Abstract
BACKGROUND
Severe traumatic brain injury (TBI) requires individualized, physiology-based management to avoid secondary brain injury. Recent improvements in quantitative assessments of metabolism, oxygenation, and subtle examination changes may potentially allow for more targeted, rational approaches beyond simple intracranial pressure (ICP)-based management. The authors present a case in which multimodality monitoring assisted in decision-making for decompressive craniectomy.
OBSERVATIONS
This patient sustained a severe TBI without mass lesion and was monitored with a multimodality approach. Although imaging did not seem grossly worrisome, ICP, pressure reactivity, brain tissue oxygenation, and pupillary response all began worsening, pushing toward decompressive craniectomy. All parameters normalized after decompression, and the patient had a satisfactory clinical outcome.
LESSONS
Given recent conflicting randomized trials on the utility of decompressive craniectomy in severe TBI, precision, physiology-based approaches may offer an improved strategy to determine who is most likely to benefit from aggressive treatment. Trials are underway to test components of these strategies.
Keywords: multimodality monitoring, traumatic brain injury, decompressive craniectomy, intracranial pressure, cerebral autoregulation, pupillary response
ABBREVIATIONS : CBF = cerebral blood flow, CPP = cerebral perfusion pressure, DC = decompressive craniectomy, GCS = Glasgow Coma Scale, ICP = intracranial pressure, MAP = mean arterial pressure, MMM = multimodality monitoring, NPi = Neurological Pupil index, PbO2 = brain tissue oxygenation, PRx = pressure reactivity, TBI = traumatic brain injury
Severe traumatic brain injury (TBI) represents a significant public health problem and is characterized by complex physiology, making individualized, targeted care desirable.1 Although neurocritical care and surgical strategies seek to minimize the secondary brain injury that follows the initial injury,2 it is increasingly being recognized that the physiology of the injured brain can change between patients and even within a given patient over the course of observation. This necessitates a precision medicine approach in which therapy is directed toward the individual patient’s physiology.3 Multimodality monitoring (MMM) may provide better insight into the physiological changes occurring in real time, supplementing the bedside neurological examination and allowing use of directed therapies. Ideally, MMM could provide indications of a problem before it occurs, unlike a neurological examination in which the manifestation has already occurred and may be irreversible.
Various neuromonitoring tools are used to access physiological changes, with intracranial pressure (ICP) monitoring being the most common. Increased ICP can lead to a higher rate of mortality if left untreated by impairing cerebral perfusion, resulting in secondary brain injury.4 ICP (and cerebral perfusion pressure [CPP])-directed therapy is therefore currently the cornerstone of management of severe TBI.5 When ICP becomes refractory to lower tiers of treatment, decompressive craniectomy (DC) can be used as a late-tier procedure. Two recent trials, however, arrived at complex and somewhat contradictory results regarding the efficacy of this intervention, which led to complex new recommendations.6 The authors of the updated guidelines ultimately concluded that “choosing to perform a DC is not a simple decision and that the potential benefits should be balanced against the complications and likely outcomes on a case-by-case basis.”6
The addition of other MMM variables besides ICP may provide more insight into both baseline salvageability and the risk of further deterioration and could direct interventions. Quantitative, standardized variables such as pupillary reactivity as measured by pupillometer,7 autoregulatory measures such as pressure reactivity,8 and brain tissue oxygenation (PbO2)9 may offer additional insight into impending herniation events and need for aggressive intervention. We present a case of a patient with severe TBI managed with MMM in whom these variables assisted in decision-making for DC with improvement in both monitoring variables and neurological function after intervention.
Illustrative Case
A 25-year-old male pedestrian was struck by an automobile and lost consciousness. He was brought to the University of New Mexico Emergency Department with an initial Glasgow Coma Scale (GCS) score of 4. Computed tomography of the patient’s head revealed traumatic, multifocal, multicompartmental hemorrhage with mild left predominance in the middle fossa (Fig. 1). The basal cisterns remained patent without radiographic signs of severely elevated ICP.
FIG. 1.
Noncontrast axial CT scans of the patient’s head. A and B: Initial studies with scattered contusions in the left temporal and frontal areas. There is no sulcal or basal cistern compression. C: Interval placement of the MMM bolt with external ventricular drain (arrow).
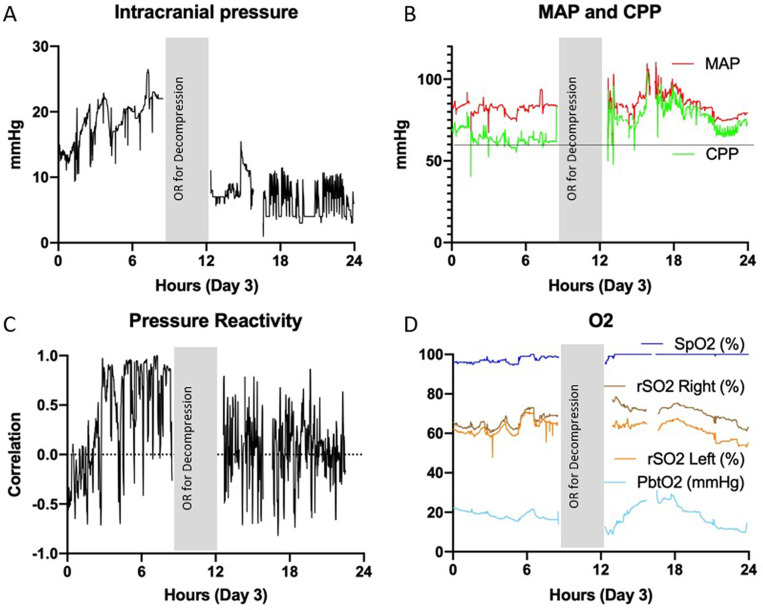
Because of the relatively benign initial appearance, a parenchymal ICP monitor was initially placed (Hummingbird Solo, IRRAS). Initial ICP was 30 mm Hg, and the patient began to have spontaneous posturing. After standard tier 1 interventions for ICP, the patient was administered a 3% NaCl bolus, which resulted in improved ICP <20 mm Hg. Due to persistent need for medical interventions for ICP, the parenchymal monitor was replaced with a single-hole MMM bolt/external ventricular drain (Hummingbird Quad, IRRAS).10 Repeated ICP elevation episodes into the mid–20-mm Hg range were treated with hypertonic saline boluses and sedation. Eventually, pentobarbital sedation was introduced because of persistent elevations despite a relatively benign radiographic appearance. Because of this benign appearance and the ability to keep the ICP range in the upper teens to low 20s, surgical intervention was not deemed necessary. On hospital day 3 (Fig. 2), the patient’s ICP continued with only moderate spikes >20 mm Hg (Fig. 2A); however, review of MMM data revealed several worsening trends. PbO2, which had consistently been >20 mm Hg, began to decrease below this threshold despite ventilator changes (Fig. 2D). Pressure reactivity (PRx; which was being continuously monitored per the Component Neuromonitoring System [Moberg Research]) had been fluctuating around 0 and began trending significantly higher toward 1 over the course of approximately 6 hours (Fig. 2C). During this time, the automated pupillometry results showed slight asymmetry and decreased reactivity (Neurological Pupil index [NPi]) on the left, which essentially became nonreactive (NPi 0) during the night between days 2 and 3 (Fig. 3). The mean arterial pressure (MAP) and CPP were within normal ranges at 70–100 mm Hg and 60–70 mm Hg, respectively (Fig. 2B). Based on these rapidly worsening indicators of impending herniation despite maximal medical management, the decision was made to perform left DC. The left side was chosen due to the predominance of contusions on the left as well as the left-sided pupillary changes. This was done in the usual fashion with a large bone flap5 placed in the abdominal subcutaneous tissue.11
FIG. 2.
MMM data on day 3. The vertical gray bar represents the time the patient was in the operating room (OR) for DC. A: Recurring ICP spikes over 20 mm Hg. B: MAP and CPP within normal ranges, 70–100 mm Hg and 60–70 mm Hg, respectively. Horizontal line represents the threshold level of 60 mm Hg for CPP. C: PRx fluctuating less than 0 with a significant increase toward 1 over the course of 6 hours. Dotted line is at PRx of 0. D: Brain tissue oxygenation (PbtO2) decreasing below 20 mm Hg. Note significant improvement in ICP and PRx after decompression. rSO2 = regional oxygen saturation; SpO2 = oxygen saturation.
FIG. 3.
Automated pupillometry measurements during the first 5 days of admission. Upper: Pupillary size. Note slight asymmetry, which improved after decompression. Lower: Pupillary reactivity (NPi). Left-eye NPi became absent (despite maintained size) between days 2 and 3. Reactivity normalized after decompression. OR = operating room.
After the DC, the patient’s ICP stayed less than 10 mm Hg without need for drainage or other interventions. Remarkably, the PRx returned to normal nearly immediately, PbO2 increased within several hours of surgery, and the left pupil NPi and size returned to normal and symmetric with the right pupil within hours of surgery.
The patient’s neurological examination results improved, and he underwent early tracheostomy. The bone flap was replaced uneventfully 3 weeks after DC, and he was discharged 27 days after injury to an inpatient rehabilitation facility with a GCS score of E4VTM6. At a 4-month follow-up visit, the patient was awake and oriented but unable to attend to his own affairs, requiring assistance in activities of daily living.
Discussion
Observations
The decision to perform DC in this patient was influenced largely by MMM in addition to standard ICP measurement. We believe that rapidly worsening PRx, PbO2, and pupillary reactivity were evidence of impending herniation, and all these physiological measures rapidly improved after DC. Such measures represent additional factors that may help case-by-case decision-making urged in this disease.6
Management of TBI with MMM remains understudied. There is a need for an effective means to monitor TBI to guide individualized interventions.5 There are many different approaches that may all give some insight into damaging brain physiology that may include inadequate perfusion, inadequate PbO2, impaired autoregulation, spreading depolarization and seizures, and impaired metabolism. Although ICP alone is the most commonly used determining factor for medical or surgical intervention, the use of PbO2 (which may be a better estimate of cerebral blood flow [CBF]12) has been evaluated. Brain Oxygen Optimization in Severe TBI Phase II (BOOST-2) demonstrated the safety and feasibility of PbO2-directed therapy,9 and BOOST-3 is a currently enrolling, double-blind randomized controlled study assessing the efficacy of such an approach.
PRx has been proposed as a surrogate measure of autoregulatory status, which has been associated with outcome and is an attractive target for individualized management based on the optimal CPP. This approach uses the highly time-resolved fluctuations of ICP as a surrogate for CBF, calculating a moving correlation coefficient of ICP and MAP. Retrospective studies have demonstrated worse outcomes in patients with a PRx threshold greater than approximately 0.25.13 By measuring PRx across an individual patient’s range of spontaneously fluctuating blood pressure or CPP, the level at which the PRx is lowest (blood flow most stable relative to MAP) can be identified and may offer an improved CPP target to individualize care. A clinical trial targeting the feasibility of this approach (COGiTATE) is underway.14
The use of automated pupillary measurement and indices is becoming a topic of significant interest, given the noninvasive nature of this approach. Abnormal NPi (<3) in comatose patients was found to be predictive of the need for surgical intervention.7 Furthermore, NPi has been associated with both increased ICP and subsequent spikes, supporting the concept that worsening NPi may be a harbinger of herniation and therefore may be used to guide earlier intervention.15
Lessons
This case demonstrates the utility of physiological MMM in addition to ICP when determining management of severe TBI, particularly the need for surgical intervention. Understanding the disturbances in underlying physiology may better allow precision care for these patients, given the complex and conflicting state of the current literature. In addition, such an approach allows better identification of patients with initially more normal physiology whose conditions subsequently deteriorate, which is the optimum group most likely to benefit from aggressive interventions such as DC.6
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Carlson, Robinson. Acquisition of data: Carlson, Robinson, Shin. Analysis and interpretation of data: Carlson, Robinson. Drafting the article: Carlson, Robinson, Cole. Critically revising the article: Carlson, Robinson, Alunday, Cole, Torbey. Reviewed submitted version of manuscript: Carlson, Robinson, Shin, Alunday, Torbey. Approved the final version of the manuscript on behalf of all authors: Carlson. Statistical analysis: Carlson. Administrative/technical/material support: Robinson.
References
- 1. Hawryluk GW, Manley GT. Classification of traumatic brain injury: past, present, and future. Handb Clin Neurol. 2015;127:15–21. doi: 10.1016/B978-0-444-52892-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 2. McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- 3. Shrestha GS, Suarez JI, Hemphill JC., III Precision medicine in neurocritical care. JAMA Neurol. 2018;75(12):1463–1464. doi: 10.1001/jamaneurol.2018.3157. [DOI] [PubMed] [Google Scholar]
- 4. Chesnut RM, Petroni G, Rondina C. Intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2013;368(18):1751–1752. doi: 10.1056/NEJMc1301076. [DOI] [PubMed] [Google Scholar]
- 5. Carney N, Totten AM, O’Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 6. Hawryluk GWJ, Rubiano AM, Totten AM, et al. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87(3):427–434. doi: 10.1093/neuros/nyaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Ahmadieh TY, Bedros N, Stutzman SE, et al. Automated pupillometry as a triage and assessment tool in patients with traumatic brain injury. World Neurosurg. 2021;145:e163–e169. doi: 10.1016/j.wneu.2020.09.152. [DOI] [PubMed] [Google Scholar]
- 8. Copplestone S, Welbourne J. A narrative review of the clinical application of pressure reactivity indices in the neurocritical care unit. Br J Neurosurg. 2018;32(1):4–12. doi: 10.1080/02688697.2017.1416063. [DOI] [PubMed] [Google Scholar]
- 9. Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. 2017;45(11):1907–1914. doi: 10.1097/CCM.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chohan MO, Akbik OS, Ramos-Canseco J, et al. A novel single twist-drill access device for multimodal intracranial monitoring: a 5-year single-institution experience. Neurosurgery. 2014;10(suppl 3):400–411. doi: 10.1227/NEU.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 11. Ernst G, Qeadan F, Carlson AP. Subcutaneous bone flap storage after emergency craniectomy: cost-effectiveness and rate of resorption. J Neurosurg. 2018;129(6):1604–1610. doi: 10.3171/2017.6.JNS17943. [DOI] [PubMed] [Google Scholar]
- 12. Valadka AB, Hlatky R, Furuya Y, Robertson CS. Brain tissue PO2: correlation with cerebral blood flow. Acta Neurochir Suppl (Wien) 2002;81:299–301. doi: 10.1007/978-3-7091-6738-0_76. [DOI] [PubMed] [Google Scholar]
- 13. Riemann L, Beqiri E, Younsi A, et al. Predictive and discriminative power of pressure reactivity indices in traumatic brain injury. Neurosurgery. 2020;87(4):655–663. doi: 10.1093/neuros/nyaa039. [DOI] [PubMed] [Google Scholar]
- 14. Beqiri E, Smielewski P, Robba C, et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open. 2019;9(9):e030727. doi: 10.1136/bmjopen-2019-030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen JW, Gombart ZJ, Rogers S, et al. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the Neurological Pupil index. Surg Neurol Int. 2011;2:82. doi: 10.4103/2152-7806.82248. [DOI] [PMC free article] [PubMed] [Google Scholar]