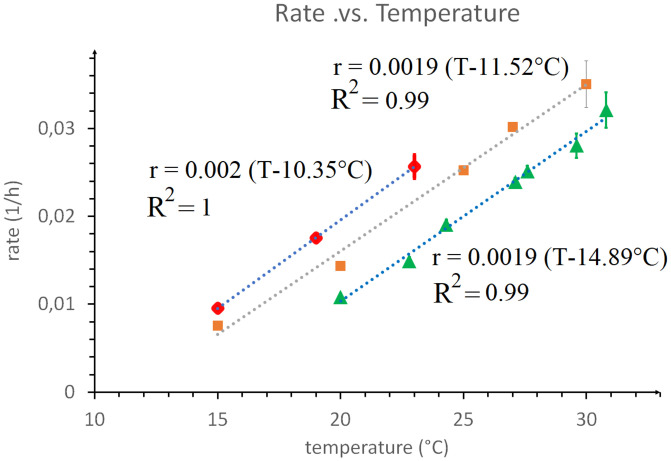
While the rate of development of poikilotherms can vary widely with temperature (and other external variables such as food availability, diet, etc.), the gene expression, morphology, and function of the organs of the embryo at all stages of development is independent of these external factors (1). In PNAS, Filina et al. (2) show that this is valid also in the development of Caenorhabditis elegans when the stage of development is defined relative to the overall developmental time, a property they call “temporal scaling.” We would like to point out here that this robustness could be a result of development occurring near a critical point, where all rates scale as θ − θc, where θ is some external variable. Particularly, if development occurs near a critical temperature Tc all developmental rates (growth rate, oscillatory frequencies, gene expression dynamics, etc.) should scale as T − Tc. We have observed this to be valid during somitogenesis in zebrafish (1, 3). From the limited data in Filina et al. (2) it appears that this is also valid for the developmental rate of C. elegans (defined as the inverse of the development duration TS) (Fig. 1). Notice, though, that while “temporal scaling” is a consequence of criticality, the reverse is not true. The linear variation of developmental rates with temperature implied by the proximity to a critical point has also been observed in the development of the various larval stages in the fly (4, 5). It is thus tempting to hypothesize that a common mechanism is at work in these various organisms, possibly an essential transcription factor displaying critical behavior (1). If such a mechanism is shared by so distant organisms as C. elegans, fly, and zebrafish, it must be evolutionary very old, dating to the last bilaterian common ancestor appearing during the Cambrian explosion.
Fig. 1.
The developmental rate r of C. elegans (defined as the inverse of the developmental duration TS) as a function of temperature [red points are data from Filina et al. (2)] and a linear best fit (blue dots). Compare with the developmental rate of the fruit fly (B. carambolae) egg [brown points are data from Danjuma et al. (4)] and linear best fit (gray dots) and with the frequency (divided by 100) of the somitogenetic clock (in hours−1) in zebrafish as a function of temperature [green points are data from Schröter et al. (3)] and linear best fit (blue dots).
Footnotes
The authors declare no competing interest.
References
- 1.Zhang W., et al. , Fgf8 dynamics and critical slowing down may account for the temperature independence of somitogenesis. Commun. Biol. 5, 113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filina O., Demirbas B., Haagmans R., van Zon J. S., Temporal scaling in C. elegans larval development. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2123110119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schröter C., et al. , Dynamics of zebrafish somitogenesis. Dev. Dyn. 237, 545–553 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Danjuma S., Thaochan N., Permkam S., Satasook C., Effect of temperature on the development and survival of immature stages of the carambola fruit fly, Bactrocera carambolae, and the Asian papaya fruit fly, Bactrocera papayae, reared on guava diet. J. Insect Sci. 14, 126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S. S., Meng X. D., Modelling development time of Lipaphis erysimi (Hemiptera: Aphididae) at constant and variable temperatures. Bull. Entomol. Res. 90, 337–347 (2000). [DOI] [PubMed] [Google Scholar]