Abstract
Central nervous system (CNS) injury, induced by ischemic/hemorrhagic or traumatic damage, is one of the most common causes of death and long-term disability worldwide. Reactive oxygen and nitrogen species (RONS) resulting in oxidative/nitrosative stress play a critical role in the pathological cascade of molecular events after CNS injury. Therefore, by targeting RONS, antioxidant therapies have been intensively explored in previous studies. However, traditional antioxidants have achieved limited success thus far, and the development of new antioxidants to achieve highly effective RONS modulation in CNS injury still remains a great challenge. With the rapid development of nanotechnology, novel nanomaterials provided promising opportunities to address this challenge. Within these, nanoceria has gained much attention due to its regenerative and excellent RONS elimination capability. To promote its practical application, it is important to know what has been done and what has yet to be done. This review aims to present the opportunities and challenges of nanoceria in treating CNS injury. The physicochemical properties of nanoceria and its interaction with RONS are described. The applications of nanoceria for stroke and neurotrauma treatment are summarized. The possible directions for future application of nanoceria in CNS injury treatment are proposed.
Keywords: nanoceria, reactive oxygen species, central nervous system injury, stroke, neurotrauma
Graphical Abstract
Introduction
As a well-known catalytic metal oxide, ceria has been widely and successfully utilized in industrial catalysis, especially for the fabrication of three-way catalytic converters for vehicle exhaust gas control [1–3]. Cerium has a specific [Xe]4f15d16s2 electron configuration, where the energy of the inner 4f level is nearly equal to that of the 6s level, allowing electrons to occupy these valence subshells variably. Due to this property, cerium ions can easily and reversibly switch between valence states of Ce3+ and Ce4+. Therefore, ceria is a crystal mixture of CeO2 and Ce2O3, and could partially exchange between CeO2 and Ce2O3 with structural integrity. During the Ce4+/Ce3+ transition, the electrons from oxygen ions localize in the 4f subshell of Ce3+, accompanied by the formation of oxygen vacancies (Fig. 1a) [4, 5], rendering ceria an excellent oxygen storage capacity [6]. Moreover, when ceria particle size decreases to nanoscale, the oxygen vacancy level and Ce3+/Ce4+ ratio get a sharp increase in the crystal structure. Correspondingly, the catalytic activities of nanoceria were significantly enhanced [7, 8]. Up to now, the engineered nanoceria can be prepared by diverse synthetic processes, such as precipitation [9, 10], hydrothermal [11], microemulsion [12], green synthesis [13], sonochemical and microwave-assisted [14] methods. Accordingly, nanoceria with different sizes, shapes and catalytic activities have been produced. More comprehensive reviews on the synthesis and properties of nanoceria were published [15]. Inspired by their intriguing physicochemical properties and catalytic performances, many studies have been conducted to explore the biological effects of nanoceria. Notably, nanoceria exhibits multiple enzyme-mimetic activities (Fig. 1b), including superoxide dismutase (SOD) [16], catalase [17], peroxidase [18], oxidase [19], DNase I [20] and photolyase [21] like activities. Benefited from these activities, nanoceria has shown great potential for biomedical applications in neurology [22–24], oncology [25–27], nephrology [28–30], hepatology [31–33], cardiology [34], ophthalmology [35–37], odontology [38–40], orthopedics [41–43] and others.
Figure 1.
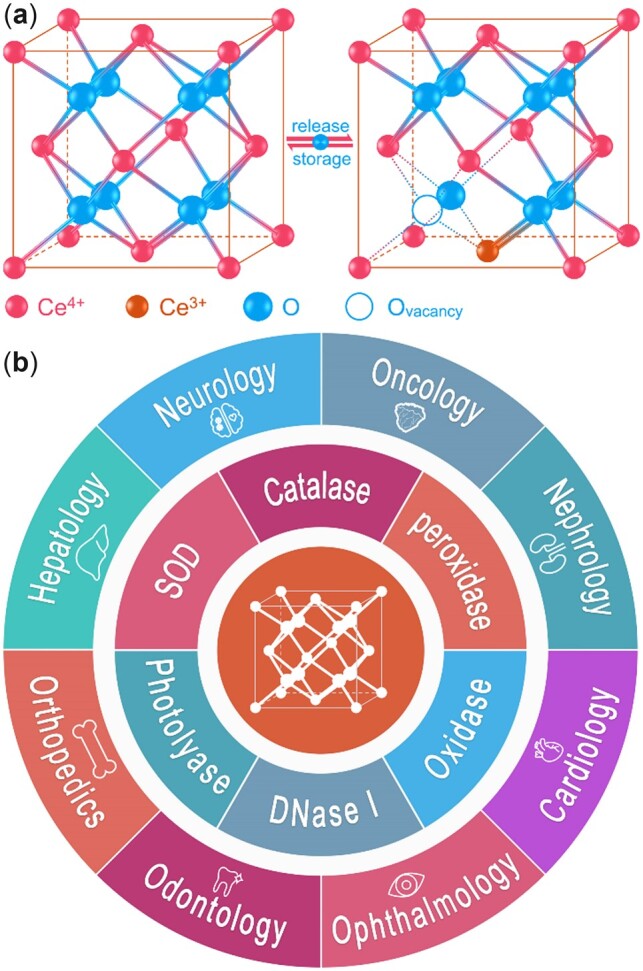
(a) Nanoceria owns a fluorite structure and can change between two valence states, accompanied by the formation of the oxygen vacancies. (b) Enzyme mimetic activities and biomedical applications of nanoceria.
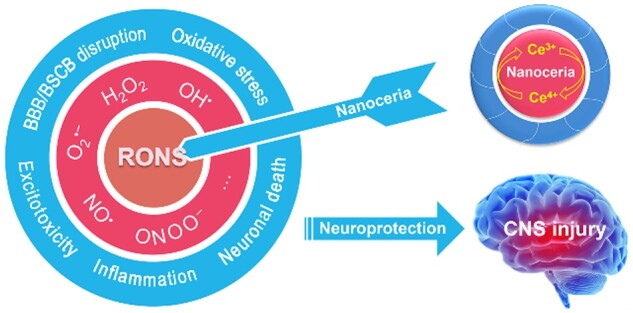
In the field of neurological diseases, central nervous system (CNS) injury (including stroke and neurotrauma) is associated with high morbidity and mortality worldwide [44–46]. Reactive oxygen and nitrogen species (RONS) play an important role in the pathological cascade of molecular events after CNS damage [47]. Because the human brain is the most oxygen supply-dependent organ [48, 49], consuming nearly 20% of the oxygen provided by the vasculature while accounting for only ∼2% of the body’s weight [50], the oxygen consumption disorder may significantly increase the RONS level in the CNS. Therefore, treatment with antioxidants can be a promising strategy against oxidative/nitrosative stress induced by RONS after CNS injury [51–53]. However, current antioxidants (e.g. vitamins, lipoic acids, polyphenols and carotenoids) utilized in RONS-related disease treatment have obtained limited therapeutic effects due to the innate drawback that these organic drugs can participate in only one redox cycle after which they inactivate, i.e. a single dose is quickly depleted in vivo and repetitive doses are still inadequate to control oxidative/nitrosative stress in many cases. Thus, more effective strategies in modulating the balance of RONS need to be developed. In the last few decades, nanotechnology has opened a new chapter in regenerative medicine and a variety of nanosized biomaterials with RONS regulating ability are springing up [54, 55]. Of these, some nanozymes possessing antioxidant activity including platinum [56], manganese [57], fullerene [58], melanin [59] and ceria [23] have shown neuroprotective effects in CNS disorders. Featured with many superiorities such as low cost, low toxicity and simple synthetic procedures, nanoceria is the most extensively explored RONS modulating nanozyme for CNS injury treatment. In addition, it is one of the first nanoparticles utilized as therapeutic agent [60, 61] and even the first material tested as an antioxidant in the space [62]. In this context, nanoceria is selected here as a representative antioxidant nanoparticle. With regenerative ability, nanoceria has an obvious advantage over the other antioxidants and may be a novel option against oxidative/nitrosative stress in the future. Hereinafter, this review discusses the RONS modulating ability of nanoceria, presents up-to-date advances in stroke and neurotrauma treatment and provides some future directions to bridge the gap between experiments and the clinic.
Nanoceria as a RONS modulator
RONS are implicated in many physiological and pathologic processes of aging and disease. They are widely formed particularly through the electron transport chains in mitochondria, including free radicals with unpaired electrons (e.g. superoxide anion (), hydroxyl radical (OH•)) and some biologically important non-radicals (e.g. hydrogen peroxide (H2O2), peroxynitrite (ONOO–)) [63]. These species are essential for regulating internal homeostasis in living systems. However, excessive RONS production can induce oxidative/nitrosative stress, leading to important biomolecules (DNA, protein, lipids) damage and cell death, which is always involved in the early onset of disease [64].
The RONS neutralizing capacities of nanoceria have been discovered for only a dozen years. At the beginning of this century, Rzigalinski et al. [61, 65] serendipitously observed that nanoceria could prolong the lifespan of neuronal cells during their pioneering works, where nanoceria might function as a nanozyme or antioxidant modulating RONS. Since then, the enzyme-like activities and biomedical applications of nanoceria have gained extensive interest in the scientific literature. is the precursor of most RONS and can be scavenged by SOD, then the SOD mimetic activity of nanoceria has been confirmed. The reaction mechanism follows by Equations (1) and (2) [16]. Further, there was a close correlation between Ce3+/Ce4+ ratio and SOD mimetic efficiency, while nanoceria with higher Ce3+/Ce4+ ratio was more effective than lower ratio ones for clearance [16, 66].
| (1) |
| (2) |
During the dismutation process of , H2O2 is generated and can be degraded by catalase to water and oxygen in aerobic organisms. Soon after the discovery of SOD mimetic activity, nanoceria has also found catalase mimetic activity [17], under which the mechanism follows by Equations (3) and (4) [67]. It is worth noting that the decomposition of H2O2 accelerated by nanoceria is also correlated with the Ce3+/Ce4+ ratio but contrary to the SOD mimetic activity. For example, nanoceria with a lower Ce3+/Ce4+ ratio (7%) was more effective in H2O2 decomposition than those with a higher Ce3+/Ce4+ ratio (28%) [17]. Nevertheless, in a study comparing the catalytic activity of three different nanostructures (nanorods, nanocubes and nanooctahedra, with Ce3+ concentrations of 18.3%, 17.6% and 15.5%, respectively), the decomposition of H2O2 was positively correlated to the Ce3+/Ce4+ ratio of nanoceria [68]. These results indicate that in addition to Ce3+ concentration, the catalase mimetic activity of nanoceria may be influenced by other factors, e.g. shape and size.
| (3) |
| (4) |
Because and H2O2 are two dominant RONS implicated in disease processes, SOD and catalase are indispensable in the construction of the first line of defense against oxidative stress [69]. In vivo, the combination of NO• and leads to the formation of ONOO–, a highly reactive molecule that can induce enzyme inactivation, DNA damage and lipid peroxidation [70]. Hence, the interaction between ONOO– and nanoceria has been tested. As a result, nanoceria significantly promoted the decay of this destructive molecule, while the decay rate seemed irrelevant to the Ce3+/Ce4+ ratio according to the experimental data [71]. In addition, it was reported that nanoceria could scavenge NO• radicals directly or indirectly, which might contribute to less production of ONOO– [72].
Hydroxyl radical (OH•), one of the strongest RONS that must be mentioned, is generated primarily by the Fenton reaction in vivo. Nanoceria acting as OH• scavenger has been demonstrated by establishing a photometric system in vitro. The OH• clearance activity was size-dependent and closely correlated with Ce3+ ions at the surface of the nanoparticles, while the smaller nanoceria (size, 5–10 nm; Ce3+/Ce4+ ratio, 30%) captured more OH• than the larger (size, 15–20 nm; Ce3+/Ce4+ ratio, 20%) [73]. It was shown as well that nanoceria could inactivate stable nitroxyl radical, and the inactivation rate increased with the particle size decreasing [74].
Generally, nanoceria can modulate multiple RONS types via an autocatalytic mechanism of reversible Ce4+/Ce3+ transition (Fig. 2). Thus, a single dose of nanoceria can maintain long-lasting antioxidant effect in biological systems. For example, in an in vitro ischemia model of hippocampal injury, 5.8 µM nanoceria achieved the same neuroprotective effects as 10 mM (nearly 1700-fold concentration of nanoceria) n-acetylcysteine, a benchmarked antioxidant commonly used to evaluate the activity of other antioxidants [75, 76]. Notably, the mechanism of RONS clearance is different between nanoceria and traditional organic antioxidants, since the former directly acts as an oxygen receptor while the latter is actually a hydrogen donor. In addition, the organic antioxidants scavenge RONS by single electron exchange with these species and in turn transform themselves into radicals acting as ‘prooxidants’. Therefore, nanoceria is a more efficient antioxidant for oxidative stress.
Figure 2.
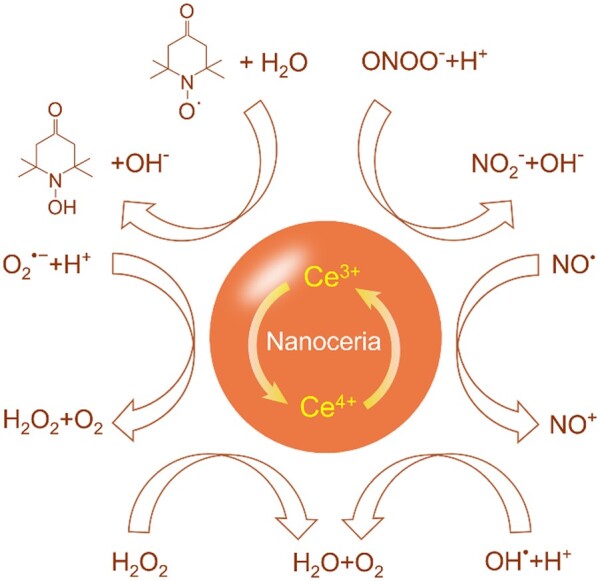
The main types of RONS can be modulated by nanoceria via an autocatalytic mechanism of reversible Ce4+/Ce3+ transition.
Nanoceria in CNS injury treatment: targeting RONS
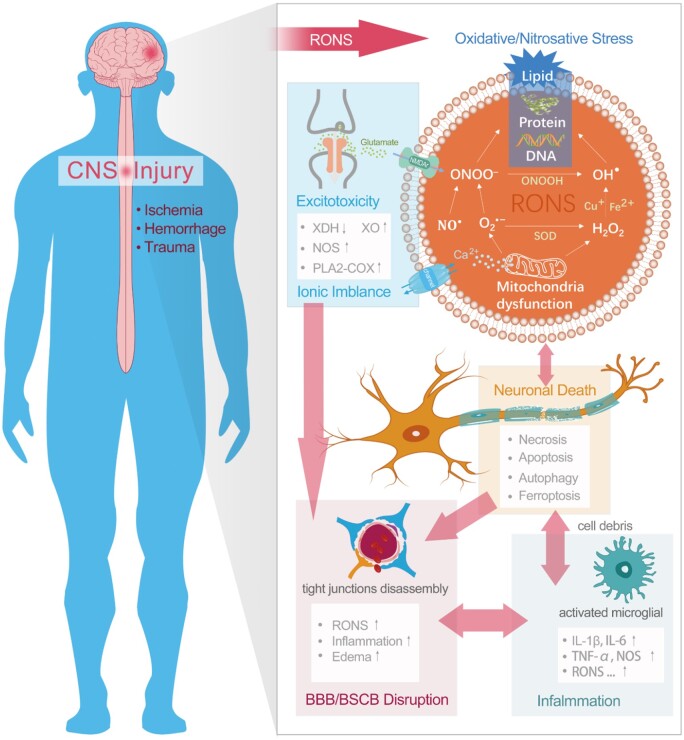
The most challenging and leading types of CNS injury are stroke and neurotrauma. Stroke, a brain attack, is induced by either blockage (ischemic stroke) or leakage (hemorrhagic stroke) of blood vessels carrying oxygen and nutrients to the brain [77]. Traumatic injury to the brain or spinal cord shares many similar pathological features with stroke, including ischemia, hemorrhage, blood–brain barrier (BBB) or blood–spinal cord barrier (BSCB) disruption, neuronal death and inflammation. When stroke or neurotrauma occurs, part of the CNS may suffer from ischemia and hypoxia, followed by mitochondrial dysfunction and oxidative/nitrosative stress, leading to further neurological disorders. Additionally, during the injury progression, many other pathways (e.g. excitotoxicity, BBB/BSCB disruption, inflammation, neuronal death) are involved, and extensive crosstalk among these pernicious pathways dramatically increases as shown in Fig. 3 [47, 77]. In this crosstalk, excessive RONS play a pivotal and important role in triggering cell death (e.g. necrosis [78, 79], apoptosis [80], autophagy [81, 82], ferroptosis [83]) and delayed neurological deficits. With excellent RONS modulating ability, nanoceria has been utilized in various CNS-related diseases, ranging from Alzheimer’s disease [24, 84–87], Parkinson’s disease [22, 88], multiple sclerosis [89, 90], amyotrophic lateral sclerosis [76], and have we focused on stroke and neurotrauma [23, 91–105].
Figure 3.
The major pathways involved in CNS injury: oxidative/nitrosative stress, excitotoxicity, ionic imbalance, inflammation, BBB/BSCB disruption and neuronal death. There is extensive crosstalk among these pathways, in which RONS play a critical role. XDH, xanthine dehydrogenase; XO, xanthine oxidase; NOS, nitric oxide synthase; PLA2, phospholipase A2; COX, cyclooxygenase; NMDAr, N-methyl-d-aspartic acid receptor; IL-1β, interleukin-1β; IL-6, interleukin-6; TNF-α, tumor necrosis factor α.
Ischemic stroke
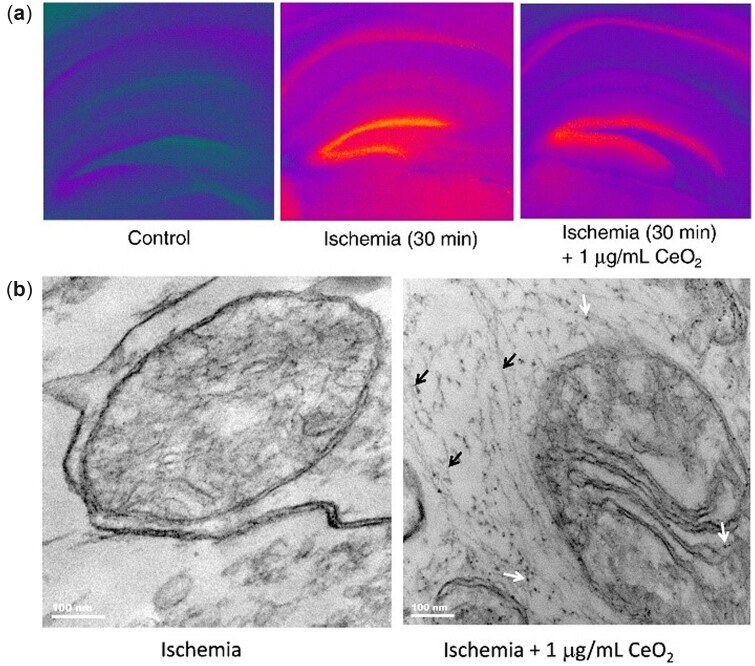
Ischemic stroke is the most common subtype of stroke, accounting for more than 80% of all strokes [106, 107]. In a mouse hippocampal brain slice model of ischemia, many nanoparticles localized to the mitochondria, in which the mitochondrial cristae were highly organized after treatment with commercially available nanoceria (Fig. 4). Besides, a significant reduction (50%) in ischemic cell death was observed with a modest reduction (15%) in and NO•. Interestingly, 3-nitrotyrosine induced by ONOO– displayed a remarkable reduction (70%), which was far greater than that of and NO•. Considering that both and NO• are precursors of ONOO–, their reductions may contribute to a significant decrease in ONOO–. These results imply that direct or indirect clearance of ONOO– is an important mechanism underlying the neuroprotective effects of nanoceria after ischemia. Moreover, it is worth mentioning that the optimal dose of nanoceria in the study is 1 µg/ml, while a higher concentration (2 µg/ml) leads to agglomeration in artificial cerebrospinal fluid (CSF), which should be considered in vivo [75]. The agglomeration is a common phenomenon for nanomaterials under physiological conditions, especially in the bloodstream. When the particle size is smaller, the surface-to-volume ratio is larger, and nanoparticles are more likely to agglomerate and adsorb plasma proteins. Then, rapid clearance by macrophages can happen before nanoceria is transported to target cells [108]. In the case of stroke, small particles (<5 nm) are more favorable to cross the BBB into the brain [109, 110]. This dilemma is a major barrier to the design of novel nanomedicine in brain disease treatment. One possible approach for nanoceria overcoming this problem is surface modification to decrease agglomeration and protein adsorption, and simultaneously increase the circulation time in the blood [111]. Recently, ∼2.5-nm nanoceria modified with different ratios of citrate acid (CA) and ethylenediamine tetraacetic acid (EDTA) was applied in the brain slice model. As a result, the antioxidant activity of nanoceria was influenced by the surface coatings and nanoparticles with a 1:1 ratio of CA/EDTA exerted a stronger neuroprotective performance. After being injected into a rat model of ischemia–reperfusion 72 h in advance, the CA/EDTA-coated nanoceria achieved a 52% reduction of superoxide accumulation in the hippocampus [94].
Figure 4.
Neuroprotective effects of nanoceria in ischemia treatment. (a) The cell death of ischemic brain slice was significantly decreased by nanoceria. (b) T EM (transmission electron microscopy) micrographs of hippocampal brain slices 24 h post-ischemia. Nanoceria were located in the mitochondria and associated with neurofilaments. Adapted with permission from Ref. [75], Elsevier 2011.
The first case of nanoceria protecting against stroke in vivo has been reported by Kim et al. In this work, uniform 3-nm nanoceria with phospholipid-PEG modification was well dispersed in both phosphate-buffered saline (PBS) and plasma, exhibiting dose-dependent SOD/catalase mimetic activities. After the ischemia–reperfusion rat received a 0.5 mg/kg intravenous injection of nanoceria, the brain infarct area was considerably reduced by 50%. Additionally, the phospholipid-PEG-capped nanoparticles accumulated in the ischemic hemisphere were far more abundant than those in the contralateral hemisphere, suggesting BBB disruption in the damaged area which promoted the entrance of nanoceria into the brain [23]. The higher accumulation of nanoceria in the brain is at least partly due to PEGylation and small particle size, which ameliorate its dispersion and prolong the circulation time in the vasculature. As PEG possesses uncharged hydrophilic residues and high surface mobility, the biocompatibility of PEGylated nanoceria in living systems is significantly improved [112] while the radical scavenging ability is little reduced compared to bare nanoceria [113]. Although BBB disruption after stroke facilitates nanoparticle permeation into the damaged area of the brain, the passage rate is still very low compared to the total injection dosage. Consequently, the therapeutic effect is quite limited, and a huge portion of nanoparticles may be transported to other organs, possibly inducing side effects.
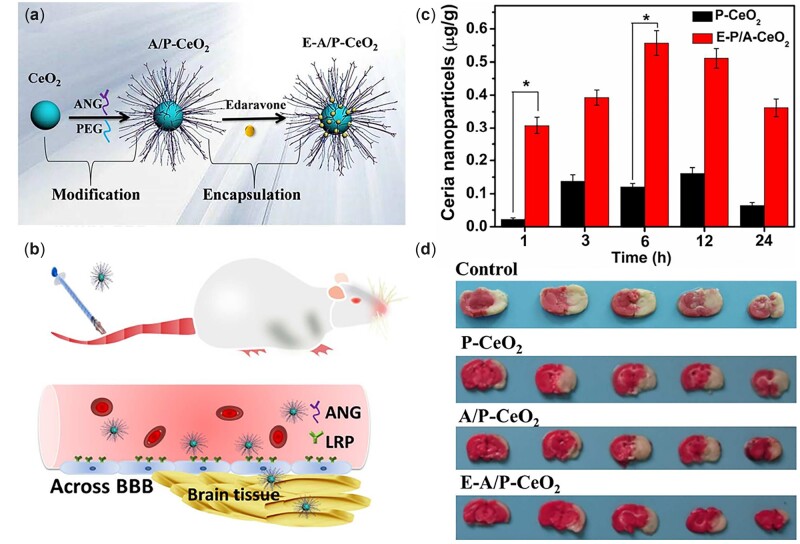
More importantly, the breakdown of the BBB may allow hazardous molecules and compounds to enter the brain as well, resulting in additional BBB damage and neurological dysfunction. Concerning this issue, functionalized core-shell nanoceria coated with angiopep-2/PEG and loaded with edaravone have achieved simultaneous intracerebral uptake and BBB protection in stroke treatment (Fig. 5). There are three key takeaway points in the nanocomposite system: (i) PEGylation improves biocompatibility, (ii) angiopep-2 targets the BBB and (iii) nanoceria and edaravone scavenge RONS [91]. Angiopep-2 is a specific ligand that can be recognized by low-density lipoprotein receptor-related protein (LRP) overexpressed on cells at the BBB, which facilitates the passage of nanoceria into brain tissue via a transcytosis process [114]. PEGylated nanoceria with angiopep-2 modification obtained much higher accumulation in the brain tissue of healthy rats after injection indicating that angiopep-2 played a critical role in BBB targeting and crossing. Moreover, when loaded with edaravone (a clinical drug applied in stroke treatment), angiopep-2/PEGylated nanoceria exhibited the most protective effects on BBB integrity. The synergetic clearance of RONS by nanoceria and edaravone could alleviate cell death and disassembly of the tight junctions [91].
Figure 5.
Schematic illustration of (a) the synthetic procedure of E-a/P-CeO2 and (b) the ANG-targeting to the overexpressed LRP on BCECs, (c) which facilitated the nanoparticles to penetrate BBB into brain tissue and thus (d) reduced the infarct volume of the ischemic brain most effectively. Adapted with permission from Ref. [91], © American Chemical Society 2018.
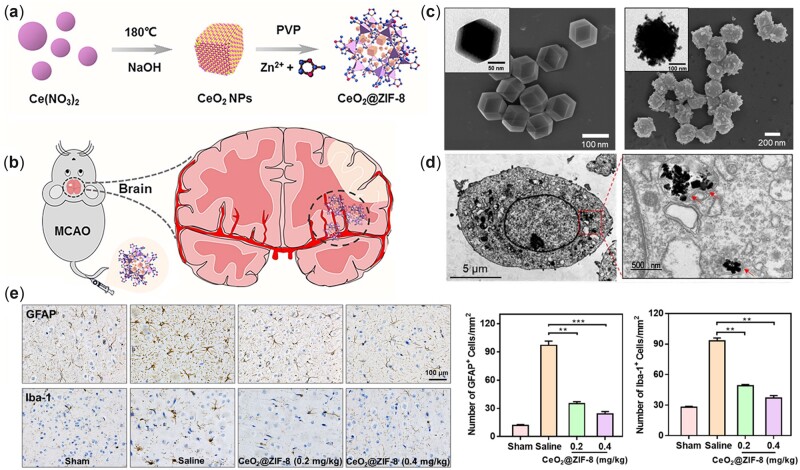
Notably, ischemic stroke can promote BBB leakage and active angiogenesis of microvessels in the penumbra [115], during which vascular endothelial growth factor (VEGF) is known to play an important role [116]. Interestingly, nanoceria with high surface area and Ce3+/Ce4+ ratio led to robust angiogenesis by modulating oxygen in the intracellular environment [117]. In a study by our group, it was observed that nanoceria enhanced vascularization by triggering high expression of the angiogenic factor VEGF [41], which could shed light on the leakage and angiogenesis of the BBB. In other experiments, nanoceria showed benefits in alleviating BBB disruption after ischemia [92, 93]. By targeting integrin αvβ3 (a cell-surface receptor protein selectively upregulated after ischemia), custom synthesized biotinylated-LXW7-nanoceria could effectively arrive at the ischemic penumbra region and prevent brain damage [92]. LXW7, incorporated into this nanocomposite system, not only facilitates nanoparticles targeting damaged areas but also inhibits integrin αvβ3, preserving the BBB in the early stage [118]. In addition to the BBB, oxidative/nitrosative stress during stroke can damage endothelial cells, inducing vascular lesions and additional cerebral hemorrhage. Nanoceria coated with phosphonic acid PEG copolymers exhibited protective effects by reducing glutamate-induced production of RONS in cerebral endothelial cells [119]. More recently, following an in situ synthetic method, nanoceria was capped with ZIF-8, a porous metal-organic framework comprised imidazolate linkers and zinc ions (Fig. 6). Through a lysosome-mediated endocytic pathway, CeO2@ZIF-8 could be delivered into cells, after which the outer ZIF-8 shell decomposed and the inner core of CeO2 was released in the acidic lysosomal environment. The CeO2@ZIF-8 nanocomposite gained enhanced BBB penetration and RONS clearance, which promoted the stroke treatment compared to the free CeO2 group [95]. In addition, CeO2@ZIF-8 also decreased the activation of microglia and astrocytes after ischemia (Fig. 6e) suggesting its involvement in the immune response, which was consistent with other reports [99, 120–122]. Although CeO2@ZIF-8 was relatively large (240 nm) to some extent, it penetrated the BBB successfully due to the ZIF-8 encapsulation. In another study, 8-nm nanoceria was loaded onto poly-(lactide-co-glycolide)-PEG copolymer matrix to form nanocomposites. These composites resulted in an effective decrease in the infarct volume and edema level of the ischemic brain [93].
Figure 6.
(a) Schematic illustration of CeO2@ZIF-8 synthesis and (b) its application in ischemic stroke treatment. (c) TEM and SEM (scanning electron microscope) images of ZIF-8 (left) and CeO2@ZIF-8 (right) nanocomposites. (d) TEM image of CeO2@ZIF-8 internalized in PC12 cells. The CeO2 nanopolyhedra was found in the lysosome but the outer ZIF-8 framework mostly degraded in the acidic lysosomal environment. © Treatment with CeO2@ZIF-8 significantly down-regulated the expression of GFAP (glial fibrillary acidic protein; a marker of astrocytes) and iba-1 (ionized calcium-binding adaptor molecule-1; a marker of microglia) in ischemic brain sections. Adapted with permission from Ref. [95], CC by-NC 4.0, © The Author(s) 2020.
Hemorrhagic stroke
Hemorrhagic stroke accounts for ∼20% of all strokes, including two nontraumatic subtypes, intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) [106]. In the early stage of hemorrhage, brain damage develops with the formation of hematoma and edema, followed by increased intracranial pressure and inflammatory response, inducing secondary brain injury and further neurological deficits [77, 123, 124]. To date, effective treatment that significantly improves the poor outcomes has yet to be discovered. As a regenerative RONS modulator, nanoceria has achieved considerable effects in animal models of hemorrhagic stroke [97–100].
ICH is characterized by intraparenchymal bleeding followed by the development of hematoma, leading to initial mass effect and subsequent inflammation [125]. However, therapies focused on hematoma resolution have achieved little progress, and surgical removal is difficult due to its special location. Perihematomal inflammation sparking secondary brain injury is a potential target for therapeutic intervention [126]. Nanoceria exhibits anti-inflammatory activity in vitro [120], as well as in vivo ICH models [97, 98]. When PEGylated biocompatible nanoceria was intravenously administered to ICH rats, high accumulation in the hemorrhagic hemisphere was observed, indicating the nanoparticles crossed the damaged BBB. The perihematomal edema caused by ICH was significantly reduced by 68% after treatment with nanoceria, while the hematoma volume was scarcely influenced. In addition, treatment with nanoceria effectively decreased the RONS level and suppressed the recruitment of CD68-positive microglia/macrophages, thus alleviating the inflammation response [97].
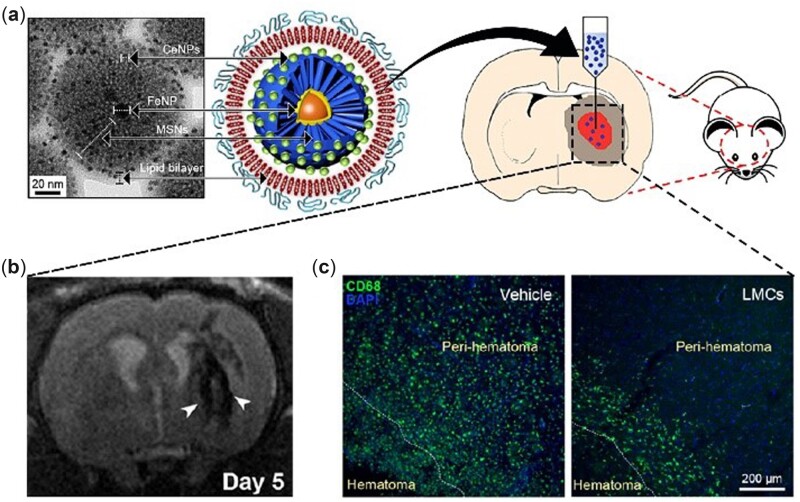
After ICH, resident microglia are activated, and a large number of macrophages migrate to the hemorrhagic area due to chemotaxis, followed by phagocytosis of cellular debris and secretion of proinflammatory cytokines. Moreover, with the activation of the nuclear factor-kappa B signaling pathway, inducible nitric oxide synthase is subsequently expressed, resulting in excessive NO generation [127]. Nanoceria can scavenge excessive NO [72, 120], which acts as a dual mediator of neurotransmission (under normal physiological conditions) and inflammation (under pathological conditions) [128]. In light of the anti-inflammatory capacity, nanoceria was loaded on lipid-coated mesoporous silica nanoparticles for theragnosis of ICH (Fig. 7a). In the synthesis, nanoceria was absorbed by magnetic mesoporous silica nanoparticles (FeNP@MSNs) through electrostatic interactions and exquisitely embedded into the 3.2-nm pores of MSNs. After coating with lipids, FeNP@MSNs doped with nanoceria were intracerebrally injected into ICH rats. Magnetic resonance imaging (MRI) clearly showed that these nanoparticles were successfully recruited to the perihematomal region and spontaneously internalized by microglia/macrophages (Fig. 7b), which was necessary for attenuating the inflammatory response. As expected, treatment with custom synthesized nanoparticles significantly reduced CD68-positive inflammatory cells (Fig. 7c) and brain edema in the perihematomal region after ICH, which was attributed to the antioxidative capacity of nanoceria. In addition to these therapeutic effects, taking advantage of iron oxide nanoparticles, the nanocomposites increased the efficacy of MRI enhancement in vivo, which was beneficial for ICH diagnosis [98]. Other examples have been reported utilizing nanoceria for MRI contrast enhancement in disease theragnosis [129, 130].
Figure 7.
(a) Custom synthesized nanocomposite (FeNP@MSNs doped with nanoceria) and its utilization in theragnosis of ICH. (b) MRI of ICH brain 5 days after nanocomposite injection. The arrow head points to low signal intensities in the peri-hematomal region, indicating uptake of these nanoparticles by the recruited inflammatory cells. (c) Activated microglia/macrophages in vehicle-treated (left) and nanocomposite-treated (right) groups. Adapted with permission from Ref. [98], Springer Nature 2018.
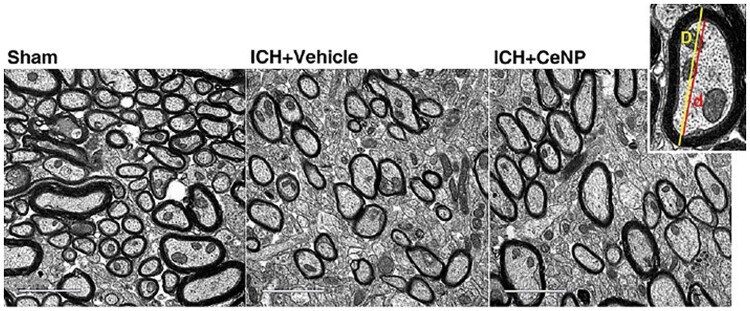
During ICH, RONS accumulation can induce severe damage to white matter, which accounts for at least 50% of the whole human brain volume [131]. A more recent study has reported that PEGylated nanoceria treatment could effectively ameliorate white matter injury and promote the regeneration of myelin sheaths, which surround the axons of neurons and enable the electrical impulses between nerve cells to transmit rapidly. In the ICH group treated with nanoceria, the myelin sheaths at the perihematomal site were thicker than those of the nontreated group (Fig. 8). Namely, PEGylated nanoceria treatment promoted remyelination and improved the integrity of myelinated fibers [99]. In addition, after intraperitoneal injection of nanoceria into a rat model of sciatic nerve crush injury, more myelinated fibers and thicker myelin sheaths were observed [132]. Since crosstalk among oligodendrocytes, microglia and astrocytes is involved in remyelination [133–135], nanoceria may modulate the crosstalk via RONS clearance. After the injury, nanoceria treatment induces a lower expression of M1 microglia and A1 astrocytes and promotes the differentiation of oligodendrocyte progenitor cells, all of which contribute to remyelination and white matter repair. It is consistent with the report that nanoceria might regulate the phenotype of microglia, i.e. from proinflammatory M1 to anti-inflammatory M2 under pathological conditions [121].
Figure 8.
Representative electron micrographs showing myelin sheaths at the perihematomal sites in the striatum after ICH. Adapted with permission from Ref. [99], CC by-NC 4.0, © The Author(s) 2021.
SAH accounts for ∼5% of all strokes. Approximately 85% of cases are caused by rupture of an intracranial aneurysm, followed by extravasation of blood into the subarachnoid space. Due to advances in theranostic techniques, the case fatality rates of SAH have markedly decreased during the past several decades. However, many survivors of SAH experience a variety of sequelae, including neurological, cognitive or functional deficits [136]. After blood invades the subarachnoid space, a series of events occur, including a sharp rise in intracranial pressure, a decrease in cerebral blood flow, overproduction of oxyhemoglobin, and other changes in physiology, neurochemistry, molecules and ions. RONS are widely implicated in these damage cascades [137–139].
It was shown that nanoceria synthesized in the aqueous phase and modified with aminocaproic acid/PEG obtained therapeutic effects in the SAH model. In vitro, bespoke ceria nanoparticles with high Ce3+ concentrations (43–57%) effectively protected macrophages from death when treated with cytotoxic hemin. The blood clot itself could generate RONS via hemoglobin autoxidation, the Fenton reaction of heme and the toxic effects of thrombin. In vivo, 1-h after SAH onset, nanoceria was intravenously injected into rats resulting in markedly improved neurological scores and survival rates [100]. It is worth noting that nanoceria might contribute to the aggravation of vasospasm via scavenging NO, which is the endothelium-derived relaxing factor generated from blood vessels [140]. Recently, nanoceria has been shown to induce NO generation from S-nitrosoglutathione (one of the most biologically abundant NO donors), which overturns the conventional concept that nanoceria acts widely as a NO-scavenging agent [141]. Therefore, the interaction between NO and nanoceria under SAH conditions needs to be more clearly elucidated.
Neurotrauma
Traumatic brain injury (TBI) is the most common cause of death and disability in young people [142]. More than 50 million people experience TBI every year worldwide [143], leading to direct loss of properties and even lives along with indirect impacts on families, friends and society. The primary injury, caused by sudden traumatic damage to the cerebral tissue (vascular breakage and neuronal death), occurs immediately and is almost inevitable. Therefore, therapeutic interventions are mainly focused on the secondary injury, which involves a cascade of biochemical and molecular events, including ionic homeostasis disturbance, release of excitatory neurotransmitters, mitochondrial dysfunction, as well as overproduction of RONS [53].
It was reported that nanoceria improved outcomes after mild TBI. In vitro, 10 nM nanoceria added to cell culture 1 h post-injury significantly reduced neuronal death and attenuated glutamate-mediated calcium signaling dysregulation. Furthermore, when applied in a rodent model induced by mild lateral fluid percussion to the rat brain, nanoceria protected the activity of endogenous antioxidants (SOD, catalase and glutathione), thus reducing oxidative damage to the brain. Even though repetitive doses were administered and the most effective dose was the highest dosing paradigm (2.5 mg/kg in total), the distribution of 10-nm nanoparticles in the brain was relatively low [101]. As the mimetic activity of nanoparticles varies with shape [68], ceria nanorods have exhibited better therapeutic effects on alleviation of brain edema than ceria nanospheres after TBI [102].
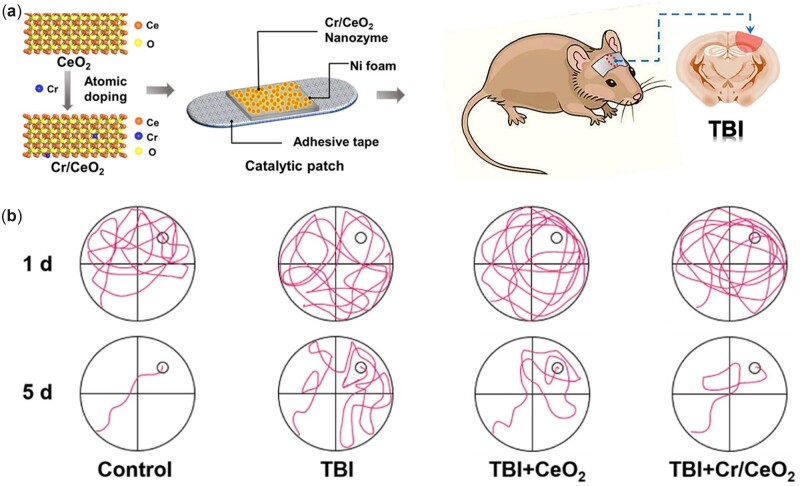
The conventional delivery route of intravenous injection is likely to generate potential biosafety issues related to the toxicity of nanoparticles, most of which will be transported to other organs through blood circulation. Therefore, it is essential to explore a new solution for TBI treatment to minimize the potential risk. Non-invasive bandage and patch with nanozymes for topical treatment in TBI have been designed [103, 104]. Although traditional antioxidant-based bandages could decrease infection triggered by RONS and inflammation around the wound area, they maintain efficacy for only a short period and need to be frequently renewed, which is inconvenient for brain trauma. To address this issue, a single-atom Pt/CeO2-based bandage was constructed by dispersing and trapping single Pt atoms in the CeO2 (111) matrix, leading to lattice expansion and catalytic activity enhancement. With the help of Pt single-atom catalyst, the Pt/CeO2 system showed several-fold higher activities in RONS clearance than CeO2 clusters. In addition, the wound dressing bandage sustained long-term catalytic activity for up to 30 days with little decay. When pasted on the wound of moderate TBI mice for 8 days, it effectively accelerated wound healing to healthy levels while the untreated group only recovered partially [103]. Likewise, the Cr/CeO2 nanozyme-based catalytic patch was utilized for TBI treatment as well as shown in Fig. 9. The monodisperse Cr/CeO2 nanozyme prepared by the coprecipitation method was loaded onto nickel foams. When doped with Cr3+ ions, nanoceria exhibited lattice distortion and a higher Ce3+/Ce4+ ratio, displaying a several-fold enhancement in enzyme-like activity. In addition, the Cr/CeO2 nanozyme at a 10% doping concentration achieved the highest Ce3+/Ce4+ ratio and the best enzyme-mimetic activity. The patch pasted on TBI mice resulted in excellent wound recovery and improved cognitive function. Furthermore, some of the nanoparticles released from the patch diffused into the brain tissue through the disrupted BBB after TBI, which played a crucial role in modulating RONS and alleviating inflammation [104]. Interestingly, the lattice of nanoceria expanded or contracted when doped with other metal ions because the atomic radius of doping ions is larger or smaller than that of Ce. Through such a doping method, the Ce3+/Ce4+ ratio of nanoceria could increase, and correspondingly, the enzyme-mimetic activity changes.
Figure 9.
(a) Design of non-invasive patch loaded with Cr/CeO2 nanozyme for topical treatment in TBI. (b) Path of the mice searching platform in Morris water maze test. After 5 days of training, TBI mice with Cr/CeO2 nanozyme patch treatment were able to find the platform in a short time, while non-treated TBI mice still could not quickly locate the position of platform. Reprinted from Ref. [104], CC by 4.0, © The Authors 2021.
Traumatic spinal cord injury, another type of neurotrauma, is an acute injury to the spinal cord caused by external physical impacts similar to TBI [144]. It was initially reported that nanoceria offered neuroprotection to spinal cord neurons isolated from adult rats. Through a microemulsion synthetic process, well-dispersed nanoparticles obtained high biocompatibility and neuroprotection capability in vitro. Nanoceria-treated neurons exhibited normal electrical activity and higher survival compared to the control ones [145]. Similarly, nanoceria was capped by plant extracts [146] or loaded onto biopolymer [147, 148] to improve its biocompatible activity. When cocultured with spinal cord cells, these nanocomposites exhibited auto-regenerative and neuroprotective activity, suggesting the therapeutic potential of nanoceria in spinal cord injury treatment. Furthermore, in a rat model of spinal cord contusion, 20-nm nanoceria was locally injected into the lesion cavity 30 min post-injury. Through modulation of RONS, nanoceria treatment alleviated the inflammatory response by downregulating the expression of inducible nitric oxide synthase and proinflammatory cytokines. The cystic cavity size was substantially reduced (Fig. 10), which consequently contributed to locomotor functional recovery [105]. Locally injected nanoceria could be internalized into microglia around the lesion area and modulate their phenotype from proinflammatory M1 to anti-inflammatory M2, as previously reported [121]. A more effective strategy to target microglia and modulate the inflammatory response could be achieved by coating nanoparticles with microglia-specific antibodies. With conjugation of CD11b antibody, ceria–zirconia nanocomposites were intrathecally administered into the spinal canal of mice, resulting in a significantly higher accumulation in microglia other than astrocytes and neurons [149]. Nanoceria may translocate into neurons through axons, which depends on both axonal integrity and electrical activity. In an experiment conducted on frog sciatic nerve fibers, 120-nm nanoceria translocated along the nerve fibers with a speed closer to the slow axonal transport rate [150], which might contribute to the recovery of the damaged nerves [132]. To be mentioned, the bacterial infection commonly occurs after neurotrauma, which seems innocuous and can be treated by antibiotics in most cases. Interestingly, nanoceria has also exhibited antibacterial activity [151, 152], which might promote the recovery of infected neurons and nerves. Normally, CNS is protected from most infections by immune responses and multilayer barriers. Thus, neuron infections mainly occur in the peripheral nervous system and rarely spread into CNS. However, some infections can innervate the peripheral area, resulting in direct or immune-mediated pathology in CNS. With powerful anti-inflammatory activities [120, 121], nanoceria may be effective against neuroinflammation caused by bacterial or viral infection.
Figure 10.
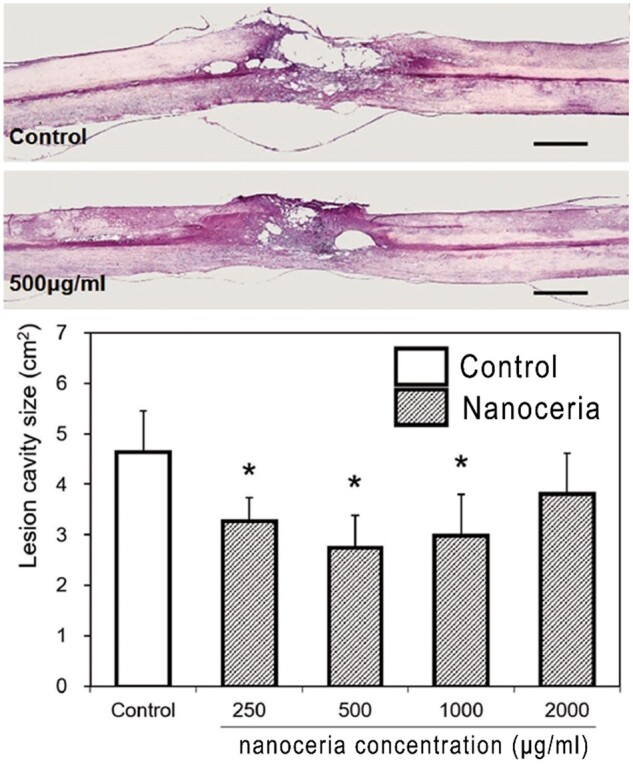
Local injection of nanoceria at concentration of 500 μg/ml significantly reduced the lesion cavity size. Adapted from Ref. [105], CC by 4.0, © The Authors 2021.
Challenges and future directions
Hereinabove, we have summarized up-to-date applications of nanoceria in acute CNS injury treatment. In general, studies commonly show significant benefits in animal models of CNS injury, which highlights great opportunities that nanoceria and its related biomaterials could be a potential option against oxidative stress in clinical practice. To move this nanomaterial from the bench to the bedside, some challenges remain to be met, leaving future directions for us to follow.
First, the safety problems concerning nanoceria in vivo must be considered. Apart from its beneficial RONS-scavenging ability, nanoceria exhibits toxic activities in some cases [153–159]. There are some controversial results regarding the anti- and pro-oxidant activities of nanoceria, which are affected by many factors including particle size, shape, surface chemistry, coatings, local pH and ligands [160]. As these factors greatly influence the biological activity of nanoceria, particles must be well characterized before being delivered into the body, which has been ignored during some works (Table 1).
Table 1.
Applications of nanoceria in various experimental models of CNS injury
| Disease type | Nanoceria size (nm) | Ce3+/Ce4+ ratio | Coatings | Optimal dosage | Experimental model | Delivery method | Main effects | Date and reference |
|---|---|---|---|---|---|---|---|---|
| Ischemia | 10 nm | Not mentioned | None | 1 µg/ml | Brain slice | Cocultured | 30% reduction of ROS in brain slice. | 2011 [75] |
| Protecting the structure of mitochondria. | ||||||||
| 3 nm | Not mentioned | Phospholipid-PEG | 0.5 mg/kg | MCAO | Intravenous | 50% reduction of infarct volumes in brain. | 2012 [23] | |
| 4.3 nm | 34% | Angiopep-2-PEG/edaravone | 0.6 mg/kg | MCAO | Intravenous | 30% reduction of infarct volumes in brain. | 2018 [91] | |
| Protecting the BBB integrity. | ||||||||
| Not mentioned | Not mentioned | Biotinylated-LXW7 | 0.5 mg/kg | MCAO | Intravenous | 30% reduction of infarct volumes in brain. | 2018 [92] | |
| Improving neurologic deficit. | ||||||||
| 40 nm | Not mentioned | PEG/PLGA | 10 mg/kg | MCAO | Not mentioned | Decreasing infarct volumes and brain edema. | 2018 [93] | |
| 2–2.5 nm | Not mentioned | Citric acid/EDTA | 60 mg/kg | MCAO | Intraperitoneal | 52% reduction of superoxide in hippocampus. | 2019 [94] | |
| 20 nm | Not mentioned | ZIF-8 | 0.4 mg/kg | MCAO | Intravenous | 25% reduction of infarct volumes in brain. | 2020 [95] | |
| Decreasing inflammation. | ||||||||
| Hemorrhage | 3 nm | 43% | Phospholipid-PEG | 0.5 mg/kg | ICH | Intravenous | 68% reduction of brain edema. | 2017 [97] |
| Decreasing inflammation. | ||||||||
| 3 nm | Not mentioned | Phospholipid-PEG | 10 µg | ICH | Intracerebral | Decreasing microglia/macrophage recruitment and brain edema. | 2018 [98] | |
| 3.4 nm | 43% | Oleylamine-PEG-DSPE | 0.5 mg/kg | ICH | Intravenous | Decreasing inflammation. | 2021 [99] | |
| Promoting remyelination. | ||||||||
| 3 nm | 43–57% | Aminocaproic acid-PEG | 0.5 mg/kg | SAH | Intravenous | Improving neurologic deficit and survival rates. | 2018 [100] | |
| TBI | 10 nm | 33% | None | 0.5 mg/kg | Mild TBI | Intravenous | Reducing calcium dysregulation. | 2020 [101] |
| Improving cognitive function. | ||||||||
| 3 nm/9 nm | 40%/27% | None | 11.6 mM | Mild TBI | Retro-orbital | Decreasing cell death and cerebral edema. | 2021 [102] | |
| Improving cognitive function. | ||||||||
| Not mentioned | Not mentioned | None | Not mentioned | Mild TBI | Non-invasive topical | Decreasing inflammation and wound size. | 2019 [103] | |
| Improving cognitive function. | ||||||||
| 8–12 nm | 27% | None | Not mentioned | Mild TBI | Non-invasive topical | Decreasing inflammation and wound size. | 2021 [104] | |
| Improving cognitive function. | ||||||||
| TSCI | 19.5 nm | 34% | None | 10 µg | Spinal cord contusion | Local injected | Decreasing inflammation and cavity size. | 2017 [105] |
| Improving locomotor function. | ||||||||
| 3–5 nm | Not mentioned | None | 10 nM | Spinal cord cell | Cocultured | Neuro-protective effect on the spinal cord neurons. | 2007 [145] | |
| 15 nm | Not mentioned | Leaf extract | 10 nM | Spinal cord cell | Cocultured | Neuro-protective effect on the spinal cord neurons. | 2021 [146] | |
| 15–25 nm | Not mentioned | Chitosan | Not mentioned | Spinal cord cell | Cocultured | Neuro-protective effect on the spinal cord neurons. | 2018 [147] | |
| 40 nm | Not mentioned | PCL/RVL | Not mentioned | Spinal cord cell | Cocultured | Neuro-protective effect on the spinal cord neurons. | 2020 [148] |
PEG, polyethylene glycol; EDTA, ethylene diamine tetraacetic acid; PLGA, poly lactic-co-glycolic acid; ZIF, zeolitic imidazolate framework; DSPE, distearoyl phosphoethanolamine; PCL, poly (e-caprolactone); RVL, resveratrol; MCAO, middle cerebral artery occlusion; ICH, Intracerebral hemorrhage; SAH, subarachnoid hemorrhage; TSCI, traumatic spinal cord injury.
In an experiment exploring the interaction with OH•, nanoceria could convert from exhibiting antioxidant to oxidant activity as the concentration of OH• and nanoparticles increased. At high concentrations, more Ce3+ ions were introduced into the system, and nanoceria might catalyze the production of OH• similar to Fe2+ in a Fenton reaction [161]. When polymer-coated ceria nanoparticles with different surface charges were added to diverse cell lines, they exhibited charge-dependent subcellular localization and cytotoxicity. Nanoceria with a positive or neutral charge entered most of the cell lines, while negatively charged particles internalized mostly in the cancer cell lines. Furthermore, the internalization and localization of nanoceria were closely related to its cytotoxicity. After localized in the lysosomes of these cells, nanoceria exhibited obvious toxicity due to the acidic microenvironment; but little toxicity was shown in the cytoplasm or outside the cells [162]. Based on a large body of literature, nanoceria only displays toxicity in animals when injected at high doses (more than tenths of mg of CeO2 per kg of body weight) [163, 164]. For example, rat brain pro-oxidant effects were reported after peripheral administration of 85 mg/kg nanoceria. Without permeating the BBB, 5-nm nanoceria indirectly decreased the ratio of reduced to oxidized glutathione in the hippocampus and cerebellum, which was an indicator of oxidative stress [165]. To date, there is little evidence of toxicity in response to CNS injury treatment with nanoceria in vivo, as the administration doses are generally very low, mostly lower than 1 mg/kg (Table 1). ‘When it is not toxic, it is not a medicine’, as the saying goes. Every medicine has side effects, what we should focus on is the safe range of concentrations. Thus, nanoceria is typically safe when injected intravenously at therapeutic doses.
Another issue is regarding how to cross the BBB/BSCB. Many biodistribution studies have unanimously found that the major organs of nanoceria accumulation are the liver and spleen, while a minimal fraction could penetrate the BBB/BSCB into the brain or spinal cord [109, 157, 159]. The most characteristic structure of the BBB/BSCB is the tight junction, an intercellular barrier between endothelial cells [166], which blocks the paracellular passage of most particles. Under normal conditions, the pore size of tight junctions is speculated to be 1.4–1.8 nm; thus, only particles sized ∼1 nm or less can be passively transported through ultrasmall pores [167]. Several methods have been proposed to cross the barrier, including paracellular transport, passive diffusion, cell/carrier-mediated transport and receptor/adsorptive-mediated transcytosis [168, 169]. Specifically, acute CNS injury is usually accompanied by BBB or BSCB disruption, leading to the transient splitting of the junction, which renders a discrete path for nanoparticles. The most conventional solution to facilitate the penetration of larger nanoparticles into the CNS is surface modification. However, the results are not satisfactory in most cases. Another feasible method bypassing the BBB/BSCB is the direct injection of the nanomedicine into the CSF instead of the bloodstream [170]. Through intrathecal administration, nanoparticles sized <10 nm could passively traffic from CSF to the parenchymal tissue [171]. With the help of CSF flow, nanoceria can be dispersed over the whole CNS. In a previous study, PEGylated ceria–zirconia nanocomposites sized 9 nm were intrathecally injected into mice to determine whether the nanoparticles could reach the spinal cord cells. These nanoparticles were widely delivered into the CNS area from the brain to different regions of the spinal cord, and nanoceria was identified in a large part of the spinal cord cells but only in 7% of the brain cells [149]. However, the biodistribution and pharmacokinetics of nanoceria through intrathecal administration have rarely been reported. Considering that these inorganic nanoparticles may be maintained in living bodies for years, long-term experiments are needed to investigate the biochemical changes in vivo. Therefore, it is essential to explore these aspects more thoroughly.
How much RONS can each dose of nanoceria scavenge? Furthermore, how much scavenging is too much? To solve these problems, the precise molecular mechanisms by which nanoceria scavenges RONS need to be completely elucidated and understood. Most preclinical studies designed for medical applications of nanoceria are mainly focused on the therapeutic outcome rather than the underlying mechanisms of action. Many results indicate that the enzyme-mimetic activity of nanoceria is closely related to the Ce3+/Ce4+ ratio, as smaller particles with a larger Ce3+ fraction present stronger catalytic effects [172]. However, the Ce3+/Ce4+ ratio is strongly influenced by the intracellular environment, and what happens to this ratio in the milieu of cells and tissues remains an enigma that has yet to be explored. When internalized into cells, nanoceria was reported to possess a lower Ce3+/Ce4+ ratio than outside the cells, indicating a net reduction of its oxidation state in the intracellular environment. In addition, a similar ratio was observed in the organelles (cytoplasm, lysosome), which suggested that the net reduction might occur earlier in the process of cellular internalization [173]. Even though nanoceria protects cells against oxidative/nitrosative stress caused by RONS, it seems more rational to treat nanoceria not as a pure antioxidant, but as a mediator of signal transduction partaking in the process of neuronal death and protection. Hence, nanoceria presents somewhat different effects after localized into cells of different types or physiological states [162]. When delivered into the CNS, nanoceria might change the levels of some neurotransmitters, e.g. dopamine, glutamate and NO. It was reported that nanoceria could oxidize dopamine under acidic conditions in aqueous solutions [19] and in human serum [174], while another study reported its promotive effects on dopamine secretion in PC12 neuronal-like cells [175].
In summary, although some work still needs to be accomplished, nanoceria by virtue of its powerful antioxidant activity is considered to be quite promising in the treatment of CNS injury as well as other RONS-related diseases.
Funding
This work was supported by the Pre-research Project (2020XYY15) of Army Medical University.
Conflicts of interest statement. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Wang Yang, School of Biomedical Engineering and Medical Imaging, Army Medical University, Chongqing 400038, China; Army Health Service Training Base, Army Medical University, Chongqing 400038, China.
Maoting Zhang, School of Biomedical Engineering and Medical Imaging, Army Medical University, Chongqing 400038, China.
Jian He, School of Biomedical Engineering and Medical Imaging, Army Medical University, Chongqing 400038, China.
Mingfu Gong, Xinqiao Hospital, Army Medical University, Chongqing 400038, China.
Jian Sun, School of Biomedical Engineering and Medical Imaging, Army Medical University, Chongqing 400038, China.
Xiaochao Yang, School of Biomedical Engineering and Medical Imaging, Army Medical University, Chongqing 400038, China.
References
- 1. Trovarelli A. Catalytic properties of ceria and CeO2-containing materials. Catal Revi 1996;38:439–520. [Google Scholar]
- 2. Trovarelli A, De Leitenburg C, Boaro M, Dolcetti G.. The utilization of ceria in industrial catalysis. Catal Today 1999;50:353–67. [Google Scholar]
- 3. Trovarelli A. Catalysis by Ceria and Related Materials. Singapore: World Scientific, 2002. [Google Scholar]
- 4. Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, Comelli G, Rosei R.. Electron localization determines defect formation on ceria substrates. Science 2005;309:752–5. [DOI] [PubMed] [Google Scholar]
- 5. Skorodumova NV, Simak SI, Lundqvist BI, Abrikosov IA, Johansson B.. Quantum origin of the oxygen storage capability of ceria. Phys Rev Lett 2002;89:166601. [DOI] [PubMed] [Google Scholar]
- 6. Campbell CT, Peden CH.. Oxygen vacancies and catalysis on ceria surfaces. Science 2005;309:713–4. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande S, Patil S, Kuchibhatla S, Seal S.. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett 2005;87:133113. [Google Scholar]
- 8. Trovarelli A, Llorca J.. Ceria catalysts at nanoscale: how do crystal shapes shape catalysis? ACS Catal 2017;7:4716–35. [Google Scholar]
- 9. Terribile D, Trovarelli A, Llorca J, de Leitenburg C, Dolcetti G.. The synthesis and characterization of mesoporous high-surface area ceria prepared using a hybrid organic/inorganic route. J Catal 1998;178:299–308. [Google Scholar]
- 10. Vincent A, Babu S, Heckert E, Dowding J, Hirst SM, Inerbaev TM, Self WT, Reilly CM, Masunov AE, Rahman TS, Seal S.. Protonated nanoparticle surface governing ligand tethering and cellular targeting. ACS Nano 2009;3:1203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masui T, Hirai H, Imanaka N, Adachi G, Sakata T, Mori H.. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J Mater Sci Lett 2002;21:489–91. [Google Scholar]
- 12. Patil S, Kuiry S, Seal S, Vanfleet R.. Synthesis of nanocrystalline ceria particles for high temperature oxidation resistant coating. J Nano Res 2002;4:433–8. [Google Scholar]
- 13. Arumugam A, Karthikeyan C, Hameed ASH, Gopinath K, Gowri S, Karthika V.. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C 2015;49:408–15. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Zhu J-J, Zhu J-M, Liao X-H, Xu S, Ding T, Chen H-Y.. Preparation of nanocrystalline ceria particles by sonochemical and microwave assisted heating methods. Phys Chem Chem Phys 2002;4:3794–9. [Google Scholar]
- 15. Melchionna M, Trovarelli A, Fornasiero P.. Synthesis and properties of cerium oxide-based materials. In: Scirè S, Palmisano L (ed.). Cerium Oxide (CeO2): Synthesis, Properties and Applications. Elsevier, 2020, 13–43. [Google Scholar]
- 16. Korsvik C, Patil S, Seal S, Self WT.. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun 2007;10:1056–8. [DOI] [PubMed] [Google Scholar]
- 17. Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JE, Seal S, Self WT.. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun (Camb) 2010;46:2736–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiao X, Song H, Zhao H, Bai W, Zhang L, Lv Y.. Well-redispersed ceria nanoparticles: promising peroxidase mimetics for H2O2 and glucose detection. Anal Methods 2012;4:3261–7. [Google Scholar]
- 19. Asati A, Santra S, Kaittanis C, Nath S, Perez JM.. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem 2009;121:2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu F, Lu Q, Huang PJ, Liu J.. Nanoceria as a Dnase I mimicking nanozyme. Chem Commun (Camb) 2019;55:13215–8. [DOI] [PubMed] [Google Scholar]
- 21. Tian Z, Yao T, Qu C, Zhang S, Li X, Qu Y.. Photolyase-like catalytic behavior of CeO2. Nano Lett 2019;19:8270–7. [DOI] [PubMed] [Google Scholar]
- 22. Kwon HJ, Kim D, Seo K, Kim YG, Han SI, Kang T, Soh M, Hyeon T.. Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angew Chem Int Ed Engl 2018;57:9408–12. [DOI] [PubMed] [Google Scholar]
- 23. Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ, Jang H, Yang HS, Kim JY, Park HK, Park SP, Park S, Yu T, Yoon BW, Lee SH, Hyeon T.. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl 2012;51:11039–43. [DOI] [PubMed] [Google Scholar]
- 24. Kwon HJ, Cha MY, Kim D, Kim DK, Soh M, Shin K, Hyeon T, Mook-Jung I.. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 2016;10:2860–70. [DOI] [PubMed] [Google Scholar]
- 25. Tian Z, Li J, Zhang Z, Gao W, Zhou X, Qu Y.. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015;59:116–24. [DOI] [PubMed] [Google Scholar]
- 26. Li H, Liu C, Zeng Y-P, Hao Y-H, Huang J-W, Yang Z-Y, Li R.. Nanoceria-mediated drug delivery for targeted photodynamic therapy on drug-resistant breast cancer. ACS Appl Mater Interfaces 2016;8:31510–23. [DOI] [PubMed] [Google Scholar]
- 27. Xiao Y-F, Li J-M, Wang S-M, Yong X, Tang B, Jie M-M, Dong H, Yang X-C, Yang S-M.. Cerium oxide nanoparticles inhibit the migration and proliferation of gastric cancer by increasing DHX15 expression. Int J Nanomedicine 2016;11:3023–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu H, Jin F, Liu D, Shu G, Wang X, Qi J, Sun M, Yang P, Jiang S, Ying X, Du Y.. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020;10:2342–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weng Q, Sun H, Fang C, Xia F, Liao H, Lee J, Wang J, Xie A, Ren J, Guo X, Li F, Yang B, Ling D.. Catalytic activity tunable ceria nanoparticles prevent chemotherapy-induced acute kidney injury without interference with chemotherapeutics. Nat Commun 2021;12:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang D-Y, Liu H, Li C, Younis MR, Lei S, Yang C, Lin J, Li Z, Huang P.. Ceria nanozymes with preferential renal uptake for acute kidney injury alleviation. ACS Appl Mater Interfaces 2020;12:56830–8. [DOI] [PubMed] [Google Scholar]
- 31. Ni D, Wei H, Chen W, Bao Q, Rosenkrans ZT, Barnhart TE, Ferreira CA, Wang Y, Yao H, Sun T, Jiang D, Li S, Cao T, Liu Z, Engle JW, Hu P, Lan X, Cai W.. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater 2019;31:1902956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang X, Hu J, Becker KV, Engle JW, Ni D, Cai W, Wu D, Qu S.. Antioxidant and C5a-blocking strategy for hepatic ischemia-reperfusion injury repair. J Nanobiotechnol 2021;19:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oró D, Yudina T, Fernández-Varo G, Casals E, Reichenbach V, Casals G, González de la Presa B, Sandalinas S, Carvajal S, Puntes V, Jiménez W.. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol 2016;64:691–8. [DOI] [PubMed] [Google Scholar]
- 34. Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE.. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardio Res 2007;73:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo L-J, Nguyen DD, Lai J-Y.. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics 2021;11:5447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Patil S, Seal S, McGinnis JF.. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 2006;1:142–50. [DOI] [PubMed] [Google Scholar]
- 37. Luo L-J, Nguyen DD, Lai J-Y.. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: a push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020;243:119961. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Li C, Wan Y, Qi M, Chen Q, Sun Y, Sun X, Fang J, Fu L, Xu L, Dong B, Wang L.. Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small 2021;17:2101505. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Qi M, Li C, Dong B, Wang J, Weir MD, Imazato S, Du L, Lynch CD, Xu L, Zhou Y, Wang L, Xu HHK.. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J Mater Chem B 2019;7:6955–71. [DOI] [PubMed] [Google Scholar]
- 40. Ren S, Zhou Y, Zheng K, Xu X, Yang J, Wang X, Miao L, Wei H, Xu Y.. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact Mater 2022;7:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiang J, Li J, He J, Tang X, Dou C, Cao Z, Yu B, Zhao C, Kang F, Yang L, Dong S, Yang X.. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces 2016;8:4489–99. [DOI] [PubMed] [Google Scholar]
- 42. Dou C, Li J, He J, Luo F, Yu T, Dai Q, Chen Y, Xu J, Yang X, Dong S.. Bone-targeted pH-responsive cerium nanoparticles for anabolic therapy in osteoporosis. Bioact Mater 2021;6:4697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Kang F, Gong X, Bai Y, Dai J, Zhao C, Dou C, Cao Z, Liang M, Dong R, Jiang H, Yang X, Dong S.. Ceria nanoparticles enhance endochondral ossification–based critical–sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. FASEB J 2019;33:6378–89. [DOI] [PubMed] [Google Scholar]
- 44. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CMM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C.. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet 2014;383:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langlois JA, Rutland-Brown W, Wald MM.. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–8. [DOI] [PubMed] [Google Scholar]
- 46. Lee BB, Cripps RA, Fitzharris M, Wing PC.. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014;52:110–6. [DOI] [PubMed] [Google Scholar]
- 47. Lewén A, Matz P, Chan PH.. Free radical pathways in CNS injury. J Neurotrauma 2000;17:871–90. [DOI] [PubMed] [Google Scholar]
- 48. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 2006;97:1634–58. [DOI] [PubMed] [Google Scholar]
- 49. Gilgun-Sherki Y, Melamed E, Offen D.. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40:959–75. [DOI] [PubMed] [Google Scholar]
- 50.McKenna MC, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A. Energy metabolism of the brain. In: Brady ST (ed.). Basic neurochemistry. Amsterdam: Elsevier, 2012, 200–231. [Google Scholar]
- 51. Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D.. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 2002;54:271–84. [DOI] [PubMed] [Google Scholar]
- 52. Shirley R, Ord ENJ, Work LM.. Oxidative stress and the use of antioxidants in stroke. Antioxidants (Basel) 2014;3:472–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pietro VD, Yakoub KM, Caruso G, Lazzarino G, Signoretti S, Barbey AK, Tavazzi B, Lazzarino G, Belli A, Amorini AM.. Antioxidant therapies in traumatic brain injury. Antioxidants 2020;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang B, Chen Y, Shi J.. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev 2019;119:4881–985. [DOI] [PubMed] [Google Scholar]
- 55. Ferreira CA, Ni D, Rosenkrans ZT, Cai W.. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res 2018;11:4955–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takamiya M, Miyamoto Y, Yamashita T, Deguchi K, Ohta Y, Ikeda Y, Matsuura T, Abe K.. Neurological and pathological improvements of cerebral infarction in mice with platinum nanoparticles. J Neurosci Res 2011;89:1125–33. [DOI] [PubMed] [Google Scholar]
- 57. Li C, Zhao Z, Luo Y, Ning T, Liu P, Chen Q, Chu Y, Guo Q, Zhang Y, Zhou W, Chen H, Zhou Z, Wang Y, Su B, You H, Zhang T, Li X, Song H, Li C, Sun T, Jiang C.. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci (Weinh) 2021;8:e2101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee HJ, Park J, Yoon OJ, Kim HW, Lee DY, Kim DH, Lee WB, Lee N-E, Bonventre JV, Kim SS.. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol 2011;6:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Ai K, Ji X, Askhatova D, Du R, Lu L, Shi J.. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J Am Chem Soc 2017;139:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Callahan P, Colon J, Merchant S, Kuiry S, Patil S, Seal S, Rzigalinski B.. Deleterious effects of microglia activated by in vitro trauma are blocked by engineered oxide nanoparticles. J Neurotrauma 2003;20:1053. [Google Scholar]
- 61. Rzigalinski BA, Bailey D, Chow L, Kuiry S, Patil S, Merchant S, Seal S.. Cerium oxide nanoparticles increase the lifespan of cultured brain cells and protect against free radical and mechanical trauma. FASEB J 2003;17:A606. [Google Scholar]
- 62. Genchi GG, Degl’Innocenti A, Martinelli C, Battaglini M, De Pasquale D, Prato M, Marras S, Pugliese G, Drago F, Mariani A, Balsamo M, Zolesi V, Ciofani G.. Cerium oxide nanoparticle administration to skeletal muscle cells under different gravity and radiation conditions. ACS Appl Mater Interfaces 2021;13:40200–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Halliwell B, Gutteridge JM.. Free Radicals in Biology and Medicine. Oxford: Oxford University Press, 2015. [Google Scholar]
- 64. D’Autréaux B, Toledano MB.. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007;8:813–24. [DOI] [PubMed] [Google Scholar]
- 65. Rzigalinski BA. Nanoparticles and cell longevity. Technol Cancer Res Treat 2005;4:651–9. [DOI] [PubMed] [Google Scholar]
- 66. Heckert EG, Karakoti AS, Seal S, Self WT.. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008;29:2705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shcherbakov AB, Zholobak NM, Ivanov VK.. Biological, biomedical and pharmaceutical applications of cerium oxide. In: Scirè S, Palmisano L (ed.). Cerium Oxide (CeO2): Synthesis, Properties and Applications. Elsevier, 2020, 279–358. [Google Scholar]
- 68. Fisher TJ, Zhou Y, Wu T-S, Wang M, Soo Y-L, Cheung CL.. Structure-activity relationship of nanostructured ceria for the catalytic generation of hydroxyl radicals. Nanoscale 2019;11:4552–61. [DOI] [PubMed] [Google Scholar]
- 69. Sies H, Jones DP.. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363–83. [DOI] [PubMed] [Google Scholar]
- 70. Alvarez B, Radi R.. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003;25:295–311. [DOI] [PubMed] [Google Scholar]
- 71. Dowding JM, Seal S, Self WT.. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−). Drug Deliv Transl Res 2013;3:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dowding JM, Dosani T, Kumar A, Seal S, Self WT.. Cerium oxide nanoparticles scavenge nitric oxide radical (NO). Chem Commun (Camb) 2012;48:4896–8. [DOI] [PubMed] [Google Scholar]
- 73. Xue Y, Luan Q, Yang D, Yao X, Zhou K.. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C 2011;115:4433–8. [Google Scholar]
- 74. Ivanov VK, Shcherbakov AB, Ryabokon’ IG, Usatenko AV, Zholobak NM, Tretyakov YD.. Inactivation of the nitroxyl radical by ceria nanoparticles. Dokl Chem 2010;430:43–6. [Google Scholar]
- 75. Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ, Ludington JS, Chatani P, Mosenthal WP, Leiter JC, Andreescu S, Erlichman JS.. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 2011;51:1155–63. [DOI] [PubMed] [Google Scholar]
- 76. DeCoteau W, Heckman KL, Estevez AY, Reed KJ, Costanzo W, Sandford D, Studlack P, Clauss J, Nichols E, Lipps J, Parker M, Hays-Erlichman B, Leiter JC, Erlichman JS.. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine: NBM 2016;12:2311–20. [DOI] [PubMed] [Google Scholar]
- 77. Lo EH, Dalkara T, Moskowitz MA.. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003;4:399–415. [DOI] [PubMed] [Google Scholar]
- 78. Fujikawa DG. The role of excitotoxic programmed necrosis in acute brain injury. Comput Struct Biotechnol J 2015;13:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y.. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol 2018;16:1396–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Raghupathi R, Graham DI, McINTOSH TK.. Apoptosis after traumatic brain injury. J Neurotrauma 2000;17:927–38. [DOI] [PubMed] [Google Scholar]
- 81. Wang P, Shao B-Z, Deng Z, Chen S, Yue Z, Miao C-Y.. Autophagy in ischemic stroke. Prog Neurobiol 2018;163–164:98–117. [DOI] [PubMed] [Google Scholar]
- 82. Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G.. Autophagy in acute brain injury. Nat Rev Neurosci 2016;17:467–84. [DOI] [PubMed] [Google Scholar]
- 83. Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J.. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 2019;56:4880–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim D, Kwon HJ, Hyeon T.. Magnetite/ceria nanoparticle assemblies for extracorporeal cleansing of amyloid-beta in Alzheimer’s disease. Adv Mater 2019;31:e1807965. [DOI] [PubMed] [Google Scholar]
- 85. D’Angelo B, Santucci S, Benedetti E, Loreto SD, Phani R, Falone S, Amicarelli F, Ceru MP, Cimini A.. Cerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. CNANO 2009;5:167–76. [Google Scholar]
- 86. Cimini A, D’Angelo B, Das S, Gentile R, Benedetti E, Singh V, Monaco AM, Santucci S, Seal S.. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of abeta aggregates modulate neuronal survival pathways. Acta Biomater 2012;8:2056–67. [DOI] [PubMed] [Google Scholar]
- 87. Guan Y, Li M, Dong K, Gao N, Ren J, Zheng Y, Qu X.. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-beta peptide. Biomaterials 2016;98:92–102. [DOI] [PubMed] [Google Scholar]
- 88. Li Y, Li Y, Wang H, Liu R.. + Er3+ codoped cerium oxide upconversion nanoparticles enhanced the enzymelike catalytic activity and antioxidative activity for Parkinson’s disease treatment. ACS Appl Mater Interfaces 2021;13:13968–77. [DOI] [PubMed] [Google Scholar]
- 89. Heckman KL, DeCoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D, Leiter JC, Clauss J, Knapp K, Gomez C, Mullen P, Rathbun E, Prime K, Marini J, Patchefsky J, Patchefsky AS, Hailstone RK, Erlichman JS.. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013;7:10582–96. [DOI] [PubMed] [Google Scholar]
- 90. Eitan E, Hutchison ER, Greig NH, Tweedie D, Celik H, Ghosh S, Fishbein KW, Spencer RG, Sasaki CY, Ghosh P, Das S, Chigurapati S, Raymick J, Sarkar S, Chigurupati S, Seal S, Mattson MP.. Combination therapy with lenalidomide and nanoceria ameliorates CNS autoimmunity. Exp Neurol 2015;273:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bao Q, Hu P, Xu Y, Cheng T, Wei C, Pan L, Shi J.. Simultaneous blood-brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano 2018;12:6794–805. [DOI] [PubMed] [Google Scholar]
- 92. Zhang T, Li C-Y, Jia J-J, Chi J-S, Zhou D, Li J-Z, Liu X-M, Zhang J, Yi L.. Combination therapy with LXW7 and ceria nanoparticles protects against acute cerebral ischemia/reperfusion injury in rats. Curr Med Sci 2018;38:144–52. [DOI] [PubMed] [Google Scholar]
- 93. Gao Y, Chen X, Liu H.. A facile approach for synthesis of nano-CeO particles loaded co-polymer matrix and their colossal role for blood-brain barrier permeability in cerebral ischemia. J Photochem Photobiol B 2018;187:184–9. [DOI] [PubMed] [Google Scholar]
- 94. Estevez AY, Ganesana M, Trentini JF, Olson JE, Li G, Boateng YO, Lipps JM, Yablonski SER, Donnelly WT, Leiter JC, Erlichman JS.. Antioxidant enzyme-mimetic activity and neuroprotective effects of cerium oxide nanoparticles stabilized with various ratios of citric acid and EDTA. Biomolecules 2019;9:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. He L, Huang G, Liu H, Sang C, Liu X, Chen T.. Highly bioactive zeolitic imidazolate framework-8–capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci Adv 2020;6:eaay9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou D, Fang T, Lu L-Q, Yi L.. Neuroprotective potential of cerium oxide nanoparticles for focal cerebral ischemic stroke. J Huazhong Univ Sci Technol [Med Sci] 2016;36:480–6. [DOI] [PubMed] [Google Scholar]
- 97. Kang D-W, Kim CK, Jeong H-G, Soh M, Kim T, Choi I-Y, Ki S-K, Kim DY, Yang W, Hyeon T, Lee S-H.. Biocompatible custom ceria nanoparticles against reactive oxygen species resolve acute inflammatory reaction after intracerebral hemorrhage. Nano Res 2017;10:2743–60. [Google Scholar]
- 98. Cha BG, Jeong H-G, Kang D-W, Nam M-J, Kim CK, Kim DY, Choi I-Y, Ki SK, Kim SI, Han J, Kim J, Lee S-H.. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res 2018;11:3582–92. [Google Scholar]
- 99. Zheng J, Lu J, Mei S, Wu H, Sun Z, Fang Y, Xu S, Wang X, Shi L, Xu W, Chen S, Yu J, Liang F, Zhang J.. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: microglia-astrocyte involvement in remyelination. J Neuroinflammation 2021;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jeong H-G, Cha BG, Kang D-W, Kim DY, Ki SK, Kim SI, Han JH, Yang W, Kim CK, Kim J, Lee S-H.. Ceria nanoparticles synthesized with aminocaproic acid for the treatment of subarachnoid hemorrhage. Stroke 2018;49:3030–8. [DOI] [PubMed] [Google Scholar]
- 101. Bailey ZS, Nilson E, Bates JA, Oyalowo A, Hockey KS, Sajja V, Thorpe C, Rogers H, Dunn B, Frey AS, Billings MJ, Sholar CA, Hermundstad A, Kumar C, VandeVord PJ, Rzigalinski BA.. Cerium oxide nanoparticles improve outcome after and mild traumatic brain injury. J Neurotrauma 2020;37:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Youn DH, Tran NM, Kim BJ, Kim Y, Jeon JP, Yoo H.. Shape effect of cerium oxide nanoparticles on mild traumatic brain injury. Sci Rep 2021;11:15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yan R, Sun S, Yang J, Long W, Wang J, Mu X, Li Q, Hao W, Zhang S, Liu H, Gao Y, Ouyang L, Chen J, Liu S, Zhang X-D, Ming D.. Nanozyme-based bandage with single-atom catalysis for brain trauma. ACS Nano 2019;13:11552–60. [DOI] [PubMed] [Google Scholar]
- 104. Zhang S, Liu Y, Sun S, Wang J, Li Q, Yan R, Gao Y, Liu H, Liu S, Hao W, Dai H, Liu C, Sun Y, Long W, Mu X, Zhang XD.. Catalytic patch with redox Cr/CeO2 nanozyme of noninvasive intervention for brain trauma. Theranostics 2021;11:2806–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim J-W, Mahapatra C, Hong J-Y, Kim MS, Leong KW, Kim H-W, Hyun JK.. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv Sci (Weinh) 2017;4:1700034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V.. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69. [DOI] [PubMed] [Google Scholar]
- 107. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 108. Patil S, Sandberg A, Heckert E, Self W, Seal S.. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007;28:4600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hardas SS, Butterfield DA, Sultana R, Tseng MT, Dan M, Florence RL, Unrine JM, Graham UM, Wu P, Grulke EA, Yokel RA.. Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicol Sci 2010;116:562–76. [DOI] [PubMed] [Google Scholar]
- 110. Yokel RA, Tseng MT, Dan M, Unrine JM, Graham UM, Wu P, Grulke EA.. Biodistribution and biopersistence of ceria engineered nanomaterials: size dependence. Nanomedicine 2013;9:398–407. [DOI] [PubMed] [Google Scholar]
- 111. Zhang Y, Kohler N, Zhang M.. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002;23:1553–61. [DOI] [PubMed] [Google Scholar]
- 112. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM.. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016;99:28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Karakoti AS, Singh S, Kumar A, Malinska M, Kuchibhatla S, Wozniak K, Self WT, Seal S.. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc 2009;131:14144–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Demeule M, Currie JC, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne JP, Béliveau R.. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem 2008;106:1534–44. [DOI] [PubMed] [Google Scholar]
- 115. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM.. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994;25:1794–8. [DOI] [PubMed] [Google Scholar]
- 116. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M.. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 2000;106:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Das S, Singh S, Dowding JM, Oommen S, Kumar A, Sayle TXT, Saraf S, Patra CR, Vlahakis NE, Sayle DC, Self WT, Seal S.. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials 2012;33:7746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J.. Inhibition of integrin αvβ3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke 2006;37:1902–9. [DOI] [PubMed] [Google Scholar]
- 119. Goujon G, Baldim V, Roques C, Bia N, Seguin J, Palmier B, Graillot A, Loubat C, Mignet N, Margaill I, Berret J-F, Beray-Berthat V.. Antioxidant activity and toxicity study of cerium oxide nanoparticles stabilized with innovative functional copolymers. Adv Healthcare Mater 2021;10:2100059. [DOI] [PubMed] [Google Scholar]
- 120. Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM.. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009;5:2848–56. [DOI] [PubMed] [Google Scholar]
- 121. Zeng F, Wu Y, Li X, Ge X, Guo Q, Lou X, Cao Z, Hu B, Long NJ, Mao Y, Li C.. Custom-made ceria nanoparticles show a neuroprotective effect by modulating phenotypic polarization of the microglia. Angew Chem 2018;130:5910–4. [DOI] [PubMed] [Google Scholar]
- 122. Hekmatimoghaddam S, Iman M, Shahdadi Sardo H, Jebali A.. Gelatin hydrogel containing cerium oxide nanoparticles covered by interleukin-17 aptamar as an anti-inflammatory agent for brain inflammation. J Neuroimmunol 2019;326:79–83. [DOI] [PubMed] [Google Scholar]
- 123. Van Gijn J, Kerr RS, Rinkel GJ.. Subarachnoid haemorrhage. Lancet 2007;369:306–18. [DOI] [PubMed] [Google Scholar]
- 124. Xi G, Keep RF, Hoff JT.. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006;5:53–63. [DOI] [PubMed] [Google Scholar]
- 125. Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ.. Progression of mass effect after intracerebral hemorrhage. Stroke 1999;30:1167–73. [DOI] [PubMed] [Google Scholar]
- 126. Aronowski J, Zhao X.. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011;42:1781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen J-C, Ho F-M, Pei-Dawn Lee C, Chen C-P, Jeng K-CG, Hsu H-B, Lee S-T, Tung WW, Lin W-W.. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 2005;521:9–20. [DOI] [PubMed] [Google Scholar]
- 128. Ding R, Chen Y, Yang S, Deng X, Fu Z, Feng L, Cai Y, Du M, Zhou Y, Tang Y.. Blood-brain barrier disruption induced by hemoglobin in vivo: involvement of up-regulation of nitric oxide synthase and peroxynitrite formation. Brain Res 2014;1571:25–38. [DOI] [PubMed] [Google Scholar]
- 129. Wu Y, Zhang R, Tran HD, Kurniawan ND, Moonshi SS, Whittaker AK, Ta HT.. Chitosan nanococktails containing both ceria and superparamagnetic iron oxide nanoparticles for reactive oxygen species-related theranostics. ACS Appl Nano Mater 2021;4:3604–18. [Google Scholar]
- 130. Eriksson P, Tal AA, Skallberg A, Brommesson C, Hu Z, Boyd RD, Olovsson W, Fairley N, Abrikosov IA, Zhang X, Uvdal K.. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci Rep 2018;8:6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Smith E, Gurol M, Eng J, Engel C, Nguyen T, Rosand J, Greenberg S.. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 2004;63:1606–12. [DOI] [PubMed] [Google Scholar]
- 132. Soluki M, Mahmoudi F, Abdolmaleki A, Asadi A, Sabahi Namini A.. Cerium oxide nanoparticles as a new neuroprotective agent to promote functional recovery in a rat model of sciatic nerve crush injury. Br J Neurosurg 2020;1–6. [DOI] [PubMed] [Google Scholar]
- 133. Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD.. Astrocytes promote myelination in response to electrical impulses. Neuron 2006;49:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hughes AN, Appel B.. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci 2020;23:1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Domingues HS, Portugal CC, Socodato R, Relvas JB.. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE.. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009;8:635–42. [DOI] [PubMed] [Google Scholar]
- 137. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014;10:44–58. [DOI] [PubMed] [Google Scholar]
- 138. Cahill J, Cahill WJ, Calvert JW, Calvert JH, Zhang JH.. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2006;26:1341–53. [DOI] [PubMed] [Google Scholar]
- 139. Sehba FA, Hou J, Pluta RM, Zhang JH.. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 2012;97:14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G.. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 1987;84:9265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Luo Z, Zhou Y, Yang T, Gao Y, Kumar P, Chandrawati R.. Ceria nanoparticles as an unexpected catalyst to generate nitric oxide from S-nitrosoglutathione. Small 2022;18:2105762. [DOI] [PubMed] [Google Scholar]
- 142. Ghajar J. Traumatic brain injury. Lancet 2000;356:923–9. [DOI] [PubMed] [Google Scholar]
- 143. Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime A-C, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang J-Y, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Söderberg J, Stanworth SJ, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K; InTBIR Participants and Investigators. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017;16:987–1048. [DOI] [PubMed] [Google Scholar]
- 144. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG.. Traumatic spinal cord injury. Nat Rev Dis Primers 2017;3:17018. [DOI] [PubMed] [Google Scholar]
- 145. Das M, Patil S, Bhargava N, Kang JF, Riedel LM, Seal S, Hickman JJ.. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007;28:1918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yiling W, Murakonda GK, Jarubula R.. Application of green-synthesized cerium oxide nanoparticles to treat spinal cord injury and cytotoxicity evaluation on paediatric leukaemia cells. Mater Res Express 2021;8:075006. [Google Scholar]
- 147. Fang X, Song H.. Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair. J Photochem Photobiol B 2019;191:83–7. [DOI] [PubMed] [Google Scholar]
- 148. Dong L, Kang X, Ma Q, Xu Z, Sun H, Hao D, Chen X.. Novel approach for efficient recovery for spinal cord injury repair via biofabricated nano-cerium oxide loaded PCL with resveratrol to improve in vitro biocompatibility and autorecovery abilities. Dose Response 2020;18:1559325820933518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Choi B, Soh M, Manandhar Y, Kim D, Han SI, Baik S, Shin K, Koo S, Kwon HJ, Ko G, Oh J, Hwang H, Hyeon T, Lee SJ.. Highly selective microglial uptake of ceria-zirconia nanoparticles for enhanced analgesic treatment of neuropathic pain. Nanoscale 2019;11:19437–47. [DOI] [PubMed] [Google Scholar]
- 150. Kastrinaki G, Samsouris C, Kosmidis EK, Papaioannou E, Konstandopoulos AG, Theophilidis G.. Assessing the axonal translocation of CeO2 and SiO2 nanoparticles in the sciatic nerve fibers of the frog: an ex vivo electrophysiological study. Int J Nanomedicine 2015;10:7089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shah V, Shah S, Shah H, Rispoli FJ, McDonnell KT, Workeneh S, Karakoti A, Kumar A, Seal S.. Antibacterial activity of polymer coated cerium oxide nanoparticles. PLoS One 2012;7:e47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sun Y, Sun X, Li X, Li W, Li C, Zhou Y, Wang L, Dong B.. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials 2021;268:120614. [DOI] [PubMed] [Google Scholar]
- 153. Lin W, Huang YW, Zhou XD, Ma Y.. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol 2006;25:451–7. [DOI] [PubMed] [Google Scholar]
- 154. Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM.. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 2006;40:6151–6. [DOI] [PubMed] [Google Scholar]
- 155. Park EJ, Choi J, Park YK, Park K.. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008;245:90–100. [DOI] [PubMed] [Google Scholar]
- 156. Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE.. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008;2:2121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yokel RA, Florence RL, Unrine JM, Tseng MT, Graham UM, Wu P, Grulke EA, Sultana R, Hardas SS, Butterfield DA.. Biodistribution and oxidative stress effects of a systemically-introduced commercial ceria engineered nanomaterial. Nanotoxicology 2009;3:234–48. [Google Scholar]
- 158. Yokel RA, Hussain S, Garantziotis S, Demokritou P, Castranova V, Cassee FR.. The yin: an adverse health perspective of nanoceria: uptake, distribution, accumulation, and mechanisms of its toxicity. Environ Sci Nano 2014;1:406–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yokel RA, Au TC, MacPhail R, Hardas SS, Butterfield DA, Sultana R, Goodman M, Tseng MT, Dan M, Haghnazar H, Unrine JM, Graham UM, Wu P, Grulke EA.. Distribution, elimination, and biopersistence to 90 days of a systemically introduced 30 nm ceria-engineered nanomaterial in rats. Toxicol Sci 2012;127:256–68. [DOI] [PubMed] [Google Scholar]
- 160. Grulke E, Reed K, Beck M, Huang X, Cormack A, Seal S.. Nanoceria: factors affecting its pro- and anti-oxidant properties. Environ Sci Nano 2014;1:429–44. [Google Scholar]
- 161. Lu M, Zhang Y, Wang Y, Jiang M, Yao X.. Insight into several factors that affect the conversion between antioxidant and oxidant activities of nanoceria. ACS Appl Mater Interfaces 2016;8:23580–90. [DOI] [PubMed] [Google Scholar]
- 162. Asati A, Santra S, Kaittanis C, Perez JM.. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010;4:5321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Rzigalinski BA, Carfagna CS, Ehrich M.. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. WIREs Nanomed Nanobiotechnol 2017;9:e1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Casals E, Zeng M, Parra-Robert M, Fernández-Varo G, Morales-Ruiz M, Jiménez W, Puntes V, Casals G.. Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small 2020;16:e1907322. [DOI] [PubMed] [Google Scholar]
- 165. Hardas SS, Sultana R, Warrier G, Dan M, Florence RL, Wu P, Grulke EA, Tseng MT, Unrine JM, Graham UM, Yokel RA, Butterfield DA.. Rat brain pro-oxidant effects of peripherally administered 5 nm ceria 30 days after exposure. Neurotoxicology 2012;33:1147–55. [DOI] [PubMed] [Google Scholar]
- 166. Anderson JM, Van Itallie CM.. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009;1:a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang T-T, Li W, Meng G, Wang P, Liao W.. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci 2016;4:219–29. [DOI] [PubMed] [Google Scholar]
- 168. Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F.. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater 2018;30:1801362. [DOI] [PubMed] [Google Scholar]
- 169. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016;15:275–92. [DOI] [PubMed] [Google Scholar]
- 170. Naseri Kouzehgarani G, Feldsien T, Engelhard HH, Mirakhur KK, Phipps C, Nimmrich V, Clausznitzer D, Lefebvre DR.. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv Drug Deliv Rev 2021;173:20–59. [DOI] [PubMed] [Google Scholar]
- 171. Fowler M, Cotter J, Knight B, Sevick-Muraca E, Sandberg D, Sirianni R.. Intrathecal drug delivery in the era of nanomedicine. Adv Drug Deliv Rev 2020;165:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Baldim V, Bedioui F, Mignet N, Margaill I, Berret J-F.. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018;10:6971–80. [DOI] [PubMed] [Google Scholar]
- 173. Szymanski CJ, Munusamy P, Mihai C, Xie Y, Hu D, Gilles MK, Tyliszczak T, Thevuthasan S, Baer DR, Orr G.. Shifts in oxidation states of cerium oxide nanoparticles detected inside intact hydrated cells and organelles. Biomaterials 2015;62:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hayat A, Andreescu D, Bulbul G, Andreescu S.. Redox reactivity of cerium oxide nanoparticles against dopamine. J Colloid Interface Sci 2014;418:240–5. [DOI] [PubMed] [Google Scholar]
- 175. Ciofani G, Genchi GG, Liakos I, Cappello V, Gemmi M, Athanassiou A, Mazzolai B, Mattoli V.. Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: proliferation, differentiation, and dopamine secretion. Pharm Res 2013;30:2133–45. [DOI] [PubMed] [Google Scholar]