Significance
We found that bitter substances–induced airway smooth muscle relaxation was mediated by Gαt release of Tas2R activation and the released Gαt blocked contractile responses by efficiently suppressing the Gαq cycling of AChR signaling. Given the Gαq coupled with various G protein–coupled receptors, this mechanism may be manipulated to intervene in multiple pathological processes. As an example, the bitter substance kudinoside A simultaneously attenuated airway constriction in rodent models of both asthma and chronic obstructive pulmonary disease (COPD) and decreased pulmonary inflammation and tissue remodeling in the COPD rat model.
Keywords: bitter receptor, acetylcholine receptor, COPD, asthma
Abstract
Both chronic obstructive pulmonary disease (COPD) and asthma are severe respiratory diseases. Bitter receptor–mediated bronchodilation is a potential therapy for asthma, but the mechanism underlying the agonistic relaxation of airway smooth muscle (ASM) is not well defined. By exploring the ASM relaxation mechanism of bitter substances, we observed that pretreatment with the bitter substances nearly abolished the methacholine (MCh)-induced increase in the ASM cell (ASMC) calcium concentration, thereby suppressing the calcium-induced contraction release. The ASM relaxation was significantly inhibited by simultaneous deletion of three Gαt proteins, suggesting an interaction between Tas2R and AChR signaling cascades in the relaxation process. Biochemically, the Gαt released by Tas2R activation complexes with AChR and blocks the Gαq cycling of AChR signal transduction. More importantly, a bitter substance, kudinoside A, not only attenuates airway constriction but also significantly inhibits pulmonary inflammation and tissue remodeling in COPD rats, indicating its modulation of additional Gαq-associated pathological processes. Thus, our results suggest that Tas2R activation may be an ideal strategy for halting multiple pathological processes of COPD.
Bitter substances widely exist in the natural world and cover a large range of chemical structures such as trepenoids, quinine, and alkaloids (1–3). To sense these substances, a bitter taste receptor–based sensing system has been established in oral gustatory buds. Tas2R receptors are also expressed in other tissues, including the gut, airway, brain, and immune system, which enable the activation of multiple physiological processes to adapt to bitter environments (4–7). Airway smooth muscle cells (ASMCs) are reported to abundantly express an array of Tas2R receptors, and agonistic activation of these receptors can efficiently release bronchoconstriction (8–10). As current beta2-adrenergic agonist receptors are subject to tachyphylaxis and other side effects (9), Tas2R agonists are considered a new generation of antiasthma drugs (11–13). We previously identified a class of bitter substances, kudinosides, that has a unique structure of trepenoids and shows an extraordinary capacity to relax ASM (14). We thus used the canonical bitter substances chloroquine and kudinoside A (we here called KE-A) as Tas2R agonists in this study.
As a class of G protein–coupled receptors (GPCRs), Tas2Rs couple to heterotrimeric G protein consisting of Gαts and β- and γ-subunits. The Gαts include α-gustducin (also named Gnat3, encoded by Gnat3) and its homolog α-transducins (Gnat1 and Gnat2, encoded by Gnat1 and Gnat2) (15). Canonically, upon Tas2R activation, Gnats are disassociated from the Gβγ/Gnat complex, and the released Gβγ activates phospholipase β (PLCβ) to produced inositol 1,4,5-triphosphate (IP3). The resultant IP3 mediates the release of Ca2+ from the endoplasmic reticulum. Regarding signal transduction in ASM, Deshpande et al. proposed that bitter substances activate a canonical pathway (Tas2R-gustducin-PLCβ-IP3/IP3R) to increase the release of intracellular calcium ([Ca2+]i), then induces the membrane hyperpolarization via large-conductance potassium (BK) channels (9). However, patch-clamp recordings of smooth muscle cells show no activation of BK channels upon chloroquine treatment (16). Alternatively, Zhang et al. proposed that bitter tastants reverse the increase in [Ca2+]i evoked by bronchoconstrictors and this reversal effect is mediated by the suppression of L-type voltage-dependent calcium channels (VDCCs) (8). However, by analyzing human ASM, Grassin-Delyle established that none of the above signaling pathways can explain the Tas2R agonist–induced relaxation of human isolated bronchi (17). Thus, the mechanism of bitter substance–mediated bronchodilation has not yet been fully elucidated.
It is well known that an increase in calcium level is required for the smooth muscle contractile responses (18–20). In the case of Tas2R activation, the intracellular calcium level is also elevated, but the resultant effect is relaxation. We here found that the elevated calcium by Tas2R activation was not sufficient to phosphorylate the myosin light chain. Importantly, activation of Tas2R almost abolished the calcium elevation induced by methacholine (MCh). By comparing the Gnats and Gαq sequences of helix 5 (H5, the main site of interaction with GPCRs) (21) and establishing the Gnats triple knockout (TKO) mice, we revealed a cross-talk between these cascades that was mediated by the released Gnats from activated Tas2R to interrupted Gq cycling of acetylcholine receptor (AChR) activation. To assess the role of bitter substance in multiple GPCR-associated processes, we determined the therapeutic effect of kudinoside A on chronic obstructive pulmonary disease (COPD) rats. The administration of kudinoside A not only attenuated airway constriction but also improved pulmonary inflammation and bronchial remodeling, suggesting that bitter substances such as kudinoside A have a unique advantage for asthma/COPD therapies.
Results
Tas2R Activation Prevents Acetylcholine-Induced Increases in the Calcium Level in ASM.
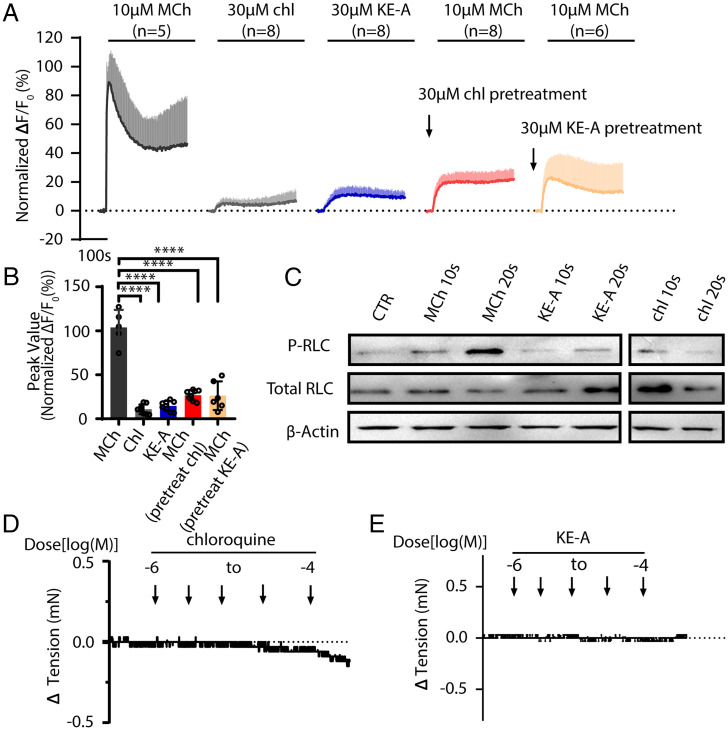
In human ASM, Tas2R10 and Tas2R14 are considered to be critical for airway relaxation (17). As canonical chloroquine and other compounds are considered to be nonselective for Tas2R receptors, we investigated a family of bitter substances, kudinosides, for experiments. These compounds were extracted from Ilex kudingcha (used as bitter tea in China), and they are pentacyclic triterpenes and feature the typical structure of lactones (14). We established TAS2R10 and TAS2R14 bitter receptor reporter cells by respectively cotransfecting HEK293T cells with the expressive vectors of human TAS2R10-Gα16/gust44 and human TAS2R14-Gα16/gust44 (22, 23). Measurement for the Ca2+ release induced by bitter substances indicated that KE-A was a potent TAS2R10 agonist (SI Appendix, Fig. S1 E and F). Note that KE-A might also be able to activate other TAS2Rs because KE-A treatment also led to a moderate increase for the control cells transfected with Gα16/gust44 only. To test whether KE-A activates Tas2R in airway smooth muscle, we isolated mouse ASMCs and primarily cultured them in dulbecco's modified eagle medium (DMEM). Upon exposure to KE-A, the mouse ASM primary cells showed a typical increase in intracellular calcium in a similar manner as chloroquine did (Fig. 1A). However, the calcium levels were much smaller than the MCh-induced calcium release level (average ΔF/F0: 14.56% (KE-A)/10.76% (chloroquine) vs. ΔF/F0 of MCh: 103.9%, both P < 0.001) (Fig. 1 A and B). To determine whether the elevated calcium level was sufficient to evoke smooth muscle contraction, we measured myosin regulatory light chain (RLC) phosphorylation in ASM tissues. The RLC phosphorylation in ASM strips treated with MCh alone was significantly increased by 20 s, but not after treatment with KE-A or chloroquine (Fig. 1C). Consistently, KE-A and chloroquine treatment did not induce ASM contraction (Fig. 1 D and E).
Fig. 1.
Bitter substances affect increases in the cytosolic Ca2+ concentration. ASMCs were isolated from mouse airways and cultured in vitro. The primary cells were then subjected to the measurement of intracellular calcium. (A) Ca2+ release upon stimulation with 10 µM MCh, 30 µM chloroquine, and 30 µM KE-A. The calcium release induced by MCh was significantly inhibited by pretreatment with chloroquine or KE-A. (B) Quantification of the peak values of the released calcium, as shown in A. (C) After respective stimulation with MCh or bitter substances, the airway smooth muscle tissues were applied to measure the phosphorylation level of RLC. (D and E) Treatment with chloroquine or KE-A did not initiate ASM contraction. ****P < 0.0001, n > 5.
Because cytosolic calcium and its resultant RLC phosphorylation are required for smooth muscle contraction, we speculated that Tas2R activation plays a critical role in the release of calcium from MCh-stimulated mouse ASMCs. We incubated ASMCs with bitter substances (30 µM chloroquine and 30 µM KE-A) and then stimulated the cells with MCh, revealing that the calcium elevation was significantly inhibited (P < 0.001) (Fig. 1 A and B). Collectively, our results showed that Tas2R activation induced a slight increase in the intracellular calcium levels that was insufficient to induce RLC phosphorylation, whereas it significantly suppressed MCh-mediated calcium release.
Gnats Are Necessary for the Inhibitory Effect of Tas2R Activation on Acetylcholine-Induced Calcium Elevation.
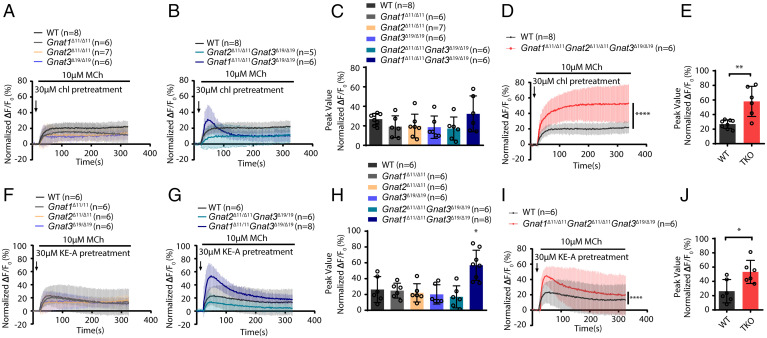
The suppressive effect of Tas2R activation on MCh-mediated calcium release clearly implied an interaction between the Tas2R and AChR pathways. To confirm this interaction, we deleted gustducin and two α-transducins by establishing compound Gnat1/2/3 KO mice (Gnat1, Gnat2, and Gnat3) using CRISPR-Cas9 technology (SI Appendix, Figs. S2A and S3). All of the mice with Gnat KO appeared normal in terms of their physiological activities, body growth, fur appearance, etc. The smooth muscle and epithelium of the mutant airways appeared normal also (SI Appendix, Fig. S2 B–D). To measure the inhibitory effect of bitter substances on MCh-induced calcium release, we then prepared primary smooth muscle cells from wild-type (WT) and mutant airways. Upon stimulation with MCh, the KE-A– and chloroquine-pretreated ASMCs with individual deletions of Gnat1, Gnat2, or Gnat3 exhibited significantly increased cytosolic calcium levels to an extent similar to that in the control cells (Fig. 2 A and F). The cytosolic calcium level was not increased in the muscle cells with double KO of Gnat2/3, but the calcium release was slightly increased in muscle cells with double KO of Gnat1 and Gnat3 in the KE-A group (Fig. 2 B, C, G, and H). However, when Gnat1, Gnat2, and Gnat3 were deleted simultaneously (TKO), the calcium levels in KE-A– and chloroquine-pretreated cells were increased to a significantly greater extent than that in control cells (ΔF/F0 of the fluo-4 intensity: TKO = 50.39% vs. WT = 20.17% for chloroquine, and TKO = 44.15% vs. WT = 21.26% for KE-A) (Fig. 2 D, E, I, and J). This result indicates that the three Gnat members are necessary for the counteractive effect of Tas2R activation on calcium elevation.
Fig. 2.
The suppressive effects of KE-A and chloroquine on MCh-induced Ca2+ elevation in Gnat1/2/3 knockout mouse ASMCs. (A) Pretreatment with chloroquine did not suppress the MCh-induced calcium release in ASMCs deficient for Gnat1, Gnat2, or Gnat3. (B) Pretreatment with chloroquine did not suppress the MCh-induced calcium release in ASMCs with double knockout of Gnat2/Gnat3 or Gnat1/Gnat3, although the levels in the cells with Gnat1/Gnat3 double knockout were slightly increased. (C) Quantification of A and B. (D) Triple knockout of Gnat1/2/3 counteracted the suppressive effects of chloroquine, quantification of the peak calcium levels in E. (F) KE-A did not suppress the MCh-induced calcium release in ASMCs deficient for Gnat1, Gnat2, or Gnat3. (G) KE-A did not suppress the MCh-induced calcium release in ASMCs with double knockout of Gnat2/Gnat3, but the levels were slightly increased in ASMCs with Gnat1/Gnat3 double knockout. (H) Quantification of the peak calcium levels in F and G. (I) Triple knockout of Gnat1/2/3 counteracted the suppressive effects of KE-A. (J) Quantification of the peak calcium levels in I. Cytosolic calcium levels are expressed as ΔF/F0 (%). *P < 0.05, **P < 0.01, ****P < 0.0001; unpaired Student's t test and two-way ANOVA.
Gnats Are Necessary for ASM Relaxation Induced by Tas2R Activation.
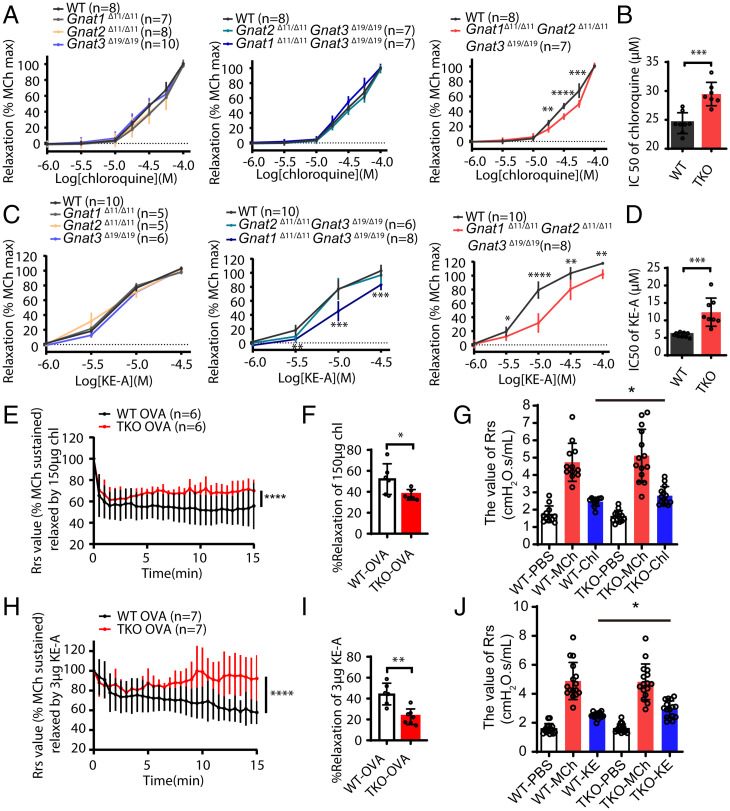
We then measured the relaxation responses of Gnat-deficient ASMs to bitter substances. The responses of all muscles with single or double KO of the Gnat 1/2/3 genes were comparable to those of the control, except that the muscle with Gnat1/3 double KO showed a reduced relaxation response to KE-A (Fig. 3 A–D and SI Appendix, Fig. S4). In the triple KO muscle, however, the relaxation responses to KE-A and chloroquine were significantly inhibited. In particular, when the mutant muscle was treated with 10 μM KE-A, only ∼25% of the force evoked by MCh was relaxed, while ∼80% was relaxed in the control muscle. The half maximal inhibitory concentration (IC50) values of KE-A increased by ∼200% (from 5.85 ± 0.22 μM to 12.36 ± 1.42 μM), while the IC50 of chloroquine increased by ∼125% (from 24.43 ± 0.64 μM to 29.46 ± 0.76 μM). This result indicated that the deletion of Gnats suppressed Tas2R activation–mediated relaxation. We also measured the contractile responses of these smooth muscles and found that the maximum contraction force induced by MCh had no difference between WT and Gnat knockout mice. This indicated that the suppression effect of Gnats deletion on relaxation was unlikely contributed by contractile alteration after gene deletion.
Fig. 3.
Bitter substance–induced ASM relaxation is inhibited by the deletion of Gnat1/2/3. Upon stimulation of left extrapulmonary bronchial smooth muscle with 10 μM MCh, the evoked force tension was relaxed by chloroquine or KE-A. (A) The dose-responsive relaxation of chloroquine in Gnat-deficient and control left extrapulmonary bronchi. (B) The IC50 values (concentration at which relaxation was inhibited by 50%) corresponding to A. (C) The dose-responsive relaxation of KE-A in Gnat-deficient and control tracheal tissues. (D) The IC50 values corresponding to C. (E) Asthmatic airway constriction in the mutant mice was resistant to chloroquine (150 μg) (E and F) and KE-A (3 μg) (H and I). (G and J) The change of Rrs of the mice with different treatments: phosphate-buffered saline (PBS), methacholine,150 µg Chl and 3 µg KE-A (Chl treatment: WT n = 12, TKO n = 14; KE-A treatment: WT n = 16, TKO n = 16). The bars represent the means ± SDs; the sample sizes (n) are indicated in the panel; KO OVA versus control (CTR) OVA as determined by two-way ANOVA. (F and I) Percentage of relaxation induced by chloroquine and KE-A in WT and TKO mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; unpaired Student’s t test.
To measure this effect in vivo, we sensitized wild-type and Gnat1/2/3 triple KO mice with ovalbumin (OVA) and established an asthmatic animal model as previously reported (9, 24). While all of the animals had comparable airway hyperresponsiveness, as evidenced by a three- to fourfold increase in respiratory system resistance (Rrs) values after MCh challenge (SI Appendix, Fig. S6A), the asthmatic animals exhibited more acidic mucin protein and inflammatory infiltration of cells particularly of eosinophils around the airways, greater impairment, and swelling of epithelial goblet cells (SI Appendix, Fig. S5 A–D). The amount of eosinophil cells in the bronchoalveolar lavage fluid (BALF) and eosinophilic inflammation–related cytokines were also increased after OVA challenge (SI Appendix, Fig. S5 E–K). Upon treating each asthmatic animal with 150 µg chloroquine, the wild-type mice showed significantly reduced Rrs values during the observation period, while the TKO mice showed no reduction in Rrs or even moderately increased Rrs values at ∼5 min after treatment (Fig. 3 E–G). Similarly, when the mice were treated with KE-A (3 µg for each animal), the TKO mice showed no apparent reduction in Rrs values, which were significantly higher than those of the wild-type mice (Fig. 3 H–J) (P < 0.01). We also measured central airway resistance (Rn) of these animals, but no difference was observed between WT and TKO groups after bitter substances treatment, indicating that Tas2R activation by the substances had an effect predominantly on bronchioles (SI Appendix, Fig. S6 B and C). This observation showed that the deletion of Gnat1/2/3 also impaired the relaxation response under asthmatic conditions.
Bitter Substances Also Relax the Depolarization-Induced Contraction, which Is Dependent on AChR Signaling.
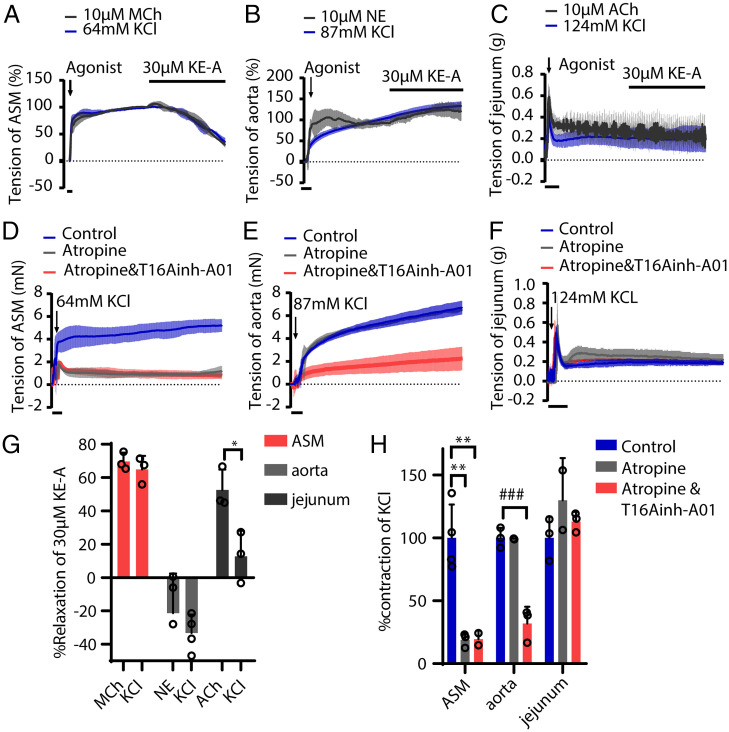
Smooth muscle contraction may be also initiated by membrane depolarization through L-type calcium channels, which is distinct from AChR signaling cascade. Previous reports have shown that bitter substances also relax depolarization-induced ASM contraction (8). We herein observed that KE-A effectively relaxed KCl-evoked ASM (Fig. 4 A, C, and G) but did not relax other smooth muscle (Fig. 4B). Given that agonistic relaxation is mediated by Gnats, as observed above, we wanted to elucidate the mechanism underlying the relaxation of depolarization-induced contraction. Before addressing this, we pretreated ASM with atropine, an antagonist of M-type AChRs and found more than 80% of the force induced by 60 mM KCl was reduced (Fig. 4 D and H); however, this inhibition was not observed in other tissues such as the aorta and jejunum (Fig. 4 D–F and H). This observation indicated that in ASM, depolarization-induced contraction was dependent on AChR signaling. Although the mechanistic formation of this dependency effect has to be explored, our result provides convincing evidence that bitter substances relax depolarization-induced contraction through AChR signaling also.
Fig. 4.
Kudinoside A relaxed the KCl-evoked contraction of the airway but not of the aorta or jejunum smooth muscle. (A) KE-A (30 μM) relaxed the ASM contraction evoked by 10 μM MCh or 64 mM KCl. (B and C) KE-A did not relax the tension force of the aorta and jejunum smooth muscle evoked by agonists and KCl depolarization. (D) The depolarization-evoked contraction of ASM was significantly induced by atropine. (E) The depolarization-evoked aortic contraction was partially inhibited by atropine. (F) The depolarization-evoked contraction of jejunum smooth muscle was not relaxed by atropine. (G) Quantitation of the data in A–C. (H) Quantitation of the data in D–F. *P < 0.05, **P < 0.01, unpaired Student’s t test; ###P < 0.001, unpaired Student’s t test. NE: norepinephrine.
Tas2R Activation Inhibits AChR Signaling via the Interaction of Gnats with AChR.
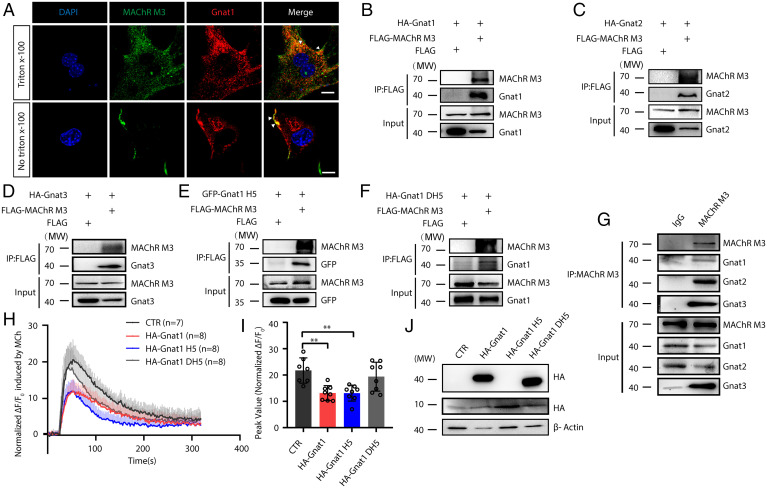
The suppressive effects of Gnats on MCh-induced contraction and calcium release prompted us to assess whether an interaction exists between the Tas2R and AChR signaling cascades. As Gnats are highly similar to Gq, we hypothesized that this interaction might be mediated by the binding of Gnats to AChR at the Gq binding site. To test this hypothesis, we first stained the MAChR M3 and Gnats simultaneously in primary ASMCs, revealing that the M3 receptor overlapped well with Gnat1 (Fig. 5A). However, Gαi, another important G protein coupled with GPCR, did not overlap with the M3 receptor (SI Appendix, Fig. S7F), suggesting that the overlapping pattern of the M3 receptor varied with different G proteins. We then constructed MAChR M3 and Gnats plasmids to transfect HEK293T cells for coimmunoprecipitation assay (SI Appendix, Fig. S7 A–C). Expectedly, the Gnat1/2/3 and M3 receptor proteins simultaneously appeared in the precipitated fraction (Fig. 5 B–D). To assess this interaction in vivo, we precipitated the lysate of fresh mouse ASM tissues with an anti-MAChR M3 antibody. Gnat1, Gnat2, and Gnat3 signals were clearly detected in the precipitated pellet (Fig. 5G). We next wanted to determine which structural features of Gnats are responsible for this interaction. The C-terminal H5 region of Gαq was the main binding site with GPCRs, and the Gnat1/2/3 proteins were shown to share an identical H5 sequence (IYSHMTCATDTQNVKKFVFDAVTDIIIKENLKDCGLF), which showed a 61.11% identity with the Gαq H5 sequence (SI Appendix, Fig. S7 D and E). We also could detect the EGFP-H5Gnat protein in the MAChR M3 precipitated fraction (Fig. 5E), but the truncated Gnat1 without H5 just showed a weak interaction (Fig. 5F). To functionally confirm this H5 interaction, we overexpressed Gnat1 and H5Gnat in HEK293T cells and then assessed the suppressive effect of Tas2R activation on calcium release. The calcium evoked by MCh was significantly inhibited when Gnat1 and H5Gnat were overexpressed (Fig. 5 H–J), while overexpressing Gnat1 without H5 slightly counteracted the inhibition of calcium release. Altogether, our observations strongly suggest that bitter substances inhibit AChR signaling through the physical interaction of Gɑts with AChR via the H5 region.
Fig. 5.
Interaction of Gnats with AChR. (A) Immunofluorescence confocal images showing the overlapping (yellow) expression of transducin (red) and AChR (green) in intact and Triton-treated ASMCs. (Scale bar, 10 μm.) (B–F) Coimmunoprecipitation of FLAG-MAChR M3 with HA-Gnat1, HA-Gnat2, HA-Gnat3, GFP-C-Gnat1 H5 domain and HA-Gnat1 DH5 domain in HEK293T cells. (G) Coimmunoprecipitation of endogenous MAChR M3 and Gnat1/Gnat2/Gnat3 in rat ASM tissues. (H–J) Calcium release in HEK293T cells transfected with the pCMV HA, HA-Gnat1, HA-Gnat1 H5 domain and HA-Gnat1 DH5 domain plasmids. Western blot and statistical analyses of transfected cells were performed. **P < 0.01, n > 7.
Inhalation of Kudinoside A Attenuates COPD in Rats.
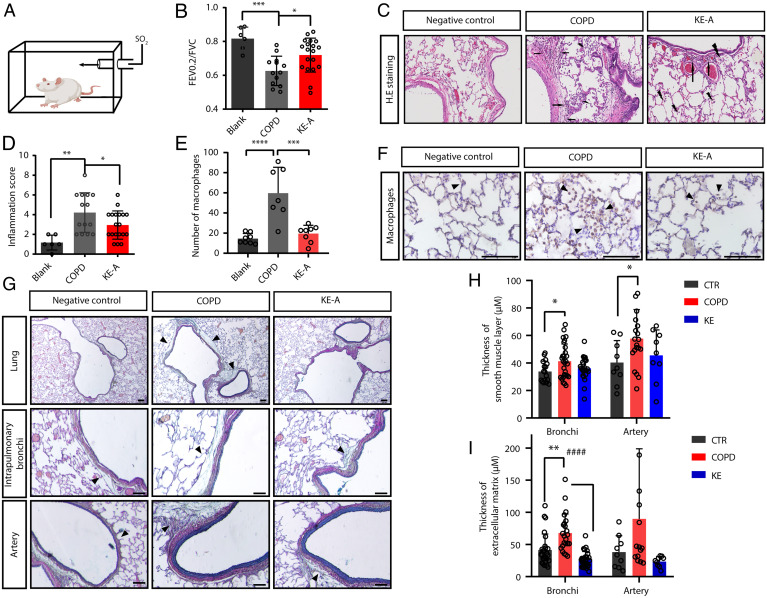
In addition to bronchoconstriction, pulmonary remodeling and inflammation are typical pathological features of COPD and are caused by multiple factors such as inflammatory mediators, cytokines, and the overproduction of ACh (25–27). Most of these factors are agonists of Gq-coupled GPCRs that are also expressed in various nonmuscle cells. We thus assessed the therapeutic efficacy of bitter substances in COPD rats. We first established COPD rats, which exhibited increased airway resistance, inflammatory infiltration, and mucus secretion (Fig. 6 A and D). The administration of KE-A could increase the ratio of forced expiratory volume in 0.2 second to forced vital capacity (FEV0.2/FVC) compared with COPD rats (Fig. 6B). Importantly, after treatment with KE-A, the lung inflammation scores of rats with COPD were significantly reduced (P < 0.05) (Fig. 6 C and D). Especially, the macrophage level in pulmonary alveoli was reverted to that in control rats (Fig. 6 E and F). Movat’s staining of the lung tissues showed that the smooth muscle layers of the bronchi and arteries of rats with COPD were significantly thicker than those of the controls and that KE-A treatment ameliorated this effect (Fig. 6 G–H). Furthermore, the extracellular matrix layers around the intrapulmonary bronchi and arteries of rats with COPD were significantly thicker than those of the controls but were restored significantly after KE-A treatment (Fig. 6I). In conclusion, KE-A effectively attenuates COPD inflammation and remodeling and shows therapeutic potential for the disease.
Fig. 6.
KE-A attenuates COPD airway constriction as well as pulmonary inflammation and remodeling. (A) Schematic diagram of the rat COPD atomization model. (B) Upon treatment with KE-A, the rats with COPD showed increased FEV0.2/FVC ratios. (C) Improved pulmonary inflammation in rats after KE-A treatment. The white position in the black ellipse indicates hyperplasia of bronchial goblet cells, the hexagon indicates congestion, the black arrow indicates neutrophil infiltration, and the black lightning indicates macrophage infiltration. (D) Total immune cell infiltration scores of plane C. (E) Statistical analysis of macrophage numbers showing that KE-A improved macrophage infiltration. (F) Representative images of EMR1 staining in alveolar macrophages are shown in the lungs of rats treated with different agents (40×). (Scale bar, 100 μm.) (G) Movat's staining of the lung sections of negative control, COPD rats, and KE-A–treated COPD rats. Quantitation of the thickness of the smooth muscle layers (H) and extracellular matrix layers around intrapulmonary bronchi and blood vessels (I). (Scale bar, 100 μm.) *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ####P < 0.0001, n > 10.
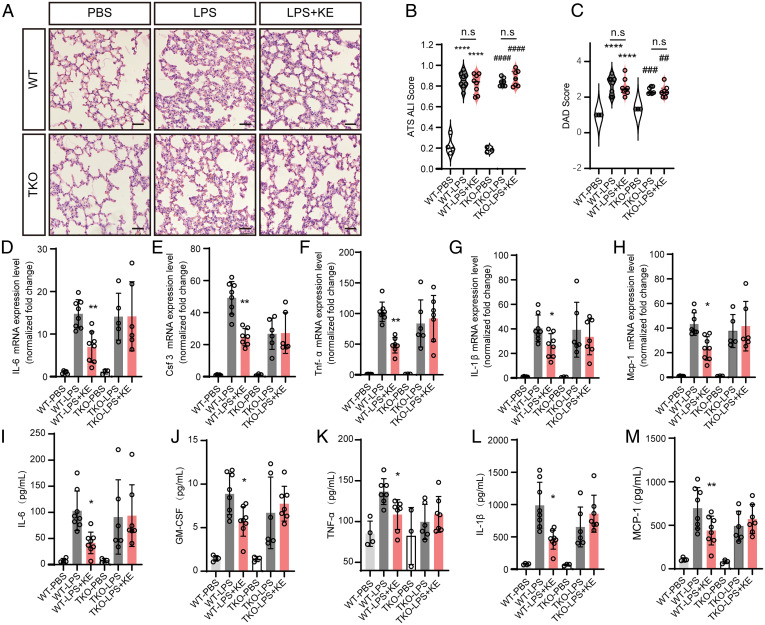
To further investigate the effect of Tas2R activation on inflammation, we established lipopolysaccharide (LPS)-induced acute pulmonary inflammation mouse model (28, 29). Forty-eight hours after treatment with LPS inhalation, pulmonary inflammation was significantly evoked as evidenced by apparent inflammatory infiltration of immune cells and significantly elevated expression of cytokines (Fig. 7 and SI Appendix, Fig. S8). When these mice were simultaneously treated with KE-A, the inflammatory infiltration of the cells was comparable to the control (Fig. 7 A–C), whereas the expressions of IL-1β, CSF-3 (colony stimulating factor 3), MCP-1 (monocyte chemoattractant protein-1), IL-6, and TNF-α were significantly attenuated (Fig. 7 D–M). However, this suppressing effect was significantly inhibited by Gnats deletion. This result convincingly showed an essential role of Tas2R activation in acute pulmonary inflammation.
Fig. 7.
Agonistic activation of Tas2R by KE-A attenuates LPS-induced acute pulmonary inflammation. (A) The histology of the lung tissues of LPS- or PBS-inhaled mice with and without KE-A treatment (H&E staining, 20×). (Scale bar, 50 μm.) The alveolar walls of PBS-inhaled mice are very thin, and the alveoli contain no infiltrated cells, while the LPS-inhaled lungs show apparent inflammatory infiltration. Transient treatment with KE-A does not change such inflammation infiltration and other pulmonary structures. Blinded histopathological evaluation of lung damage was performed by using the acute lung injury (ALI) scoring systems (B) and defuse alveolar damage (DAD) histological scoring systems (C) that were generated by the American Thoracic Society. ****P < 0.0001, * compared with WT PBS ; ##P < 0.01, ###P < 0.001, ####P < 0.0001, # compared with TKO PBS; n.s.: no significance. Two-way ANOVA. (D–H) The expression levels of inflammation-associated genes of mice (n = 3 to 8). (I–M) The concentrations of the multiple inflammation cytokines in the lung lysates were measured by a Luminex assays kit (n = 3 to 8). *P < 0.05, **P < 0.01, compared with WT LPS group; two-way ANOVA.
Discussion
In this report, we investigated the mechanism of ASM relaxation induced by Tas2R activation. Our results showed that, chloroquine and KE-A slightly increased the intracellular calcium levels in ASMCs through canonical Tas2R signaling. Pretreating the ASM with these substances nearly abolished the calcium elevation evoked by MCh. This observation suggests that the inhibition of calcium signaling during ASM contraction underlies Tas2R activation–mediated relaxation. Because the deletion of Gnats counteracted the inhibitory effect and Gnats had the capacity to bind the AChR, we suggest that the direct interaction of Gnats with AChR also underlies the inhibitory effect in addition to ASM relaxation. Based on our observations, we proposed a working model for ASM relaxation induced by Tas2R activation (SI Appendix, Fig. S9). When Tas2Rs and AChR are activated simultaneously within ASMCs, the released Gnats ectopically bind AChR and thus block Gq cycling, leading to the inhibition of signal transduction required for ASM contraction.
According to our model, ASM relaxation is mediated by released Gnats rather than by calcium elevation. This result together with the result that the elevated calcium level induced by Tas2R activation did not induce RLC phosphorylation suggest that the calcium elevation induced by bitter substances is irrelevant to the development of force tension. Another paradox is that bitter substances can relax depolarization-evoked ASM (8). Here, we observed that atropine significantly inhibited the depolarization-evoked force, suggesting that the depolarization pathway depends on the AChR, at least in ASM; however, the underlying mechanism remains unknown. Thus, the relaxation effect of bitter substances on the depolarization-evoked force of ASM is also mediated by ACh signaling. Note that this is not true for other smooth muscles because the depolarization-evoked force of these muscles did not depend on ACh signaling. Thus, this model not only explains why Tas2R activation can relax depolarization-evoked force but also why bitter substances display differential relaxation in different muscles.
As several GPCR receptors couple with Gq, Tas2R activation theoretically influences numerous GPCR-mediated signaling pathways other than the muscarinic pathway. In asthma and COPD, a vast number of acute inflammatory mediators (e.g., bradykinin, leukotrienes, and histamine) and ACh are overproduced and exert their effects on pulmonary inflammation and remodeling through GPCRs present in ASM, epithelial, and immune cells (30–35). Here, we showed that repeated treatments with kudinoside A not only significantly attenuated airway constriction but also inhibited pulmonary inflammation and remodeling. We also used an acute lung injury mice model to further examine the effect of KE-A to inhibit pulmonary inflammation. As Tas2R signaling serves as an intrinsic physiological inhibitor of various GPCRs, Tas2R agonists have a unique advantage over current COPD/asthma medicines in terms of their diverse beneficial effects. In addition, since Tas2R activation has a greater effect on relaxing small airways rather than the central airway as we observed in this report, the bitter substances could have a better effect to treat COPD in contrast to asthma.
Kudinosides are isolated from kudingcha and are primarily made of ilex plants, and kudingcha may have various pharmaceutical activities in different systems. For example, in the vascular system, crude extracts of kudingcha may improve blood circulation in the heart and brain (36). In the immune system, kudingcha extracts and saponins may effectively modulate the functions of macrophages and lymphocytes, thereby inhibiting different inflammatory reactions. These diverse pharmacological effects of kudingcha also support the role of kudinoside A in altering COPD pathological processes.
Materials and Methods
All animal manipulations in this study were conducted in accordance with the criteria of the Institutional Animal Care and Use Committee of the Model Animal Research Center of Nanjing University (Nanjing, China). We established Gnats knockout mice by CRISPR-Cas9 technology, and animals used for experiments were 8 to 10 weeks of age. ASM isometric contraction was recorded by a PowerLab recording device (AD Instruments). A Fluo-4 Direct Calcium assay kit (Invitrogen, F10472) was used to measure the calcium signal at an excitation wavelength of 494 nm and an emission wavelength of 516 nm with a microplate reader (BioTek, Synergic H1). The asthma mice model was sensitized with 100 μg of OVA (Sigma-Aldrich) by intraperitoneal injection, and the COPD rat model was administered SO2 via inhalation. Respiratory function was measured with a flexivent forced concussion pulmonary function detector with a negative pressure–driven forced expiration module (SCIREQ, FX-4).
Supplementary Material
Acknowledgments
We thank all staff members of the State Key Laboratory of Pharmaceutical Biotechnology and Medical School of Nanjing University for invaluable discussions and comments. This work was supported by the National Natural Science Funding of China (32071121 to M.-S.Z., and 82192862 to X.G., which M.-S.Z. is a main member), Fundamental Research Funds for the Central Universities (2021300242), and Shanghai KE Pharmaceutical Ltd, Co.
Footnotes
Competing interest statement: M.M., L.-Y.Y., and Z.-L.Z. are affiliated with Shanghai KE Pharmaceutical Ltd, Co.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2121513119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Kluska M., Marciniuk-Kluska A., Prukała D., Prukała W., Analytics of quinine and its derivatives. Crit. Rev. Anal. Chem. 46, 139–145 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Fabiano-Tixier A.-S., et al. , Rapid and green analytical method for the determination of quinoline alkaloids from Cinchona succirubra based on Microwave-Integrated Extraction and Leaching (MIEL) prior to high performance liquid chromatography. Int. J. Mol. Sci. 12, 7846–7860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwood K. M., et al. , Raloxifene prevents stress granule dissolution, impairs translational control and promotes cell death during hypoxia in glioblastoma cells. Cell Death Dis. 11, 989 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders C. J., Christensen M., Finger T. E., Tizzano M., Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl. Acad. Sci. U.S.A. 111, 6075–6080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah A. S., Ben-Shahar Y., Moninger T. O., Kline J. N., Welsh M. J., Motile cilia of human airway epithelia are chemosensory. Science 325, 1131–1134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finger T. E., et al. , Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc. Natl. Acad. Sci. U.S.A. 100, 8981–8986 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasteva G., et al. , Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl. Acad. Sci. U.S.A. 108, 9478–9483 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C. H., et al. , The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 11, e1001501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande D. A., et al. , Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan S., Sharma P., Shah S. D., Deshpande D. A., Bitter taste receptor agonists alter mitochondrial function and induce autophagy in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L154–L165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu M. S., A “bitter” end to asthma revealed. Protein Cell 2, 433–434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinett K. S., et al. , Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 50, 678–683 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clifford R. L., Knox A. J., Future bronchodilator therapy: A bitter pill to swallow? Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L953–L955 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Zhu M., Sun J., Pan Y., . Methods and compositions for treatment of COPD diseases, US Patent 10,786,521 B2. https://uspto.report/patent/grant/10,786,521. (2014).
- 15.Grassin-Delyle S., et al. , The expression and relaxant effect of bitter taste receptors in human bronchi. Respir. Res. 14, 134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C.-H., et al. , Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat. Med. 18, 648–650, author reply 650–651 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Grassin-Delyle S., The expression and relaxant effect of bitter taste receptors in human bronchi. Respir. Res. 14, 134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P., Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J. Biol. Chem. 276, 16365–16373 (2001). [DOI] [PubMed] [Google Scholar]
- 19.He W. Q., et al. , Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135, 610–620 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somlyo A. P., Somlyo A. V., Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Flock T., et al. , Universal allosteric mechanism for Ga activation by GPCRs. Nature 524, 173–179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki T., et al. , Bitter taste receptor activation by hop-derived bitter components induces gastrointestinal hormone production in enteroendocrine cells. Biochem. Biophys. Res. Commun. 533, 704–709 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Ueda T., Ugawa S., Yamamura H., Imaizumi Y., Shimada S., Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptor cells. J. Neurosci. 23, 7376–7380 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P., et al. , Inflammatory mediators mediate airway smooth muscle contraction through a G protein-coupled receptor-transmembrane protein 16A-voltage-dependent Ca2+ channel axis and contribute to bronchial hyperresponsiveness in asthma. J. Allergy Clin. Immunol. 141, 1259–1268.e11 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Barnes P. J., Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 18, 454–466 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Houghton A. M., Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer 13, 233–245 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Barnes P. J., Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8, 183–192 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Matute-Bello G., Frevert C. W., Martin T. R., Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L379–L399 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittet J. F., Mackersie R. C., Martin T. R., Matthay M. A., Biological markers of acute lung injury: Prognostic and pathogenetic significance. Am. J. Respir. Crit. Care Med. 155, 1187–1205 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Kistemaker L. E., Gosens R., Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol. Sci. 36, 164–171 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Koarai A., Ichinose M., Possible involvement of acetylcholine-mediated inflammation in airway diseases. Allergol. Int. 67, 460–466 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Gosens R., Zaagsma J., Grootte Bromhaar M., Nelemans A., Meurs H., Acetylcholine: A novel regulator of airway smooth muscle remodelling? Eur. J. Pharmacol. 500, 193–201 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Kistemaker L. E. M., Oenema T. A., Meurs H., Gosens R., Regulation of airway inflammation and remodeling by muscarinic receptors: Perspectives on anticholinergic therapy in asthma and COPD. Life Sci. 91, 1126–1133 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Humbles A. A., et al. , A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Decramer M., Janssens W., Miravitlles M., Chronic obstructive pulmonary disease. Lancet 379, 1341–1351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo A. Y., et al. , Inotropic and chronotropic actions of Ilex latifolia inhibition of adenosine-5'-triphosphatases as a possible mechanism. Life Sci. 68, 1259–1270 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.