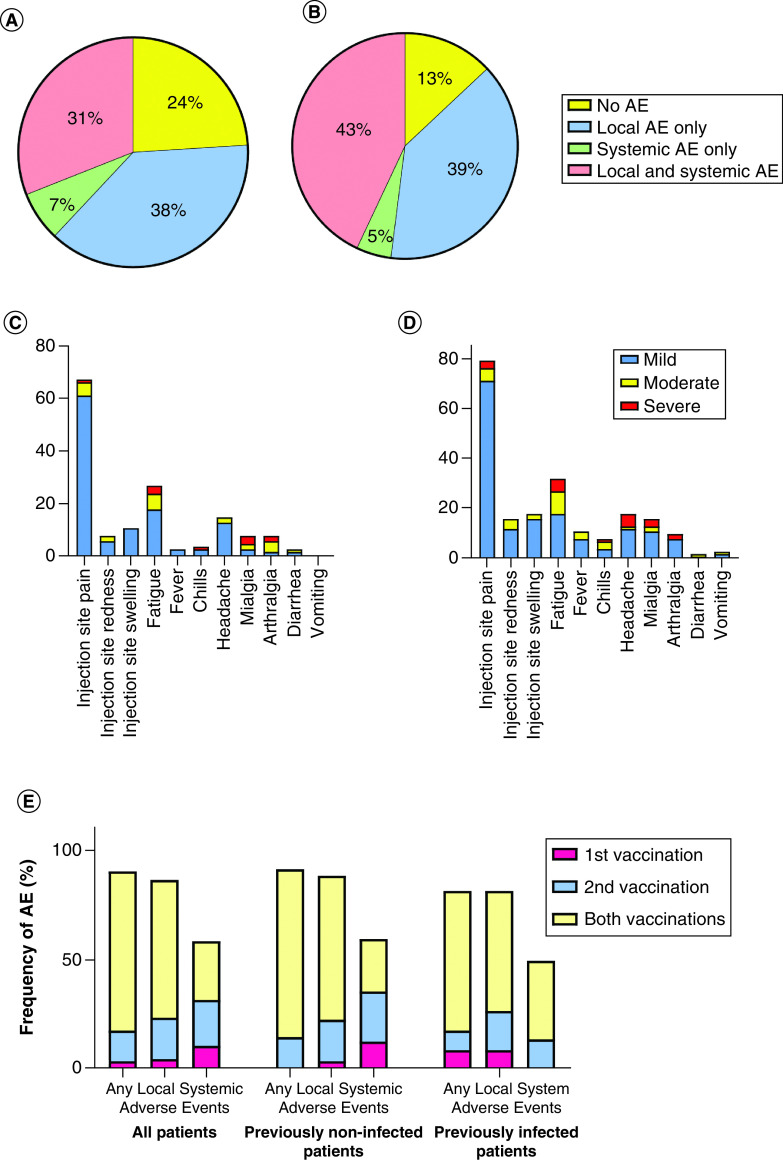
There were no new safety concerns after vaccine administration, and none of the patients had life-threatening AEs. Patients with local, systemic or both types of AE were recorded in 38, 7 and 31% after the first vaccination and 39, 5 and 43% after the second vaccination, respectively. Most of the AEs were local – pain, redness or swelling at the injection site, with 69% of patients experiencing them after the first and 82% of patients after the second vaccination. The proportion of AEs and their severity are shown in Figure 5. No major differences were noted in terms of AE frequency when comparing patients not previously infected with COVID-19 with those who were previously infected; 78% of non-previously infected patients reported AEs after the first, 91% after the second and 73% after both vaccinations. Similarly, 73% of previously infected patients reported AEs after the first, 73% after the second and 64% after both vaccinations, respectively.
Figure 5. Proportion of patients experiencing local, systemic or both types of adverse events after vaccination.
Proportion of cancer patients experiencing local, systemic or both types of AEs after the (A) first and (B) second vaccinations. Percentage of cancer patients, severity and types of AEs experienced after (C) first and (D) second vaccinations; (E) frequency of AEs reported after the first and second vaccinations and the proportion of patients reporting AEs after both vaccination doses for the entire group of cancer patients (left), those not previously infected with SARS-CoV-s (middle) and those previously infected with SARS-CoV-2 (right).
AE: Adverse event.
COVID-19 infection after vaccination
After reviewing all patient electronic health records on 15 December 2021, there were three cases of PCR-confirmed COVID-19 infection among this group of vaccinated patients. The first patient was infected 3 months after the second vaccine dose; his anti-SARS-CoV-2 S1 IgG value was at the upper limit (2020 ng/ml), but he also had disease progression during the infection and died, probably due to cancer progression and not to severe COVID-19 course. The second patient was infected just before the 6-month time point; her anti-SARS-CoV-2 S1 IgG value after 3 months was 1648 ng/ml, but she then tested negative at the time of infection and received an antibody cocktail with indevimab and casivirimab. She had a moderate disease course with cough and fatigue. The third patient was infected 6 months after the vaccination and had only a sore throat with no other symptoms; her anti-SARS-CoV-2 S1 IgG level was at the upper limit (2020 ng/ml) at the time of infection. Both patients survived the COVID-19 disease without sequelae and are now receiving anticancer treatment normally.
Discussion
This study sheds light on the immunogenicity of cancer patients on active treatment for solid malignancies and provides both immediate data about seroconversion after the complete primary course of vaccination with mRNA-based SARS-CoV-2 vaccines and also extended data 3 months after. Compared with healthy controls, cancer patients on anticancer treatment achieved adequate seroconversion rates after completing the primary course of vaccination regardless of the type of anticancer therapy received. However, anti-SARS-CoV-2 S1 IgG antibody production was built more gradually than in healthy controls, especially after the first vaccination dose, where differences were also statistically significant. Three months after vaccination, there was a decline in anti-SARS-CoV-2 S1 IgG levels of patients receiving chemotherapy and ICI, but not targeted therapy, compared with healthy controls.
The COVID-19 pandemic has had devastating consequences in the frail population of cancer patients, and thus it is vital to gather reliable data to demonstrate whether vaccination benefits this population [2–7,9]. Due to the paucity of available data, patients are often reluctant to be vaccinated because the efficacy and safety profile of vaccines in this population is unknown, leaving them at low vaccination levels – from 40 to 60% [19–22].
From reports available so far, it is evident that patients with hematological malignancies do poorly in terms of seroconversion rates compared with patients treated for solid tumors, due to both the impaired immune system caused by the disease itself and to immunosuppressive therapy [15,23–26].
Similar to our study, others also showed more gradual anti-SARS-CoV-2 S1 IgG antibody buildup after the first vaccination [15,23,27]. Also, levels of anti-SARS-CoV-2 antibodies are lower in cancer patients compared with healthy individuals irrespective of the timing of anticancer therapy [27,28]. This is further confirmed in our group of patients, where the seroconversion rate after the first dose was significantly lower than after the second (64 vs 96%; p < 0.0001). In the largest prospective trial reported so far, cancer patients performed exceptionally well in terms of seroconversion after vaccination [29]. A recent systematic review and meta-analysis provided data on promising antibody response after COVID-19 vaccination in cancer patients with seroconversion rates >90% [30].
This study indicates that patients receiving ICIs have the lowest anti-SARS-CoV-2 antibody production, especially after the first dose of the vaccine. After vaccination with two doses, antibody levels seem to be comparable regardless of the anticancer therapy received. Similarly, data of 102 patients with solid cancer receiving chemo-immunotherapy or immunotherapy plus targeted therapy and being vaccinated achieved the lowest anti-SARS-CoV-2 IgG titers [31]. These data are further supported by a group of solid cancer patients that had significantly lower antibody production after only one dose of vaccine in patients receiving ICIs [32]. Because ICIs are showing an immunomodulatory mode of action in patients infected with SARS-CoV-2, thus reducing cytotoxic effects and cytokine release, there could also be a potential effect on delaying anti-SARS-CoV-2 S1 IgG antibody production after vaccination. Accumulating evidence also suggests that SARS-CoV-2 vaccination may even improve the efficacy of ICI treatment in cancer patients and that side effects associated with vaccination may be expressed less in cancer patients treated with monotherapy with an ICI [33,34].
The present study indicates that antibody levels against SARS-CoV-2 are sufficient for most cancer patients on active anticancer treatment after vaccination with both doses; out of this cohort of 112 patients, only three were infected with COVID-19 and symptomatic after a complete vaccination course. Most of the patients were adequate responders after vaccination with both doses and were considered to have a high probability of protection against severe COVID-19 infection, having anti-SARS-CoV-2 S1 IgG Ab >880 ng/ml (44 BAU/ml). Nevertheless, there was a marked decline in anti-SARS-CoV-2 S1 IgG 3 months after vaccination with both doses, especially in the group of patients receiving chemotherapy or ICI. That further supports the idea of a third vaccination dose in this patient population, which is also safe and feasible as shown by recent publications [35].
In this study, there were no new safety concerns in terms of reactogenicity or AEs after vaccination. Other reports also show less frequent emergence of AEs after vaccination in cancer patients than in healthy subjects [10,11,15,29,36]. Significantly fewer local or systemic AEs are reported, especially after the second vaccination. It should be noted that cancer patients are already affected by their disease, symptoms and possible AEs of anticancer treatment, thus making AEs due to vaccination less easy to perceive.
Because the anti-SARS-CoV-2 S1 IgG assay has high sensitivity and specificity, it is easily reproducible, quick and inexpensive and it correlates well with immunogenicity achieved after infection, it is reasonable to expect that a high level of anti-SARS-CoV-2 S1 IgG antibodies after vaccination correlates with protection against worse disease outcome, as shown in the early phases of vaccine development [37,38]. However, antibody levels only represent humoral immunity, whereas cellular immunity may be preserved despite lower antibody levels in cancer patients compared with controls. In this study, the upper limit of antibody measurements was at 2020 ng/ml, so the differences between different groups in our cohort could be even greater than observed here. Another shortcoming might be the underrepresentation of certain groups of patients or malignancies, mirroring the frequency of certain patient groups being treated at certain centers. The strength of the present study is a centralized laboratory for analysis of antibody levels, which reduces the chances of differences in methodology and execution of the assay. In addition, the study was prospectively planned with every time point for blood sampling prearranged, thus reducing the inconsistency in study execution. Also, only patients with solid cancers were included, which further harmonizes the group under investigation and emphasize potential other variables that play a role in the antibody production.
Conclusion
In conclusion, in this study, patients with solid malignancies on active cancer treatment produced high levels of anti-SARS-CoV-2 S1 IgG antibodies after a complete primary course of vaccination. However, the dynamic of antibody production after vaccination is highly influenced by type of anticancer therapy, with patients receiving ICI having the slightest and most gradual antibody production. This supports the incentive for cancer patients to receive vaccination – not only to protect them from worse disease outcomes in case of infection but also to apply cancer therapy safely in the ongoing COVID-19 pandemic, while considering all other safety measures. Furthermore, because anti-SARS-CoV-2 S1 IgG levels start to decline after 3 months, initiatives for a third vaccination dose to provide adequate protection against severe COVID-19 disease in case of infection are warranted.
Summary points.
Vaccination against SARS-CoV-2 is crucial to protect cancer patients.
Data on efficacy and safety in this patient population is scarce.
This study reports high seroconversion in cancer patients, but only after a complete primary course of vaccination.
Lower levels of adequate antibody production were observed in patients treated with immune checkpoint inhibitors.
Three months after complete vaccination, SARS-CoV-2 S1 IgG antibodies start to decline rapidly.
Most adverse events after vaccination were mild, and no new safety concerns were raised.
Author contributions
All authors were involved in study design and execution. M Rijavec and UB Stojkovic supervised antibody detection and data collection. U Janzic, M Rijavec, J Debeljak, and UB Stojkovic were involved in data interpretation and graphical imaging processing. U Janzic and M Rijavec were the major contributors to writing the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
The authors thank all of the patients recruited to this study, the nursing staff and the study coordinators V Martinc and E Raztresen for their invaluable dedication. The authors also thank U Žibert, Ž Kogovšek, L Dejanović and I Hasanović for their excellent laboratory work.
Ethical conduct of research
All methods and procedures were carried out in accordance with Declaration of Helsinki. The study was approved by the Slovenian National Ethics Committee (no. 0120-39/2021/6). The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Footnotes
Financial & competing interests disclosure
This research was supported by the Slovenian Research Agency (grant no. P3-0360). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Worldometers.info. COVID-19 Coronavirus pandemic (2021). (Accessed 1 December 2021) www.worldometers.info/
- 2.Garassino MC, Whisenant JG, Huang LC et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 21(7), 914–922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer NM, Choueiri TK, Shah DP et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 395(10241), 1907–1918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee LYW, Cazier JB, Starkey T et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 21(10), 1309–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fillmore NR, La J, Szalat RE et al. Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study. J. Natl. Cancer Inst. 113(6), 691–698 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V, Goel S, Kabarriti R et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 10(7), 935–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui W, Yousaf N, Bhosle J et al. Real-world outcomes in thoracic cancer patients with severe acute respiratory syndrome coronavirus 2 (COVID-19): single UK institution experience. Cancer Treat. Res. Commun. 25, 100261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimgaonkar V, Aggarwal C, Berman AT et al. Impact of telemedicine adoption on accessibility and time to treatment in patients with thoracic malignancies during the COVID-19 pandemic. BMC Cancer 21(1), 1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Marcke C, Honoré N, van der Elst A et al. Safety of systemic anti-cancer treatment in oncology patients with non-severe COVID-19: a cohort study. BMC Cancer 21(1), 578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384(5), 403–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pivotal phase III trial of efficacy and safety of anti-SARS-CoV-2 mRNA vaccine.
- 11.Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383(27), 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pivotal phase III trials of efficacy and safety of anti-SARS-CoV-2 mRNA vaccine.
- 12.Voysey M, Clemens SAC, Madhi SA et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269), 99–111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadoff J, Gray G, Vandebosch A et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 384(23), 2187–2201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchette PS, Chung H, Pritchard KI et al. Influenza vaccine effectiveness among patients with cancer: a population-based study using health administrative and laboratory testing data from Ontario, Canada. J. Clin. Oncol. 37(30), 2795–2804 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Monin L, Laing AG, Muñoz-Ruiz M et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 22(6), 765–778 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garassino MC, Vyas M, de Vries EGE et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor. Educate. Ann. Oncol. 32(5), 579–581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamposioras K, Mauri D, Papadimitriou K et al. Synthesis of recommendations from 25 countries and 31 oncology societies: how to navigate through Covid-19 labyrinth. Front. Oncol. 10, 575148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhardt KA, Dewald F, Heger E et al. Evaluation of a new spike (S)-protein-based commercial immunoassay for the detection of anti-SARS-CoV-2 IgG. Microorganisms 9(4), 733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brko GM, Popovic M, Jovic M et al. COVID-19 vaccines and cancer patients: acceptance, attitudes and safety. J. BUON 26(5), 2183–2190 (2021). [PubMed] [Google Scholar]
- 20.Barriere J, Gal J, Hoch B et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann. Oncol. 32(5), 673–674 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodziak A, Sigorski D, Osmola M et al. Attitudes of patients with cancer towards vaccinations-results of online survey with special focus on the vaccination against Covid-19. Vaccines 9(5), 411 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moujaess E, Zeid NB, Samaha R et al. Perceptions of the COVID-19 vaccine among patients with cancer: a single-institution survey. Futur. Oncol. 17(31), 4071–4079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Addeo A, Shah PK, Bordry N et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 39(8), 1091–1098.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakkar A, Gonzalez-Lugo JD, Goradia N et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 39(8), 1081–1090.e2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrière J, Chamorey E, Adjtoutah Z et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 32(8), 1053–1055 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corti C, Antonarelli G, Scotté F et al. Seroconversion rate after vaccination against COVID-19 in cancer patients-a systematic review. Ann. Oncol. 33(2), 158–168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palich R, Veyri M, Marot S et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. 32(8), 1051–1053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters M, Verbruggen L, Teuwen L et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 6(5), 100274 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 22(12), 1681–1691 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A large, multicenter trial of cancer patient population highlighting efficacy and safety of SARS-CoV-2 mRNA vaccines.
- 30.Cavanna L, Citterio C, Toscani I. Covid-19 vaccines in cancer patients. Seropositivity and safety. Systematic review and meta-analysis. Vaccines 9(9), 1048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massarweh A, Eliakim-Raz N, Stemmer A et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 7(8), 1133–1140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terpos E, Zagouri F, Liontos M et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J. Hematol. Oncol. 14(1), 86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Switzer B, Haanen J, Lorigan PC et al. Clinical and immunologic implications of COVID-19 in patients with melanoma and renal cell carcinoma receiving immune checkpoint inhibitors. J. Immunother. Cancer 9(7), e002835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo B, Li J, Hou X et al. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 17(26), 3477–3484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro LC, Thakkar A, Campbell ST et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 40(1), 3–5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important trial of cancer patients receiving booster doses of anti SARS-CoV-2 vaccines and developing adequate immune response.
- 36.Naranbhai V, Pernat CA, Gavralidis A et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J. Clin. Oncol. 40(1), 12–23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A large, multicenter trial highlighting not only efficacy but also safety of anti SARS-CoV-2 vaccines in a cancer patient population.
- 37.Mulligan MJ, Lyke KE, Kitchin N et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586(7830), 589–593 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Sahin U, Muik A, Derhovanessian E et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586(7830), 594–599 (2020). [DOI] [PubMed] [Google Scholar]