Significance
The β3-adrenergic receptor (β3-AR) is a major regulator of energy expenditure that triggers lipolysis and thermogenesis in adipose tissue, thereby significantly attenuating the risk of obesity. Obesity is associated with diminished β3-AR expression and decreased β-adrenergic responses, but the underlying molecular mechanisms remain elusive. Here, we show that ten-eleven translocation (TET) proteins, the crucial modifiers of DNA methylation, act as epigenetic suppressors of β3-AR in adipocytes by recruiting histone deacetylases to its promoter, thereby reducing the sensitivity to β-adrenergic stimulation. Adipose-specific ablation of all TET proteins in mice prevents body weight gain and metabolic disorders under high-fat diet challenge via enhanced fat browning, lipolysis, and thermogenesis. Modulation of adipocyte TET proteins may provide a new therapeutic avenue to treat obesity.
Keywords: TET proteins, β3-AR, catecholamine resistance, obesity, HDACs
Abstract
β-adrenergic receptor (β-AR) signaling plays predominant roles in modulating energy expenditure by triggering lipolysis and thermogenesis in adipose tissue, thereby conferring obesity resistance. Obesity is associated with diminished β3-adrenergic receptor (β3-AR) expression and decreased β-adrenergic responses, but the molecular mechanism coupling nutrient overload to catecholamine resistance remains poorly defined. Ten-eleven translocation (TET) proteins are dioxygenases that alter the methylation status of DNA by oxidizing 5-methylcytosine to 5-hydroxymethylcytosine and further oxidized derivatives. Here, we show that TET proteins are pivotal epigenetic suppressors of β3-AR expression in adipocytes, thereby attenuating the responsiveness to β-adrenergic stimulation. Deletion of all three Tet genes in adipocytes led to increased β3-AR expression and thereby enhanced the downstream β-adrenergic responses, including lipolysis, thermogenic gene induction, oxidative metabolism, and fat browning in vitro and in vivo. In mouse adipose tissues, Tet expression was elevated after mice ate a high-fat diet. Mice with adipose-specific ablation of all TET proteins maintained higher levels of β3-AR in both white and brown adipose tissues and remained sensitive to β-AR stimuli under high-fat diet challenge, leading to augmented energy expenditure and decreased fat accumulation. Consequently, they exhibited improved cold tolerance and were substantially protected from diet-induced obesity, inflammation, and metabolic complications, including insulin resistance and hyperlipidemia. Mechanistically, TET proteins directly repressed β3-AR transcription, mainly in an enzymatic activity-independent manner, and involved the recruitment of histone deacetylases to increase deacetylation of its promoter. Thus, the TET–histone deacetylase–β3-AR axis could be targeted to treat obesity and related metabolic diseases.
Beta-adrenergic signaling is a crucial modulator of energy expenditure that triggers lipolysis and heat dissipation in brown and beige adipocytes (1, 2). Upon cold exposure, sympathetic nerve terminals release the catecholamines norepinephrine and epinephrine, which bind to β-adrenergic receptors (β-ARs) and activate the intracellular signaling cascade involving adenylyl cyclase/cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) (3, 4). This event ultimately triggers hydrolysis of stored triglycerides (TGs) by activating key lipases, including hormone-sensitive lipase (HSL). Fatty acids derived from lipolysis are transported to the mitochondria and consumed as fuels to produce thermal energy (1, 2). Furthermore, free fatty acids are proposed to be allosteric activators of uncoupling protein 1 (UCP1) (5). During adaptive thermogenesis, β-AR signals also activate key thermogenic genes, including Ppargc1a (encoding PPARγ coactivator-1α [Pgc-1α]), Ucp1, and various components of the electron transport chain, thus promoting mitochondrial biogenesis, fatty acid oxidation, and thermogenesis (2–4). Additionally, β-ARs are important regulators of fat browning. There are three β-AR subtypes: β1-, β2-, and β3-AR. In mice, β3-AR plays important roles in inducing beige adipocytes from preexisting white adipocytes in response to β-adrenergic agonists, whereas β1-AR mainly mediates beige adipocyte generation following cold exposure (6). β3-AR is also important for browning, lipolysis, and thermogenesis in human adipocytes (7, 8).
In addition to cold-induced thermogenesis, β-ARs also play essential roles in diet-induced thermogenesis. A Trp64Arg mutation in the β3-AR gene is associated with abdominal obesity and increased susceptibility to weight gain and insulin resistance (9, 10). Mice lacking all three β-ARs subtypes (known as “β-less” mice) are prone to diet-induced obesity, due to a significantly lower metabolic rate (11). Strikingly, the brown adipose tissue (BAT) from β-less mice display white adipose tissue (WAT)-like features, mainly populated by markedly enlarged adipocytes with unilocular lipid droplets that lack UCP1 expression. Consistently, the β-less mice are highly susceptible to cold-induced hypothermia and are unable to increase oxygen consumption in response to β-agonist treatment. Mice lacking β3-AR alone are also susceptible to depositing more fat compared to wild-type (WT) mice (12). Notably, in both humans and rodents, obesity is associated with blunted β-AR–triggered responses in adipose tissue (AT), a state known as catecholamine resistance (13–15), presumably due to a significant reduction of β3-AR expression and function in response to chronic nutrient overload (16–19). Furthermore, β-ARs, particularly β1-AR and β2-AR, undergo agonist-induced desensitization (20, 21). In rodents, selective β3-AR agonists exert excellent antiobesity and antidiabetic effects by increasing energy expenditure, due to their abilities to induce lipolysis, mitochondrial biogenesis, fat oxidation, and the thermogenic program in AT (22–26). However, results of most clinical studies targeting β3-AR have been disappointing (27), although mirabegron, a selective β3-AR agonist approved for the treatment of overactive bladder syndrome (2), activated human brown and beige adipocytes and improved glucose metabolism in obese individuals when treated chronically or at high doses (7, 28–31). This might be attributed to the poor selectivity of the human β3-AR agonists and different expression patterns of β-ARs in rodents and human ATs. Indeed, β3-AR is abundantly expressed in both WAT and BAT in rodents and thus acts as the principal receptor of catecholamine signals. However, its levels are very low in human ATs. Instead, β-adrenergic stimulation of human adipocytes is mainly mediated by β1-AR and β2-AR, whose agonists often induce undesired complications, including hypertension and tachycardia (27). Together, these results strongly suggest that strategies to potentiate β3-AR expression and catecholamine sensitivity with minimal side effects in AT may be promising in obesity treatments.
Ten-eleven translocation (TET) proteins are dioxygenases that reprogram or erase the methylation status of DNA by oxidizing 5-methylcytosine to 5-hydroxymethylcytosine (5hmC) and further oxidized derivatives (32). They are crucial regulators of gene expression that serve as either transcriptional activators or repressors, depending on the context (32, 33). In many cell types, including mouse embryonic stem cells, immune cells, or neuronal cells, TET proteins utilize their enzymatic activities to sequentially oxidize 5-methylcytosine to 5hmC and further oxidation products at promoters or enhancers, followed by DNA demethylation, to control gene transcription (34, 35). However, they can also control gene expression independently of their enzymatic activity, which is mainly achieved by partnering with numerous transcription factors and associated coregulators, including chromatin modifiers (36–39). For example, TET proteins can be implicated in the formation of transcriptional corepressor complex with histone deacetylase (HDAC) to repress transcription (37–39).
Previous studies have suggested that TET proteins may have regulatory roles in adipocyte differentiation and function. Depletion of TET1 or TET2 substantially inhibits adipogenic differentiation of 3T3-L1 preadipocytes or mouse embryonic fibroblasts (40, 41). However, a recent study showed that TET1 is dispensable for adipogenesis of primary preadipocytes isolated from mice (42). Moreover, in mature adipocytes, TET proteins are involved in the PPARγ-regulated transcriptional program by being recruited to PPARγ response elements and inducing their demethylation to activate PPARγ target genes (40). In mice, all three TET proteins are expressed in various adipose depots, although at different levels (39), and TET1 suppresses thermogenesis by repressing Ucp1 and Ppargc1a in a beige adipocyte–specific manner (39). As a result, Tet1 knockout mice are cold tolerant and partially protected from diet-induced obesity and metabolic dysfunction. In contrast, another recent report showed that Tet1 haploinsufficiency promotes diet-induced obesity and aggravates glucose intolerance (42). Thus, the exact roles of TET proteins in adipocyte biology and metabolism remain unclear.
We show here that chromatin modification via TET-HDAC interaction constitutes a crucial regulatory component of adipocyte plasticity and adaptive thermogenesis by modulating β3-AR expression and catecholamine sensitivity. TET proteins acted as pivotal epigenetic suppressors of β3-AR expression in adipocytes, thereby reducing the sensitivity to β-adrenergic stimulation in vitro and in vivo. Thus, TET loss resulted in enhanced β-adrenergic responses, including browning, lipolysis, thermogenic gene induction, mitochondrial respiration, and fatty acid oxidation. Moreover, mice lacking all TET proteins in adipocytes resisted β3-AR down-regulation and remained sensitive to β-AR stimuli during chronic high-fat diet (HFD) challenge. Consequently, the knockout mice maintained a higher capacity for fat lipolysis, thermogenesis, and energy expenditure, and were substantially resistant to diet-induced obesity and associated metabolic complications.
Results
TET Deficiency Increases β-Adrenergic Responsiveness by Up-Regulating β3-AR Expression in Adipocytes.
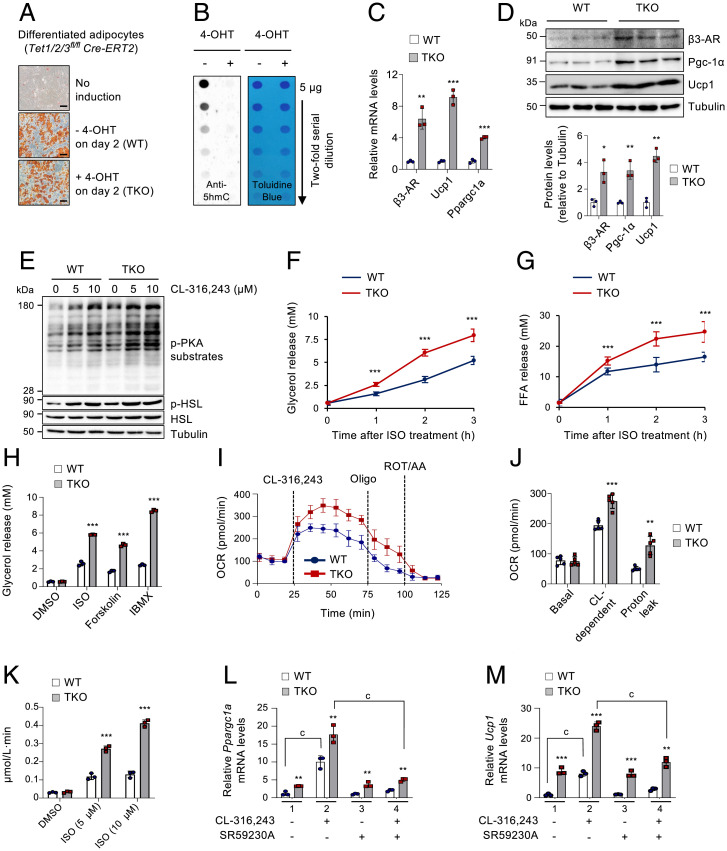
It was previously reported that TET1 directly repressed the expression of crucial thermogenic genes, including Ucp1 and Ppargc1a, in beige adipocytes (39). As a result, its loss increased energy expenditure and partially protected mice from diet-induced obesity and related metabolic comorbidities. β3-AR is the principal regulator of lipolysis and thermogenesis that acts upstream of Ucp1 and Ppargc1a (1, 2). Thus, we aimed to investigate whether TET proteins could control the expression of β3-ARs, thereby modulating β-adrenergic responsiveness to indirectly control the downstream thermogenic program. To test this, we established immortalized stromal vascular fraction cells isolated from the subcutaneous inguinal WAT (iWAT) of Tet1fl/fl Tet2fl/fl Tet3fl/fl Cre-ERT2 mice (SI Appendix, Fig. S1A). All three Tet genes were successfully deleted after 48 h of 4-hydroxytamoxifen (4-OHT) treatment that activated the Cre-ERT2 recombinase (SI Appendix, Fig. S1B). Notably, deletion of all Tet genes at the early phase of adipogenic differentiation (achieved by treating preadipocytes with 4-OHT at day −3 or day 0) almost completely blocked adipogenesis in vitro, as assessed by quantification of accumulated lipids and expression of adipogenic marker genes such as Pparg and Fabp4 (SI Appendix, Fig. S1 C–E). However, Tet deletion at a later stage (achieved by treating cells with 4-OHT at day 2 of differentiation) did not significantly affect adipogenesis (SI Appendix, Fig. S1 C–E), thus indicating a stage-specific requirement for TET proteins in adipogenesis. Based on these results, we used WT and Tet1, Tet2, Tet3 triple knockout (hereafter, Tet TKO) adipocytes under similar differentiation states for subsequent studies (Fig. 1A), where Tet deletion was also monitored by 5hmC loss using a dot blot assay (43) (Fig. 1B). Importantly, the β3-AR mRNA and protein levels were significantly elevated in Tet TKO adipocytes (Fig. 1 C and D and SI Appendix, Fig. S1F). Among the three β-AR subtypes, β3-AR up-regulation was most prominent upon Tet deletion (SI Appendix, Fig. S1G). As expected, both mRNA and protein levels of Pgc-1α and Ucp1 were also increased in Tet TKO adipocytes (Fig. 1 C and D).
Fig. 1.
TET deficiency increases β-adrenergic responsiveness by up-regulating β3-AR expression in adipocytes. (A) Oil Red O staining of differentiated WT and Tet TKO adipocytes. Preadipocytes were treated with or without 4-OHT on day 2 of differentiation. n = 3. (Scale bar, 100 μm.) (B) Dot blot analysis to quantify genomic 5hmC in WT and Tet TKO adipocytes shown in A. Toluidine blue staining confirmed blotting of equal amounts of DNA. (C) mRNA expression of β3-AR, Ucp1, and Ppargc1a relative to Gapdh in WT and Tet TKO adipocytes. n = 3. (D) Immunoblot analysis (Top) and quantification (Bottom) of β3-AR, Pgc-1α, and Ucp1 proteins in WT and Tet TKO adipocytes. α-Tubulin served as a loading control. n = 3. (E) Immunoblot analysis of p-PKA substrates, p-HSL, and total HSL proteins in WT and Tet TKO adipocytes stimulated with or without CL-316,243 for 5 min at the indicated concentrations. α-Tubulin served as a loading control. (F and G) In vitro lipolysis in WT and Tet TKO adipocytes. The released glycerol (F) and free fatty acid (G) were quantified at the indicated time points after stimulation with ISO (5 μM). n = 3. (H) In vitro lipolysis in WT and Tet TKO adipocytes stimulated with ISO (5 μM), forskolin (20 μM), or isobutylmethylxanthine (IBMX) (0.2 mM). n = 3. (I and J) Oxygen consumption rate (OCR) (I) and average OCR (J) in WT and Tet TKO adipocytes sequentially stimulated with CL-316,243 (10 µM), oligomycin (oligo; 1.25 µM), and rotenone/antimycin A (ROT/AA, 1 µM). n = 5. (K) In vitro fatty acid oxidation in WT and Tet TKO adipocytes stimulated with ISO at the indicated concentrations. n = 3. (L and M) mRNA expression of Ppargc1a (L) and Ucp1 (M) relative to Gapdh in WT and Tet TKO adipocytes stimulated with or without CL-316,243 (1 μM, 24 h). Cells were either untreated or pretreated for 1 h with SR59230A (10 μM), a β3-AR antagonist, prior to CL-316,243 treatments. n = 3. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. **P < 0.005, ***P < 0.0005 versus WT; cP < 0.0005. DMSO, dimethyl sulfoxide; CL, CL-316,243.
Stimulation of β-ARs activates the cAMP/PKA signaling cascade that ultimately triggers lipolysis by activating key lipases, including HSL (1–4). Furthermore, activated β-ARs also stimulate the transcription of key thermogenic genes, including Ppargc1a, Ucp1, and various genes implicated in mitochondrial respiration (1–4). Therefore, we next asked whether elevated β3-AR expression by TET deficiency influenced adipocyte sensitivity to β-adrenergic stimuli. Treatment of WT and Tet TKO adipocytes with CL-316,243, a selective β3-AR agonist, revealed that the intracellular β-adrenergic signaling was more potently activated in Tet TKO adipocytes compared to WT controls, as determined by higher levels of phosphorylated forms of PKA substrates and HSL in the stimulated Tet TKO adipocytes (Fig. 1E). Consistent with these results, Tet TKO significantly increased the rate of lipolysis stimulated by isoproterenol (ISO), a pan–β-AR agonist, as assessed by the increased release of ISO-stimulated glycerol and free fatty acids in the Tet TKO adipocytes, when compared to WT controls (Fig. 1 F and G). TET deficiency also significantly promoted lipolysis in adipocytes stimulated with forskolin or isobutylmethylxanthine (IBMX) that increased the concentration of intracellular cAMP (Fig. 1H), presumably due to the enhancement of precoupled stable complexes between the β3-AR and adenylyl cyclase as a result of β3-AR up-regulation (7). Furthermore, the oxygen consumption rate remained comparable between WT and Tet TKO adipocytes under basal conditions, but the CL-316,243–dependent oxygen consumption rate and proton leak were significantly increased in Tet TKO adipocytes, when compared to WT controls (Fig. 1 I and J). TET deficiency also substantially enhanced fatty acid oxidation in ISO-stimulated adipocytes (Fig. 1K). Thus, these results suggest that TET deficiency renders adipocytes more sensitive to β3-AR signals by up-regulating β3-AR expression.
We next determined whether TET deficiency elicited enhanced thermogenic gene expression by up-regulating β3-AR expression. In agreement with a previous report (39), TET deficiency alone could significantly up-regulate a broad panel of thermogenesis-related genes (SI Appendix, Fig. S2A; control), confirming that TET proteins directly regulated their expression. Indeed, Tet TKO adipocytes possessed approximately fourfold higher Ppargc1a promoter–driven reporter activity than did WT adipocytes (SI Appendix, Fig. S3A). TET proteins suppressed Ppargc1a promoter activity through the CREB-binding element (CRE) that was previously shown to be responsive to β-AR signaling (SI Appendix, Fig. S3 A and B) (44), and this suppression did not require their catalytic activities (SI Appendix, Fig. S3C). Instead, chromatin immunoprecipitation followed by qRT-PCR (ChIP-qPCR) showed that TET deficiency blocked the recruitment of HDAC1 to the Ppargc1a and Ucp1 gene loci, leading to an accumulation of acetylated H3K27, the active histone mark (SI Appendix, Fig. S3 D and E). In both WT and Tet TKO adipocytes, forskolin considerably up-regulated Ppargc1a, Ucp1, Ppara, Elovl3, and Cidea; however, this up-regulation was more robust in Tet TKO cells (SI Appendix, Fig. S2A). Neither Tet deficiency nor forskolin affected the expression of adipogenic Pparg and Fabp4 marker gene expressions (SI Appendix, Fig. S2B). Furthermore, acute stimulation with CL-316,243 also increased the mRNA levels of these thermogenic genes in WT adipocytes, and this was further enhanced in Tet TKO adipocytes (Fig. 1 L and M and SI Appendix, Fig. S3F; panels 1 and 2). Notably, pretreatment with SR59230A, a β3-AR antagonist, substantially blocked the stimulatory effect of CL-316,243 in both WT and Tet TKO adipocytes (Fig. 1 L and M and SI Appendix, Fig. S3F; compare panels 2 and 4), confirming that β3-AR up-regulation by TET deficiency was mainly responsible for the increased thermogenic gene expression during β-adrenergic stimulation.
TET Proteins Suppress β3-AR Expression and Signaling, Which Is Mainly Independent of Enzymatic Activity.
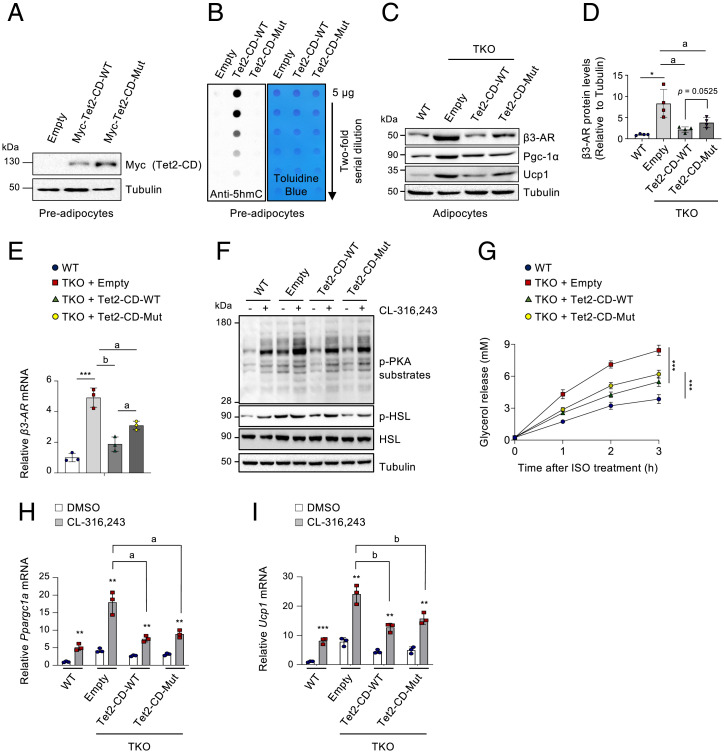
To further confirm whether the enzymatic activities of TET proteins were implicated in the control of thermogenic gene expression, the immortalized preadipocytes derived from iWAT of Tet1fl/fl Tet2fl/fl Tet3fl/fl Cre-ERT2 mice were transduced with lentiviruses expressing WT or a catalytically inactive mutant version of the Myc-tagged Tet2 catalytic domain (Tet2-CD), which was previously shown to reverse loss of 5hmC and the effects by TET deficiency (45–47). Immunoblotting and dot blot assays validated appropriate Tet2 expression and activity (Fig. 2 A and B). As previously shown, both mRNA and protein levels of β3-AR were up-regulated in differentiated adipocytes after induction of Tet deletion at day 2 of differentiation (Fig. 2 C–E). However, this up-regulation was substantially inhibited by ectopic expression of both WT and mutant Tet2-CD, although the catalytic mutant exhibited slightly weaker inhibitory effects (Fig. 2 C–E). Consistently, both WT and mutant Tet2-CD similarly attenuated intracellular β-adrenergic signaling (Fig. 2F), lipolysis (Fig. 2G), and thermogenic gene induction (Fig. 2 H and I and SI Appendix, Fig. S4 A–C) in Tet TKO adipocytes upon β-agonist treatment. As expected, the Pgc-1α and Ucp1 protein levels were also significantly down-regulated by Tet2-CD regardless of its enzymatic activity (Fig. 2C and SI Appendix, Fig. S4 D–F).
Fig. 2.
TET proteins control β3-AR expression and signaling in an enzymatic activity–independent manner. (A) Immunoblot analysis of Myc-tagged WT and mutant (Mut) Tet2 catalytic domain (Tet2-CD) in preadipocytes derived from iWAT of Tet1fl/fl Tet2fl/fl Tet3fl/fl Cre-ERT2 mice. α-Tubulin served as a loading control. n = 3. (B) Dot blot analysis to quantify genomic 5hmCs in cells shown in A. Toluidine blue staining confirmed blotting of equal amounts of DNA. n = 3. (C) Immunoblot analysis of β3-AR, Pgc-1α, and Ucp1 proteins in differentiated WT adipocytes or Tet TKO adipocytes expressing empty vector, WT, or mutant Tet2-CD. α-Tubulin served as a loading control. n = 4. (D) Quantification of β3-AR proteins presented in C. n = 4. (E) mRNA levels of β3-AR relative to Gapdh in adipocytes shown in C. n = 3. (F) Immunoblot analysis of phosphorylated (p)-PKA substrates, p-HSL, and total HSL proteins in adipocytes shown in C after stimulation with or without CL-316,243 (10 µM, 5 min). α-Tubulin served as a loading control. (G) In vitro lipolysis in adipocytes shown in C. Plasma glycerol was quantified after stimulation with or without ISO (5 μM) at the indicated time points. n = 6. (H and I) mRNA levels of Ppargc1a (H) and Ucp1 (I) relative to Gapdh in adipocytes shown in C after stimulation with or without CL-316,243 (1 μM, 24 h). n = 3. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT; aP < 0.05, bP < 0.005.
Adipocyte-Specific TET Ablation Increases the Responsiveness to β3-AR Signals In Vivo.
β3-AR stimulation triggers beige adipocyte induction and activation in both murine and human ATs (6–8). To test whether elevated levels of β3-AR in Tet TKO adipocytes affect fat browning under adrenergic stimulation, we generated adipose-specific Tet TKO mice by crossing Tet1fl/fl Tet2fl/fl Tet3fl/fl mice with Adiponectin-Cre mice (48). We deleted all three Tet genes to exclude potential functional compensation among TET family members that is commonly observed in various tissues (32, 49, 50). We verified successful Tet deletion and the resulting 5hmC diminution specifically in adipose tissues but not in the liver, kidney, pancreas, and other nonadipose tissues (SI Appendix, Fig. S5 A–C). Furthermore, Tet deletion was induced only in mature adipocytes within adipose depots and not in stromal vascular fraction cells containing preadipocytes and leukocytes (SI Appendix, Fig. S5 D and E).
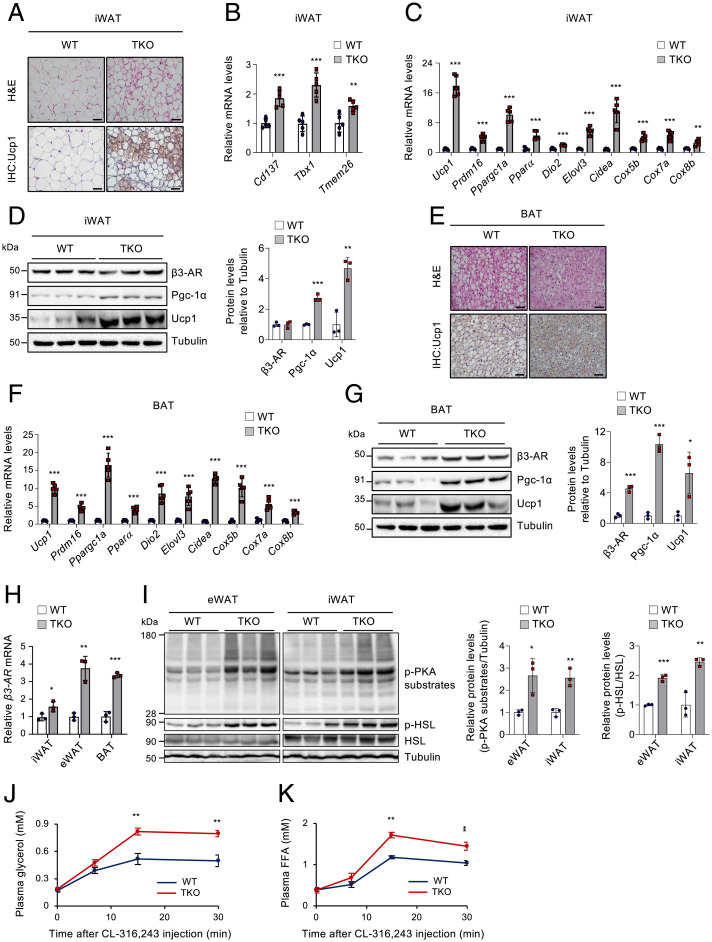
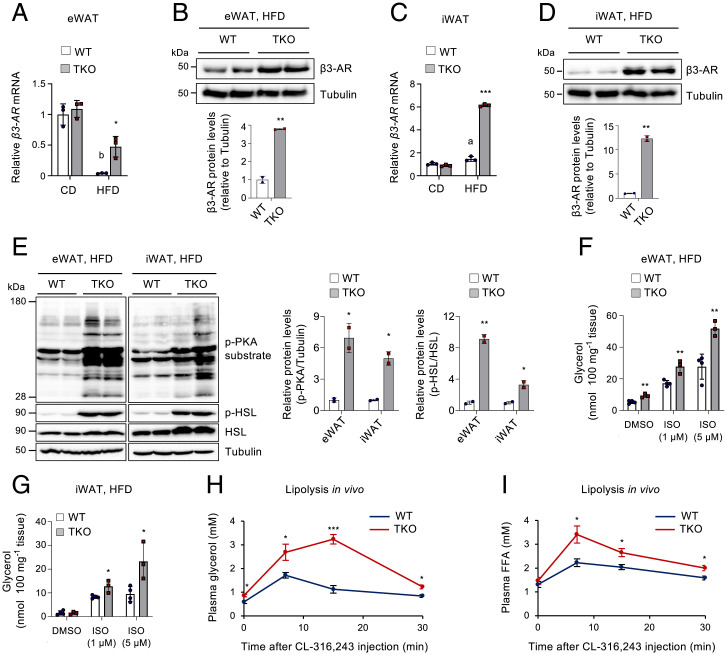
To assess whether TET proteins influence β3-AR agonist–triggered browning in vivo, WT and Tet TKO mice were administered CL-316,243 daily for three consecutive days, a condition that induces mild fat browning (6). Histological analyses revealed that the iWAT of Tet TKO mice possessed more abundant Ucp1+ multilocular adipocytes following CL-316,243 treatment compared to WT control (Fig. 3A). The iWAT of Tet TKO mice also displayed higher mRNA levels of genes associated with beiging (namely, Cd137, Tbx1, and Tmem26), thermogenesis (e.g., Ucp1, Ppargc1a, Ppara), lipolysis (e.g., Pnpla2, Lipe), and fatty acid oxidation (e.g., Acadm, Acadvl, Acox1) (Fig. 3 B and C and SI Appendix, Fig. S6 A and B). Levels of Pgc-1α and Ucp1 proteins were also higher in the iWAT of Tet TKO mice (Fig. 3D). A previous report showed that mice with Fabp4-Cre–mediated deletion of Tet1 exhibited a depot-specific effect; they displayed increased thermogenic gene expression only in iWAT but not in BAT (39). However, the interscapular BAT of Tet TKO mice showed similar phenotypes as those observed in iWAT, including enhanced browning (Fig. 3E) and an elevated expression of β3-AR and genes associated with thermogenesis, lipolysis, and fatty acid oxidation (Fig. 3 F and G and SI Appendix, Fig. S6 C and D). Furthermore, both iWAT and BAT from Tet TKO mice contained higher amounts of mitochondrial DNAs compared to those from WT controls (SI Appendix, Fig. S6 E and F). Together, these results suggest that TET loss promotes BAT activation as well as WAT browning. Notably, the mRNA levels of β3-AR were higher in adipose depots (i.e., iWAT, eWAT, and BAT) from Tet TKO mice, more prominently in the latter two, when compared to WT controls (Fig. 3H). Consistently, Tet TKO mice displayed enhanced β-adrenergic signaling and in vivo lipolytic responses upon β3-AR agonist treatment, as determined by increased phosphorylation of PKA substrates and HSL (Fig. 3I), and more robust release of plasma glycerol and free fatty acids (Fig. 3 J and K). Collectively, these results suggest that adipose TET deficiency leads to elevated β3-AR expression and increases the sensitivity to β3-AR stimulation in vivo.
Fig. 3.
Adipose TET deficiency increases the responsiveness to β3-AR agonist in vivo. WT and Tet TKO mice were administered CL-316,243 (1 mg/kg body weight/d) intraperitoneally daily for three consecutive days. (A) Representative hematoxylin and eosin (H&E) (Top) and immunohistochemical staining for Ucp1 (Bottom) in iWAT of CL-316,243–treated mice. n = 4. (Scale bar, 50 μm.) (B and C) mRNA levels of beige adipocyte–selective genes (B) or thermogenesis-related genes (C) relative to Gapdh in iWAT of CL-316,243–treated mice. n = 5. (D) Immunoblot analysis (Left) and quantification (Right) of β3-AR, Pgc-1α, and Ucp1 proteins in iWAT of CL-316,243–treated mice. α-Tubulin served as a loading control. n = 3. (E) Representative H&E (Top) and immunohistochemical staining for Ucp1 (Bottom) in BAT of CL-316,243–treated mice. n = 4. (Scale bar, 50 μm.) (F) mRNA levels of thermogenesis-related genes relative to Gapdh in BAT of CL-316,243–treated mice. n = 5. (G) Immunoblot analysis (Left) and quantification (Right) of β3-AR, Pgc-1α, and Ucp1 proteins in BAT of CL-316,243–treated mice. α-Tubulin served as a loading control. n = 3. (H) β3-AR mRNA levels relative to Gapdh in ATs (iWAT, eWAT, or BAT) of CL-316,243–treated mice. n = 3. (I) Immunoblot analysis (Left) and quantification (Right) of phosphorylated (p)-PKA substrates, p-HSL, and total HSL proteins in eWAT or iWAT of CL-316,243–treated mice. α-Tubulin served as a loading control. n = 3. (J and K) In vivo lipolysis assay in WT and Tet TKO mice stimulated with CL-316,243. Plasma glycerol (J) and free fatty acid (FFA) (K) levels were quantified at the indicated time points. n = 3. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT. IHC, immunohistochemistry.
Adipocyte-Specific Tet TKO Mice Are Resistant to Diet-Induced Obesity.
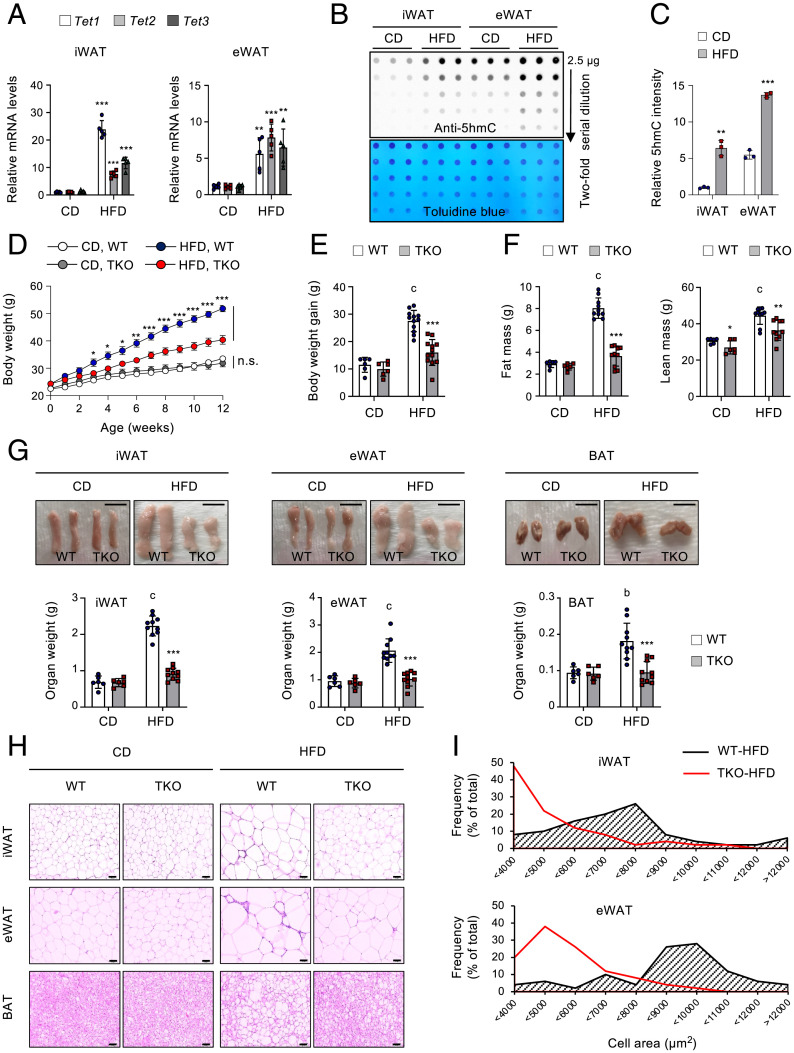
β3-AR is substantially repressed in various animal models of obesity, and this β3-AR down-regulation has been considered a key contributing factor that causes catecholamine resistance in obesity (16–18). As TET proteins negatively regulated β3-AR, we next asked whether Tet expression was altered under persistent nutrient surplus. Notably, levels of Tet1, Tet2, and Tet3 mRNAs (Fig. 4A) and those of 5hmCs (Fig. 4 B and C) were significantly elevated in iWAT and epididymal WAT (eWAT) of mice fed a HFD (60% energy as fat) compared to the levels in mice fed a chow diet (CD; 10% energy as fat). TET1, TET2, and TET3 mRNA levels also tended to increase in patients with obesity or diabetes, compared to lean individuals, although this difference did not reach statistical significance, possibly due to negative feedback on TET expression in humans under obese or diabetic conditions (SI Appendix, Fig. S7A). These results suggest that Tet expression and function are nutritionally regulated in ATs and that TET proteins may play a role in diet-induced obesity.
Fig. 4.
Adipocyte Tet expression is elevated in obesity, and Tet TKO mice resist diet-induced obesity. (A) Tet1, Tet2, and Tet3 mRNA levels relative to Gapdh in iWAT and eWAT from C57BL/6 male mice fed either CD or HFD for 12 wk. n = 5. (B) Genomic 5hmC levels in iWAT and eWAT from the mice shown in A. Toluidine blue staining confirmed blotting of equal amounts of DNAs. (C) Quantification of relative 5hmC intensity from the image shown in B. (D) Changes in body weight of WT and Tet TKO mice fed either CD or HFD. n = 6–12. (E) Summary of body weight gain in WT and Tet TKO mice fed either CD (n = 6) or HFD (n = 12) for 12 wk. (F) Absolute amount of fat mass (Left) and lean mass (Right) from WT and Tet TKO mice fed either CD (n = 6) or HFD (n = 10). (G) Representative photographs of iWAT, eWAT, and BAT of WT and Tet TKO mice fed either CD or HFD (Top). A summary of the weights of each fat pad is also shown (Bottom). n = 6 mice fed CD; n = 10 mice fed HFD. (Scale bar, 1 cm.) (H and I) Hematoxylin and eosin staining (H; n = 10) and frequency distribution of adipocyte cell size (I) in iWAT and eWAT of WT and Tet TKO mice fed either CD or HFD. (Scale bar, 100 μm.) All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT; bP < 0.005, cP < 0.0005 versus CD. n.s., not significant.
To clarify the physiological function of TET proteins in the development of obesity, Tet TKO and WT littermate mice were fed either a CD or HFD for 12 wk, starting at 8 wk of age. There was no difference in weight gain in response to the CD (Fig. 4 D and E and SI Appendix, Fig. S7B). However, Tet TKO mice were more resistant to weight gain in response to a HFD than were WT mice, despite comparable food intakes (Fig. 4 D and E and SI Appendix, Fig. S7 B and C). The lower body weight of Tet TKO mice primarily resulted from a reduction in fat mass with only a marginal alteration in lean body mass (Fig. 4F and SI Appendix, Fig. S7 D and E). Examination of dissected fat tissues confirmed that iWAT, eWAT, and BAT were significantly smaller in these mice (Fig. 4G). Histologically, both WAT and BAT from HFD-fed Tet TKO mice contained adipocytes possessing smaller lipid droplets compared with those of WT controls, whereas there was no discernible difference in adipocyte size of mice fed a CD (Fig. 4 H and I). Upon HFD feeding, the average adipocyte size increased by ∼3.56-fold in iWAT of WT mice but only ∼1.68-fold in Tet TKO mice, and similar reductions were also observed in eWAT (by ∼1.41-fold) and BAT (by ∼3.25-fold) of Tet TKO mice (SI Appendix, Fig. S7F). These data indicate that adipose TET deficiency robustly inhibits the development of obesity and obesity-induced adipocyte hypertrophy. Previous reports suggested that TET proteins were required for early adipogenic differentiation in vitro (40, 41). However, the observed difference in body weight change was not attributable to impaired adipogenesis in vivo because mRNA levels of known markers or regulators of adipogenesis were comparable between WT and Tet TKO mice on either diet, consistent with the fact that adiponectin-Cre we used was active in mature adipocytes (48) (SI Appendix, Fig. S7 G–J). Furthermore, we confirmed that Tet TKO female mice were also resistant to diet-induced obesity, mainly due to a reduction in fat mass (SI Appendix, Fig. S8).
Adipocyte-Specific Tet TKO Mice Resist β3-AR Reduction and Catecholamine Resistance during Diet-Induced Obesity.
Activation of the β3-adrenergic signaling increases energy expenditure by triggering lipolysis, fatty acid oxidation, and thermogenesis, ultimately leading to a decline in fat accumulation and obesity resistance (1, 2). Thus, we next sought to determine whether the restricted AT hypertrophy in the HFD-fed Tet TKO mice resulted from increased β-adrenergic responsiveness through enhanced β3-AR expression. β3-AR was substantially suppressed in eWAT of WT mice after 12 wk on a HFD, but this was substantially blocked in Tet TKO mice (Fig. 5A). Protein levels of β3-AR also remained significantly higher in HFD-fed Tet TKO mice (Fig. 5B). HFD feeding did not affect β3-AR mRNA levels in iWAT and BAT of WT mice, but TET deficiency led to an elevated β3-AR expression in these ATs following HFD feeding (Fig. 5 C and D and SI Appendix, Fig. S9A). Consistent with the higher levels of β3-AR expression, both eWAT and iWAT of HFD-fed Tet TKO mice displayed significantly enhanced β-AR signaling, as determined by increased phosphorylation of PKA substrates and HSL (Fig. 5E).
Fig. 5.
TET deficiency resists obesity-associated catecholamine resistance by blocking β3-AR reduction during diet-induced obesity. (A) β3-AR mRNA levels relative to Gapdh in eWAT from WT and Tet TKO mice fed either CD or HFD. n = 3. (B) Immunoblot analysis (Top) and quantification (Bottom) of β3-AR proteins in eWAT from WT and Tet TKO mice fed an HFD. α-Tubulin served as a loading control. (C) β3-AR mRNA levels relative to Gapdh in iWAT from WT and Tet TKO mice fed either CD or HFD. n = 3. (D) Immunoblot analysis (Top) and quantification (Bottom) of β3-AR proteins in iWAT from WT and Tet TKO mice fed an HFD. α-Tubulin served as a loading control. (E) Immunoblot analysis (Left) and quantification (Right) of phosphorylated (p)-PKA substrates, p-HSL, and total HSL proteins in eWAT or iWAT from HFD-fed WT and Tet TKO mice. α-Tubulin served as a loading control. (F and G) Ex vivo lipolysis in eWAT (F) and iWAT (G) isolated from HFD-fed WT and Tet TKO mice after stimulation with or without ISO at the indicated concentrations. n = 3–4. (H and I) In vivo lipolysis in HFD-fed WT and Tet TKO mice. Plasma glycerol (H) and free fatty acid (I) levels were quantified after administration of CL-316,243. n = 3–4. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT; aP < 0.05, bP < 0.005 versus CD. DMSO, dimethyl sulfoxide.
Fig. 6.
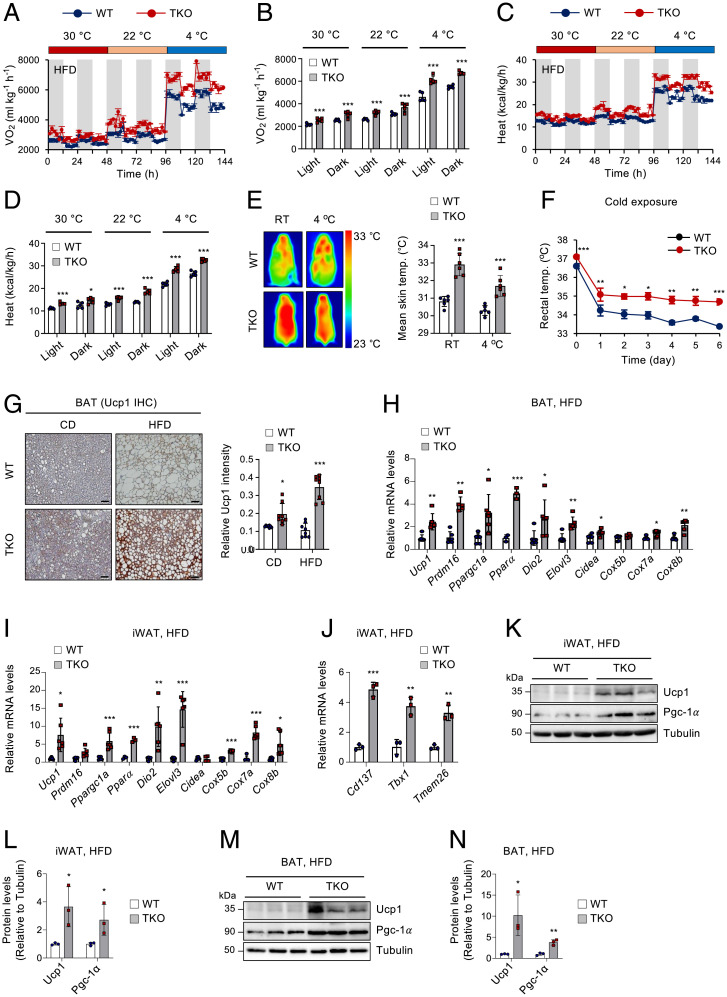
TET deficiency leads to the maintenance of a higher capacity for thermogenesis and energy expenditure under chronic overnutrition. (A−D) Indirect calorimetry of HFD-fed WT and Tet TKO mice maintained at 30 °C, 22 °C, and 4 °C to obtain Vo2 (A), average Vo2 (B), heat production (C), and average heat production (D) data. n = 5. (E) Representative dorsal infrared thermal images (Left) and mean skin temperature (Right) of HFD-fed WT and Tet TKO mice housed at room temperature (RT) or 4 °C for 1 wk. n = 6. (F) Changes in rectal temperatures of HFD-fed WT and Tet TKO mice during cold exposure. n = 7 WT mice; n = 9 Tet TKO mice. (G) Representative immunohistochemical staining for Ucp1 (n = 6; Left) in BAT sections from WT and Tet TKO mice fed either CD or HFD for 12 wk. Quantification results are also shown (Right). n = 8. (Scale bar, 100 μm.) (H and I) mRNA levels of thermogenesis-related genes relative to Gapdh in BAT (H) or iWAT (I) from HFD-fed WT and Tet TKO mice. n = 5–6 (except Pparα, n = 3). (J) mRNA levels of beige adipocyte-selective genes relative to Gapdh in iWAT from HFD-fed WT and Tet TKO mice. n = 3. (K–N) Immunoblot analysis and quantification of Ucp1 and Pgc-1α proteins in iWAT (K and L) or BAT (M and N) from WT and Tet TKO mice fed an HFD. α-Tubulin served as a loading control. n = 3. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT. IHC, immunohistochemistry.
To further confirm whether increased β3-AR expression and signaling affect lipolytic responses, eWAT and iWAT were isolated from HFD-fed WT or Tet TKO mice and treated with ISO. The ISO-stimulated lipolysis, as assessed by the release of glycerol and free fatty acids, was significantly enhanced in these explants of Tet TKO mice compared to those from WT mice (Fig. 5 F and G and SI Appendix, Fig. S9B). Moreover, TET deficiency also significantly promoted lipolysis in isolated primary ATs treated with forskolin or IBMX (SI Appendix, Fig. S9C). We also observed a mild increase in lipolytic responses in the BAT from Tet TKO mice (SI Appendix, Fig. S9 D and E). To further confirm the lipolytic activity in vivo, we injected mice intraperitoneally with CL-316,243. Consistent with data obtained in vitro, the serum glycerol and free fatty acid levels were significantly higher in Tet TKO mice than in WT mice (Fig. 5 H and I). Together, these results suggest that TET deficiency resisted β3-AR reduction during chronic HFD challenges and thereby rendered adipocytes more sensitive to catecholamines. This may have contributed to the maintenance of higher levels of β-agonist–stimulated lipolytic capacity and resistance to adipocyte hypertrophy.
Adipose TET Deficiency Leads to the Maintenance of Higher Capacity for Thermogenesis and Energy Expenditure during Chronic Overnutrition.
Excess caloric intake activates thermogenesis as a means to increase energy expenditure to prevent excess fat accumulation and obesity (3, 4). This diet-induced thermogenesis is critically dependent on β-AR signaling (3, 4). However, energy expenditure fails to fully compensate for chronic overnutrition during the development of obesity. Indeed, consistent with β-AR reduction and decreased β-AR responses, obese humans and animals display diminished adipose thermogenesis (51). As β3-AR expression and β-adrenergic responsiveness were maintained at higher levels in Tet TKO mice fed an HFD, we next examined whether TET deficiency resists the reduction of energy expenditure and thermogenesis during chronic overnutrition. To test this possibility, we first performed indirect calorimetry on HFD-fed WT and Tet TKO mice maintained at different ambient temperatures (30 °C, 22 °C, and 4 °C). HFD-fed Tet TKO mice exhibited significantly higher rates of oxygen consumption (Vo2), carbon dioxide production (Vco2), and heat dissipation under all conditions, and these alterations became more pronounced as the temperature dropped (Fig. 6 A–D and SI Appendix, Fig. S10 A and B). However, the levels of motor activity were comparable between both groups of mice (SI Appendix, Fig. S10C). The respiratory exchange rate (Vco2/Vo2) indicated that a greater proportion of carbohydrate metabolism was preserved in HFD-fed Tet TKO mice during dark periods at 22 °C and 4 °C (SI Appendix, Fig. S10D), which was likely due to reduced fat deposition. To further correct metabolic rates for body mass, we performed regression-based ANCOVA using body weights as a covariate. We observed a group-specific effect between WT and Tet TKO mice, which was independent of a difference in body mass at 30 °C and 4 °C, whereas both genotypes fell on a similar regression line at 22 °C (SI Appendix, Fig. S10E). It is possible that the effect was stronger at 4 °C because levels of catecholamine released by sympathetic nerve terminals were up-regulated upon cold exposure. As Tet-deficient adipocytes express higher β3-AR compared to WT cells, they would exhibit stronger responsiveness to the catecholamines. When we plotted ANCOVA-predicted metabolic rates at a given body mass of 40 g (kcal/h/40 g), as suggested previously as an alternative analysis when there is absolutely no overlap in the data from two groups on the mass axis (52, 53), as observed in our cases (Fig. 4), Tet TKO mice displayed significantly higher metabolic rates compared to WT mice under all conditions (SI Appendix, Fig. S10F). Together, these results indicate that Tet TKO mice maintained a higher capacity of thermogenesis despite chronic overnutrition. Consistent with these observations, HFD-fed Tet TKO mice maintained significantly higher surface and core (rectal) temperatures than did WT littermates under basal conditions and after cold exposure (Fig. 6 E and F and SI Appendix, Fig. S10G). Thus, these results suggest that TET proteins restrict adaptive thermogenesis and energy expenditure during periods of chronic overnutrition.
Fig. 7.
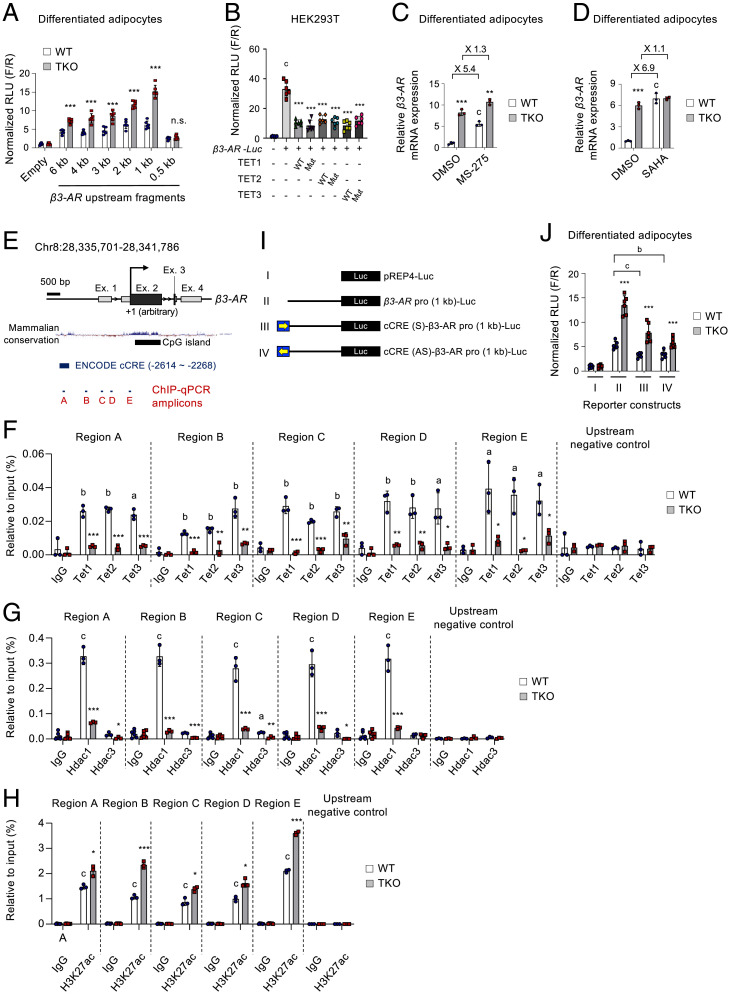
TET proteins cooperate with HDACs to directly suppress β3-AR transcription. (A) pREP4-based luciferase reporter activities driven by serially truncated β3-AR promoters in WT and Tet TKO adipocytes. Results were normalized to Renilla luciferase activity. n = 6. (B) pREP4-based luciferase reporter activities driven by the β3-AR (1 kb) promoter in HEK293T cells transfected with WT or mutant TET1, TET2, and TET3 plasmids. n = 6. (C and D) mRNA expression of β3-AR relative to Gapdh in WT and Tet TKO adipocytes stimulated with or without MS-275 (10 μM, 24 h) (C) or a pan-HDAC inhibitor, SAHA (20 μM, 6 h) (D). n = 3. (E) The genomic configuration of the mouse β3-AR gene locus. Locations of ChIP-qPCR amplicons, mammalian conservation, CpG islands, and Encyclopedia of DNA Elements (ENCODE) cis-CRE (cCRE) are shown. (F−H) ChIP-qPCR assays for Tet1, Tet2, and Tet3 (F); HDAC1 and HDAC3 (G); and acetylated H3K27 (H3K27ac) (H) for regions A−E shown in E. Enrichment at the nonbinding β3-AR upstream region was used as a negative control. n = 3. (I) Schematic representation of the β3-AR (1 kb) promoter-driven luciferase constructs. Yellow arrows indicate the direction of the incorporated ENCODE cCRE element shown in E. (J) pREP4-based luciferase reporter assay for the β3-AR promoters shown in (I) in WT and Tet TKO adipocytes. n = 6. All data are presented as the mean ± SD. The P values were determined by unpaired Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 versus WT; aP < 0.05, bP < 0.005, cP < 0.0005 versus reporter only (B), dimethyl sulfoxide (DMSO) (C), immunoglobulin G (IgG) (F–H), and reporter II in WT (J). Ex, exon; Mut, catalytically inactive mutant; n.s., not significant; RLU, relative light units; F/R, firefly/renilla.
Consistent with these observations, HFD-fed Tet TKO mice possessed smaller brown adipocytes with increased Ucp1 expression in BAT compared to WT controls (Fig. 6G) and exhibited elevated levels of thermogenic genes in both BAT and iWAT (Fig. 6 H and I). They also showed higher mRNA expressions of beiging marker genes in iWAT (Fig. 6J). The levels of Ucp1 and Pgc-1α proteins were also significantly higher in both iWAT and BAT of HFD-fed TKO mice (Fig. 6 K–N). Furthermore, HFD consumption significantly reduced the expression of Ppargc1a, a critical regulator of mitochondrial biogenesis and energy metabolism (54), in iWAT and eWAT, which was potently blocked by TET deficiency (SI Appendix, Fig. S11A). Ppargc1a was also maintained at a higher level in BAT from HFD-fed Tet TKO mice. Consistently, various adipose depots from HFD-fed Tet TKO mice maintained higher mitochondrial DNA content and fatty acid oxidation capacity (SI Appendix, Fig. S11 B and C). Together, these data suggest that elevated TET expression in chronic overnutrition may promote obesity by limiting the energy expenditure and thermogenic potentials of both beige and BAT adipocytes.
TET Proteins Cooperate with HDACs to Directly Repress β3-AR Transcription in Adipocytes.
Given the inverse correlation between β3-AR and Tet expressions, we assessed whether TET proteins could directly regulate β3-AR promoter activity. We cloned serially truncated versions of the promoter regions (from 0.5 kb to 6 kb) of the mouse β3-AR gene linked to a luciferase reporter (β3-AR-Luc) into pREP4 plasmid that forms chromatin in mammalian cells (55, 56). Except for the 0.5-kb proximal region, all fragments larger than 1 kb significantly stimulated reporter activities in WT adipocytes, and this was further enhanced in Tet TKO cells (Fig. 7A), indicating TET-mediated suppression of the β3-AR promoter activity. In another reporter assay in HEK293T cells, both WT and catalytically inactive mutants of TET proteins were capable of suppressing the β3-AR promoter activity, confirming that TET proteins controlled β3-AR transcription, mainly in a catalytic activity–independent manner (Fig. 7B).
TET proteins often mediate transcriptional silencing through their incorporation into HDAC complexes (37–39). Thus, we next investigated whether HDACs are implicated in TET-mediated thermogenic gene regulation. MS-275, a selective inhibitor of HDAC1 and HDAC3, up-regulated various thermogenic genes in both WT and Tet TKO adipocytes; however, the fold induction by MS-275 was significantly decreased in Tet TKO cells compared to WT cells (Fig. 7C and SI Appendix, Fig. S12 A–C), suggesting the possible implication of HDACs, including HDAC1 and HDAC3, in TET-mediated β3-AR suppression. However, upon MS-275 treatment, Tet TKO adipocytes still expressed higher levels of thermogenic genes compared to WT controls. Thus, it is possible that additional HDACs other than class I HDACs may also be implicated in the TET-mediated suppression of these thermogenic genes. To test this possibility, we treated WT and Tet TKO adipocytes with suberoylanilide hydroxamic acid (SAHA; also known as vorinostat), a pan-HDAC inhibitor that potently inhibits class I and class II HDACs. SAHA exhibited little effects on thermogenic gene expression in Tet TKO adipocytes, whereas it robustly up-regulated it in WT controls (Fig. 7D and SI Appendix, Fig. S12 A and B). Furthermore, when treated with SAHA, both WT and Tet TKO adipocytes expressed thermogenic genes at comparable levels. Together, these results indicate that TET proteins directly repress β3-AR and other thermogenic genes by modulating HDACs.
As previously reported (38, 39), we also confirmed that TET proteins physically associated with HDAC1 and HDAC3 (SI Appendix, Fig. S12 D–F). HDAC enzymes deacetylate histones and suppress transcription. ChIP-qPCR revealed that WT adipocytes exhibited a significant accumulation of all three TET proteins and HDAC1 on the upstream regulatory regions of the β3-AR gene locus (Fig. 7 E–G). However, the occupancy of HDAC3 was very low and not significant when compared with enrichment by immunoglobulin G controls (Fig. 7G). Notably, Tet TKO adipocytes exhibited a diminished occupancy of both TET and HDAC1 proteins in the β3-AR gene locus and thus possessed increased levels of acetylated H3K27, the active histone marker (Fig. 7 F–H). The immunoprecipitated genomic DNA samples showed no enrichment at the nonbinding region upstream of β3-AR. These results suggest that TET proteins recruit HDAC1 to the β3-AR gene promoter, ultimately leading to histone deacetylation for transcriptional suppression.
The Encyclopedia of DNA Elements project (57) predicted a 346-bp upstream DNA element located between −2,614 and −2,268 as one of the candidate cis-regulatory elements in the β3-AR locus (Fig. 7E, region A and SI Appendix, Fig. S12G). This region is well conserved among mammalian species (Fig. 7E), and its histone acetylation was controlled by TET-HDAC interaction (Fig. 7 F–H, region A). To confirm the functionality of this element, we constructed additional reporters by incorporating this element upstream of the β3-AR promoter (1 kb) in both orientations (Fig. 7I). The presence of this element significantly diminished β3-AR promoter activity in adipocytes regardless of its orientation (Fig. 7J, compare reporter activities in WT cells), and this was significantly relieved by TET deficiency (Fig. 7J), confirming its silencer-like function. Together, these results indicate that TET proteins directly repress β3-AR transcription by the epigenetic modification of its promoter via the recruitment of HDACs.
Discussion
Here, we report that TET proteins are crucial epigenetic suppressors of β3-AR expression and β-adrenergic responsiveness in adipocytes. A pan-TET deficiency significantly elevated β3-AR expression and sensitivity to β-AR agonists in cultured cells and mice, thus potentiating the β3-AR–triggered downstream responses, including lipolysis, thermogenic gene induction, mitochondrial respiration, and fat browning. Notably, adipose Tet expression was elevated by excess nutrient uptake. During the development of HFD-induced obesity, adipose-specific pan-TET deficiency led to the maintenance of higher β3-AR expression and β-adrenergic responsiveness in both WAT and BAT. As a result, Tet TKO mice were strikingly resistant to diet-induced obesity, inflammation, and related metabolic defects.
Previous studies proposed the potential regulatory roles of TET proteins in adipocyte differentiation and function. However, knockdown of Tet1 or Tet2 exerts controversial effects on adipogenic differentiation in vitro (40–42). Our data show that TET proteins differentially affect adipogenesis in vitro, depending on the differentiation stage. Ablation of all TET proteins in preadipocytes or during adipogenic induction completely blocked the formation of mature adipocytes, but its loss at a later stage did not exhibit any significant antiadipogenic effects. TET proteins may be essential for the early adipogenic commitment of progenitor cells, presumably by regulating key adipogenic regulators, including PPARγ, as previously suggested (41, 58). However, they may be largely dispensable for adipogenesis after preadipocytes are committed to adipogenic lineage. Furthermore, a previous report showed that Tet1 deletion in mice suppressed thermogenesis by repressing Ucp1 and Ppargc1a in beige adipocytes (39). Thus, the Tet1 knockout mice were partially protected from cold stress, diet-induced obesity, and associated metabolic dysfunctions. In contrast, another report showed that Tet1 haploinsufficiency predisposed mice to diet-induced obesity and aggravated glucose intolerance (42). Thus, the exact roles of TET proteins in metabolism remain unclear. Here, we deleted all three Tet genes using Adiponectin-Cre, which is expressed in a highly adipocyte-specific manner (48, 59), and confirmed that triple TET deficiency substantially increases β3-AR expression and function in both ATs and cultured adipocytes, and enhances fat lipolysis, browning, and thermogenic gene expression. The resulting phenotype was more pronounced than that of cells and mice rendered Tet1-deficient with Fabp4-Cre (39), which is expressed in both AT and nonadipose tissue (59). Furthermore, although Tet1 deficiency was previously shown to increase thermogenic gene expression only in iWAT but not in BAT (39), our Tet TKO mice showed similar phenotypes in both WAT and BAT. The weaker phenotypes in Tet1 knockout mice, compared to our Tet TKO mice, may be attributed to the functional redundancy among TET family members.
Our analysis identified β3-AR as the direct target of TET proteins that, in turn, controlled downstream β-adrenergic responses. From a molecular standpoint, we showed that TET proteins recruited HDACs, particularly HDAC1, to the β3-AR promoter to directly suppress its transcription. TET deficiency led to a dissociation of HDACs from the β3-AR gene promoter, thereby switching the repressive chromatin state to the active state. Pharmacological inactivation of HDACs led to significant induction of various thermogenic genes in WT adipocytes, but its effect was decreased in Tet TKO adipocytes. Notably, this TET-HDAC cooperation occurred via a physical interaction between TET and HDAC enzymes, and functional cooperation did not require the catalytic activity of the TET proteins in reporter assays for β3-AR promoter activities. HDACs, particularly class I HDACs, have recently emerged as promising molecular targets for treating obesity and type 2 diabetes (60). TET deficiency mimics the effects of HDAC inhibition in vitro and in vivo. Genetic or pharmacological inactivation of HDACs (particularly HDAC1 or HDAC3) simultaneously activates β3-AR, Ucp1, and Ppargc1a by increasing histone acetylation at these gene loci (61–63). Consistently, HDAC inactivation in mice facilitates systemic energy expenditure by promoting WAT browning, lipolysis, thermogenesis, and oxidative metabolism, and thus prevents obesity and insulin resistance (61, 62, 64). All these phenotypes are recapitulated in our Tet TKO mice. Thus, TET proteins may control thermogenesis and energy expenditure by modulating the genomic distribution of HDACs. As TET deficiency resists the suppressive effects of chronic overnutrition on β3-AR expression and function, targeting the TET-HDAC-β3-AR axis could provide a way to reprogram fat cell fates and functions to treat obesity and related metabolic diseases.
Our data also suggested that HFD-induced elevation of TET expression is causally linked to the initiation and development of diet-induced obesity. β3-AR expression and β-adrenergic signaling are significantly suppressed during chronic nutrient overload, ultimately leading to catecholamine resistance in obesity (13–18). Despite chronic overnutrition, TET deficiency may allow for sustained catecholamine sensitivity by blocking β3-AR down-regulation, thus enhancing β-adrenergic signaling, lipolysis, mitochondrial biogenesis, fatty acid oxidation, and thermogenesis. Ultimately, it may result in an overall increase in energy expenditure to prevent fat accumulation and obesity. Moreover, TET proteins seem to exert their primary effects at the level of lipolysis. TET loss leads to a remarkable increase in β-agonist–stimulated lipolysis in vitro and in vivo. The increased lipolysis may contribute to the maintenance of smaller and metabolically healthier adipocytes by robustly antagonizing fat storage and AT hypertrophy, despite excess nutrient uptake. AT expansion during periods of nutritional affluence promotes inflammation and adipocyte dysfunction (65). Consistent with reduced fat cell size, Tet TKO mice were markedly protected from inflammation and metabolic defects when fed an HFD (SI Appendix, Figs. S13–S15). Thus, it is likely that the improved metabolic phenotypes observed in the HFD-fed Tet TKO mice may primarily be attributable to the reduced AT hypertrophy and body weight gain. However, given the role of β3-AR signals in antagonizing ectopic fat accumulation and obesity, our data suggest that TET-mediated modulation of catecholamine sensitivity may exert more fundamental effects on the metabolic phenotypes. β3-ARs are abundantly expressed in murine ATs and serve as primary β-ARs. Agonists of β3-ARs display antiobesity and antidiabetic effects in mice. However, low levels of β3-AR expression in human white adipocytes (2, 66) seemed to limit the therapeutic efficacy of β3-AR agonists in clinical trials (27). Thus, combined administration of selective β3-AR agonists while boosting β3-AR expression with pan-TET inhibitors may offer a therapeutic opportunity for treating human obesity and metabolic diseases.
It remains unknown how TET expression is up-regulated in ATs following HFD consumption. Recent studies have suggested that TET expression or function can be controlled by obesity- or type 2 diabetes–associated hyperglycemia (67–69). However, it was variably altered depending on the context and not directly assessed in ATs. In this regard, we note that 5hmC paradoxically accumulates more effectively under hypoxia (70–72), despite limited levels of oxygen, an essential cofactor for TET enzymes (32). HIF-1α has been shown to activate TET1, TET2, and TET3 transcription by directly binding to their promoters in various cells, including adipocytes (70–72). As HFD-induced adipocyte expansion restricts oxygen availability within ATs (73), the subsequent HIF-1α accumulation may stimulate Tet expression. Furthermore, recent studies showed that hypoxia-induced HIF-1α triggers obesity-induced AT inflammation and adipocyte dysfunction, leading to insulin resistance (73). Notably, upon HFD feeding, mice lacking HIF-1α in adipocytes largely phenocopied our Tet TKO mice, as both animal models exhibited resistance to diet-induced obesity, reduced macrophage accrual and inflammation, improved insulin sensitivity, glucose tolerance, and elevated energy expenditure and fatty acid oxidation (73–75). Together, these results suggest that adipose HIF-1α and TET proteins may work in the same pathway to promote fat deposition and obesity in times of excessive nutrient uptake. More studies are warranted to determine whether and how the hypoxia–HIF-1α–TET axis works to induce obesity and metabolic defects.
In summary, we identified TET proteins as pivotal epigenetic suppressors of β3-AR in adipocytes that reduced the sensitivity to β-adrenergic stimulation in vitro and in vivo. Our findings shed light on the epigenetic control of β-adrenergic responses via TET–HDAC cooperation. Because loss of TET proteins in adipocytes robustly defended against the suppressive effects of chronic overnutrition on β-adrenergic sensitivity, thermogenesis, and energy expenditure, targeting the TET–HDAC-β3–AR axis may increase therapeutic options for treating obesity and related metabolic diseases.
Materials and Methods
Mice carrying LoxP-targeted (floxed) Tet1fl/fl, Tet2fl/fl, and Tet3fl/fl have been described previously (76–78) and were provided by Dr. Anjana Rao (La Jolla Institute, CA, USA). Adiponectin-Cre (#010803) and Cre-ERT2 (#8085) transgenic mice were obtained from the Jackson Laboratory. Detailed materials and methods can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (Grants 2018R1D1A1B07049676, 2019R1F1A1063340, 2018R1A6A1A03025810, and 2017R1C1B2005604) and the Future-leading Project Research Fund of Ulsan National Institute of Science and Technology (Grant 1.220023.01). M.K. is also supported by the Center for Genomic Integrity, Institute for Basic Science (Grant IBS-R022-D1).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2205626119/-/DCSupplemental.
Data Availability
RNA-sequencing datasets used in this study have been deposited in National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository with the accession number GSE178762.
References
- 1.Robidoux J., Martin T. L., Collins S., Beta-adrenergic receptors and regulation of energy expenditure: A family affair. Annu. Rev. Pharmacol. Toxicol. 44, 297–323 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Evans B. A., Merlin J., Bengtsson T., Hutchinson D. S., Adrenoceptors in white, brown, and brite adipocytes. Br. J. Pharmacol. 176, 2416–2432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani E. T., Kajimura S., Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowell B. B., Spiegelman B. M., Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Fedorenko A., Lishko P. V., Kirichok Y., Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y., Berry D. C., Graff J. M., Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cero C., et al. , β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6, e139160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghorbani M., Teimourian S., Farzad R., Asl N. N., Apparent histological changes of adipocytes after treatment with CL 316,243, a β-3-adrenergic receptor agonist. Drug Des. Devel. Ther. 9, 669–676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widén E., et al. , Association of a polymorphism in the beta 3-adrenergic-receptor gene with features of the insulin resistance syndrome in Finns. N. Engl. J. Med. 333, 348–351 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Clément K., et al. , Genetic variation in the beta 3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N. Engl. J. Med. 333, 352–354 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Bachman E. S., et al. , betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Susulic V. S., et al. , Targeted disruption of the beta 3-adrenergic receptor gene. J. Biol. Chem. 270, 29483–29492 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Reynisdottir S., Ellerfeldt K., Wahrenberg H., Lithell H., Arner P., Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J. Clin. Invest. 93, 2590–2599 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jocken J. W., Blaak E. E., van der Kallen C. J., van Baak M. A., Saris W. H., Blunted beta-adrenoceptor-mediated fat oxidation in overweight subjects: A role for the hormone-sensitive lipase gene. Metabolism 57, 326–332 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Guo T., et al. , Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins S., et al. , Impaired expression and functional activity of the beta 3- and beta 1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol. Endocrinol. 8, 518–527 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Muzzin P., et al. , An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J. Biol. Chem. 266, 24053–24058 (1991). [PubMed] [Google Scholar]
- 18.Collins S., Daniel K. W., Rohlfs E. M., Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int. J. Obes. Relat. Metab. Disord. 23, 669–677 (1999). [DOI] [PubMed] [Google Scholar]
- 19.De Matteis R., et al. , Immunohistochemical identification of the beta(3)-adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. Int. J. Obes. Relat. Metab. Disord. 26, 1442–1450 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Shenoy S. K., et al. , beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M., A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401, 286–290 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Ghorbani M., Claus T. H., Himms-Hagen J., Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 54, 121–131 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Granneman J. G., Li P., Zhu Z., Lu Y., Metabolic and cellular plasticity in white adipose tissue I: Effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 289, E608–E616 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Pérusse F., Bukowiecki L. J., Mechanisms of the antidiabetic effects of the beta 3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am. J. Physiol. 274, R1212–R1219 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Hao L., et al. , Beneficial metabolic effects of mirabegron in vitro and in high-fat diet-induced obese mice. J. Pharmacol. Exp. Ther. 369, 419–427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arch J. R., et al. , Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 309, 163–165 (1984). [DOI] [PubMed] [Google Scholar]
- 27.Arch J. R., Challenges in β(3)-adrenoceptor agonist drug development. Ther. Adv. Endocrinol. Metab. 2, 59–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finlin B. S., et al. , The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Invest. 130, 2319–2331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finlin B. S., et al. , Human adipose beiging in response to cold and mirabegron. JCI Insight 3, e121510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Mara A. E., et al. , Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest. 130, 2209–2219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cypess A. M., et al. , Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastor W. A., Aravind L., Rao A., TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 14, 341–356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H., et al. , Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature 473, 389–393 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hon G. C., et al. , 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol. Cell 56, 286–297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsagaratou A., et al. , Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc. Natl. Acad. Sci. U.S.A. 111, E3306–E3315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X., Li G., Xie R., Decoding the role of TET family dioxygenases in lineage specification. Epigenetics Chromatin 11, 58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams K., et al. , TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q., et al. , Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damal Villivalam S., et al. , TET1 is a beige adipocyte-selective epigenetic suppressor of thermogenesis. Nat. Commun. 11, 4313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiki K., et al. , PPARγ-induced PARylation promotes local DNA demethylation by production of 5-hydroxymethylcytosine. Nat. Commun. 4, 2262 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Wiehle L., et al. , Tet1 and Tet2 protect DNA methylation canyons against hypermethylation. Mol. Cell. Biol. 36, 452–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Y., et al. , Vitamin C inhibits the metabolic changes induced by tet1 insufficiency under high fat diet stress. Mol. Nutr. Food Res. 65, e2100417 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Huang Y., et al. , The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS One 5, e8888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herzig S., et al. , CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Lio C. W., et al. , Tet2 and Tet3 cooperate with B-lineage transcription factors to regulate DNA modification and chromatin accessibility. eLife 5, e18290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagner S., et al. , TET2 regulates mast cell differentiation and proliferation through catalytic and non-catalytic activities. Cell Rep. 15, 1566–1579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao Y., et al. , DNA demethylase Tet2 suppresses cisplatin-induced acute kidney injury. Cell Death Discov. 7, 167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eguchi J., et al. , Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An J., et al. , Acute loss of TET function results in aggressive myeloid cancer in mice. Nat. Commun. 6, 10071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawlaty M. M., et al. , Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell 24, 310–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung R. T., Shetty P. S., James W. P., Barrand M. A., Callingham B. A., Reduced thermogenesis in obesity. Nature 279, 322–323 (1979). [DOI] [PubMed] [Google Scholar]
- 52.Speakman J. R., Measuring energy metabolism in the mouse - Theoretical, practical, and analytical considerations. Front. Physiol. 4, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller T. D., Klingenspor M., Tschöp M. H., Revisiting energy expenditure: How to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Lin J., Handschin C., Spiegelman B. M., Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 (2005). [DOI] [PubMed] [Google Scholar]
- 55.van der Vlag J., den Blaauwen J. L., Sewalt R. G., van Driel R., Otte A. P., Transcriptional repression mediated by polycomb group proteins and other chromatin-associated repressors is selectively blocked by insulators. J. Biol. Chem. 275, 697–704 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Liu R., et al. , Regulation of CSF1 promoter by the SWI/SNF-like BAF complex. Cell 106, 309–318 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Moore J. E., et al. ; ENCODE Project Consortium, Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 583, 699–710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sérandour A. A., et al. , Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 40, 8255–8265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeffery E., et al. , Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte 3, 206–211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong B. X., et al. , Regulation of thermogenic adipocyte differentiation and adaptive thermogenesis through histone acetylation. Front. Endocrinol. (Lausanne) 11, 95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galmozzi A., et al. , Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 62, 732–742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrari A., et al. , HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat. Commun. 8, 93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li F., et al. , Histone deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of histone H3 lysine 27 (H3K27) deacetylation and methylation. J. Biol. Chem. 291, 4523–4536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Z., et al. , Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H., et al. , Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng C., et al. , Respective degree of expression of beta 1-, beta 2- and beta 3-adrenoceptors in human brown and white adipose tissues. Br. J. Pharmacol. 118, 929–934 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhliwayo N., Sarras M. P. Jr., Luczkowski E., Mason S. M., Intine R. V., Parp inhibition prevents ten-eleven translocase enzyme activation and hyperglycemia-induced DNA demethylation. Diabetes 63, 3069–3076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan E. F., et al. , Hyperglycemia affects global 5-methylcytosine and 5-hydroxymethylcytosine in blood genomic DNA through upregulation of SIRT6 and TETs. Clin. Epigenetics 11, 63 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu D., et al. , Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali M. M., Phillips S. A., Mahmoud A. M., HIF1α/TET1 pathway mediates hypoxia-induced adipocytokine promoter hypomethylation in human adipocytes. Cells 9, 134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao J. Z., Liu H., Wickrema A., Godley L. A., HIF-1 directly induces TET3 expression to enhance 5-hmC density and induce erythroid gene expression in hypoxia. Blood Adv. 4, 3053–3062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matuleviciute R., Cunha P. P., Johnson R. S., Foskolou I. P., Oxygen regulation of TET enzymes. FEBS J. 288, 7143–7161 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Lee Y. S., et al. , Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krishnan J., et al. , Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 26, 259–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang C., et al. , Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ko M., et al. , Ten-eleven-translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc. Natl. Acad. Sci. U.S.A. 108, 14566–14571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko M., et al. , TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol. Rev. 263, 6–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang J., et al. , Simultaneous deletion of the methylcytosine oxidases Tet1 and Tet3 increases transcriptome variability in early embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 112, E4236–E4245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-sequencing datasets used in this study have been deposited in National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository with the accession number GSE178762.