Abstract
Strains of Salmonella enterica (n = 212) of different serovars and phage types were used to establish a library typing computerized system for serovar Enteritidis on the basis of PCR fingerprinting, infrequent-restriction-site PCR (IRS-PCR), or pulsed-field gel electrophoresis (PFGE). The rate of PCR fingerprinting interassay and intercenter reproducibility was low and was only increased when DNA samples were extracted at the same time and amplified with the same reaction mixtures. Reproducibility of IRS-PCR technique reached 100%, but discrimination was low (D = 0.52). The PFGE procedure showed an intercenter reproducibility value of 93.3%. The high reproducibility of PFGE combined with the previously determined high discrimination directed its use for library typing. The use of PFGE with enzymes XbaI, BlnI, and SpeI for library typing of serovar Enteritidis was assessed with GelCompar 4.0 software. Three computer libraries of PFGE DNA profiles were constructed, and their ability to recognize new DNA profiles was analyzed. The results obtained pointed out that the combination of PFGE with computerized analysis could be suitable in long-term epidemiological comparison and surveillance of Salmonella serovar Enteritidis, specially if the prevalence of genetic events that could be responsible for changes in PFGE profiles in this serovar was low.
Salmonella enterica serovar Enteritidis is widely recognized as a major cause of food-borne gastroenteritis in humans and has been isolated from cases of human disease in increasing numbers worldwide during the past 20 years (25). Animals and their products, particularly meat and eggs from chickens, are considered major sources of infections with this pathogen for humans (19, 22). Phage typing (PT) has facilitated epidemiological tracing, and it has become clear that most isolates of serovar Enteritidis belong to a limited number of PTs (35). It has been suggested by many authors that PT data reveal the clonal diffusion of a limited number of strains throughout Europe and the United States (17, 25).
During the past 15 years, an expansion of techniques suited for DNA amplification has been observed. PCR procedures have confirmed their enormous potential for the diagnosis of infectious disease agents. Besides, Williams et al. (37) and Welsh and McClelland (36) developed a general PCR typing procedure, based on the use of a short single arbitrary oligonucleotide and low annealing temperatures. These variations in conventional PCR procedure allowed for the generation of genotypic markers, and validation at taxonomic and epidemiological levels has been achieved for a number of microorganisms (31). We have demonstrated (13) the usefulness of combining PCR fingerprinting with PT in the epidemiological characterization of Salmonella serovar Enteritidis.
Infrequent-restriction-site PCR (IRS-PCR) is a recently developed typing method, based on the selective amplification of DNA fragments generated by the double restriction of DNA. The method is carried out in four steps: DNA digestion with two restriction enzymes, DNA ligation of oligonucleotide adapters to the cohesive ends of the restricted fragments, selective amplification by PCR, and separation of PCR-generated fragments by gel electrophoresis. The method has been shown to be easy to perform and to have good reproducibility (15, 24). Results of the application of this new technology, in relation with the epidemiological typing of Salmonella serovar Enteritidis, have not been reported in the literature yet.
Pulsed-field gel electrophoresis (PFGE) is an established method for the analysis of large fragments generated by restriction endonuclease digestion of genomic DNA (14) and is currently considered to be one of the most reliable typing procedures (16). The PFGE method has been shown to be highly effective for epidemiological studies of some serovars of S. enterica (2, 3, 17, 20, 21). The discrete number of well-resolved band fragments allows a visual comparison of the restriction profiles. Furthermore, the adequate typeability and discriminatory power of this method have led to the conclusion that PFGE is, at present, one of the most valuable epidemiological tools available for the molecular analysis of this important pathogen (10, 11, 23). However, to further evaluate the potential of this molecular typing system, it must be demonstrated to be sufficiently reproducible or amenable to standardization for use in definitive library typing (33).
For the establishment of an international database of Salmonella DNA profiles, it is necessary to build up large databases containing fragment patterns from a wide variety of strains, to which unknown strains can be compared. Computerized gel analysis allows the comparison of band profiles presented in different gels and the construction of databases that could be useful in the development of library typing systems of microorganisms (28). Band profiles are compared and clustered in dendrograms on the basis of calculated similarity coefficients. The objective of this study was to explore the possibilities of establishing such a library typing system for Salmonella serovar Enteritidis on the basis of PCR fingerprinting, IRS-PCR, or PFGE. The study was designed to determine the intralaboratory or intercenter reproducibility of the methods in three different laboratories, comparing the DNA patterns obtained with an established protocol. Computerized gel analysis was used for the construction of DNA profile databases, for assessment of the similarities between band profiles, and for the detection and identification of new strains through their DNA profiles.
MATERIALS AND METHODS
Bacteria.
Strains of S. enterica (n = 212) were divided into five bacterial collections and were used for several purposes (Table 1). The isolates were identified by conventional biochemical methods and serotyped with respect to cell wall (O) and flagellar (H) antigens. The isolates belonging to serovar Enteritidis were phage typed by standard methods and assigned to phage types according to the scheme of Ward et al. (35).
TABLE 1.
Characteristics of the Salmonella strain collections used in this study
| Collection | No. of strains | Geographic origin | Description of strain collection | Serovars of Salmonella | Purpose of the study | Application centers | Source or reference |
|---|---|---|---|---|---|---|---|
| 1 | 51 | Spain | Strains from outbreaks and epidemiologically unrelated strains; Salmonella ATCC 49214 | Enteritidis | Test the PCR fingerprinting reproducibility | A | 13 |
| B | |||||||
| 2 | 9 | Spain | Epidemiologically unrelated strains | Enteritidis, Typhimurium, Dublin, Arizonae, Litchfield, Miami, Virchow, Abony, serogroup 11 | Optimization of IRS-PCR | B | This study |
| 3 | 36 | Denmark, England, Spain | Epidemiologically unrelated strains | Enteritidis | Determination of the typability, reproducibility, and discriminatory power of IRS-PCR | B | This study |
| 4 | 15 | Denmark, England, Spain | Epidemiologically unrelated strains | Enteritidis | Intercenter reproducibility of PFGE | B | This study |
| C | |||||||
| 5 | 101 | Denmark, England, Spain | Epidemiologically unrelated strains | Enteritidis | Establish a library typing system | B | 11 |
PCR fingerprinting.
Fifty isolates of Salmonella serovar Enteritidis, isolated from humans, foods, and environmental sources at the Public Health Laboratory, Bilbao, Spain, were included in the study of reproducibility of PCR fingerprinting (Table 1, collection 1). Twenty-seven of the isolates were derived from eight outbreaks of human salmonellosis in the Basque Country, Spain, in the period between 1983 and 1994. The rest of the isolates were epidemiologically unrelated strains recovered from food and water reservoirs, rivers, and beaches in the same region. The type strain of Salmonella serovar Enteritidis ATCC 49214 from the American Type Culture Collection was also included. The strains were analyzed separately in two laboratories: Laboratory of Microbiology, Public Health Laboratory, Bilbao, Spain (center A), and Department of Immunology, Microbiology, and Parasitology, Basque Country University, Vitoria-Gasteiz, Spain (center B). The isolates were grown overnight at 37°C in tryptone soy agar (BBL, Cockeysville, Md.). A small loopful of bacteria was used for the DNA extractions. After being heated at 100°C for 10 min and centrifuged in a Micro Centaur centrifuge (MSE, Sanyo, Leicester, United Kingdom) (13,000 rpm, 10 min), the DNA concentration of the supernatant was measured by spectrophotometry at an absorbance of 260 nm. A stock solution of 50 ng of DNA per μl was kept at −20°C. The following three individual primers were used in this study (Table 2): ERIC2, M13, and OPS-19 (synthetized by PE Applied Biosystems, Madrid, Spain). The amplification temperatures and the controls used were as described previously by López-Molina et al. (13). In order to optimize the PCR procedures, variable amounts per reaction of the following reagents were evaluated: primer (10 to 90 pmol), MgCl2 (0 to 4 mM), and DNA template (100 to 325 ng). Finally, PCR was carried out in a 50-μl volume with 10 mM Tris HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 200 μM each deoxynucleoside triphosphate (PE Applied Biosystems), 1 U of Ampli-Taq polymerase (PE Applied Biosystems), 250 ng of template DNA, and primer (50 pmol for ERIC2, 40 pmol for M13, and 20 pmol for OPS-19). Two brands of thermocyclers were used in this study. A 9600 thermocycler (PE Applied Biosystems) was located in center A and an Autocycler 32 thermocycler (Linus, Mortlake, Australia) was used in center B. After amplification, DNA was subjected to horizontal electrophoresis on 2% agarose (Bio-Rad Laboratories, Richmond, Calif.) in Tris-borate-EDTA (TBE) buffer for 1 h and 30 min at 150 V. pGEM (Promega, Barcelona, Spain) was used as a molecular weight standard and was allocated along the gels several times in order to normalize the gels in the computerized analysis.
TABLE 2.
Oligonucleotides used in this study
| Designation | Sequence | Method |
|---|---|---|
| Primer ERIC-2 | 5′-AAGTAAGTGACTGGGGTGAGCG-3′ | PCR fingerprinting |
| Primer M13 | 5′-GTAAAACGACGGCCAGT-3′ | PCR fingerprinting |
| Primer OPS-19 | 5′-GAGTCAGCAG-3′ | PCR fingerprinting |
| Adapter AX | ||
| AX1 | PO4 5′-CTAGTACTGGCAGACTCT-3′ | IRS-PCR |
| AX2 | 5′-GCCAGTA-3′ | IRS-PCR |
| Adapter AH | ||
| AH1 | 5′-AGAACTGACCTCGACTCGCACG-3′ | IRS-PCR |
| AH2 | 5′-TGCGAGT-3′ | IRS-PCR |
| Adapter AT | ||
| AT1 | 5′-CCTGATGAGTCCTGAC-3′ | IRS-PCR |
| AT2 | 5′-CGGTCAG-3′ | IRS-PCR |
| Primer PX | 5′-AGAGTCTGCCAGTACTAGA-3′ | IRS-PCR |
| Primer AH1 | 5′-AGAACTGACCTCGACTCGCACG-3′ | IRS-PCR |
| Primer AT1 | 5′-CCTGATGAGTCCTGAC-3′ | IRS-PCR |
IRS-PCR.
A collection of nine strains belonging to different Salmonella serovars recovered from human and environmental sources at the Public Health Laboratory, Bilbao, Spain, was used to optimize the IRS-PCR method (Table 1, collection 2). A bacterial collection of 36 strains of Salmonella serovar Enteritidis was used to study the typability, the discriminatory power, and the reproducibility of the IRS-PCR method (Table 1, collection 3). The strains were recovered from unrelated animal, human, or food sources and identified as described above. Ten strains were isolated in Denmark between 1985 and 1997 and were provided by the Danish Veterinary Laboratory (Copenhagen, Denmark) (center C). S. Chappell, from the Central Veterinary Laboratory, Surrey, United Kingdom, kindly provided 13 strains isolated in 1996 and 1997. The remaining 13 strains were isolated between 1984 and 1992 at the Public Health Laboratory, Bilbao, Spain. The IRS-PCR study was performed at center B.
Two DNA extraction procedures were used and compared. The first procedure was as described above in the PCR fingerprinting method. A second procedure was as described previously by Garaizar et al. (5). The DNA concentration was estimated as described in “PCR fingerprinting,” and stock solutions of 0.4 μg of DNA per μl were kept at −20°C. The adapters and primers used in IRS-PCR are shown in Table 2, and the IRS-PCR method was performed according to the protocol previously described by Mazurek et al. (15). Two combinations of restriction enzymes (Boehringer-Mannheim, Barcelona, Spain) were evaluated: XbaI-HhaI and XbaI-TaqI. DNA restriction was carried out with 1 μg of bacterial DNA, 10 U of XbaI, and 10 U of HhaI (or the same amount of TaqI) and was performed for 1 h at 37°C. The following reagents were added to the reaction mixture: 2 U of T4 DNA ligase (Bioline, London, United Kingdom), 1 mM ATP (Bioline), 20 pmol of adapter AX (AX1-AX2), and 20 pmol of adapter AH (AH1-AH2) (or the same amount of adaptor AT [AT1-AT2] when the XbaI-TaqI combination was used). All the oligonucleotide adapters and primers were synthesized by PE Applied Biosystems. The DNA ligation was carried out at 16°C for 1 h. In order to inactivate the T4 ligase, the samples were heated at 65°C for 20 min. A second round of DNA restriction was carried out with 5 U of each restriction enzyme for 15 min at 37°C. The PCR was carried out in a final volume of 50 μl containing the following reagents: 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 1 μM primer PX (Table 2), 1 μM primer AH1 (or 1 μM primer AT1 when the XbaI-TaqI combination was used), 1 U of Ampli-Taq polymerase, and 1 μl of the restricted ligated DNA. An Autocycler 32 thermocycler (Linus) was used with the following amplification temperatures: 1 cycle at 94°C for 6 min and 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min. The amplified DNA fragments were electrophoresed in 8% polyacrylamide gel in TBE buffer at a constant 150 V for 45 min. pGEM was used as the molecular weight standard.
PFGE.
The bacterial collection used to study the intercenter reproducibility of PFGE was composed of 15 unrelated Salmonella serovar Enteritidis strains recovered from human, food, and animal sources, isolated in Denmark, England, and Spain between 1984 and 1997 (Table 1, collection 4). The set of strains was analyzed separately in two laboratories, center B and center C. In both centers, DNA preparations and fragmentation with restriction endonucleases XbaI, BlnI, and SpeI were performed according to the protocol previously described by Olsen et al. (17). Each laboratory prepared the required solutions and used different batches of commercial restriction endonucleases (Boehringer-Mannheim and Amersham Life Science, Ltd., Buckinghamshire, England, respectively). Samples were subjected to PFGE on 1.2% agarose (wt/vol) in 0.5× TBE buffer. The running conditions for different enzymes were as described by Laconcha et al. (11). The electrophoresis apparatus used was a CHEF-DR III (Bio-Rad) in center C and a CHEF-DR II in center B. Lambda DNA (Bio-Rad and Sigma, St. Louis, Mo.) served as a molecular size standard.
Computerized gel analysis.
After electrophoresis, the gels with the DNA were stained with ethidium bromide and photographed with Polaroid film. Photographs of PFGE fingerprints from the different gels were interpreted visually according to published guidelines (27, 29). A capital letter was used to define a distinct type, and subtype profiles were indicated by a numerical suffix. The index of discrimination was calculated according to Simpson's index (D) of diversity as described by Hunter and Gaston (9). For computer analysis, images were scanned, saved in a TIFF format as described by Garaizar et al. (6), and sent intercenter by file transfer protocol, and then analyzed by GelCompar version 4.0 software (Applied Maths, Kortrijk, Belgium). After conversion and normalization of gels, the degrees of similarity of DNA profiles were determined by the Dice coefficient or the maximum-correlation coefficient, and dendrograms were generated by the unweighted pair group method using arithmetic averages. Three computerized DNA profile libraries were generated with data obtained from 101 strains of Salmonella serovar Enteritidis (Table 1, collection 5), previously analyzed by PFGE with the restriction enzymes XbaI, BlnI, and SpeI (11). The identity of the 15 duplicated strains used previously in the reproducibility studies of the PFGE method (Table 1, collection 4) were matched against the constructed computerized libraries to test their ability to recognize new strains.
RESULTS
PCR fingerprinting.
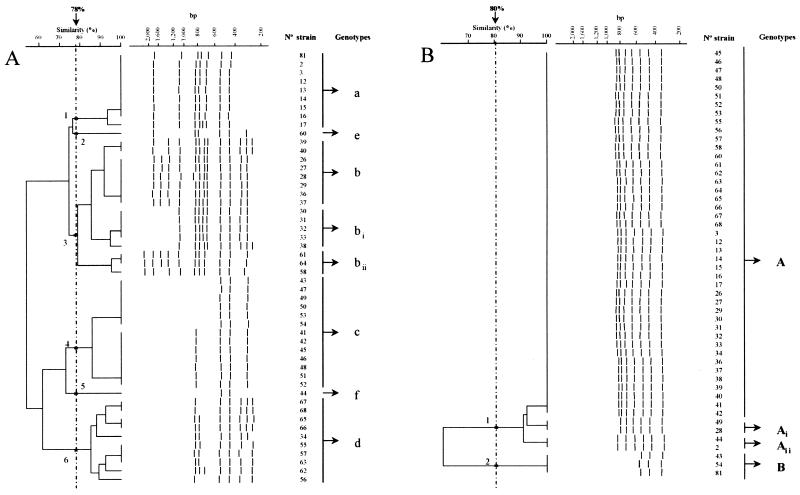
In order to determine the intra- and interassay reproducibility of PCR fingerprinting in the same laboratory, groups of duplicate strains were analyzed. For the intraassay study, DNA extractions and PCR procedures of the duplicates were done at the same time, whereas the amplicons were placed in different agarose gels. Different profiles were not obtained, although differences in band intensity were sometimes observed. In the interassay analysis of reproducibility, duplicates were cultivated and their DNA was extracted and amplified at different times during the study. In this case, some of the duplicates showed differences in band profiles, mainly associated with faint bands with various grades of intensity. Consequently, interassay reproducibility values were low, ranging from 44 to 73%. To assess the intercenter reproducibility of PCR fingerprinting, strain collection 1 (Table 1) was duplicated and separately processed and analyzed in two laboratories (centers A and B). The reagents and the preparation of the samples were identical in both centers and carried out by the same person. In center A, amplification of the DNA was carried out in a single batch with the PE Applied Biosystems 9600 thermocycler. In center B, amplification of the DNA from the isolates was carried out in four batches, due to the impossibility of running all the samples together in a single experiment with the Linus Autocycler 32 thermocycler, and with replicative purposes to assess interassay reproducibility. In center A, the results of the PCR fingerprints with the three-primer set showed that the most of the strains clustered in a single cluster in the dendrograms. In center B, the banding profiles of the strains extracted and amplified in each batch clustered together. When the results obtained in both centers were compared, a large number of discrepancies between duplicate strains were observed, in number, intensity, and position of the bands as is shown with the primer M13 in Fig. 1.
FIG. 1.
Similarity dendrograms of band patterns of Salmonella serovar Enteritidis strains produced by PCR fingerprinting and M13 primer. (A) Amplification of DNA was performed with a Linus Autocycler 32 thermocycler in several batches. (B) Amplification of DNA was performed with a PE Applied Biosystems 9600 thermocycler in one batch.
IRS-PCR.
The procedure of DNA extraction described by Garaizar et al. (5) was chosen for the IRS-PCR method, because of the poorly defined DNA bands obtained with the boiling procedure used in PCR fingerprinting (data not shown). Optimization of the ligation step was carried out by testing variable amounts of DNA (range, 0.4 to 1 μg) against variable amounts of adapters (range, 15 to 30 pmol). The PCR variables were used while testing variable amounts of restricted ligated DNA (range, 0.5 to 1 μl), MgCl2 (range, 1 to 4 mM), and primers (range, 35 to 75 pmol). In the ranges studied, no significant differences in band profiles were obtained, except with MgCl2, where low concentrations inhibited amplification. The optimal conditions are described in Materials and Methods. Reproducibility of IRS-PCR was assessed in duplicate strains in which their DNA were extracted and amplified at different times. Identical band profiles were obtained, in terms of number, intensity, and position, and therefore the reproducibility values reached 100%.
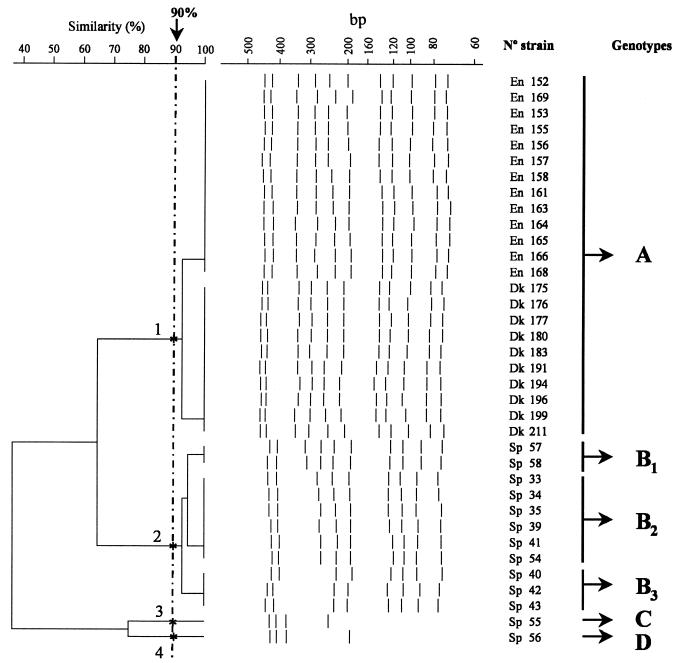
Nine strains belonging to different serovars of Salmonella (Table 1 collection 2) were analyzed by IRS-PCR with a combination of the restriction enzymes XbaI-HhaI, and XbaI-TaqI. Amplification of fragments was carried out in duplicate, and identical results were obtained. With the enzyme combination XbaI-HhaI, the number of bands obtained ranged from 7 to 16 (Fig. 2), and with the combination XbaI-TaqI, this number ranged from 3 to 8 (data not shown). Typability and discrimination values between serovars of Salmonella reached 100%. When 36 strains of Salmonella serovar Enteritidis (Table 1, collection 3) were processed by IRS-PCR, amplification of the DNA from the isolates was carried out in three batches with the Linus Autocycler 32 thermocycler. The number of bands obtained with the enzyme combination XbaI-HhaI was 4 to 11 and divided the collection into four clusters as is shown in Fig. 3, obtaining an index of discrimination of 0.52. Interestingly, strains from Denmark and England were grouped together in the dendrogram at 90% similarity in cluster A, but Spanish strains were grouped in clusters B, C, and D. This increase in discrimination in the group of the Spanish strains was not corroborated by their PFGE profile data. A subgroup of nine strains (three for each country) were further processed with selective primers PX-A, PX-C, PX-G, and PX-T. These primers had an extension of a nucleotide at the 3′ end (A, C, G, and T respectively) of primer X (Table 2). With selective primers, a lower number of bands was obtained, and discrimination between the selected strains was not increased (data not shown).
FIG. 2.
IRS-PCR of different serovars of Salmonella obtained with the enzyme combination XbaI-HhaI. (A) Lane M, molecular mass marker (pGEM). Lane 1, serovar Arizonae; lane 2, serovar Litchfield; lane 3, serovar Virchow; lane 4, serovar Miami; lane 5, Salmonella serogroup 11; lane 6, serovar Abony; lane 7, serovar Virchow; lane 8, serovar Dublin; lane 9, serovar Enteritidis; lane 10, serovar Typhimurium. Numbers on the left indicate the size of the marker in base pairs. (B) Similarity dendrogram of the IRS-PCR patterns.
FIG. 3.
Similarity dendrogram of the IRS-PCR patterns of 36 epidemiologically unrelated Salmonella serovar Enteritidis strains obtained with enzyme combination XbaI-HhaI. En, England; Dk, Denmark; Sp, Spain.
PFGE.
The reproducibility of the method was tested by independently examining the same set of strains (Table 1, collection 4) in centers B and C (Fig. 4). Table 3 shows the visual and automatic computerized analyses of PFGE profiles generated by both centers. When visual analysis was performed, a very high agreement in intensity, number, and position of bands in most of the strains analyzed was observed, reaching 93.33% of reproducibility value. In only one strain, strain 15, a disagreement occurred after digestion with XbaI and BlnI enzymes. In each case, an extra macrorestriction band of 40 kb was found when the experiments were carried out in center B. Therefore, two different subtype profiles were assigned to this strain, depending on the place where the analysis was made (with the XbaI enzyme A10 or A12 and BlnI enzyme A10 or A12 in center B or C, respectively). In contrast, only profile A1 was found with SpeI enzyme in both laboratories for this strain. Computerized analysis of these profiles confirmed the high reproducibility of the PFGE method.
FIG. 4.
Agarose gels of the PFGE profiles of Salmonella serovar Enteritidis obtained in the intercenter reproducibility study of PFGE. Fifteen duplicated strains were processed in two laboratories. Pictures on the left were obtained in center C (Denmark) and those on the right in center B (Spain). (A) DNA digested with restriction enzyme XbaI. (B) DNA digested with restriction enzyme SpeI. (C) DNA digested with restriction enzyme BlnI. Lanes M, lambda ladder used as a molecular marker. Numbers on the right indicate the size of the marker in kilobases.
TABLE 3.
Reproducibility of PFGE method in the analysis of 15 epidemiologically unrelated Salmonella serovar Enteritidis strains in two laboratories; correlation between visual and computerized analysis
| Strain no. | Countrya; year; origin | PFGE profile analysis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual
|
Computerized
|
||||||||||
| PT | XbaI | BlnI | SpeI | XbaIb | Similarity (%) | BlnIb | Similarity (%) | SpeIc | Similarity (%) | ||
| 1 | DK; 1997; hatchery | 4 | A1 | A8 | A9 | A1 | 100 | A1, A8 | 100 | A1 | 93.3 |
| 2 | DK; 1995; avian | 8 | A10 | A10 | A1 | A10 | 100 | A10 | 100 | A1 | 93.2 |
| 3 | EN; 1996; avian | 6 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 94.2 |
| 4 | SP; 1995; egg | 1 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 93.1 |
| 5 | SP; 1996; human | 6 | A1 | A1 | A3 | A1 | 100 | A1 | 100 | A3 | 90.6 |
| 6 | DK; 1988; avian | 32 | A13 | B | A1 | A13 | 100 | B | 100 | A1 | 91.2 |
| 7 | DK; 1997; human | 1 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 92.4 |
| 8 | EN; 1996; cattle | 8 | A10 | A10 | A1 | A10 | 100 | A10 | 100 | A1 | 94.4 |
| 9 | EN; 1996; avian | 1 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 92.3 |
| 10 | SP; 1996; avian | 4 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 95.0 |
| 11 | DK; 1987; human | 2 | A10 | A10 | A1 | A10 | 100 | A10 | 100 | A1 | 92.5 |
| 12 | DK; 1996; human | 6 | A1 | A1 | A2 | A1 | 100 | A1 | 100 | A2 | 91.2 |
| 13 | DK; 1984; human | 14b | D | G | E | A12 | 92.9 | F | 75 | A1 | 51.9 |
| 14 | EN; 1996; avian | 4 | A1 | A1 | A1 | A1 | 100 | A1 | 100 | A1 | 95.2 |
| 15 | SP; 1996; human | 8 | A12, A10 | A12, A10 | A1 | A12, A10 | 100, 100 | A12, A10 | 100 | A1 | 89.3 |
DK, Denmark; EN, England; SP, Spain.
The comparison with the Xba and Bln libraries was made with the Dice coefficient.
The comparison with the Spe library was made with the maximum-correlation coefficient.
Library database systems.
On basis of the high intercenter reproducibility values of PFGE reported here and the high discrimination values reported previously, the PFGE method was chosen to establish a library computerized system for Salmonella serovar Enteritidis. For this purpose, bacterial collection 5 (Table 1) was used. The collection was composed of 101 strains belonging to the most prevalent phage types, isolated from human, food, and animal sources, and were previously analyzed by PFGE (11). Three computerized libraries of PFGE DNA profiles were constructed from the data obtained with restriction enzymes XbaI, BlnI, and SpeI. After library construction, the databases' abilities to recognize and analyze new profiles were assessed when the PFGE results obtained with bacterial collection 4 (Table 1) were analyzed. The comparisons between profiles assigned automatically by the computer and visually are shown in Table 3. When comparison with the Xba library was carried out, full agreement existed between visual and computer comparison for most of the strains, and only two strains showed different data. Strain 15 was automatically assigned to profile A12 as well as to profile A10 (100% of similarity), independently of the laboratory where the analysis was made. The profile of strain 13 was recognized as A12 with a similarity of 92.9% by computer, whereas visual comparison assigned the strain to profile D. Also, the results of the profile recognition study with the Bln library showed concordant results for most strains. In this case, strain 1 was recognized as A1 as well as A8 (100% of similarity) and strain no. 15 was assigned to profile A10 as well as to A12 (100% of similarity) by automatic comparison, as the computer was unable to distinguish between the profiles. When results obtained by the SpeI enzyme were compared with to the database in the Spe library, more difficulties were found, and the index of analysis had to be modified from the Dice coefficient to the maximum-correlation coefficient in order to get a better concordance between visual and automatic comparisons. The low levels of similarity obtained in strain 13 revealed that these profiles were not previously recorded at the three libraries.
DISCUSSION
For the establishment of an international database of Salmonella DNA profiles, it is necessary to build up large databases containing patterns from a wide variety of strains, to which unknown strains could be compared. Computerized gel analysis allows the comparison of band profiles allocated in different gels and the construction of databases that could be useful in developing a library typing systems of microorganisms. These techniques could allow communication between centers and rapid detection of the spread of pathogens and emerging infections. The objective of this study was to explore the possibilities of establishing such a library typing system on the basis of several molecular epidemiological techniques that allow strain characterization of Salmonella serovar Enteritidis. As a result of the application of such molecular techniques, a not-obvious relation between the source of the isolates (i.e., food, human, or environmental) with their genotypes was found.
PCR fingerprinting method.
In a previous study (13), we showed the usefulness of PCR fingerprinting procedures in the epidemiological typing of Salmonella serovar Enteritidis. The primary objective of the present study was to explore the possibilities of establishing a library typing system for serovar Enteritidis on the basis of PCR fingerprinting, assessing the intraassay, interassay, and interlaboratory reproducibility of the method. When PCR fingerprinting was performed with the Linus Autocycler 32 thermocycler, the isolates were processed in different assays or batches and were electrophoresed in several agarose gels. Clustering of band profiles was clearly associated with the different assays. This association was not detected when all the strains were processed in one assay using the PE Applied Biosystems 9600 thermocycler. In these interassay comparisons, important differences in intensity and band number were observed. Following the strict criteria of Ellsworth et al. (4), distinguishing between specific, reproducible, and constant bands and nonreproducible bands, the reproducibility of our method was 44%. Analyzing the profiles more permissively and not taking into account the faint bands of some gels, reproducibility increased to 73%. Nevertheless, when samples from which DNA was extracted at the same time and amplified using the same reaction mixture were compared at the intraassay studies, a reproducibility of 100% was obtained.
There are some studies designed to assess intercenter comparison of PCR fingerprinting results. Penner et al. (18), analyzing the results obtained in several laboratories, concluded that the main differences in banding profiles were due to the use of different thermocyclers. van Belkum et al. (32), analyzing 60 strains of Staphylococcus aureus in seven laboratories with the same protocol, DNA templates, and primers, obtained discordant results as well. Grundmann et al. (8), analyzing PCR fingerprinting of Acinetobacter isolates in seven laboratories, obtained better reproducibility using commercially available “PCR pellets” and standardized primers. In our study, the instability of band profiles that we observed with different thermocyclers could be due to differences in the transition between melting and annealing temperatures. As reported by Schweder et al. (26), these differences could influence the sizes and amounts of the fragments obtained. Nevertheless, in our study other factors such as minor differences in procedures or PCR mixtures could not be discarded as causes of variability between band profiles. Our data have shown that PCR fingerprinting could be useful as an epidemiological marker when strictly defined conditions are maintained, isolates are processed in a single batch with identical reagents, and interassay or intercenter comparisons are not usually required. With this observation, we could conclude that with PCR fingerprinting no library-definitive typing system is feasible at present.
IRS-PCR method.
A new typing method, such as IRS-PCR, has to be evaluated prior to being considered valuable in epidemiological typing. We have optimized the procedure for Salmonella serovar Enteritidis, and the results are encouraging. Assay reproducibility values were high, but the discrimination of the method could not be considered elevated (D = 0.52), even taking into consideration the high degree of clonality revealed by most of the epidemiological typing methods of this serovar. In our search of the literature on IRS-PCR, few studies have shown data from this method. Mazurek et al. (15), described the method and used the combination of XbaI-HhaI restriction enzymes to characterize epidemiologically related and unrelated clinical isolates of Mycobacterium avium and Mycobacterium intracellulare, using PX-G selective primer to obtain unique genotypes for the nonrelated strains. Riffard et al. (24) obtained high discrimination results (D = 0.996) with IRS-PCR, comparable to that obtained with PFGE in an epidemiological study of Legionella pneumophila. The amplified fragment length polymorphism (AFLP) procedure described by Vos et al. (34) has several similar steps in common with IRS-PCR, but the adapters used for IRS-PCR are different. In AFLP analysis, the adapters are composed of oligonucleotides of 18 to 22 bases, while in IRS-PCR, both adapters are composed of two chains, one 18 to 22 bases long and the other 7 bases long. This characteristic of adapters in IRS-PCR simplifies the method, reducing the number of bands and making the analysis simpler. Aarts et al. (1) obtained good discrimination with AFLP when analyzing 62 serovars of Salmonella, although the discrimination values decreased when analyzing strains of serovar Enteritidis. Our data demonstrated that IRS-PCR is a rapid, simple, and reproducible method for discriminating between Salmonella serovars, but discriminating between strains of serovar Enteritidis with these enzymes yielded relatively low values. The use of new combinations of restriction enzymes to increase the strain discrimination and the performance of interlaboratory multicenter studies should address their possible use in definitive library typing in the near future.
PFGE.
The reproducibility of the PFGE method when analyzing strains of serovar Enteritidis was high (93.33%). Although only some of the reagent batches, such as agarose and enzymes, and different equipment were used, nearly the same results were generated in this interlaboratory study. The only exception was one strain that showed different profiles after cleavage with XbaI or BlnI. In both cases, an extra band of approximately 40 kb was detected when the analysis was carried out in center C. This observed difference may be due to the carriage of a plasmid, although in previous studies, plasmid bands did not account for the restriction fragment length polymorphism detected in PFGE experiments (12, 30). In order to corroborate the presence or absence of plasmids in that strain, PFGE was performed without restriction enzymes, and only the band of chromosomal DNA was observed (data not shown). Therefore, the observed variation in PFGE profiles could reflect a chromosomal rearrangement or a point mutation of the chromosomal DNA during in vitro growth. We are considering the possibility of cloning and sequencing such DNA fragments in order to determine the molecular basis of that genotypic divergence. Careful surveillance of changes in PFGE profiles would help in the determination of the prevalence of such genetic events, responsible for this biological variability of genotypes.
Similarity between DNA profiles in the bacterial duplicates was quantified through the use of GelCompar software. Our study highlighted the importance of adequate normalization and selection of the parameters used for analysis and matching in computerized gel analysis. This observation correlates well with previous studies (7). The software was efficient at recognizing and grouping strains with closely similar genotypic fingerprints, and results were similar to those expected from visual inspection of the gels.
Library typing.
Once the interlaboratory study showed that the PFGE method was reproducible, our aim was to assess the possibility of constructing computerized libraries of DNA restriction enzyme profiles and perform identity searches of new DNA profiles in these databases. Three libraries were constructed with data obtained from a previous analysis of 101 Salmonella serovar Enteritidis strains isolated in three European countries (11). The PFGE profiles of the 15 strains used for the reproducibility study were compared and matched with these created libraries. The libraries consisted of defined units which each represented a homogeneous electrophoretic type, containing one or more representative profiles. For the creation of such libraries, it is important to emphasize that only gels with identical molecular weight standards and similar image resolutions could be used. The possibility of adding a new unit to the library when a new DNA profile appeared was open. When the PFGE profiles of these new strains of Salmonella serovar Enteritidis were compared to the libraries, the program showed the best matching units with the corresponding correlation in decreasing order. The program was efficient at recognizing and comparing unknown patterns with Xba and Bln libraries with the same mathematical coefficient but in the case of the Spe library, different calculation methods had to be used to get better results.
The program examined in this study, GelCompar, proved to be a useful tool for indicating the similarity between strains and was able to establish computerized libraries for epidemiological comparison. As the PFGE method has been shown to be reproducible, discriminative, and capable of feasible intercenter standardization, we recommended the use of PFGE for epidemiological comparisons, such as constructing a typing library, in combination with software that allows construction of computerized databases of DNA profiles. The determination of the prevalence of the genetic events that could be responsible for changes in PFGE profiles in this serovar could help address the question of the potential long-term use of this system.
ACKNOWLEDGMENTS
This work was supported by Research Project grant 093.123-EA 125/96 from the Basque Country University and Education, University, and Investigation Department grant PI-1998-52 from the Basque Government, Spain.
REFERENCES
- 1.Aarts H J M, van Lith L A J T, Keijer J. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. doi: 10.1046/j.1472-765x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Baggesen D L, Wegener H C, Madsen M. Correlation of conversion of Salmonella enterica serovar Enteritidis phage type 1, 4, or 6 to phage type 7 with loss of lipopolysaccharide. J Clin Microbiol. 1997;35:330–333. doi: 10.1128/jcm.35.1.330-333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggesen D L, Sandvang D, Aarestrup F M. Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J Clin Microbiol. 2000;38:1581–1586. doi: 10.1128/jcm.38.4.1581-1586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellsworth D L, Rittenhouse K D, Honeycutt R L. Artifactual variation in randomly amplified polymorphic DNA banding patterns. BioTechniques. 1993;14:214–217. [PubMed] [Google Scholar]
- 5.Garaizar J, Kaufmann M E, Pitt T L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991;29:1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garaizar J, Latorre M, López-Molina N, Laconcha I, Alberdi L, Rementeria A, Audicana A, Uliarte R, Cisterna R. Computerized restriction endonuclease analysis compared with O-serotype and phage type in the epidemiological fingerprinting of Pseudomonas aeruginosa strains. Clin Microbiol Infect. 1997;3:222–228. doi: 10.1111/j.1469-0691.1997.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Gerner-Smidt P, Graves L M, Hunter S, Swaminathan B. Computerized analysis of restriction fragment length polymorphism patterns: comparative evaluation of two commercial software packages. J Clin Microbiol. 1998;36:1318–1323. doi: 10.1128/jcm.36.5.1318-1323.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundmann H J, Towner K J, Dijkshoorn L, Gerner-Smidt P, Maher M, Seifert H, Vaneechoutte M. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J Clin Microbiol. 1997;35:3071–3077. doi: 10.1128/jcm.35.12.3071-3077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter P R, Gaston M A. Numerical index of discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laconcha I, López-Molina N, Rementeria A, Audicana A, Perales I, Garaizar J. Phage typing combined with pulsed-field gel electrophoresis and random amplified polymorphic DNA increases discrimination in the epidemiological analysis of Salmonella enterica strains. Int J Food Microbiol. 1998;40:27–34. doi: 10.1016/s0168-1605(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 11.Laconcha I, Baggesen D L, Rementeria A, Garaizar J. Genotypic characterization by PFGE of Salmonella enterica serotype Enteritidis phage type 1, 4, 6, and 8 isolated from animal and human sources in three European countries. Vet Microbiol. 2000;75:155–165. doi: 10.1016/s0378-1135(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 12.Liebisch B, Schwarz S. Molecular typing of Salmonella enterica subsp. enterica serovar Enteritidis isolates. J Med Microbiol. 1996;44:52–59. doi: 10.1099/00222615-44-1-52. [DOI] [PubMed] [Google Scholar]
- 13.López-Molina N, Laconcha I, Rementeria A, Audicana A, Perales I, Garaizar J. Typing of Salmonella enteritidis of different phage types of PCR fingerprinting. J Appl Microbiol. 1998;84:877–882. doi: 10.1046/j.1365-2672.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 14.Maslow J N, Mulligan M E, Arbeit R D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993;17:153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek G H, Reddy V, Marston B J, Haas W H, Crawford J T. DNA fingerprinting by infrequent-restriction-site amplification. J Clin Microbiol. 1996;34:2386–2390. doi: 10.1128/jcm.34.10.2386-2390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase T, Okitsu T, Suzuki R, Morozumi H, Matsushima A, Nakamura A, Yamai S. Evaluation of DNA fingerprinting by PFGE as an epidemiologic tool for Salmonella infections. Microbiol Immunol. 1995;39:673–676. doi: 10.1111/j.1348-0421.1995.tb03255.x. [DOI] [PubMed] [Google Scholar]
- 17.Olsen J E, Skov M N, Threlfall E J, Brown D J. Clonal lines of Salmonella enterica serotype Enteritidis documented by IS200-, ribo-, pulsed-field gel electrophoresis and RFLP typing. J Med Microbiol. 1994;40:15–22. doi: 10.1099/00222615-40-1-15. [DOI] [PubMed] [Google Scholar]
- 18.Penner G A, Bush A, Wise R, Kim W, Domier L, Kasha K, Laroche A, Scoles G, Molnar S J, Fedak G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 1993;2:341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- 19.Perales I, Audicana A. Salmonella enteritidis and eggs. Lancet. 1988;ii:1133. doi: 10.1016/s0140-6736(88)90542-9. [DOI] [PubMed] [Google Scholar]
- 20.Powell N G, Threlfall E J, Chart H, Rowe B. Subdivision of Salmonella enteritidis PT 4 by pulsed-field gel electrophoresis: potential for epidemiological surveillance. FEMS Microbiol Lett. 1994;119:193–198. doi: 10.1111/j.1574-6968.1994.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 21.Punia P, Hampton M D, Ridley A M, Ward L R, Rowe B, Threlfall E J. Pulsed-field electrophoretic fingerprinting of Salmonella indiana and its epidemiological applicability. J Appl Microbiol. 1998;84:103–107. doi: 10.1046/j.1365-2672.1997.00325.x. [DOI] [PubMed] [Google Scholar]
- 22.Rampling A. Salmonella enteritidis five years on. Lancet. 1993;342:317–318. doi: 10.1016/0140-6736(93)91466-y. [DOI] [PubMed] [Google Scholar]
- 23.Ridley A M, Threlfall E J, Rowe B. Genotypic characterization of Salmonella enteritidis phage types by plasmid analysis, ribotyping, and pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:2314–2321. doi: 10.1128/jcm.36.8.2314-2321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riffard S, Lo Presti F, Vandenesch F, Forey F, Reyrolle M, Etienne J. Comparative analysis of infrequent-restriction-site PCR and pulsed-field gel electrophoresis for epidemiological typing of Legionella pneumophila serogroup 1 strains. J Clin Microbiol. 1998;36:161–167. doi: 10.1128/jcm.36.1.161-167.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue D C, Tauxe R V, Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990;105:21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweder M E, Shatters R G, Jr, West S H, Smith R L. Effect of transition interval between melting and annealing temperatures on RAPD analyses. BioTechniques. 1995;19:38–42. [PubMed] [Google Scholar]
- 27.Struelens M J, Bauernfeind A, van Belkum A, Blanc D, Cookson B D, Dijkshoorn L, El Sohl N, Etienne J, Garaizar J, Gerner-Smidt P, Legakis N, de Lencastre H, Nicolas M H, Pitt T L, Römling U, Rosdahl V, Witte W ESGEM. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 28.Struelens M J, De Gheldre Y, Deplano A. Comparative and library epidemiological typing systems: outbreak investigations versus surveillance systems. Infect Control Hosp Epidemiol. 1998;19:565–569. doi: 10.1086/647874. [DOI] [PubMed] [Google Scholar]
- 29.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terajima J, Nakamura A, Watanabe H. Epidemiological analysis of Salmonella enterica Enteritidis isolates in Japan by phage-typing and pulsed-field gel elctrophoresis. Epidemiol Infect. 1998;120:223–229. doi: 10.1017/s0950268898008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Belkum A. DNA fingerprinting of medically important microorganisms by use of PCR. Clin Microbiol Rev. 1994;7:174–184. doi: 10.1128/cmr.7.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Belkum A, van Leeuwen W, Kaufmann M E, Cookson B, Forey F, Etienne J, Goering R, Tenover F, Steward C, O'Brien F, Grubb W, Tassios P, Legakis N, Morvan A, El Solh N, de Ryck R, Struelens M, Salmenlinna S, Vuopio-Varkila J, Kooistra M, Talens A, Witte W, Verbrugh H. Assessment of resolution and intercenter reproducibility of results of genotyping Staphylococcus aureus by pulsed-field gel electrophoresis of SmaI macrorestriction fragments: a multicenter study. J Clin Microbiol. 1998;36:1653–1659. doi: 10.1128/jcm.36.6.1653-1659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward L R, de Sa J D H, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]