Significance
In striking contrast to chimpanzees, tolerant between-group interactions in bonobos, our other closest living relative, have been suggested as a pathway to understanding the evolution of human between-group peacemaking and cooperation. However, due to the lack of data on bonobo group dynamics and partitioning, bonobo between-group tolerance has been routinely challenged. Here, we compared the social systems of bonobos and chimpanzees to find distinct and stable social groups in bonobos that interact regularly and peacefully. Despite their tolerant meetings that exceed the rates previously described between human groups, bonobo groups maintained exclusive social and spatial borders. Tolerance occurring between socially distinct groups highlights the potential of bonobos as a referential model for the evolution of between-group cooperation in humans.
Keywords: bonobo, intergroup relations, grouping patterns, chimpanzee, social structure
Abstract
Human between-group interactions are highly variable, ranging from violent to tolerant and affiliative. Tolerance between groups is linked to our unique capacity for large-scale cooperation and cumulative culture, but its evolutionary origins are understudied. In chimpanzees, one of our closest living relatives, predominantly hostile between-group interactions impede cooperation and information flow across groups. In contrast, in our other closest living relative, the bonobo, tolerant between-group associations are observed. However, as these associations can be frequent and prolonged and involve social interactions that mirror those within groups, it is unclear whether these bonobos really do belong to separate groups. Alternatively, the bonobo grouping patterns may be homologous to observations from the large Ngogo chimpanzee community, where individuals form within-group neighborhoods despite sharing the same membership in the larger group. To characterize bonobo grouping patterns, we compare the social structure of the Kokolopori bonobos with the chimpanzee group of Ngogo. Using cluster analysis, we find temporally stable clusters only in bonobos. Despite the large spatial overlap and frequent interactions between the bonobo clusters, we identified significant association preference within but not between clusters and a unique space use of each cluster. Although bonobo associations are flexible (i.e., fission–fusion dynamics), cluster membership predicted the bonobo fission compositions and the spatial cohesion of individuals during encounters. These findings suggest the presence of a social system that combines clear in-group/out-group distinction and out-group tolerance in bonobos, offering a unique referential model for the evolution of tolerant between-group interactions in humans.
Humans are thought to be unique in the extent to which interactions between groups are highly variable, ranging from tolerant and cooperative to hostile and violent (1–3). While hostile between-group interactions are thought to shape human within-group cooperation and favoritism (4, 5), between-group tolerance likely facilitates information transfer and large-scale cooperation (2, 6, 7). The evolutionary origins of the highly variable nature of human between-group relations are puzzling, given the relatively inflexible nature of between-group interactions observed in most other species, including one of our closest living relatives, chimpanzees (8).
Chimpanzees (Pan troglodytes) are a highly territorial species with nearly ubiquitous hostile, sometimes lethal, interactions between individuals of different communities (9–14), which impedes the emergence of affiliative or cooperative interactions across communities. Within chimpanzee communities, individuals maintain affiliative and cooperative relationships but do not permanently associate. Instead, chimpanzee within-community associations are flexible and vary in size, composition, and duration (i.e., “atomistic” fission–fusion dynamics) (15, 16). Furthermore, in large chimpanzee communities, like Ngogo, both sexes tend to form “neighborhoods”: subgroups of community members that associate more closely (17, 18). Males and females of different neighborhoods still share the same community membership and cooperate with one another, in striking contrast to their exclusively aggressive behavior toward other communities (10, 13). Given the intense competition between chimpanzee communities and their close relatedness with humans, chimpanzees are often used as a model species for the evolution of human warfare (12).
In contrast, our other closest living relative, the less-studied bonobo (Pan paniscus), is often regarded as a nonterritorial and xenophilic species (19). Bonobos seem to engage in tolerant between-group encounters (20–25), which can be nontransient (up to approximately 30% of the total observation time in certain populations) (21, 22, 24). These prolonged meetings provide opportunities for between-group interaction, and members of different groups are observed to groom, form coalitions, share food, and hunt with one another (23, 25, 26). While tolerant between-group meetings are also evident in other nonhuman primate species—like western lowland gorillas (27), geladas (28), and guinea (29) and hamadryas baboons (30)—those are typically characterized by aggregations near sleeping or feeding sites and lack the between-group cooperative exchange seen in bonobos. The bonobo, therefore, was proposed as an evolutionary model for the human capacity for tolerance and cooperation across groups (31).
Like chimpanzees, within-group associations in bonobos are characterized by fission–fusion dynamics. However, in contrast to chimpanzees, the large home-range overlap, tolerant and affiliative associations, and the lack of a clear behavioral distinction of in-group from out-group make defining group membership in bonobos challenging. For example, whereas aggressive patterns during chimpanzee encounters offer a clear-cut way to differentiate communities, it does not appear to be the case in bonobos. Bonobos in Wamba performed more coalitionary aggression during encounters than nonencounters but, in contrast to chimpanzees, these coalitions included individuals from both the same and different groups, and targeted both in-group and out-group members with similar physical intensity (25). In bonobos at Kokolopori, aggression rates increased when groups met but tended to be less coalitionary and, similar to Wamba, targeted both in-group and out-group members (22, 32). Therefore, in the absence of clear behavioral markers of bonobo group membership how can we be certain that what observers perceive as different bonobo groups are homologous to chimpanzee communities? It is possible that bonobo groups that frequently associate are instead homologous to chimpanzee neighborhoods, where subgrouping patterns are visible but occur under membership in the same large group. This would offer an alternative explanation for the affiliative and cooperative nature of their interactions that does not involve tolerance between members of different groups.
To advance a comparative, evolutionary perspective on human social structure, we have studied the bonobo social system in more detail. Specifically, we have tested whether bonobo groups are similar to the neighborhoods in large chimpanzee communities or whether, instead, they provide evidence for a system of tolerant between-community relations. We focus on the bonobo population of Kolokopori, where previous research has identified four groups, and we test whether these four groups are better described as neighborhoods or communities (21–23). To do so, we compare the association patterns of the Kokolopori bonobos with those of the unusually large Ngogo chimpanzee community, which has distinct neighborhoods (17, 18). Following a gradual increase in their community size, the Ngogo community recently separated into two distinct communities with defined territories and hostile interactions between them (33). Therefore, the Ngogo chimpanzees before the community split represent the extreme of within-group neighborhood partitioning so far observed in the genus Pan, and thus offer an ideal population to compare with the bonobos.
As a first step to compare the chimpanzees and bonobos association patterns, we conducted a cluster analysis to determine whether clusters (subgroups) can be identified within each population. We expected that if different groups exist in the bonobo population, then the compositions of the bonobo clusters would be more robust and consistent than in Ngogo chimpanzees. If robust clusters appeared, we additionally examined the characteristics of those clusters, allowing us to evaluate the degree of in-group/out-group social and spatial distinction. Specifically, we examined variation in characteristics that would reveal the presence of distinct communities: 1) migration patterns between groups; 2) strength of dyadic associations; 3) spatial partitioning: home-range uniqueness and home-range overlap between clusters; 4) fission decisions: who individuals associate with after a party fission; and 5) spatial cohesion: what spatial distance individuals maintain from their own cluster members in the presence of individuals from a different cluster. If the bonobo social dynamics revealed clearly distinct and consistent group membership patterns in all these domains, then we could confirm community distinction and between-group tolerance in Kokolopori bonobos.
Results
To identify and compare the social structure of the Kokolopori bonobos and Ngogo chimpanzees, we calculated a dyadic measure of association strength, the observed simple ratio index (SRIobs), from 30-min association scans of party membership. We calculated the annual SRIobs for each dyad involving individuals >10 y in each population (nKokolopori = 59 individuals and 1,575 dyads, nNgogo = 108 individuals and 5,770 dyads) over 3 consecutive years (2017 to 2019 for Kokolopori or 2011 to 2013 for Ngogo). During 2017 we did not record the presence of bonobos from two of the Kokolopori groups, Fekako and Bekako, as they were not yet well-habituated enough. Overall sizes of bonobo parties showed an average ± SD of 8.06 ± 4.03 individuals in 2017, 7.21 ± 3.57 individuals in 2018, and 6.56 ± 2.94 individuals in 2019. In comparison, sizes of Ngogo chimpanzee parties averaged 9.37 ± 7.92 individuals in 2011, 8.91 ± 7.68 individuals in 2012, and 9.64 ± 7.49 individuals in 2013.
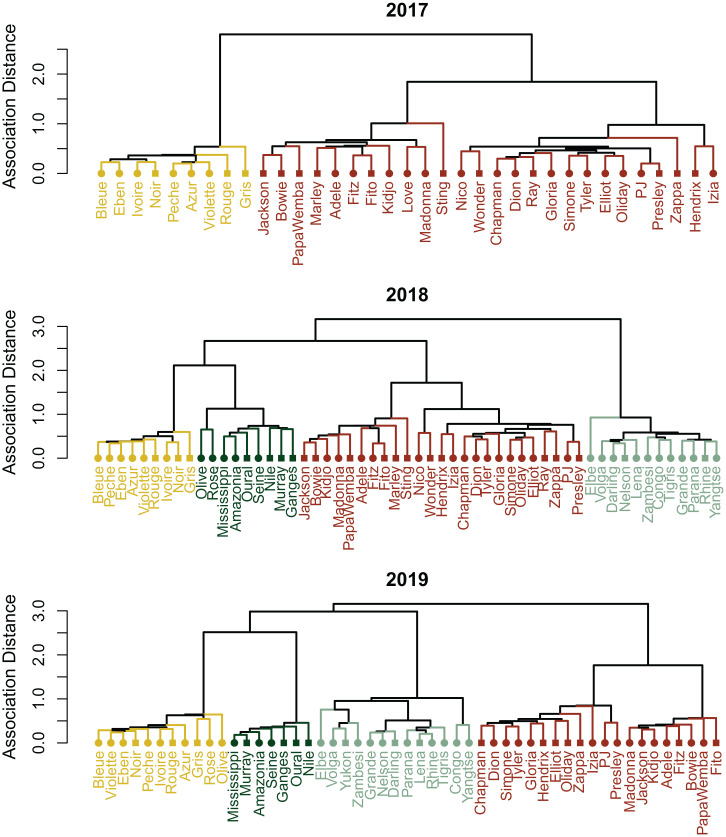
We performed hierarchical clustering analysis (34) on the yearly SRIobs and generated association distance dendrograms (Fig. 1). To evaluate clustering robustness within Kokolopori and Ngogo, we then used a method that indicates clustering fit quality—how well each individual fits within their respective cluster—the “silhouette coefficient” (35). Silhouette values closer to 1 indicate better clustering fit, whereas values lower than 0.3 indicate overall weak clustering. We found that cluster membership was more robust in bonobos than in chimpanzees (maximum average yearly silhouette coefficient [averaging the silhouette values of all individuals]: bonobos: 0.33 to 0.46; chimpanzees: 0.14 to 0.16) (Table 1 and SI Appendix, Fig. S1). Furthermore, the percentage of negative silhouette values, indicating that individuals may be assigned to the wrong cluster (observation mismatches), was substantially lower in Kokolopori (0 to 3%) compared with Ngogo (2 to 27%). The only observation mismatches in Kokolopori, 3% during 2018, were attributed to two newly immigrant nulliparous females (Olive and Rose), who appeared in the population during that year and regularly moved between the groups (see SI Appendix, Fig. S2 for migration patterns of nulliparous females in Kokolopori). We also documented visits of a single male from the Fekako group into the Ekalakala group (SI Appendix, Fig. S2) (4-d visits in both 2018 and 2019) but this did not result in an observation mismatch. Unlike the bonobos, observation mismatches in Ngogo chimpanzees involved males and parous females, and not the three newly immigrant nulliparous females.
Fig. 1.
Dendrogram visualization of Kokolopori bonobos hierarchical clustering analyses across years, with shorter distances on the y axis reflecting stronger associations. Different clusters are depicted in colors. Circles indicate females and squares males.
Table 1.
Kokolopori bonobo and Ngogo chimpanzee clustering tendencies
| Year | Population size* | Mean silhouette coefficient† | % Misclassification rates‡ | Optimal cluster numbers§ | |
|---|---|---|---|---|---|
| Kokolopori | 2017¶ | 35 (0.66) | 0.40 (2) | 0 | 2, 3, 3 (0.34) |
| 2018 | 55 (0.57) | 0.33 (4) | 3 | 4, 5, 4 (0.52) | |
| 2019 | 51 (0.5) | 0.46 (4) | 0 | 4, 5, 4 (0.66) | |
| Ngogo | 2011 | 104 (0.70) | 0.14 (3) | 27 | 3, 7, 4 (0.33) |
| 2012 | 106 (0.68) | 0.16 (2) | 2 | 2, 7, 3 (0.31) | |
| 2013 | 105 (0.69) | 0.15 (2) | 6 | 2, 7, 4 (0.32) |
*In parentheses the population sex ratio (male/female).
†In parentheses the optimal number of clusters.
‡Percentage of observations with negative silhouette values.
§Optimal number of clusters by different methods: from left to right, the silhouette coefficient, affinity propagation, and modularity. In parentheses is the modularity value.
¶Only individuals of two potential groups (Ekalakala and Kokoalongo) included.
To further determine the robustness of the identified clusters, we applied two additional methods to evaluate the optimal yearly number of clusters per population, affinity propagation and modularity (see descriptions in Materials and Methods). We found high consistency between the three methods in Kokolopori (Table 1). The optimal cluster membership detected by the different methods corresponded to the four Kokolopori groups: that is, Ekalakala, Kokoalongo, Fekako, and Bekako. The only disagreement between the methods was whether Kokoalongo was a single group or two separate ones (hereafter referred to as Kokoalongo subclusters: KokoalongoN and KokoalongoS). In Ngogo, the optimal number of clusters detected by the different methods varied to a greater extent than in Kokolopori (Table 1). Specifically, in 2011 to 2012 cluster number variation did not follow a clear pattern of nested structures, since a lower number of clusters detected by one method was not a result of the merging of two or more clusters detected by another method, as in Kokolopori. However, in 2013 the clusters detected by the different methods were almost entirely nested, and only seven individuals were assigned to clusters that were not nested (SI Appendix, Fig. S3).
While the higher average silhouette coefficient value, lower observation mismatches, and higher comparability between clustering methods in Kokolopori as compared to Ngogo suggest a higher-quality clustering pattern in the bonobo population, they do not inform us on differences in the temporal stability of cluster membership. We therefore evaluated cluster membership consistency across the 3 y in both samples using the silhouette coefficient method. We found that 93% of dyads in Kokolopori showed cluster membership consistency across years, with dyadic inconsistencies stemming from a change in cluster membership for the two newly immigrant females. In contrast, in Ngogo only 43% of dyads showed cluster membership consistency across years, and inconsistencies included female–female (8%), male–male (23%), and female–male (69%) dyads.
Overall, bonobo groups in Kokolopori are more consistent, robust, and stable than chimpanzee neighborhoods in Ngogo. Given the absence of stable and consistent clusters in Ngogo, in the following set of analyses we only examined the bonobo fission–fusion characteristics within and between the detected clusters. As the different methods identified the Kokoalongo cluster as either a single cluster or two separate ones, we investigated both scenarios. Specifically, to investigate whether the clustering structure in Kokolopori yielded groups that were comparable to chimpanzee communities, we examined whether 1) association patterns within and between clusters differed from random expectation, 2) cluster members showed spatially distinct ranging patterns (15), and 3) fission patterns were cluster specific.
The amount of time and days individuals of different clusters associated together varied greatly across cluster combinations and years (Table 2), from 1% of party scans and 4% of observation days for Kokoalongo and Fekako in 2019, to 27% of party scans and 52% of observation days for Kokoalongo and Ekalakala in 2018. Kokoalongo and Ekalakala showed high association rates that were stable across years (averaged at 35% of observation days per year), comparable to previously described association rates between them [24% of observation time (21) and 36% of observation days (22)]. Association rates between the two Kokoalongo subclusters were higher than all other between-cluster associations, varying between 45% and 54% of party scans and 67% and 74% of observation days. The duration in consecutive days of between-cluster associations varied between 1 and 13 (1 to 11 d in 2017, 1 to 13 d in 2018, 1 to 12 d in 2019), and 68% to 76% of between-cluster dyads were observed together at least once across the 3 y (100% of dyads between the Kokoalongo subclusters).
Table 2.
Association preference, home-range overlap, and encounter rates within and between clusters in Kokolopori bonobos
| Clusters | No. Dyads | % Significant associates* | Home-range overlap† | % Encounter scans (d)‡ | ||
|---|---|---|---|---|---|---|
| 2017 | Ekalakala | Ekalakala | 36 | 100 (36) | 0.97 ± 0.01 | — |
| Ekalakala | Kokoalongo | 234 | 0 | 0.54 ± 0.03 | 16 (28) | |
| Kokoalongo | Kokoalongo | 325 | 70 (230) | 0.87 ± 0.06 | — | |
| KokoalongoN | KokoalongoN | 105 | 89 (94) | 0.94 ± 0.03 | — | |
| KokoalongoS | KokoalongoS | 55 | 98 (54) | 0.88 ± 0.04 | — | |
| KokoalongoS | KokoalongoN | 165 | 49 (82) | 0.83 ± 0.04 | 47 (73) | |
| 2018 | Ekalakala | Ekalakala | 55 | 83 (46) | 0.93 ± 0.07 | — |
| Ekalakala | Kokoalongo | 275 | 0 | 0.74 ± 0.09 | 27 (52) | |
| Kokoalongo | Kokoalongo | 300 | 67 (202) | 0.88 ± 0.07 | — | |
| KokoalongoN | KokoalongoN | 105 | 85 (90) | 0.90 ± 0.08 | — | |
| KokoalongoS | KokoalongoS | 45 | 77 (35) | 0.93 ± 0.03 | — | |
| KokoalongoS | KokoalongoN | 150 | 51 (77) | 0.86 ± 0.06 | 54 (74) | |
| 2019 | Ekalakala | Ekalakala | 55 | 100 (55) | 0.96 ± 0.03 | — |
| Ekalakala | Kokoalongo | 220 | 0 | 0.60 ± 0.04 | 12 (25) | |
| Ekalakala | Fekako | 77 | 0 | 0.35 ± 0.07 | 7 (16) | |
| Fekako | Fekako | 21 | 100 (21) | 0.96 ± 0.02 | — | |
| Fekako | Kokoalongo | 140 | 0 | 0.36 ± 0.08 | 1 (4) | |
| Kokoalongo | Kokoalongo | 190 | 87 (67) | 0.91 ± 0.05 | — | |
| KokoalongoN | KokoalongoN | 66 | 95 (63) | 0.92 ± 0.06 | — | |
| KokoalongoS | KokoalongoS | 28 | 100 (28) | 0.95 ± 0.02 | — | |
| KokoalongoS | KokoalongoN | 96 | 79 (76) | 0.90 ± 0.05 | 45 (67) | |
*Percentage (and number) of dyads that are significant associates, defined as SRIobs > SRIexp in at least 95% of cases (randomizations were conducted at the level of the population).
†Mean ± SD 95% kernel dyadic overlap (using 200-m grid).
‡Percentage of scans or days (in parentheses) with mixed cluster parties, defined as parties containing at least one member of each of the two clusters.
To examine whether cluster associations were significantly stronger than random expectations, we used party composition permutations and detected the occurrence of “significant associates” (i.e., dyadic SRIobs higher than expected in at least 95% of cases). See Materials and Methods for the permutation procedure and for the treatment of cluster membership inconsistencies across methods. A permutation procedure, including all known adult bonobos, revealed no significant associates between clusters, and nearly complete significant associates within-cluster (100% in Ekalakala and Fekako) (Table 2). We also found more significant associates within than between the two Kokoalongo subclusters (Table 2). Since nearly all within-cluster dyads and no between-cluster dyads showed nonrandom associations, we confirmed that bonobo clusters are robust, characterized by strong within-cluster and weak between-cluster associations.
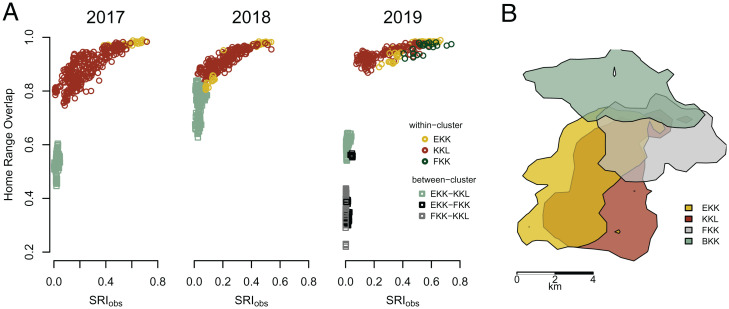
To evaluate whether the Kokolopori clusters can also be identified by space use—indicating cluster-specific home-range uniqueness—we assessed the home-range overlap of all within- and between-cluster dyadic combinations using the GPS locations and compositions of party scans (Materials and Methods). Home-range overlap of 1 represents a 100% spatial overlap between individuals. Dyadic home-range overlap varied with a yearly average of 0.88 to 0.98 within clusters and 0.35 to 0.76 between clusters (Table 2). Visualization of the relationship between the dyadic home-range overlaps and the SRIobs (the association value upon which clustering was determined) showed spatial partitioning by cluster membership (Fig. 2A). Spatial partitioning was less differentiated between the Kokoalongo subclusters and home-range overlap appeared as a continuum (SI Appendix, Fig. S4). Despite large home-range overlap between members of different clusters, each cluster still ranged exclusively in areas that were not used by members of other clusters (Fig. 2B).
Fig. 2.
Ranging patterns of Kokolopori bonobos. (A) The yearly home-range overlap (y axis) as a function of the SRIobs (x axis) for all bonobo dyads from cluster combinations that were regularly followed by human observer (2017 to 2019: EKK, Ekalakala and KKL, Kokoalongo; 2019: FKK, Fekako). Circles or squares represent values for within- or between-cluster dyads, respectively. (B) The 95% kernel home-range estimates of the four Kokolopori bonobo clusters, Ekalakala (EKK, 35 km2), Kokoalongo (KKL, 40 km2), Fekako (FKK, 18 km2), and Bekako (BKK, 21 km2).
As a next step to characterize the bonobo social system, we evaluated their fission patterns. Bonobo within-cluster associations showed “atomistic” fission–fusion dynamics as not all within-cluster members permanently associated with one another. This was reflected in the party size relative to cluster size in Kokolopori, which varied between 0.33 in Kokoalongo and 0.71 in Fekako (SI Appendix, Table S1). Furthermore, party permutations conducted separately within each cluster revealed that only about a half (46 to 52%) of cluster members were significant associates (SI Appendix, Table S1).
We investigated how cluster membership predicted who remained in association after fissions. To identify the underlying rules governing fission compositions, we used “association rule mining” (36), a method that detects relationship probabilities between different objects in a dataset. Specifically, we evaluated the probabilities of individuals from cluster A and B to appear together after a fission (i.e., using the “support” parameter) (Materials and Methods). For all cluster combinations, we found that only within cluster members had at least 5% probability (α level of 0.05) to appear together in postfission parties. The maximum probability of postfission associations of individuals from different clusters was 3% between Ekalakala and Kokoalongo and <1% between Fekako and both Ekalakala and Kokoalongo. Between the Kokoalongo subclusters, postfission patterns with at least 5% probability involved members of both subclusters (38% of dyads).
As a final investigation of the bonobo fission–fusion patterns, we examined whether cluster members maintained spatial cohesion while in the presence of members of other clusters. We evaluated spatial cohesion by identifying two co-occurring parties, each including individuals from the same cluster and at least one of them also including individuals from another cluster (i.e., mixed-cluster parties) and estimated the distance between them. The 95th distance percentile—representing the upper distance limit of 95% of these instances—was equal to or lower than 400 m for all between cluster combinations (Ekalakala–Kokoalongo: 400 m, n = 4,214 party scans; Ekalakala–Fekako: 240 m, n = 197 scans; Fekako–Kokoalongo: 350 m, n = 150 scans). In comparison, the 95th distance percentile between the two Kokoalongo subclusters—as a proxy of the typical distance maintained within different subgroups of the same group—was 1,500 m (n = 1,436 scans), and the average distance between parties composed of individuals from exclusively different clusters was >3,000 m (Ekalakala–Kokoalongo: 3,138 m, n = 12,790 scans; Ekalakala–Fekako: 6,113 m, n = 3,810 scans; Fekako–Kokoalongo: 4,824 m, n = 3,344 scans). The distances maintained between individuals from the same cluster when other clusters are present are well below the approximately 700-m audible distance of bonobo vocalizations (37), likely allowing cluster members to coordinate their movements in space.
Discussion
Our findings demonstrate that the social structure of the Kokolopori bonobos comprises stable and clearly delineated clusters (hereafter groups) with distinct ranging and fission patterns. The bonobo group membership was consistent and stable over time despite frequent and prolonged associations between different groups that lasted from a few hours to 2 wk. The identified grouping patterns in bonobos were substantially more predictable and consistent than the neighborhood grouping patterns of the large Ngogo chimpanzee community. Furthermore, bonobo group membership predicted the occurrence of significant associates, unique home-range usage, fission decisions, and spatial cohesion during encounters, indicating that within-group associations in Kokolopori are homologous to those in chimpanzee communities. Taking these data together, we demonstrate the presence of a clear distinction between regularly associating bonobo groups along which cooperative exchange across borders can emerge. Our results also indicate that within these groups, additional substructures that are more similar to chimpanzee neighborhoods (the Kokoalongo subclusters) can be formed.
Association tendencies between the four bonobo groups varied considerably, with two of the groups, Ekalakala and Kokoalongo, associating together for approximately 35% of days over the 3-y study period. This result echoes previous findings in the same population (21, 22) and findings from another bonobo population, Wamba, in which the most frequent between-group associations occurred at 27% of observation days (24). Interactions between other group combinations at Kokolopori were rarer (Table 2), indicating association preference between different bonobo groups. Prolonged and tolerant between-group associations do not seem ubiquitous across bonobo populations and are considered rare in LuiKotale and Lomako (26, 38), raising questions regarding the potential ecological and social pressures promoting tolerant between-group interactions and demonstrating flexibility in social strategies within this species. Similarly, ecological variation has long been suggested as one of the key drivers for differences in tolerance and competition between chimpanzees and bonobos (39).
Bonobo and chimpanzee societies are traditionally classified as male philopatric, with females typically migrating to other groups before adulthood. Similar to other bonobo populations, young females in Kokolopori regularly moved between different groups within the population (often during encounters) (SI Appendix, Fig. S2) before remaining and reproducing in one group. In contrast, male bonobos typically remain in their natal group and therefore their association patterns may offer a better marker of group identity. Nonetheless, evidence suggests that group membership of male bonobos is less restricted than male chimpanzees, and both bonobo male transfers (40) and visits (41) to other groups have been previously described. Our observations of temporary male visits between groups in Kokolopori add to these reports, and together suggest greater flexibility of group movements for male bonobos compared to chimpanzees.
Other social species who maintain tolerant associations between individuals of different social groups or units are typically those living in multilevel societies. Gatherings of different social units in these species often occurs near sleeping or feeding sites and are suggested to improve predation avoidance, collective group defense, and information flow (27, 30, 42–44). The optimization of foraging efficiency through improved information flow has been hypothesized as a potential benefit of between-group tolerance in bonobos, given frequent joint foraging activities and a reduced tendency to end an encounter in areas less familiar to at least one of the groups (21). However, bonobo foraging efficiency in less familiar areas (estimated by c-peptide, an energy balance biomarker) did not increase in the presence of another group that was more familiar with those areas (45). An alternative explanation is that between-group encounters in bonobos do not offer functional benefits, but instead are a by-product of the strong bonobo predisposition to interact socially, as suggested for African elephants (34). Flexibility in bonobo between-group relationships offers a context to explore the proximate and ultimate pressures that promote between-group associations and cooperation or shift from cooperation to separation.
In humans, improved information flows through high rates of interband interactions, and large numbers of social partners outside the resident group may have promoted the evolution of cumulative culture (2, 6). The yearly dyadic average association probabilities (i.e., sleeping in the same camp and engaging in a conversation at least once) of interband dyads in hunter-gatherers reached 29% in the Ache and 12% in the Hadza and occurred within a wide social network consisting of a thousand potential interaction partners over an individual’s lifespan (2). That bonobos also associate with multiple individuals outside their residential group offers a unique comparative model to the human social system (19). The probabilities of between-group dyadic associations in Kokolopori bonobos were high, with over 68% of between-group dyads associating at least once each year within a social network of 59 potential adult partners over a 3-y period. High between-group association rates in bonobo likely provide ample opportunities for affiliative and cooperative interactions between individuals of different groups, which may offer an additional benefit afforded by between-group tolerance in bonobos, but this remains to be tested.
Group membership in Kokolopori is associated with increased within-group behavioral homogeneity, for example in their choice of prey type (23), which may suggest that in addition to stable and consistent grouping patterns, bonobo groups also show group-specific behavior. In a system that combines between-group tolerance and within-group behavioral homogeneity, opportunities to share knowledge across groups can promote the proliferation of cumulative cultural practices (2). However, given that the size of the bonobo social network is more limited than humans, with far fewer potential interaction partners during an individual’s lifetime (in the hundreds instead of thousands), information flow and subsequent accumulation of knowledge are likely more restricted. Furthermore, as observable behavioral diversity is biased toward tool-use (46), and since evidence for tool-use in bonobos is limited (47, 48), it is likely challenging to detect cultural practices in bonobos. Natural variation in association tendencies between different bonobo groups and group-specific behavioral expressions offers a valuable platform to examine the role of tolerance and social network size in supporting knowledge sharing and the emergence of cultural practices in nonhumans.
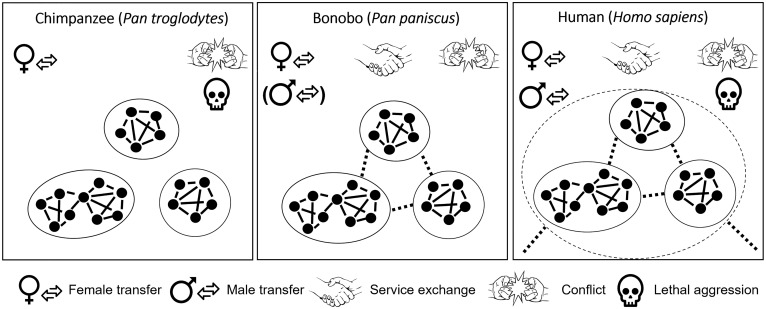
While bonobos and chimpanzees interact with fewer partners over their lifespan, they do share with humans a multimale/multifemale social structure, high association flexibility, and a wide diversity of relationship types and cooperative exchange among related and unrelated individuals (2, 11, 38, 49–53). The tolerant between group relationships observed in bonobos may serve as the building blocks upon which the interband social structures in humans could have evolved. As such, the bonobo social system may represent the transitional state from an ancestral state of closed multimale/multifemale groups to the aggregation of groups into the multilevel structure observed in humans (Fig. 3). The evolution of cooperation between different groups in the human social system has been attributed to the advent of pair bond formation and exogamy in the human lineage (7). Given our finding that bonobos form distinct social entities (communities) with cooperation and alliance formation between them in the absence of pair bonding challenges the notion of pair bonding as a necessary element of between-group alliance emergence in the human lineage. The flexible associations, in-group/out-group categorization, and recurrent tolerant and cooperative between-group interactions in bonobos offer a unique referential model for the evolution of between-group tolerance and cooperation in humans.
Fig. 3.
Schematic representation of between-group relationships in hominids. Individuals are represented by nodes and social interactions by links, forming social networks (groups). Black circles delineate different communities in Pan and bands in modern hunter-gatherers, with human bands varying substantially in consistency of band membership across cultures. Dotted links represent tolerant associations between groups and dotted circles represent the multilevel social organization observed in humans.
Materials and Methods
Study Sites and Data Collection.
We investigated association dynamics between 59 adult bonobos at the Kokolopori Bonobo Reserve, Democratic Republic of Congo (54). The Kokolopori bonobos have been followed by a team of the Bonobo Conservation Initiative (BCI), and the local NGO Vie Sauvage (VS) since 2007. In 2016, M.H.S. established a research field site in Kokolopori and initiated a detailed data-collection protocol including demographic (i.e., birth, death, migration), association, and behavior data of two habituated bonobo groups, Ekalakala and Kokoalongo. At least two observers followed each bonobo group daily from dawn to dusk, recording behavioral data with the cybertracker program, and location data using a GPS (Garmin© 62) with the tracklog function set at 1-min intervals.
During party follows, the habituated Ekalakala and Kokoalongo groups encountered other, semihabituated bonobos belonging to the Fekako (7 adults) and Bekako (12 to 14 adults) groups. Fekako and Bekako were irregularly followed by a BCI and VS team since 2015, and data on their ranging patterns and group demography were collected at rates of ∼100 d/y, however, without the collection of detailed association data. In November 2018, M.H.S. initiated regular daily follows of the Fekako group. By February 2019, the habituation status of the Fekako individuals permitted the initiation of a full day data-collection protocol identical to the one employed in Ekalakala and Kokoalongo.
Here, we used 3 y of association and demography data, collected between January 2017 and December 2019 during party follows (55), to characterize the social system of bonobos within our study area. In total, we collected data on all adults and adolescents >10 y during 1,000 observation days in Ekalakala (9 to 11 individuals), 1,075 observation days in Kokoalongo (18 to 27 individuals), and 257 observation days in Fekako (7 individuals). We did not include in the analyses short-term visits of unknown females (SI Appendix, Fig. S2).
To contextualize potential within- and between-group association dynamics of the Kokolopori bonobos, we compared those to the within-group association patterns of the Ngogo chimpanzee group, Kibale National Park, Uganda, observed continuously since 1995 (56). Ngogo is the largest known chimpanzee group, and by early 2010 consisted of approximately 108 adult and adolescent group members (>10 y). Due to the unusual size of the group and the tendency of the Ngogo individuals to form association modules (17, 18), Ngogo provides an ideal comparative setting to evaluate the social dynamics of the Kokolopori bonobos. Here, similarly to the bonobos, we used 3 full years of data collected between 2011 and 2013 during 806 observation days (104 to 106 individuals, >10 y).
Dyadic Associations.
We recorded the cumulative party composition at 30-min intervals (Kokolopori) or 15-min intervals (Ngogo), marking every weaned individual observed during the scan and starting anew every 30- or 15-min. If the same individuals were followed by more than one observer, we randomly selected one data point, to ensure that parties followed by more than one observer were not overrepresented in the data. For comparability between the Kokolopori and the Ngogo datasets, we converted the Ngogo data into 30-min party composition scans based on all individuals observed during each two 15-min scans within the half hour. We only included individuals >10 y in the party composition analyses.
We used the 30-min party association scans to calculate the observed SRIobs for each dyad and year, in Kokolopori (n = 1,575 dyads, 2017 to 2019) and Ngogo (n = 5,770 dyads, 2011 to 2013). We calculated the SRI as PAB/(PA + PB − PAB), with PAB being the number of scans A and B were observed together, and PA and PB the number of scans in which A or B were observed, respectively.
Intrinsic Assessments of Association Cluster Quality.
To identify the underlying structure of the Kokolopori bonobo and Ngogo chimpanzee association patterns, we applied hierarchical clustering analysis (34) on the yearly SRIobs dissimilarity matrix (transformed χ2/3) (27). Based on Ward’s clustering criterion (57), we conducted yearly agglomerative hierarchical clustering using the agnes function of the “cluster” package in R.
We assessed the quality of clustering patterns within Kokolopori and Ngogo using the silhouette coefficient (35). We computed the average silhouette coefficient in R using the function silhouette of the package “cluster.” The coefficient is based on the average distance of within (compactness) and between (separation) cluster observations, revealing how well each observation (i.e., individual) fits within their respective cluster. It is a measure of cluster quality that is normalized between −1 and 1. Silhouette values close to −1 infer that individuals are likely assigned to the wrong cluster, values close to 0 infer that individuals can be equally assigned to any of the identified clusters, and values close to 1 indicate a good clustering. Overall, silhouette values < 0.3 represent weak clustering (35). We evaluated the optimal average silhouette coefficient within Kokolopori and Ngogo, by varying the number of potential clusters between 2 and 10 and identifying the solution that yields the maximum mean silhouette value per population. Misclassification rates of observations can be used as an additional measure of cluster fit quality. That is, once the optimal number of clusters within each population is evaluated by the silhouette approach, we assess the misclassification rates as the number of observations with a negative value divided by the total observation number. We compared the maximum silhouette values and the percentage of misclassifications for the respective optimal cluster number between populations.
Furthermore, we evaluated cluster membership consistency across the 3 y in both samples using the silhouette coefficient method. Because we are interested in measuring how consistent is the appearance of dyads in the same cluster over time, we focused on dyads that clustered together in at least 1 of the years. We calculated the proportion of consistent dyads in each population by dividing the number of dyads that clustered together in all the years they appeared in the data by the total number of dyads that clustered together in at least 1 of the years. Dyads had to appear in the data for at least 2 y to be included in the calculations. We multiplied the proportion by 100 and present percentages, with an outcome of 100% indicating no temporal change in cluster membership.
We applied two additional methods, affinity propagation and modularity, to determine how the optimal yearly number of clusters varies between methods as an additional mean to assess robustness. Affinity propagation is a centroid-based clustering algorithm that exchanges “messages” between all data points (here individuals) until a set of reliable clusters emerge (58). The modularity maximization approach measures the density of within-cluster connections compared to the density of between-cluster connections (59). The optimal number of clusters occurs when no improvement in modularity can be achieved. We used the “apcluster” package and function, and the cluster_louvain function of the “igraph” package (60), to assessed affinity propagation and modularity, respectively.
Quantifying Within- and Between-Cluster Associations in Bonobos.
We used the yearly bonobo cluster membership, as detected by the three different measures, to assess within- and between-cluster variation of association patterns. We only included data on bonobo clusters that were observed regularly (Ekalakala and Kokoalongo 2017 to 2019, Fekako 2019), because members of clusters not followed by an observer appeared in the data only during encounters. Assessments of clustering patterns revealed two inconsistencies between the methods in 2018. The inconsistencies involved the cluster membership identity of two nulliparous females that immigrated into the Kokolopori groups during 2018. The two females were either assigned to Ekalakala, which is a group that was regularly followed by human observers, or to Fekako, which at the time was not followed by observers. Because we only used data from clusters with regular behavioral data collection in the set of bonobo-specific analyses, we included these two females in the Ekalakala cluster during 2018. During 2019, the two females were consistently assigned to the Ekalakala cluster by all methods.
Evaluating Association Preference within and between Bonobo Clusters via Data Permutations.
To reliably discern association preference, we accounted for the nonindependence of party observations (61) by generating the pattern expected from the data given random associations (null model). To achieve random association probabilities, we bootstrapped the party compositions of each year (1,000 replicates) with replacement (62). We conducted the party composition permutations while accounting for the underlying structure of the party scan data collection by 1) keeping the party size in each scan constant (52, 63), and 2) the likelihood for each individual to be randomly selected contingent on their occurrence likelihood in the yearly data (62). Following this procedure, we conducted 1,000 permutations of the observed party scan data for each year. To be able to evaluate dyadic association preference on the different levels of the social system (i.e., population and cluster), we repeated this procedure four times, by conducting the party composition randomizations either for all clusters together or separately for each of the three clusters with regular data. We used the randomized dataset to calculate the expected simple ratio index (SRIexp, based on 1,000 randomizations) for each dyad and year, and compared these to the SRIobs to evaluate dyadic association preference (i.e., significant associates) (64). Dyads were considered as significant associates when the dyadic SRIobs was higher than the SRIexp in at least 95% of cases (minimum of 950 of 1,000 randomizations), indicating that there is less than 5% probability that the observed association is a result of random processes.
Examining Home-Range Overlap within and between Bonobo Clusters.
To evaluate variation in spatial usage within and between clusters, we measured dyadic spatial overlap using the compositions of the 30-min party scans together with their GPS locations, extracted at the scan midpoint (i.e., 15 min after the start of the scan). For every individual that was present in the data throughout each year, we determined the yearly home-range utilization distribution using the function kernelUD of the R package “AdehabitatHR” (v0.4.16), based on the assembly of their GPS party locations. We then calculated the 95% kernel dyadic home-range overlaps using the function kerneloverlaphr.
Quantifying Fission Decisions within and between Bonobo Clusters.
We investigated fission decisions via association rule mining (36), a method often used to identify an underlying rule structure that can explain a set of economic transactions, and by this informing purchasing decisions of customers. Essentially, association rule mining detects relationships between different objects in large datasets and reveals the rules that explain these associations (36). We applied this method to the bonobo party association data to identify the underlying rule structure of fission decisions of each cluster with continuous data (i.e., Ekalakala, Kokoalongo, and Fekako). For each 30-min party composition scan collected by the same observer (prioritizing one observer per cluster and therefore a total of three fission datasets) we evaluated whether that party scan was a result of a fission event from the direct previous scan. We defined fissions whenever at least one individual that was present in the direct previous 30-min scan was not present in the following one, independent of cluster membership or party size. Overall, 40% of fissions occurred on days during which individuals of different clusters associated together. We generated the set of rules underlying fissions using the apriori function of the “arules” package in R (36), and setting the support and confidence parameters to 0.01 so to not limit the detection of associations. The support parameter reflects the probability of A and B to be observed (here fission) together (i.e., probability of the condition), whereas the confidence parameter reflects the conditional probability of B given A (the fission compositions in which B is observed out of the fission compositions of A, i.e., confidence of the condition).
Additionally, for each cluster combination, we explored the maximum distance between 95% of mixed-cluster parties. When two observers simultaneously followed different clusters, we could calculate the distance between them using the GPS tracklog data. For each 30-min party composition scan with data from two observers, we calculated the spatial GPS distance at the scan midpoint and characterized each party as a mixed-cluster party or not.
Ethics Statement.
The research presented here was noninvasive and adhered to the principles for the ethical treatment of nonhuman primates of the American Society of Primatologists. The research was approved by the Max Planck Society and Harvard University.
Supplementary Material
Acknowledgments
We thank the Institut Congolais pour la Conservations de la Nature and the Ministry of Scientific Research and Technology in the Democratic Republic of the Congo for their support and permission to work in the Kokolopori Bonobo Reserve, Democratic Republic of Congo; the Uganda Wildlife Authority, and the Uganda National Council for Science and Technology for permission to work in the Kibale National Park; Sam Angedakin, Sebastian Ramirez Amaya, and Kevin Lee for assistance with the long-term database at Ngogo; John Mitani and David Watts for logistical support; the Bonobo Conservation Initiative and Vie Sauvage, especially Sally Coxe and Albert Lotana Lokasola, for supporting our work; the Kokolopori Bonobo Research Project and Ngogo Chimpanzee Project staff members; and Alexander Mielke and Erin Wessling for helpful discussions and comments. Funding was provided by Harvard University, Arizona State University, and the Max Planck Society.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201122119/-/DCSupplemental.
Data Availability
The data and R code used in the study are available via GitHub (65).
References
- 1.Zefferman M. R., Mathew S., An evolutionary theory of large-scale human warfare: Group-structured cultural selection. Evol. Anthropol. 24, 50–61 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Hill K. R., Wood B. M., Baggio J., Hurtado A. M., Boyd R. T., Hunter-gatherer inter-band interaction rates: Implications for cumulative culture. PLoS One 9, e102806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrangham R. W., Glowacki L., Intergroup aggression in chimpanzees and war in nomadic hunter-gatherers: Evaluating the chimpanzee model. Hum. Nat. 23, 5–29 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Bernhard H., Fischbacher U., Fehr E., Parochial altruism in humans. Nature 442, 912–915 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Bowles S., Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Migliano A. B., et al. , Hunter-gatherer multilevel sociality accelerates cumulative cultural evolution. Sci. Adv. 6, eaax5913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapais B., Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. 22, 52–65 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Sterelny K., The origins of multi-level society. Topoi (Dordr.) 40, 207–220 (2021). [Google Scholar]
- 9.Wilson M. L., et al. , Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Langergraber K. E., Watts D. P., Vigilant L., Mitani J. C., Group augmentation, collective action, and territorial boundary patrols by male chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 114, 7337–7342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuni L., Crockford C., Wittig R. M., Group-level cooperation in chimpanzees is shaped by strong social ties. Nat. Commun. 12, 539 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrangham R. W., Evolution of coalitionary killing. Am. J. Phys. Anthropol. (suppl. 29), 1–30 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Mitani J. C., Watts D. P., Amsler S. J., Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Williams J. M., Oehlert G. W., Carlis J. V., Pusey A. E., Why do male chimpanzees defend a group range? Anim. Behav. 68, 523–532 (2004). [Google Scholar]
- 15.Grueter C. C., et al. , Multilevel organisation of animal sociality. Trends Ecol. Evol. 35, 834–847 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Aureli F., et al. , Fission‐fusion dynamics: New research frameworks. Curr. Anthropol. 49, 627–654 (2008). [Google Scholar]
- 17.Wakefield M. L., Grouping patterns and competition among female Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 29, 907 (2008). [Google Scholar]
- 18.Mitani J. C., Amsler S. J., Social and spatial aspects of male subgrouping in a community of wild chimpanzees. Behaviour 140, 869–884 (2003). [Google Scholar]
- 19.Furuichi T., Variation in intergroup relationships among species and among and within local populations of African Apes. Int. J. Primatol. 41, 203–223 (2020). [Google Scholar]
- 20.Itani G., Relations between unit-groups of Bonobos at Wamba, Zaire: Encounters and temporary fusions. Afr. Study Monogr. 11, 153–186 (1990). [Google Scholar]
- 21.Lucchesi S., et al. , Beyond the group: How food, mates, and group size influence intergroup encounters in wild bonobos. Behav. Ecol. 31, 519–532 (2020). [Google Scholar]
- 22.Cheng L., et al. , Variation in aggression rates and urinary cortisol levels indicates intergroup competition in wild bonobos. Horm. Behav. 128, 104914 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Samuni L., Wegdell F., Surbeck M., Behavioural diversity of bonobo prey preference as a potential cultural trait. eLife 9, e59191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamaki T., Ryu H., Toda K., Tokuyama N., Furuichi T., Increased frequency of intergroup encounters in wild bonobos (Pan paniscus) around the yearly peak in fruit abundance at Wamba. Int. J. Primatol. 39, 685–704 (2018). [Google Scholar]
- 25.Tokuyama N., Sakamaki T., Furuichi T., Inter-group aggressive interaction patterns indicate male mate defense and female cooperation across bonobo groups at Wamba, Democratic Republic of the Congo. Am. J. Phys. Anthropol. 170, 535–550 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Fruth B., Hohmann G., Food sharing across borders: First observation of intercommunity meat sharing by Bonobos at LuiKotale, DRC. Hum. Nat. 29, 91–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison R. E., Groenenberg M., Breuer T., Manguette M. L., Walsh P. D., Hierarchical social modularity in gorillas. Proc. Biol. Sci. 286, 20190681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai M., Ohsawa H., Mori U., Dunbar R., Social organization of gelada baboons: Social units and definitions. Primates 24, 13–24 (1983). [Google Scholar]
- 29.Patzelt A., et al. , Male tolerance and male-male bonds in a multilevel primate society. Proc. Natl. Acad. Sci. U.S.A. 111, 14740–14745 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schreier A. L., Swedell L., The fourth level of social structure in a multi-level society: Ecological and social functions of clans in hamadryas baboons. Am. J. Primatol. 71, 948–955 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Pisor A. C., Surbeck M., The evolution of intergroup tolerance in nonhuman primates and humans. Evol. Anthropol. 28, 210–223 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Cheng L., Samuni L., Lucchesi S., Deschner T., Surbeck M., Love thy neighbour: Behavioural and endocrine correlates of male strategies during intergroup encounters in bonobos. Anim. Behav. 187, 319–330 (2022). [Google Scholar]
- 33.Sandel A. A., Watts D. P., Lethal coalitionary aggression associated with a community fission in chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 42, 26–48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittemyer G., Douglas-Hamilton I., Getz W. M., The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 69, 1357–1371 (2005). [Google Scholar]
- 35.Rousseeuw P., Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20, 53–65 (1987). [Google Scholar]
- 36.Hornik K., Grün B., Hahsler M., arules—A computational environment for mining association rules and frequent item sets. J. Stat. Softw. 14, 1–25 (2005). [Google Scholar]
- 37.Schamberg I., Cheney D. L., Clay Z., Hohmann G., Seyfarth R. M., Call combinations, vocal exchanges and interparty movement in wild bonobos. Anim. Behav. 122, 109–116 (2016). [Google Scholar]
- 38.Hohmann G., Fruth B., “Dynamics in social organization of bonobos (Pan paniscus)” in Behavioural Diversity in Chimpanzees and Bonobos, Boesch C., Hohmann G., Marchant L., Eds. (Cambridge University Press, 2002), pp. 138–150. [Google Scholar]
- 39.White F. J., Wrangham R. W., Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105, 148–164 (1988). [Google Scholar]
- 40.Hohmann G., Association and social interactions between strangers and residents in bonobos (Pan paniscus). Primates 42, 91–99 (2001). [Google Scholar]
- 41.Toda K., Tokuyama N., Ishizuka S., Furuichi T., A short-term visit of an adult male bonobo from the neighboring unit-group at Wamba. PAN 25, 22–24 (2018). [Google Scholar]
- 42.Whitehead H., et al. , Multilevel societies of female sperm whales (Physeter macrocephalus) in the Atlantic and Pacific: Why are they so different? Int. J. Primatol. 33, 1142–1164 (2012). [Google Scholar]
- 43.Grueter C. C., van Schaik C. P., Evolutionary determinants of modular societies in colobines. Behav. Ecol. 21, 63–71 (2010). [Google Scholar]
- 44.Swedell L., Plummer T., A papionin multilevel society as a model for hominin social evolution. Int. J. Primatol. 33, 1165–1193 (2012). [Google Scholar]
- 45.Lucchesi S., et al. , Better together? How intergroup associations affect energy balance and feeding behavior in wild bonobos. Behav. Ecol. Sociobiol. 75, 1–17 (2020). [Google Scholar]
- 46.Carvalho S., et al. , Using nonhuman culture in conservation requires careful and concerted action. Conserv. Lett. 15, e12860 (2022). [Google Scholar]
- 47.Samuni L., Lemieux D., Lamb A., Galdino D., Surbeck M., Tool use behavior in three wild bonobo communities at Kokolopori. Am. J. Primatol. 84, e23342 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Furuichi T., et al. , Why do wild bonobos not use tools like chimpanzees do? Behaviour 152, 425–460 (2015). [Google Scholar]
- 49.Langergraber K. E., Mitani J. C., Vigilant L., The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 104, 7786–7790 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitani J. C., Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640 (2009). [Google Scholar]
- 51.Samuni L., et al. , Social bonds facilitate cooperative resource sharing in wild chimpanzees. Proc. Biol. Sci. 285, 20181643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surbeck M., et al. , Sex-specific association patterns in bonobos and chimpanzees reflect species differences in cooperation. R. Soc. Open Sci. 4, 161081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldblum J. T., Krupenye C., Bray J., Pusey A. E., Gilby I. C., Social bonds provide multiple pathways to reproductive success in wild male chimpanzees. iScience 24, 102864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Surbeck M., Coxe S., Lokasola A. L., Lonoa: The establishment of a permanent field site for behavioural research on bonobos in the Kokolopori Bonobo Reserve. Pan Africa News 24, 13–15 (2017). [Google Scholar]
- 55.Altmann J., Observational study of behavior: Sampling methods. Behaviour 49, 227–267 (1974). [DOI] [PubMed] [Google Scholar]
- 56.Wood B. M., Watts D. P., Mitani J. C., Langergraber K. E., Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J. Hum. Evol. 105, 41–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murtagh F., Legendre P., Ward’s hierarchical agglomerative clustering method: Which algorithms implement ward’s criterion? J. Classif. 31, 274–295 (2014). [Google Scholar]
- 58.Frey B. J., Dueck D., Clustering by passing messages between data points. Science 315, 972–976 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Blondel V. D., Guillaume J.-L., Lambiotte R., Lefebvre E., Fast unfolding of communities in large networks. J. Stat. Mech. 2008, P10008 (2008). [Google Scholar]
- 60.Csardi G., Nepusz T., The igraph software package for complex network research. Int. J. Complex Syst. 1695, 1–9 (2006). [Google Scholar]
- 61.Farine D. R., A guide to null models for animal social network analysis. Methods Ecol. Evol. 8, 1309–1320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farine D. R., Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol. Evol. 4, 1187–1194 (2013). [Google Scholar]
- 63.Mielke A., Crockford C., Wittig R. M., Predictability and variability of association patterns in sooty mangabeys. Behav. Ecol. Sociobiol. 74, 46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bejder L., Fletcher D., BrÄger S., A method for testing association patterns of social animals. Anim. Behav. 56, 719–725 (1998). [DOI] [PubMed] [Google Scholar]
- 65.L. Samuni et al., Pan-social-structure. GitHub. https://github.com/LiranSamuni/Pan-social-structure. Deposited 2 June 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and R code used in the study are available via GitHub (65).