Abstract
Background
The aim of the present work was to study the influence of body position on resting and exercise pulmonary hemodynamics in patients assessed for pulmonary hypertension (PH).
Methods and Results
Data from 483 patients with suspected PH undergoing right heart catheterization for clinical indications (62% women, age 61±15 years, 246 precapillary PH, 48 postcapillary PH, 106 exercise PH, 83 no PH) were analyzed; 213 patients (main cohort, years 2016–2018) were examined at rest in upright (45°) and supine position, such as under upright exercise. Upright exercise hemodynamics were compared with 270 patients (historical cohort) undergoing supine exercise with the same protocol. Upright versus supine resting data revealed a lower mean pulmonary artery pressure 31±14 versus 32±13 mm Hg, pulmonary artery wedge pressure 11±4 versus 12±5 mm Hg, and cardiac index 2.9±0.7 versus 3.1±0.8 L/min per m2, and higher pulmonary vascular resistance 4.1±3.1 versus 3.9±2.8 Wood P<0.001. Exercise data upright versus supine revealed higher work rates (53±26 versus 33±22 watt), and adjusting for differences in work rate and baseline values, higher end‐exercise mean pulmonary artery pressure (52±19 versus 45±16 mm Hg, P=0.001), similar pulmonary artery wedge pressure and cardiac index, higher pulmonary vascular resistance (5.4±3.7 versus 4.5±3.4 Wood units, P=0.002), and higher mean pulmonary artery pressure/cardiac output (7.9±4.7 versus 7.1±4.1 Wood units, P=0.001).
Conclusions
Body position significantly affects resting and exercise pulmonary hemodynamics with a higher pulmonary vascular resistance of about 10% in upright versus supine position at rest and end‐exercise, and should be considered and reported when assessing PH.
Keywords: body position, exercise, hemodynamic, pulmonary hypertension, right heart catheterization
Subject Categories: Hemodynamics
Nonstandard Abbreviations and Acronyms
- CO
cardiac output
- HR
heart rate
- mPAP
mean pulmonary artery pressure
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- RAP
right atrial pressure
- RHC
right heart catheterization
Clinical Perspective
What Is New?
To our knowledge, this is the largest study of patients with mainly pulmonary vascular disease assessed by right heart catheterization to study the influence of supine versus 45° upright body position on pulmonary hemodynamics.
What Are the Clinical Implications?
Body position significantly affects resting and exercise pulmonary hemodynamics by revealing an about 10% higher pulmonary vascular resistance upright versus supine.
Our data underscores the importance of considering body position while interpreting and reporting pulmonary hemodynamics in pulmonary vascular disease.
Pulmonary hypertension (PH) is generally diagnosed by right heart catheterization (RHC) at rest in supine position. However, many pulmonary hemodynamic measures lack standardization and the hemodynamic definition of PH is currently debated. 1 , 2 , 3 A recent consensus suggests to lower the diagnostic cutoff of the mean pulmonary artery pressure (mPAP) to >20 mm Hg based on studies which revealed that this cutoff corresponds to 2 SD of the mean normal value of 14 mm Hg and that mortality increases with an increasing mPAP starting from this normal value. 4 , 5 , 6 To increase specificity for pulmonary vascular disease, a pulmonary vascular resistance (PVR) of ≥3 Wood units was added to the diagnostic criteria for precapillary PH, albeit the normal PVR limit is debated. 1 , 7 , 8 The pulmonary artery wedge pressure (PAWP >15 mm Hg) is used to define postcapillary PH, but is known to vary considerably according to the measurement methods and the patients’ fluid status. 9 , 10 The cardinal symptom of PH is dyspnea on exertion, thus, a pathological PVR for a given cardiac output (CO) resulting in a higher mPAP potentially precedes resting PH and is associated with worse prognosis, 11 , 12 albeit many questions about the diagnostic and prognostic value of exercise PH and the mode of assessment are still unanswered. 3 , 13 Regarding these challenges, standardization of RHC‐measures are highly warranted to diagnose and classify PH and for this, the influence of body position has to be explored. Expert consensus suggests to obtain resting measurements in supine position with pulsatory pressures averaged over several respiratory cycles and transducers of fluid‐filled catheters zeroed to the mid‐thoracic line. 9 , 14 However, as soon as hemodynamics are measured during exercise, body position may be changed to upright (≥45°), which may alter venous return and intrathoracic pressures and thus significantly change pulmonary hemodynamics. As humans spend most daytime upright, it may additionally be of interest to know the influence of this principal body position on pulmonary hemodynamic measures at rest and exercise.
The aim of the present work was therefore to investigate the influence of the upright versus supine position on main pulmonary hemodynamic parameters at rest and exercise and to explore if positional changes vary between diagnostic groups.
Methods
The data and analytic methods will be shared with other interested researchers in the field upon request to the corresponding author.
Study Design and Patients
This is a retrospective analysis of data from all adults (aged >18 years) who underwent clinically indicated RHC in the PH‐center at the University Hospital Zurich from January 2005 until July 2018. The main analysis cohort were patients investigated January 2016 to July 2018 (main cohort) who had hemodynamic assessment at rest both, supine and upright (defined as 45°), before performing upright stepwise cycle exercise. Exercise hemodynamics in upright position of this main cohort was additionally compared with patients who had supine stepwise cycling exercise from 2005 to January 2016 (historical cohort).
To further explore positional differences according to diagnostic groups, we assessed patients overall and classified into the following 4 hemodynamic groups according to guidelines or task‐force statements 2 , 13 : precapillary PH defined as mPAP ≥25 mm Hg and PAWP ≤15 mm Hg at supine rest, postcapillary PH defined as mPAP ≥25 mm Hg and PAWP >15 mm Hg at supine rest, exercise PH defined as mPAP <25 mm Hg at supine rest, thus not fitting into criteria for other groups, but mPAP >30 mm Hg and mPAP/CO >3 Wood units at end‐exercise. No PH was defined as not fulfilling any of the other groups.
All patients gave written informed consent to undergo RHC, data registration and scientific analysis. The study was approved by the cantonal ethical authorities Zurich, Switzerland (KEK 2019‐00470).
RHC at Rest and During Exercise
A balloon‐tipped, triple‐lumen, fluid‐filled 7.5 Fr Swan Ganz catheter (Baxter/Edwards, Deerfield, IL, USA) was introduced via an internal jugular vein. Transducers were set at the mid‐axillary line and zeroed to atmospheric pressure 9 , 14 to measure the mPAP, the PAWP and the right atrial pressure (RAP). CO was assessed by thermodilution (Baxter/Edwards) and cardiac index calculated as CO/body surface area. PVR was calculated as PVR= (mPAP−PAWP)/CO. The total pulmonary resistance (mPAP/CO) was calculated at the end of each step of exercise. Resting measurements were assessed in both cohorts supine as mean of 2 stable measurements after 15 minutes of rest and in 45° upright position after 5 minutes of rest in the main cohort with the transducers set at the same mid‐axillary line as in the supine position. 14 Exercise testing was performed as stepwise incremental cycle exercise starting with 10 watts followed by an increase of 10 to 20 watts every 3 minutes at a cycling rate of 60 rounds/min, until patient exhaustion at end‐exercise. Hemodynamic measurements were taken during the last 30 seconds of each step (TheraVital, Medica GmbH, Ravensburg) and averaged over several respiratory cycles. Exercise was performed in the upright position in the main cohort 2016 to July 2018 and in the supine position in the historical cohort 2005 to 2016. RHC was conducted by the same team throughout the years with similar protocols and methods.
Statistical Analysis
Data are expressed as mean±SD and mean difference (95% CI). Single missing values were rare and not imputed. As the data set was large normality was assumed. Comparisons of hemodynamics at rest within the main cohort, were done using mixed regression analysis with hemodynamic parameters as dependent variables and body position (upright versus supine), hemodynamic group (precapillary PH, postcapillary PH, exercise PH, and no PH) and their interaction term as fixed‐independent variables and grouped by patient. From these mixed regression models, the average marginal effect (mean difference) induced by change in body position on hemodynamic parameters (overall and for each hemodynamic group) was calculated. Exercise data between the main cohort and historical cohort were investigated by mixed linear regression analysis and adjusted for respective baseline values and workload in watts. Model diagnostics analyzed by QQ‐plots for residuals and random intercepts were deemed acceptable for the main outcomes. A P value <0.05 was considered statistically significant and no adjustment was made for multiplicity. Analysis was performed using SPSS 25 statistics, R‐studio (packages “nlme”, “lmerTest”, “margins”), and SigmaPlot softwares.
Results
Baseline Patient Characteristics
Characteristics and diagnostic groups of the 2 cohorts are shown in Table 1. The main cohort with resting data available in both positions consisted of 213 patients (60% women, mean±SD aged 61±15 years), and the historical cohort with exercise in supine position consisted of 270 patients (61% women, age 60±15 years). Baseline characteristics and hemodynamic group distribution were similar between the 2 cohorts. The patients in the no PH group had clinically indicated RHC because of suspected PH in patients with otherwise unexplained dyspnea or being at risk factors for PH attributable to an underlying disease, such as connective tissue disease.
Table 1.
Baseline Characteristics of Patients
| Main cohort 2016–2018 | Historical cohort 2005–2016 | |
|---|---|---|
| No. of patients | 213 | 270 |
| Women, n (%) | 127 (60%) | 170 (63%) |
| Age, y | 61±15 | 60±15 |
| BMI, kg/m2 | 27±5 | 26±6 |
| BSA, m2 | 1.9±0.2 | 1.82±0.2 |
| Position of hemodynamic assessments at rest | Supine and upright | Supine |
| Position of hemodynamic assessments during exercise | Upright | Supine |
| Diagnostic groups | ||
| No PH (not classified into any PH‐group) | 31 (15%) | 52 (19%) |
| Exercise PH | 38 (18%) | 68 (25%) |
| Precapillary PH | 120 (56%) | 126 (47%) |
| Postcapillary PH | 24 (11%) | 24 (9%) |
Values are expressed as number (%) or mean±SD. BMI indicates body mass index; BSA, body surface area; and PH, pulmonary hypertension.
Resting Hemodynamics in the Upright and Supine Position Within Patients of the Main Cohort
Main hemodynamic parameters at rest in the upright and supine position overall and separated by groups are shown in Table 2 and patterns of changes in hemodynamics are illustrated in Figure 1. Overall, mPAP, PAWP, and RAP were slightly but significantly lower upright versus supine. The cardiac index was also significant lower upright resulting in a higher PVR. Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mPAP/CO, and systemic vascular resistance (SVR) were also significantly higher upright.
Table 2.
Resting Hemodynamics in Upright and Supine Position Within the Main Cohort
| Upright | Supine |
Mean change (95% CI) |
P Value | |
|---|---|---|---|---|
| Overall patients, n=213 | ||||
| Heart rate, bpm | 76±13 | 74±12 | 2.4 (1.5 to 3.3) | <0.001 |
| Systolic blood pressure, mm Hg | 140±20 | 132±18 | 8.1 (6.6 to 9.6) | <0.001 |
| Diastolic blood pressure, mm Hg | 81±15 | 77±11 | 4.3 (2.9 to 5.7) | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 31±14 | 32±13 | −1.0 (−1.6 to −0.4) | <0.001 |
| Pulmonary artery wedge pressure, mm Hg | 11±4 | 12±5 | −1.5 (−1.8 to −1.1) | <0.001 |
| Right atrial pressure, mm Hg | 7±4 | 9±4 | −1.8 (−2.2 to −1.4) | <0.001 |
| Cardiac index, L/min per m2 | 2.9±0.7 | 3.1±0.8 | −0.2 (−0.3 to −0.2) | <0.001 |
| Pulmonary vascular resistance, WU | 4.1±3.1 | 3.9±2.8 | 0.3 (0.1 to 0.5) | <0.001 |
| mPAP/cardiac output, WU | 6.4±4.0 | 6.2±3.5 | 0.3 (0.0 to 0.5) | 0.047 |
| Systemic vascular resistance, WU | 20.4±7.3 | 17.2±5.6 | 3.2 (2.6 to 3.8) | <0.001 |
| Patients with precapillary pulmonary hypertension, n=120 | ||||
| Heart rate, bpm | 78±13 | 74±12 | 3.4 (2.2 to 4.5) | <0.001 |
| Systolic blood pressure, mm Hg | 137±19 | 128±16 | 8.4 (6.3 to 10.4) | <0.001 |
| Diastolic blood pressure, mm Hg | 81±13 | 77±11 | 4.0 (2.1 to 5.9) | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 38±13 | 39±12 | −0.4 (−1.2 to 0.4) | 0.376 |
| Pulmonary artery wedge pressure, mm Hg | 11±4 | 12±4 | −1.0 (−1.5 to −0.5) | <0.001 |
| Right atrial pressure, mm Hg | 8±5 | 10±4 | −1.7 (−2.2 to −1.1) | <0.001 |
| Cardiac index, L/min per m2 | 2.8±0.7 | 3.0±.8 | −0.2 (−0.3 to −0.1) | <0.001 |
| Pulmonary vascular resistance, WU | 5.7±3.4 | 5.3±2.9 | 0.5 (0.2 to 0.7) | <0.001 |
| mPAP/cardiac output, WU | 8.2±4.4 | 7.7±3.5 | 0.5 (0.2 to 0.9) | 0.001 |
| Systemic vascular resistance, WU | 20.7±7.5 | 17.5±5.5 | 3.2 (2.3 to 4.0) | <0.001 |
| Patients with postcapillary pulmonary hypertension n=24 | ||||
| Heart rate, bpm | 75±11 | 75±12 | −0.3 (−2.8 to 2.3) | 0.848 |
| Systolic blood pressure, mm Hg | 146±25 | 141±22 | 4.5 (0.2 to 9.4) | 0.041 |
| Diastolic blood pressure, mm Hg | 78±13 | 75±12 | 3.7 (−0.6 to 8.0) | 0.093 |
| Mean pulmonary artery pressure, mm Hg | 31±9 | 36±8 | −5.1 (−7.0 to −3.3) | <0.001 |
| Pulmonary artery wedge pressure, mm Hg | 15±4 | 19±5 | −4.4 (−5.6 to −3.3) | <0.001 |
| Right atrial pressure, mm Hg | 9±5 | 12±5 | −2.7 (−3.9 to −1.5) | <0.001 |
| Cardiac index, L/min per m2 | 3.0±0.7 | 3.1±0.8 | −0.1 (−0.3 to 0.1) | 0.387 |
| Pulmonary vascular resistance, WU | 2.7±1.2 | 3.2±1.7 | −0.3 (−0.9 to 0.3) | 0.277 |
| mPAP/cardiac output, WU | 5.4±1.6 | 6.9±3.2 | −1.2 (−2.0 to −0.5) | <0.001 |
| Systemic vascular resistance, WU | 18.9±7.0 | 17.5±6.3 | 1.7 (−0.1 to 3.6) | 0.063 |
| Patients with exercise pulmonary hypertension n=38 | ||||
| Heart rate, bpm | 74±11 | 72±11 | 1.8 (−0.3 to 3.8) | 0.088 |
| Systolic blood pressure, mm Hg | 146±19 | 134±22 | 11.8 (8.2 to 15.4) | <0.001 |
| Diastolic blood pressure, mm Hg | 84±22 | 77±10 | 7.0 (3.7 to 10.3) | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 20±4 | 20±3 | 0.1 (−1.4 to 1.5) | 0.943 |
| Pulmonary artery wedge pressure, mm Hg | 9±3 | 9±2 | −0.7 (−1.6 to 0.2) | 0.155 |
| Right atrial pressure, mm Hg | 6±3 | 7±3 | −1.4 (−2.4 to −0.5) | 0.002 |
| Cardiac index, L/min per m2 | 2.9±0.8 | 3.1±0.7 | −0.2 (−0.4 to −0.0) | 0.026 |
| Pulmonary vascular resistance, WU | 2.3±0.9 | 2.0±0.5 | 0.3 (−0.1 to 0.8) | 0.127 |
| mPAP/cardiac output, WU | 4.0±1.1 | 3.7±0.7 | 0.3 (−0.3 to 0.9) | 0.263 |
| Systemic vascular resistance, WU | 21.4±7.4 | 17.6±5.2 | 4.0 (2.5 to 5.4) | <0.001 |
| Patients without pulmonary hypertension, n=31 | ||||
| Heart rate, bpm | 73±12 | 72±12 | 1.54 (−0.7 to 3.8) | 0.176 |
| Systolic blood pressure, mm Hg | 141±15 | 137±16 | 5.0 (1.0 to 9.0) | 0.014 |
| Diastolic blood pressure, mm Hg | 80±9 | 78±9 | 2.7 (−1.0 to 6.4) | 0.147 |
| Mean pulmonary artery pressure, mm Hg | 16±4 | 18±3 | −1.5 (−3.1 to 0.1) | 0.067 |
| Pulmonary artery wedge pressure, mm Hg | 8±3 | 9±3 | −1.8 (−2.8 to −0.8) | <0.001 |
| Right atrial pressure, mm Hg | 6±3 | 8±3 | −1.9 (−2.9 to −0.9) | <0.001 |
| Cardiac index, L/min per m2 | 3.1±0.7 | 3.6±0.8 | −0.5 (−0.7 to −0.3) | <0.001 |
| Pulmonary vascular resistance, WU | 1.6±0.7 | 1.3±0.5 | 0.2 (−0.3 to 0.7) | 0.369 |
| mPAP/cardiac output, WU | 3.1±1.2 | 2.9±0.9 | 0.1 (−0.5 to 0.8) | 0.654 |
| Systemic vascular resistance, WU | 18.8±6.4 | 15.2±5.8 | 3.6 (2.0 to 5.2) | <0.001 |
Values are expressed as means±SD and mean difference (95% CI). mPAP indicates mean pulmonary artery pressure; and WU, Wood units.
Figure 1. Differences of resting hemodynamic in upright (45°) vs supine position in patients undergoing right heart catheterization because of suspected pulmonary hypertension.
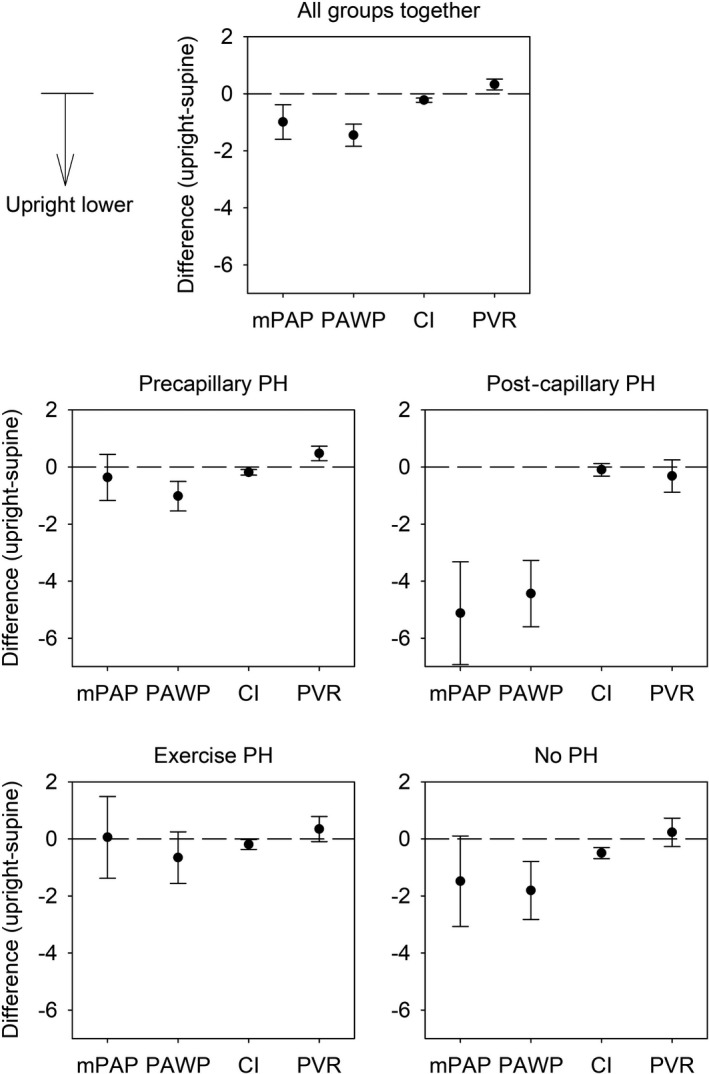
Mean differences with 95% CIs of main resting hemodynamic measures assessed by right heart catheterization in upright vs supine position within patients of the entire main cohort (all groups together) and within patients of the different hemodynamic subgroups. Differences are negative if values in upright position are lower than in supine position. Cardiac index, L/min per m2; mPAP, mean pulmonary artery pressure, mm Hg; PAWP, pulmonary artery wedge pressure, mm Hg; PH, pulmonary hypertension; and PVR, pulmonary vascular resistance.
In the precapillary PH group, PAWP, RAP, cardiac index were significantly lower upright, PVR, HR, SBP, DBP, mPAP/CO, and SVR were significantly higher upright, whereas mPAP was not different.
In the postcapillary PH group, mPAP, PAWP, RAP, and mPAP/CO were significantly lower upright, whereas HR, DBP, cardiac index, PVR, and SVR were similar. SBP was significantly higher upright.
In the exercise PH group, RAP and cardiac index were significantly lower upright, whereas SPB, DBP, and SVR were higher upright and HR, mPAP, PAWP, mPAP/CO, and PVR were similar.
In the group without PH, PAWP, RAP, and cardiac index were significantly lower upright, whereas SBP, and SVR were significantly higher and the HR, DBP, mPAP, mPAP/CO, and PVR were unchanged.
Exercise Hemodynamics in Upright Position (Main Cohort) Versus Supine (Historical Cohort)
Hemodynamic measures under exercise in the upright position (main cohort) and supine position (historical cohort) at baseline and end‐exercise, and the difference between upright and supine exercise are shown overall and separated by groups in Table 3 and illustrated in Figures 2 and 3.
Table 3.
Exercise Hemodynamics in Upright Position (Main Cohort) and Supine Position (Historical Cohort)
|
Upright exercise cohort (main cohort) |
Supine exercise cohort (historical cohort) |
Unadjusted difference at end‐exercise upright vs supine | Difference at end‐exercise upright vs supine, adjusted for baseline and work rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline rest | End‐exercise | Changes from rest to end‐exercise | Baseline rest | End‐exercise | Changes from rest to end‐exercise | Mean difference (95% CI) | P Value | Mean difference (95% CI) | P Value | |
| All patients | n=213 | n=270 | ||||||||
| Work rate, watts | 0 | 53±26 | 53±26 | 0 | 33±22 | 33±22 | 21.3 (17.1 to 25.5) | <0.001 | … | … |
| Heart rate, bpm | 76±13 | 118±22 | 42±21 | 75±13 | 110±19 | 35±18 | 8.2 (4.5 to 11.9) | <0.001 | −1.3 (−4.5 to 1.9) | 0.434 |
| Systolic blood pressure, mm Hg | 140±20 | 165±30 | 25±25 | 129±20† | 158±27 | 30±20 | 6.7 (1.5 to 11.9) | 0.012 | −8.1 (−12.7 to −3.5) | 0.001 |
| Diastolic blood pressure, mm Hg | 81±15 | 82±21 | 1±20 | 76±12† | 84±19 | 8±17 | −2.1 (−5.8 to 1.5) | 0.250 | −4.1 (−7.7 to −0.4) | 0.028 |
| Mean pulmonary artery pressure, mm Hg | 31±14 | 52±19 | 21±11 | 29±12 | 45±16 | 16±9 | 4.0 (2.0 to 5.9) | <0.001 | 2.8 (1.2 to 4.4) | 0.001 |
| Pulmonary artery wedge pressure, mm Hg | 11±4 | 15±6 | 4±4 | 11±4† | 16±7 | 4±5 | −2.0 (−3.0 to −0.9) | <0.001 | −0.4 (−1.4 to 0.6) | 0.442 |
| Right atrial pressure, mm Hg | 7±4 | 12±7 | 5±5 | 7±4 | 11±7 | 4±5 | 0.0 (−1.1 to 1.2) | 0.961 | 1.1 (0.2 to 2.0) | 0.020 |
| Cardiac index, L/min per m2 | 2.9±0.7 | 4.1±1.4 | 1.2±1.2 | 3.1±0.8* | 4.0±1.1 | 0.9±0.8 | 0.2 (−0.0 to 0.5) | 0.054 | −0.0 (−0.2 to 0.2) | 0.885 |
| Pulmonary vascular resistance, WU | 4.1±3.1 | 5.4±3.7 | 1.4±1.9 | 3.4±2.9 | 4.5±3.4 | 1.1±1.4 | 0.6 (0.0 to 1.1) | 0.034 | 0.5 (0.2 to 0.8) | 0.002 |
| mPAP/ cardiac output, WU | 6.4±4.0 | 7.9±4.7 | 1.6±2.4 | 5.6±3.4 | 7.1±4.1 | 1.4±1.7 | 0.3 (−0.4 to 0.9) | 0.391 | 0.6 (0.3 to 1.0) | 0.001 |
| Systemic vascular resistance, WU | 20.4±7.3 | 14.2±6.3 | −5.9±5.5 | 17.0±5.8† | 15.1±5.6 | −1.9±3.8 | −0.9 (−2.1 to 0.2) | 0.108 | −1.7 (−2.6 to −0.8) | <0.001 |
| Precapillary PH | n=120 | n=126 | ||||||||
| Work rate, watts | 0 | 51±26 | 51±26 | 0 | 28±19 | 28+19 | 22.8 (17.1 to 28.6) | <0.001 | … | … |
| Heart rate, bpm | 78±13 | 119±22 | 42±21 | 78±13 | 110±18 | 32±17 | 9.5 (4.4 to 14.5) | <0.001 | −0.1 (−4.4 to 4.1) | 0.948 |
| Systolic blood pressure, mm Hg | 137±19 | 165±30 | 28±26 | 129±20* | 157±27 | 28±20 | 7.0 (−0.2 to 14.3) | 0.055 | −4.9 (−10.9 to 1.0) | 0.106 |
| Diastolic blood pressure, mm Hg | 81±13 | 83±21 | 2±19 | 78±12 | 88±18 | 10±17 | −5.4 (−10.4 to −0.3) | 0.036 | −5.6 (−10.4 to −0.8) | 0.024 |
| Mean pulmonary artery pressure, mm Hg | 38±13 | 63±15 | 25±11 | 38±11 | 57±12 | 19±9 | 6.1 (3.4 to 8.7) | <0.001 | 4.4 (2.3 to 6.5) | <0.001 |
| Pulmonary artery wedge pressure, mm Hg | 11±4 | 14±5 | 3±4 | 11±3 | 16±5 | 4±5 | −1.4 (−2.9 to 0.0) | 0.056 | −0.6 (−2.0 to 0.7) | 0.361 |
| Right atrial pressure, mm Hg | 8±5 | 14±8 | 6±6 | 8±4 | 14±7 | 5±5 | −0.2 (−1.8 to 1.4) | 0.834 | 1.1 (−0.1 to 2.3) | 0.076 |
| Cardiac index, L/min per m2 | 2.8±0.7 | 3.9±1.4 | 1.1±1.1 | 3.0±0.8* | 3.7±1.1 | 0.7±0.7 | 0.2 (−0.1 to 0.5) | 0.226 | −0.1 (−0.3 to 0.1) | 0.295 |
| Pulmonary vascular resistance, WU | 5.7±3.4 | 7.6±3.7 | 1.8±2.3 | 5.4±3.2 | 6.8±3.6 | 1.4±1.7 | 0.8 (0.0 to 1.5) | 0.049 | 0.8 (0.3 to 1.2) | 0.001 |
| mPAP/cardiac output, WU | 8.2±4.4 | 10.2±4.8 | 2.1±2.7 | 7.7±3.8 | 9.5±4.3 | 1.7±2.0 | 0.7 (−0.2 to 1.6) | 0.129 | 1.0 (0.5 to 1.5) | <0.001 |
| Systemic vascular resistance, WU | 20.7±7.5 | 14.5±7.1 | −5.9±5.9 | 17.6±6.4* | 16.0±6.4 | −1.6±3.7 | −1.6 (−3.1 to 0.0) | 0.054 | −2.0 (−3.2 to −0.9) | <0.001 |
| Postcapillary PH | n=24 | n=24 | ||||||||
| Work rate, watts | 0 | 44±24 | 44±24 | 0 | 24±8 | 24±18 | 20.6 (7.4 to 33.8) | 0.002 | … | … |
| Heart rate, bpm | 75±11 | 103±16 | 28±15 | 73±11 | 102±14 | 29±14 | 0.7 (−11.0 to 12.4) | 0.910 | −9.0 (−18.3 to 0.3) | 0.058 |
| Systolic blood pressure, mm Hg | 146±25 | 158±32 | 12±23 | 144±23 | 179±35 | 35±20 | −20.4 (−36.7 to −4.0) | 0.015 | −26.8 (−39.5 to −14.0) | <0.001 |
| Diastolic blood pressure, mm Hg | 78±13 | 76±14 | −2±11 | 76±13 | 87±24 | 12±20 | −10.8 (−22.2 to 0.7) | 0.065 | −11.2 (−21.6 to −0.8) | 0.035 |
| Mean pulmonary artery pressure, mm Hg | 31±9 | 48±10 | 19±8 | 33±8 | 50±11 | 17±8 | −1.9 (−8.0 to 4.2) | 0.541 | 0.2 (−4.5 to 4.8) | 0.949 |
| Pulmonary artery wedge pressure, mm Hg | 15±4 | 23±6 | 8±5 | 18±4 | 27±5 | 8±5 | −4.2 (−7.4 to −1.0) | 0.010 | −0.5 (−3.4 to 2.3) | 0.711 |
| Right atrial pressure, mm Hg | 9±5 | 14±6 | 6±4 | 10±4 | 16±7 | 6±4 | −1.6 (−5.2 to 2.1) | 0.398 | 0.6 (−2.1 to 3.2) | 0.671 |
| Cardiac index, L/min per m2 | 3.0±0.7 | 3.7±1.0 | 0.8±0.8 | 3.4±1.2* | 4.0±1.4 | 0.5±0.4 | −0.3 (−1.10 to 0.4) | 0.402 | −0.2 (−0.7 to 0.2) | 0.365 |
| Pulmonary vascular resistance, WU | 2.7±1.2 | 3.9±2.0 | 1.3±1.5 | 2.5±1.1 | 3.6±1.8 | 1.0±1.1 | 0.4 (−1.3 to 2.0) | 0.654 | 0.5 (−0.4 to 1.4) | 0.269 |
| mPAP/ cardiac output, WU | 5.4±1.6 | 7.1±2.8 | 1.9±2.4 | 5.8±2.0 | 7.7±2.9 | 1.9±1.6 | −0.5 (−2.6 to 1.6) | 0.621 | 0.5 (−0.6 to 1.6) | 0.357 |
| Systemic vascular resistance, WU | 18.9±7.0 | 13.4±5.8 | −4.9±4.5 | 16.0±5.5 | 15.2±4.5 | −0.8±2.5 | −1.8 (−5.4 to 1.8) | 0.327 | −2.3 (−4.7 to 0.1) | 0.062 |
| Exercise PH | n=38 | n=68 | ||||||||
| Work rate, watts | 0 | 56±25 | 56±25 | 0 | 35±19 | 35±19 | 20.7 (11.5 to 29.8) | <0.001 | … | … |
| Heart rate, bpm | 74±11 | 119±22 | 45±23 | 72±12 | 111±21 | 39±19 | 8.0 (−0.1 to 16.1) | 0.052 | −2.8 (−9.3 to 3.8) | 0.406 |
| Systolic blood pressure, mm Hg | 146±19 | 171±34 | 26±28 | 128±19† | 160±24 | 33±22 | 11.0 (−0.4 to 22.3) | 0.059 | −9.4 (−18.6 to −0.3) | 0.044 |
| Diastolic blood pressure, mm Hg | 84±22 | 87±25 | 4±25 | 73±11† | 81±16 | 8±15 | 6.0 (−1.9 to 14.0) | 0.137 | 0.6 (−6.9 to 8.0) | 0.881 |
| Mean pulmonary artery pressure, mm Hg | 20±4 | 39±7 | 19±7 | 20±3 | 37±6 | 17±6 | 2.4 (−1.8 to 6.6) | 0.268 | 0.9 (−2.3 to 4.2) | 0.576 |
| Pulmonary artery wedge pressure, mm Hg | 9±3 | 14±6 | 5±5 | 10±3* | 17±7 | 7±6 | −3.9 (−6.2 to −1.6) | 0.001 | −1.7 (−3.7 to 0.4) | 0.110 |
| Right atrial pressure, mm Hg | 6±3 | 9±5 | 3±3 | 6±3 | 9±5 | 4±4 | −0.7 (−3.2 to 1.8) | 0.592 | 0.1 (−1.7 to 2.0) | 0.886 |
| Cardiac index, L/min per m2 | 2.9±0.8 | 4.2±0.9 | 1.3±0.9 | 3.2±0.8 | 4.1±1.1 | 0.9±0.8 | 0.1 (−0.4 to 0.6) | 0.746 | −0.0 (−0.3 to 0.3) | 0.976 |
| Pulmonary vascular resistance, WU | 2.3±0.9 | 3.5±1.3 | 1.0±1.1 | 1.7±0.7 | 2.8±1.4 | 1.0±1.0 | 0.7 (−0.5 to 1.8) | 0.237 | 0.3 (−0.4 to 0.9) | 0.394 |
| mPAP/ cardiac output, WU | 4.0±1.1 | 5.4±1.6 | 1.3±1.4 | 3.6±1.1 | 5.2±1.8 | 1.6±1.4 | 0.1 (−1.3 to 1.5) | 0.876 | 0.2 (−0.5 to 1.0) | 0.571 |
| Systemic vascular resistance, WU | 21.4±7.4 | 14.7±4.7 | −7.0±6.3 | 16.1±4.7† | 14.2±4.6 | −1.8±4.4 | 0.4 (−2.0 to 2.9) | 0.723 | −2.0 (−3.7 to −0.3) | 0.022 |
| No PH | n=31 | n=52 | ||||||||
| Work rate, watts | 0 | 63±23 | 63±23 | 0 | 45±31 | 45±31 | 18.0 (7.7 to 28.2) | 0.001 | … | … |
| Heart rate, bpm | 73±12 | 123±21 | 49±19 | 74±11 | 114±20 | 40±22 | 8.9 (−0.1 to 17.9) | 0.052 | 1.5 (−5.7 to 8.7) | 0.677 |
| Systolic blood pressure, mm Hg | 141±15 | 165±20 | 23±17 | 124±17† | 149±24 | 26±20 | 15.3 (2.5 to 28.1) | 0.020 | −5.3 (−15.5 to 5.0) | 0.312 |
| Diastolic blood pressure, mm Hg | 80±9 | 77±21 | −3±20 | 74±11* | 75±19 | 2±20 | 1.7 (−7.3 to 10.6) | 0.718 | −1.6 (−9.9 to 6.6) | 0.696 |
| Mean pulmonary artery pressure, mm Hg | 16±4 | 26±6 | 10±4 | 16±3 | 23±4 | 7±4 | 3.2 (−1.6 to 8.0) | 0.187 | 2.1 (−1.5 to 5.7) | 0.260 |
| Pulmonary artery wedge pressure, mm Hg | 8±3 | 11±4 | 3±4 | 9±3 | 11±5 | 2±5 | 0.3 (−2.3 to 2.8) | 0.847 | 1.9 (−0.3 to 4.2) | 0.090 |
| Right atrial pressure, mm Hg | 6±3 | 8±4 | 1±3 | 6±3 | 5±4 | −1±3 | 2.4 (−0.4 to 5.3) | 0.094 | 2.5 (0.5 to 4.6) | 0.017 |
| Cardiac index, L/min per m2 | 3.1±0.7 | 5.1±1.9 | 2.0±1.6 | 3.1±0.7 | 4.3±1.1 | 1.2±0.9 | 0.8 (0.2 to 1.3) | 0.006 | 0.4 (0.0 to 0.7) | 0.034 |
| Pulmonary vascular resistance, WU | 1.6±0.7 | 1.9±0.7 | 0.3±0.7 | 1.5±0.8 | 1.8±0.9 | 0.4±0.6 | 0.1 (−1.2 to 1.3) | 0.916 | 0.1 (−0.6 to 0.9) | 0.699 |
| mPAP/cardiac output, WU | 3.1±1.2 | 3.2±1.2 | 0.8±1.1 | 3.2±1.1 | 3.4±1.5 | 0.2±1.0 | −0.2 (−1.8 to 1.3) | 0.764 | 0.4 (−0.5 to 1.2) | 0.400 |
| Systemic vascular resistance, WU | 18.8±6.4 | 13.3±5.7 | −5.1±3.5 | 17.2±5.6 | 13.8±5.0 | −3.4±3.5 | −0.5 (−3.3 to 2.3) | 0.726 | −0.2 (−2.1 to 1.6) | 0.798 |
Values are expressed as means±SD and mean difference (95% CI). † P<0.001 and *P<0.05 comparison of baseline values in the adjusted regression analysis. mPAP indicates mean pulmonary artery pressure; and WU, Wood units.
Figure 2. Hemodynamic measures in upright (45°) and supine position at baseline rest, at 50% and 100% of individual work rate.
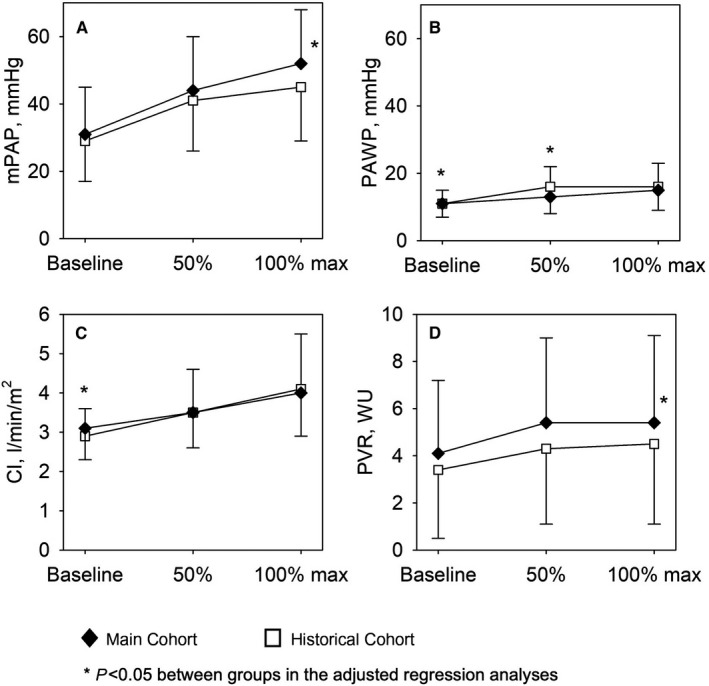
Means and SD of main hemodynamic measures assessed by right heart catheterization in upright (main cohort, n=217, black diamonds) and supine position (historical cohort, n=270, white squares) at baseline, and at 50% and 100% of individual end‐exercise work rate. *P<0.05 between groups in the adjusted regression analyses. A, Mean pulmonary artery pressure. B, Pulmonary artery wedge pressure. C, Cardiac index. D, Pulmonary vascular resistance. mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; and WU, Wood units.
Figure 3. Differences of hemodynamic measures at the end of exercise in upright (45°) vs supine position unadjusted and adjusted for baseline values and end‐exercise work rate.
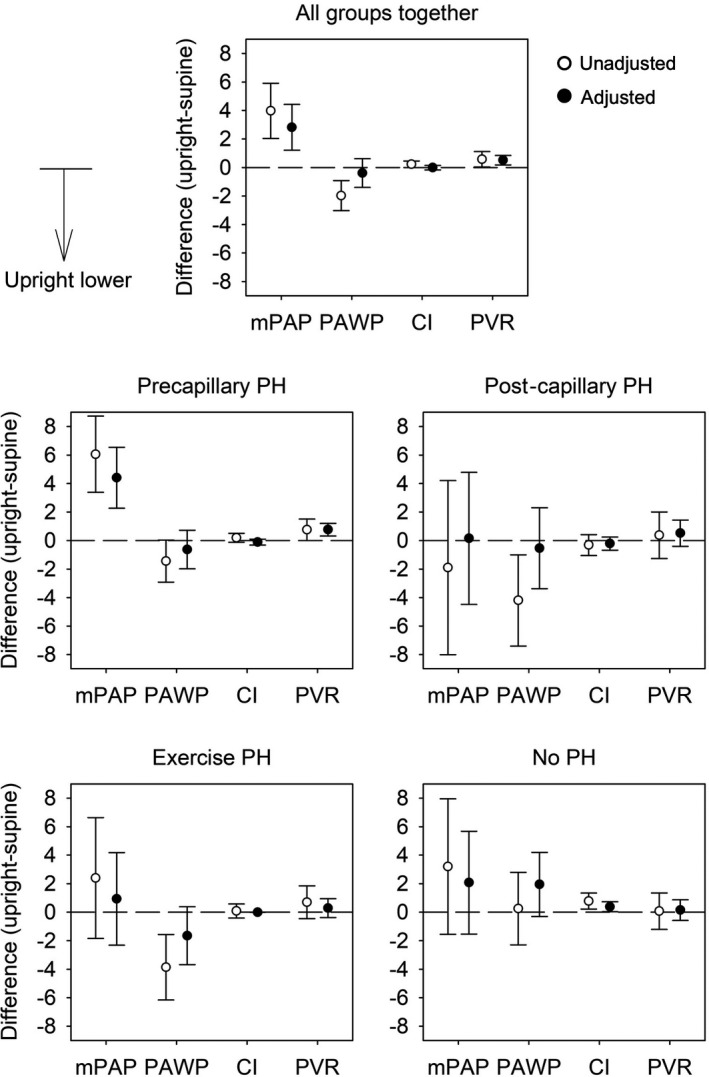
Mean differences with 95% CIs of main hemodynamic parameters assessed by right heart catheterization at the end of cycle exercise in upright (main cohort, n=217) vs supine position (historical cohort, n=270). Open circles represent unadjusted differences and filled circles represent differences adjusted for baseline values and end‐exercise work rate. Data from all patients and from patients of the different hemodynamic subgroups are displayed in separate panels. Differences are negative if values in upright position are lower than in supine position. Cardiac index, L/min per m2; mPAP, mean pulmonary artery pressure, mm Hg; PAWP, pulmonary artery wedge pressure, mm Hg; PH, pulmonary hypertension; and PVR, pulmonary vascular resistance.
Overall, patients in the upright position achieved significantly higher work rate compared with patients cycling supine and hemodynamics differed between positions already at baseline and also during exercise. Compared with the supine position, there were distinct differences at end‐exercise in the upright position, showing a higher work rate, a higher HR, SBP, mPAP, and PVR but unchanged mPAP/CO (Table 3). If adjusted for work rate and baseline, the main cohort exercising upright, revealed still a higher mPAP, PVR, and now as well mPAP/CO, whereas HR was no longer different, and the adjusted SBP and SVR were lower (Table 3).
In the largest subgroup with precapillary PH, the end‐exercise work rate achieved upright was again significantly higher, which was associated with a higher HR, mPAP, and PVR at end‐exercise. When adjusted for different baseline values between the 2 cohorts and higher work rates upright, the mPAP and PVR remained significantly higher upright versus supine, along with a higher mPAP/CO (Table 3).
In the postcapillary PH group, end‐exercise work rate was significantly higher in the cohort cycling upright versus the cohort cycling supine. Adjusted for work‐rate and baseline, the systemic blood pressure (BP) at end‐exercise was lower upright versus supine with a tendency towards lower SVR.
In the exercise PH group, end‐exercise work‐rate was again significantly higher in the cohort cycling upright, other parameters were mainly unchanged with the exception of a lower systemic BP and SVR upright versus supine at end‐exercise.
In the subgroup without PH, work rate was again higher upright versus supine, the RAP and cardiac index at end‐exercise was higher upright versus supine, no other differences were detected.
Discussion
This study provides novel comprehensive data on the influence of body position on hemodynamics in patients with suspected PH evaluated by RHC at rest and during exercise. Within a large cohort of patients assessed at rest upright and supine, we found that in the upright versus supine position both mPAP and, to a minimally greater extent, PAWP, decreased in association with a reduction in cardiac index indicating an increase in PVR of about 10%. Comparing the present main cohort cycling upright with a historical cohort cycling supine revealed that the work rate achieved upright versus supine was significantly higher, resulting in higher HR, mPAP, and PVR at end‐exercise. Adjustment for different baseline and work rates of the 2 cohorts confirmed a significantly higher mPAP, PVR, and also mPAP/CO at end‐exercise in upright versus supine position. Thus, body position at rest and during exercise should be considered when investigating and defining PH.
Resting hemodynamics assessed supine and upright within patients of the main cohort revealed that HR and systemic BP were increased in upright position, which is in line with the known baroreflex response to orthostasis with an increased sympathetic and reduced parasympathetic outflow in response to upright position. 15 , 16 Accordingly, PVR and SVR increased in the upright versus supine position as indicated by reductions in PAWP and RAP, an only minimal reduction in mPAP and a clear increase in systemic BP along with a reduction in cardiac index. Regarding the higher HR, the decreased CI indicates a significantly lower stroke volume, which is in line with lower RAP and venous return in accordance with Guytons experiments, which showed already decades ago that that the venous return determines cardiac output. 17 , 18 Bevegard et al studied healthy volunteers (10 untrained, 8 athletes) by RHC and showed that the cardiac output was lower in the sitting compared with the supine position at rest, because of a smaller stroke volume in the sitting position. 19 , 20 In line with these data and our study, 10 healthy men investigated by left and right heart catheterization in the supine and upright position, 21 revealed lower PAWP, left ventricular end‐diastolic pressure, and cardiac index in the sitting versus supine position. The mPAP in these healthy men was not different between the 2 positions at rest, which is in line with the presently investigated patients with precapillary PH, exercise PH, and no PH (Table 2 and Figure 2). In subgroup analyses, we could indeed show that the lower mPAP found upright versus supine in the analysis of the entire cohort was driven by the subgroup with postcapillary PH, which revealed a much greater reduction in PAWP and mPAP compared with patients with precapillary PH, exercise PH, and no PH (Figure 3). The major group of patients with precapillary PH responded to upright position with a significant increase in HR and SBP and a significant decrease in RAP, with consecutively lowered stroke volume and cardiac index despite increased HR, which may indicate a high dependency of pulmonary hemodynamics from cardiac pre‐ and afterload in precapillary PH. As the mPAP was only minimally reduced in upright position, the decreased PAWP and cardiac index resulted in a significantly increased PVR upright versus supine. Patients with postcapillary PH, however, did not reveal an increased HR and DBP upright versus supine and the SBP was only minimally increased, which thus may point towards a certain orthostatic dysregulation in these patients, which often reveals concomitant diastolic dysfunction and systemic hypertension. The association of autonomic dysfunction in hypertension and heart failure is known and it is currently investigated whether interventional therapies to modulate autonomic tone may be beneficial in these conditions. 22 , 23 The PAWP was significantly lower upright versus supine overall and especially in patients with postcapillary PH who were the only group who did not show a decrease in cardiac index when upright, possibly reflecting some fluid overload in supine position. Patients diagnosed with exercise PH revealed an increased systemic BP in the upright versus supine position associated with an increase in SVR, a slight elevation of HR and a reduction in CI according to the expected orthostatic reaction with lower venous return 18 but PVR was unchanged in this relatively small and heterogeneous group. In summary, our findings demonstrate that resting pulmonary hemodynamics within the same patients are significantly different according to the body position and that some positional effects vary according to pre‐ and postcapillary PH subgroups. As humans spend most of the daytime upright with constantly lower venous return and cardiac output and consecutively higher resistances, hemodynamics measured supine in the catheter laboratory may not truly reflect what pulmonary vessels withstand during activities of everyday life.
Nevertheless, for convenience and standardization, resting hemodynamics are conventionally measured supine and current PH definitions are based on supine resting measurements. 1 Pulmonary hemodynamic assessments during exercise are also possible and widely applied in the supine position with the advantage to keep the position of a patient in the catheter laboratory constant and to compare the exercise with resting measures. 3 , 13 However, exercising in a supine position does not correspond to daily activities and the necessary elevation of the legs to reach the pedals from the supine position already corresponds to a leg‐lift maneuver as used to provoke an increased venous return and uncover occult left‐heart disease. 24 In addition, leg‐lift may increase mechanoreflexes and augment peripheral sympathetic response, which may contribute to exercise limitation. 25 These arguments are in favor of investigating pulmonary hemodynamics during exercise in a more natural, upright position, which in turn calls to repeat resting measures upright as a baseline to determine the hemodynamic changes with exercise. In our PH center, we changed our daily practice to investigate patients assessed for PH in the 45° upright position in 2016. This allowed us to compare pulmonary hemodynamics during cycle exercise assessed upright in the presently described main cohort in comparison to the historical cohort, which was similarly assessed supine from 2005 to 2016.
The cohort of patients performing stepwise cycle exercise upright achieved significantly higher work rates compared with the cohort cycling supine (Table 3). This is in line with previous studies that revealed that supine exercise is associated with a reduced workload, whereas the effect of the body position on maximal oxygen uptake, HR, and respiration is more debated. 26 , 27 , 28 As hemodynamics of the 2 cohorts included in the current study already differed at rest because of different body positions and potentially other unknown factors, and as significantly higher work rates were achieved upright versus supine, comparisons at end‐exercise were performed without and with adjustment for respective baseline values and work rates. Whereas unadjusted at end‐exercise in the upright versus the supine exercise cohort, HR, systemic BP, mPAP, and PVR were higher along with the higher work rate, adjusted measures revealed a similar HR, an even lower systemic BP but still higher mPAP, PVR, and mPAP/CO. Such position‐related differences in resting and exercise hemodynamics may influence the classification of patients with PH and it is thus of importance to include body position in hemodynamic reports and definitions.
Unadjusted hemodynamic differences among the 120 patients with precapillary PH cycling upright and the 126 historical controls cycling supine were mainly the same as those in the respective entire cohorts, with higher work rates, HR, mPAP, and PVR. If adjusted for the baseline differences and work rate, HR was similar but end‐exercise mPAP, PVR, and mPAP/CO were significantly higher upright versus supine, corroborating an essential effect of body position on exercise hemodynamics in patients with precapillary PH. 3 , 13
The differences in PVR and mPAP/CO at end‐exercise between the upright versus supine cohorts overall and in the major precapillary subgroup were not present in the smaller subgroups with postcapillary PH, exercise PH, and no PH. This is in line with a previous study investigating 30 healthy young adults of both sexes by exercise echocardiography upright and semi‐recumbent, which found a similar pressure‐flow relationship despite increased work rates achieved upright. 28 Thus, different disorders as defined by hemodynamic groups may reveal distinct hemodynamic responses to exercise in different body positions. In postcapillary PH, the unadjusted PAWP at end‐exercise was significantly higher supine versus upright, but there was no significant difference after adjustment for the higher work rate and baseline. This is in line with our overall cohort and healthy subjects investigated decades ago, which revealed a higher PAWP and left ventricular end diastolic pressure when cycling supine, but a similar increase from baseline. 21
Limitations of our study are the retrospective design, the lack of randomized allocation to the different positions and that exercise hemodynamics were compared between 2 different cohorts and not within individuals cycling twice. However, the 2 cohorts were comparable on age and baseline characteristics and adjusted regression for baseline differences and distinct work rates between cohorts should minimize these biases. In addition, repetitive symptom‐limited exercise during RHC in different positions would also be prone to an order‐bias and a sufficiently long recreational period between exercises would be challenging for patients and logistics.
In conclusion, body positions distinctly affect resting and exercise hemodynamics with PVR at rest and end‐exercise in upright position exceeding values in supine position by about 10%. Body position should thus be considered when defining PH at rest and during exercise and included in reports on RHC. Upright hemodynamic may better reflect everyday life position of humans and may thus be included into hemodynamic assessments, especially under exercise.
Sources of Funding
This work was supported by the Swiss National Science Foundation and Zurich Lung. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclosures
Berlier has received a research grant from Zurich Lung and had a participation on an advisory board of Merck Sharp & Dohme SA. Ulrich and Bloch have received research grants from the Swiss National Science Foundation, Swiss‐ and Zurich Lung Leagues. Ulrich has received research grants outside of this work from Orpha Swiss, honoraria for lectures and support for attending meetings from Actelion/Janssen SA, Merck Sharp & Dohme SA and Orpha Swiss. Schwarz has received support for attending a meeting with Astra Zeneca. The remaining authors have no disclosures to report.
Acknowledgments
Berlier, Carta and Ulrich take full responsibility for the content of the manuscript, including the data analysis. Berlier, Carta and Ulrich designed the study and performed experimental work, data collection, data interpretation, and statistical analysis, including writing of the final article. Saxer, Lichtblau, Schneider, Schwarz, Bloch, and Furian participated in data collection and provided critical insights to article preparation. All authors reviewed and approved the final draft of the article.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 3. Herve P, Lau EM, Sitbon O, Savale L, Montani D, Godinas L, Lador F, Jaïs X, Parent F, Günther S, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46:728–737. doi: 10.1183/09031936.00021915 [DOI] [PubMed] [Google Scholar]
- 4. Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 5. Assad TR, Maron BA, Robbins IM, Xu M, Huang S, Harrell FE, Farber‐Eger EH, Wells QS, Choudhary G, Hemnes AR, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol. 2017;2:1361–1368. doi: 10.1001/jamacardio.2017.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xanthouli P, Jordan S, Milde N, Marra A, Blank N, Egenlauf B, Gorenflo M, Harutyunova S, Lorenz H‐M, Nagel C, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis. 2020;79:370–378. doi: 10.1136/annrheumdis-2019-216476 [DOI] [PubMed] [Google Scholar]
- 8. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, Goldstein RH, Assad T, Wertheim BM, Alba GA, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med. 2020;8:873–884. doi: 10.1016/S2213-2600(20)30317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Critical Care Med. 2014;190:252–257. [DOI] [PubMed] [Google Scholar]
- 10. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasler ED, Muller‐Mottet S, Furian M, Saxer S, Huber LC, Maggiorini M, Speich R, Bloch KE, Ulrich S. Pressure‐flow during exercise catheterization predicts survival in pulmonary hypertension. Chest. 2016;150:57–67. [DOI] [PubMed] [Google Scholar]
- 12. Lau EM, Thakkar V, Humbert M, Herve P. To stress or not to stress? Exercise pulmonary haemodynamic testing in systemic sclerosis. Eur Respir J. 2016;48:1549–1552. doi: 10.1183/13993003.01809-2016 [DOI] [PubMed] [Google Scholar]
- 13. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grünig E, Howard L, Humbert M, et al. An official European respiratory society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1700578. doi: 10.1183/13993003.00578-2017 [DOI] [PubMed] [Google Scholar]
- 14. Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42:1586–1594. doi: 10.1183/09031936.00050713 [DOI] [PubMed] [Google Scholar]
- 15. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 16. Fedorowski A, Hamrefors V, Sutton R, van Dijk JG, Freeman R, Lenders JW, Wieling W. Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res. 2017;27:167–173. doi: 10.1007/s10286-017-0409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol. 1957;189:609–615. doi: 10.1152/ajplegacy.1957.189.3.609 [DOI] [PubMed] [Google Scholar]
- 18. Henderson WR, Griesdale DE, Walley KR, Sheel AW. Clinical review: Guyton–the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care. 2010;14:243. doi: 10.1186/cc9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bevegard S, Holmgren A, Jonsson B. The effect of body position on the circulation at rest and during exercise, with special reference to the influence on the stroke volume. Acta Physiol Scand. 1960;49:279–298. doi: 10.1111/j.1748-1716.1960.tb01953.x [DOI] [PubMed] [Google Scholar]
- 20. Bevegard S, Holmgren A, Jonsson B. Circulatory studies in well trained athletes at rest and during heavy exercise. With special reference to stroke volume and the influence of body position. Acta Physiol Scand. 1963;57:26–50. doi: 10.1111/j.1748-1716.1963.tb02572.x [DOI] [PubMed] [Google Scholar]
- 21. Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol. 1978;41:52–59. doi: 10.1016/0002-9149(78)90131-5 [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee NA, Singh JP. Novel interventional therapies to modulate the autonomic tone in heart failure. JACC Heart Fail. 2015;3:786–802. [DOI] [PubMed] [Google Scholar]
- 23. Grassi G, Seravalle G, Quarti‐Trevano F, Dell'Oro R, Arenare F, Spaziani D, Mancia G. Sympathetic and baroreflex cardiovascular control in hypertension‐related left ventricular dysfunction. Hypertension. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467 [DOI] [PubMed] [Google Scholar]
- 24. Tossavainen E, Wikstrom G, Henein MY, Lundqvist M, Wiklund U, Lindqvist P. Passive leg‐lifting in heart failure patients predicts exercise‐induced rise in left ventricular filling pressures. Clin Res Cardiol. 2020;109:498–507. doi: 10.1007/s00392-019-01531-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW, Morgan DE, Stehlik J, Richardson RS. The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med Sci Sports Exerc. 2016;48:368–376. doi: 10.1249/MSS.0000000000000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hughson RL, Xing HC, Borkhoff C, Butler GC. Kinetics of ventilation and gas exchange during supine and upright cycle exercise. Eur J Appl Physiol Occup Physiol. 1991;63:300–307. doi: 10.1007/BF00233866 [DOI] [PubMed] [Google Scholar]
- 27. Cornelis N, Buys R. The effects of exercise modality on maximal and submaximal exercise parameters obtained by graded maximal exercise testing. Int J Cardiol. 2016;222:538–547. doi: 10.1016/j.ijcard.2016.07.190 [DOI] [PubMed] [Google Scholar]
- 28. Forton K, Motoji Y, Deboeck G, Faoro V, Naeije R. Effects of body position on exercise capacity and pulmonary vascular pressure‐flow relationships. J Appl Physiol. 2016;121:1145–1150. doi: 10.1152/japplphysiol.00372.2016 [DOI] [PubMed] [Google Scholar]


