Abstract
Background
There is a paucity of research describing health‐related quality of life (HRQOL) in older adults considered for advanced heart failure surgical therapies. Using data from our SUSTAIN‐IT (Sustaining Quality of Life of the Aged: Heart Transplant or Mechanical Support) study, we aimed to compare HRQOL among 3 groups of older (60–80 years) patients with heart failure before heart transplantation (HT) or long‐term mechanical circulatory support (MCS) and identify factors associated with HRQOL: (1) HT candidates with MCS, (2) HT candidates without MCS, or (3) candidates ineligible for HT and scheduled for long‐term MCS.
Methods and Results
Patients from 13 US sites completed assessments, including self‐reported measures of HRQOL (EuroQol‐5 Dimension Questionnaire, Kansas City Cardiomyopathy Questionnaire–12), depressive symptoms (Personal Health Questionnaire–8), anxiety (State‐Trait Anxiety Inventory–state form), cognitive status (Montreal Cognitive Assessment), and performance‐based measures (6‐minute walk test and 5‐m gait speed). Analyses included ANOVA, χ2 tests, Fisher’s exact tests, and linear regression. The sample included 393 patients; the majority of patients were White men and married. Long‐term MCS candidates (n=154) were significantly older and had more comorbidities and a higher New York Heart Association class than HT candidates with MCS (n=118) and HT candidates without MCS (n=121). Long‐term MCS candidates had worse HRQOL than HT candidates with and without MCS (EQ‐5D visual analog scale scores, 46±23 versus 68±18 versus 54±23 [P<0.001] and Kansas City Cardiomyopathy Questionnaire–12 overall summary scores, 35±21 versus 60±21 versus 49±22 [P<0.001], respectively). In multivariable analyses, lower 6‐minute walk distance, higher New York Heart Association class, depressive symptoms, and not being an HT candidate with MCS were significantly associated with worse overall HRQOL.
Conclusions
Our findings demonstrate important differences in overall and domain‐specific HRQOL of older patients with heart failure before HT or long‐term MCS. Understanding HRQOL differences may guide decisions toward more appropriate and personalized advanced heart failure therapies.
Keywords: advanced heart failure, quality of life, older age
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- 6MWT
6‐minute walk test
- BTC
bridge to candidacy
- BTT
bridge to transplantation
- DT
destination therapy
- EQ‐5D‐3L
EuroQol‐5 Dimension Questionnaire
- HT
heart transplantation
- KCCQ‐12
Kansas City Cardiomyopathy Questionnaire–12
- MOMENTUM 3
Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3
- OSS
overall summary score
- SUSTAIN‐IT
Sustaining Quality of Life of the Aged: Heart Transplant or Mechanical Support
Clinical Perspective
What Is New?
In a cohort of older patients with advanced heart failure, health‐related quality of life (HRQOL) was poor to fair; patients who were ineligible for heart transplantation and scheduled for implantation of long‐term mechanical circulatory support had worse HRQOL than patients awaiting heart transplantation with or without mechanical circulatory support.
Being a heart transplant candidate with mechanical circulatory support was associated with better HRQOL compared with being a heart transplant candidate without mechanical circulatory support, whereas a decreased 6‐minute walk test distance, higher New York Heart Association class, and depressive symptoms were associated with worse HRQOL in these older patients with heart failure.
What Are the Clinical Implications?
Understanding differences in HRQOL before alternative advanced surgical therapies (ie, heart transplantation and long‐term mechanical circulatory support) contributes to more informed shared decision‐making discussions for older patients with heart failure considering these treatment options.
Differences in domain‐specific HRQOL (eg, problems with mobility, usual activities, social functioning, anxiety/depression, and pain/discomfort and potentially worsening heart failure symptoms) in older patients before heart transplantation and long‐term mechanical circulatory support provide important individualized targets for treatment, especially for those awaiting long‐term mechanical circulatory support as they reported more problems than heart transplant candidates.
Approximately 6.2 million US adults have heart failure (HF), with an incidence approaching 21 per 1000 population after 65 years of age. 1 Many HF subgroups have poor survival. Among Medicare beneficiaries with HF, the 1‐year mortality rate is 29.6%. 2 Patients with advanced HF have even worse survival, with 1‐year mortality rates of 75% to 89%. 3 Treatment goals for these patients are focused not only on improving survival but also on improving health‐related quality of life (HRQOL).
Studies demonstrate a range of HRQOL outcomes in adult patients with advanced HF, varying by disease severity, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 but have rarely exclusively focused on older patients (ie, ≥60 years), despite the prevalence of HF being greatest in the elderly. In patients with ambulatory advanced HF, REVIVAL (Registry Evaluation of Vital Information for VADs in Ambulatory Life) investigators reported on outcomes in patients with a median of 60 years of age (interquartile range [IQR], 54–68 years of age), 5 whereas MedaMACS (Medical Arm of Mechanically Assisted Circulatory Support) investigators examined outcomes in patients who were on average 59 years of age (mean±SD, 59±11 years of age). 4 Studies of cohorts of patients who were more severely ill with advanced HF als o included adult patients of all ages. Patients enrolled in the MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial were a median of 64 years of age (range, 19–81 years of age; HeartMate 3) and a median of 61 years of age (range, 24–78 years of age; HeartMate II). 9 A European cross‐sectional study of patients undergoing heart transplantation (HT) by Emin et al 8 included patients whose median age in years for HT candidates with MCS was 49.6 (IQR, 39.1–55.3 years of age) and for HT candidates on medical therapy was 47.1 (IQR, 40.6–56.7 years of age). Thus, we lack an understanding of HRQOL outcomes in older patients with advanced HF, including those who may be eligible for advanced surgical therapies.
As HT and mechanical circulatory support (MCS) implantation, advanced surgical treatment options for patients with HF, are being offered more frequently to the elderly, 13 , 14 gaps in the literature also exist regarding HRQOL benefits, based on intended goal of therapy, which may inform how much these patients may benefit and which therapies may provide more HRQOL‐related benefit. White‐Williams and colleagues 15 compared baseline HRQOL in adult patients with advanced HF before left ventricular assist device (LVAD) implantation enrolled in the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) database by implant strategy (bridge to transplantation [BTT], bridge to candidacy [BTC], and destination therapy [DT]), which did not include patients awaiting HT without MCS. Similarly, in the MOMENTUM 3 trial, investigators identified baseline differences in HRQOL in adult patients with BTT and BTC versus DT implant strategies that also did not include a group of patients who were being medically treated and awaiting HT. 12 Groups in the study of HRQOL by Emin et al 8 included adult patients evaluated for HT, listed for HT on medical therapy, listed for HT on LVAD therapy, and after HT, but did not include a DT MCS group. Although these studies compared HRQOL in patients with either MCS and/or HT as an intended goal of therapy, none included all of the following 3 groups: HT (with or without MCS as a pre‐HT management strategy) and DT MCS. We examined baseline differences in HRQOL by age in 3 separate articles focused on patients undergoing HT, DT MCS, or MCS (combining patients with BTT and DT MCS), yet we did not include comparative analyses by intended goal of therapy. 7 , 16 , 17 Thus, a “head‐to‐head” comparison of baseline HRQOL in a contemporary cohort of older patients with advanced HF who are candidates for HT (with or without MCS while awaiting HT) or DT MCS remains to be elucidated. To address this gap in knowledge, as optimizing HRQOL is often a primary goal of therapy for older patients, we present findings from the SUSTAIN‐IT (Sustaining Quality of Life of the Aged: Heart Transplant or Mechanical Support) study, whose primary aim is to compare HRQOL outcomes in older patients (60–80 years of age) with advanced HF who undergo HT or DT MCS from before to 2 years after these surgeries.
In this report from the SUSTAIN‐IT study, we compared HRQOL in older patients with advanced HF at baseline (ie, before undergoing HT or DT [long‐term] MCS). HT candidates were divided into 2 groups (ie, those awaiting HT with MCS and those awaiting HT without MCS) to provide insight into differences in HRQOL between these 2 alternative pre‐HT management strategies. Thus, we aimed to compare HRQOL among the following 3 groups of patients: (1) HT candidates with MCS, (2) HT candidates without MCS, and (3) those deemed ineligible for HT and therefore candidates for long‐term MCS. We also aimed to identify patient factors associated with HRQOL. We hypothesized that (1) baseline overall and domain‐specific HRQOL of long‐term MCS candidates would be different from baseline HRQOL of the HT candidate groups, and (2) factors associated with overall HRQOL would include patient group, indicators of HF severity (eg, New York Heart Association [NYHA] class and functional capacity), and psychological variables (ie, anxiety and depressive symptoms).
Our nomenclature regarding the 3 groups of patients in this article is based on the recent Centers for Medicare and Medicaid National Coverage Decision to remove intent‐to‐treat criteria of BTT and DT by removing the requirement that patients with BTT MCS must be active on the United Network for Organ Sharing (UNOS) waiting list for HT. Thus, instead of BTT, we use the phrase “HT candidate with MCS,” and instead of DT, we use the phrase “long‐term MCS.” We defined HRQOL as “the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient” 18 and used the theoretical framework by Spilker and Revicki 18 (modified to include caregivers) to guide our research as it is focused on the influence of disease and treatment on HRQOL outcomes.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design, Settings, and Sample
We used an observational, cross‐sectional, multisite design to compare HRQOL in patients with HF before HT or long‐term MCS. Patients were recruited from 13 US medical centers with HT and MCS programs that participated in the SUSTAIN‐IT study. Study inclusion criteria were presence of advanced HF; 60 to 80 years of age; able to speak, read, and understand English; and listed with the UNOS for HT or being considered or scheduled for long‐term primary MCS, specifically LVAD implantation. Second‐generation and third‐generation US Food and Drug Administration–approved and investigational LVADs were permissible. HT candidates with MCS could have had 1 or more LVADs. Long‐term MCS candidates were recruited only if they had a low probability of becoming HT candidates (<35% chance at 2 years), per opinions of site investigators. We identified this enrollment cutoff to ensure, as best as possible, that patients in the long‐term MCS group would remain in this group for the duration of the study and not cross over to HT candidacy, at which time they would be censored. HT candidates listed for retransplant, multiple organ transplant, and/or those with right or bi‐ ventricular assist devices were excluded from the study. Long‐term MCS candidates with prior ventricular assist devices were also excluded. The study was approved by all site institutional review boards, and participants provided written informed consent.
Instruments, Data Collection, and Procedures
Patients completed the following self‐reported assessments of HRQOL: EuroQol‐5 Dimension Questionnaire (EQ‐5D‐3L 19 ; a 6‐item generic measure of HRQOL) and Kansas City Cardiomyopathy Questionnaire–12 (KCCQ‐12 20 ; a 12‐item, HF‐specific measure of HRQOL; Table S1). The EQ‐5D‐3L includes 5 dimensions of health (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) and a visual analog scale (VAS; 0 [worst]–100 [best] imaginable health state). The KCCQ‐12 has 4 domains (physical limitations, symptom frequency, social limitations, and quality of life) and an overall summary score (OSS) created from the domain scores. Clinically important differences for the EQ‐5D‐3L 17 , 21 and the KCCQ‐12 20 are 10 and 5 points, respectively. Patients also completed an 8‐item screen for depressive symptoms (Personal Health Questionnaire–8), 22 a 20‐item measure of current anxiety (State‐Trait Anxiety Inventory–state form), 23 an interviewer‐administered screen of cognitive function (Montreal Cognitive Assessment), 24 and the following 2 researcher‐monitored, performance‐based measures as surrogate markers of functional capacity and frailty, respectively (Table S1): 6‐minute walk test (6MWT) 25 and 5‐m gait speed. 26
Assessments were administered as follows: (1) in the HT candidate with MCS group after listing with UNOS while on MCS, (2) in the HT candidate without MCS group after listing with UNOS while on medical therapy, and (3) in the long‐term MCS group after being considered and/or scheduled for long‐term MCS.
Sociodemographic characteristics (eg, age, sex, race, marital status, work status, and insurance) and clinical variables (eg, etiology of HF, medical/surgical history, NYHA class, INTERMACS profile, and UNOS status) were collected by sites from patient medical records and/or directly from the Society of Thoracic Surgeons INTERMACS database via secure monthly data downloads.
Statistical Analysis
Demographic characteristics, clinical variables, and assessments were summarized using mean±SD, median (first quartile, third quartile), or count (percentage) as appropriate. Group comparisons (ie, analysis of variance [ANOVA], χ2 tests, or Fisher’s exact test) were used to test the hypothesis that baseline HRQOL of long‐term MCS candidates is different from baseline HRQOL of HT candidate groups.
Item‐level missing data on the HRQOL assessments were imputed via the within‐group respondent mean (if continuous) and mode (if categorical) to avoid group cross‐contamination. 27 This imputation method was used if <15% of item‐level data were missing, except for the KCCQ‐12, wherein imputation was not used per scoring instructions. For 58 patients with 6MWT data missing for reasons other than inability to walk or patient being too sick or too tired to walk (for whom we imputed a value of 0), we used model‐predicted single imputation based on a least squares regression model with NYHA class at enrollment and KCCQ‐12 symptom frequency as explanatory variables. We also conducted sensitivity analyses by fitting separate models for each outcome using solely observed 6MWT values (no imputation).
The second hypothesis (factors associated with overall HRQOL would include patient group, indicators of HF severity, and psychological variables) was tested using univariable and multivariable linear least squares regression models. We modeled separately overall HRQOL scores (EQ‐5D‐3L VAS and KCCQ‐12 OSS). In the univariable models, the initial pool of baseline variables included age, sex, race, marital status, working status, highest level of education more than high school, insurance type, Personal Health Questionnaire–8, State‐Trait Anxiety Inventory–state form, patient group (ie, HT candidate with MCS, HT candidate without MCS, and long‐term MCS), method of payment for medical care, history of smoking, comorbidities (myocardial infarction, diabetes, hypertension, kidney disease, arrhythmia, hyperlipidemia), Montreal Cognitive Assessment, and 6MWT. We used an entry point of α=0.3 in the univariable models to define the candidate pool for multivariable model building. Multivariable models were created by including each variable significant univariately at the 0.3 α level, excluding variables that were collinear with the outcome or poorly reported.
Between‐center differences for patient demographic and clinical characteristics and outcomes were assessed. Statistical significance was established at a 2‐sided α=0.05, and no adjustments were made for multiplicity. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Dr Andrei had full access to all data in the study and takes responsibility for its integrity and the data analysis.
Results
Between October 1, 2015 and December 31, 2018, from a pool of 1141 patients with advanced HF (649 HT candidates and 492 long‐term MCS candidates), 635 patients were approached (n=369 HT candidates and n=266 long‐term MCS candidates), and 396 (n=241 HT candidates and n=155 long‐term MCS candidates) were recruited to participate in the SUSTAIN‐IT study (Figure). A total of 2 HT candidates and 1 long‐term MCS candidate who were enrolled were deemed ineligible and immediately withdrawn, leaving a final sample size of 393 patients (239 HT candidates and 154 long‐term MCS candidates). Among those patients listed for HT, there were 118 with MCS and 121 without MCS. Reasons for not approaching candidates included timing of surgery and administrative issues (eg, staffing issues), being too sick, and other reasons. Reasons for refusal to participate for those approached included lack of interest, too sick, and other (Figure).
Figure 1. Timeline and consort flow diagram for patients before long‐term MCS or HT.
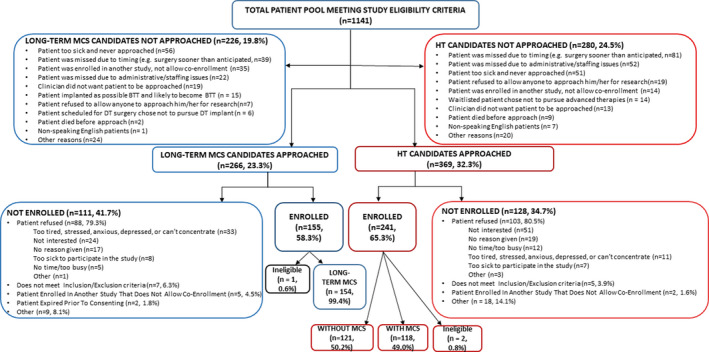
BTT indicates bridge to transplantation; DT, destination therapy; HT, heart transplantation; and MCS, mechanical circulatory support.
The majority of patients were White men and married, and 67% had more than a high school education (Table 1). Long‐term MCS candidates were older, on average, than HT candidates with and without MCS, although substantial overlap was observed between groups (Figure S1). Long‐term MCS candidates also had significantly more comorbidities and a higher NYHA class at enrollment (Table 1).
Table 1.
Patient Demographic and Clinical Characteristics at Baseline
| Variable | Available sample total, by group* | Total sample, n=393 † | Long‐term MCS candidates, n=154 † | HT candidates with MCS, n=118 † | HT candidates without MCS, n=121 † | P value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age, y | 393 (154, 118, 121) | 66.0±4.6 | 68.6±5.2 | 64.4±3.3 | 64.0±2.9 | <0.001 |
| Male sex | 393 (154, 118, 121) | 315 (80) | 122 (79) | 99 (84) | 94 (78) | 0.45 |
| White race | 390 (154, 118, 118) | 323 (83) | 126 (82) | 94 (80) | 103 (87) | 0.27 |
| Ethnicity: Hispanic or Latino | 386 (150, 118, 118) | 6 (2) | 3 (2) | 2 (2) | 1 (1) | 0.88 |
| Marital status: married/domestic partners | 388 (151, 116, 121) | 302 (78) | 118 (78) | 89 (77) | 95 (79) | 0.94 |
| Education, more than high school | 363 (133, 109, 121) | 245 (67) | 93 (70) | 69 (63) | 83 (69) | 0.52 |
| Currently working | 382 (143, 118, 121) | 67 (18) | 21 (15) | 23 (19) | 23 (19) | 0.52 |
| Working part‐time vs full‐time | 49 (15, 13, 21) | 0.45 | ||||
| Part‐time | 19 (39) | 7 (47) | 6 (46) | 6 (29) | ||
| Full‐time | 30 (61) | 8 (53) | 7 (54) | 15 (71) | ||
| Insurance type | 393 (154, 118, 121) | <0.001 | ||||
| Medicare/Medicaid | 243 (62) | 113 (73) | 69 (58) | 61 (50) | ||
| Private insurance | 150 (38) | 41 (27) | 49 (42) | 60 (50) | ||
| BMI at time of study enrollment, kg/m2 | 346 (148, 78, 120) | 28.4±5.5 | 28.4±6.5 | 29.9±4.7 | 27.5±4.3 | 0.008 |
| Clinical characteristics | ||||||
| Heart failure etiology | 393 (154, 118, 121) | 0.17 | ||||
| Ischemic cardiomyopathy | 182 (46) | 81 (53) | 55 (47) | 46 (38) | ||
| Dilated cardiomyopathy | 187 (48) | 65 (42) | 57 (48) | 65 (54) | ||
| Other | 24 (6) | 8 (5) | 6 (5) | 10 (8) | ||
| NYHA class at study enrollment | 382 (149, 113, 120) | <0.001 | ||||
| I | 18 (5) | 0 (0) | 16 (14) | 2 (2) | ||
| II | 57 (15) | 1 (1) | 43 (38) | 13 (11) | ||
| III | 128 (34) | 17 (11) | 39 (35) | 72 (60) | ||
| IV | 179 (47) | 131 (88) | 15 (13) | 33 (28) | ||
| INTERMACS profile at enrollment | 259 (146, 113, NA) | 0.005 | ||||
| Profile 1 | 33 (13) | 12 (8) | 21 (19) | NA | ||
| Profiles 2 to 3 | 189 (73) | 118 (81) | 71 (63) | NA | ||
| Profiles 4 to 7 | 37 (14) | 16 (11) | 21 (19) | NA | ||
| UNOS status at enrollment | 239 (NA, 118, 121) | <0.001 | ||||
| 1A | 44 (18) | NA | 16 (14) | 28 (23) | ||
| 1B | 138 (58) | NA | 87 (74) | 51 (42) | ||
| 2 | 44 (18) | NA | 5 (4) | 39 (32) | ||
| 7 | 13 (5) | NA | 10 (8) | 3 (2) | ||
| Length of time on UNOS waiting list at enrollment, d | 160 (NA, 67, 93) | 252 (61, 629) | NA | 507 (255, 825) | 94 (43, 330) | <0.001 |
| Length of time on VAD from implant to enrollment, d | 118 (NA, 118, NA) | 352 (171, 692) | NA | 352 (171, 692) | NA | |
| LVEF closest to date of surgery | 238 (143, 42, 53) | 0.009 | ||||
| >50, normal | 3 (1) | 0 (0) | 1 (2) | 2 (4) | ||
| .40 to 49, mild | 4 (2) | 0 (0) | 1 (2) | 3 (6) | ||
| 30 to 39, moderate | 13 (5) | 6 (4) | 1 (2) | 6 (11) | ||
| 20 to 29, moderate/severe | 75 (32) | 45 (31) | 12 (29) | 18 (34) | ||
| <20, severe | 130 (55) | 86 (60) | 22 (52) | 22 (42) | ||
| Not recorded/documented | 13 (5) | 6 (4) | 5 (12) | 2 (4) | ||
| Temporary MCS at enrollment | 393 (154, 118, 121) | 10 (3) | 4 (3) | 4 (3) | 2 (2) | 0.69 |
| ICD device in place at enrollment | 352 (150, 81, 121) | 303 (86) | 129 (86) | 64 (79) | 110 (91) | <0.001 |
| History of smoking | 390 (153, 116, 121) | 116 (30) | 25 (16) | 37 (32) | 54 (45) | <0.001 |
| Number of comorbidities | 393 (154, 118, 121) | 4.4±2.1 | 5.0±2.2 | 4.2±2.1 | 3.9±1.8 | <0.001 |
| Hypertension | 393 (154, 118, 121) | 239 (61) | 102 (66) | 67 (57) | 70 (58) | 0.21 |
| Hyperlipidemia | 393 (154, 118, 121) | 234 (60) | 95 (62) | 68 (58) | 71 (59) | 0.77 |
| Arrhythmia | 393 (154, 118, 121) | 229 (58) | 99 (64) | 69 (58) | 61 (50) | 0.07 |
| Diabetes | 393 (154, 118, 121) | 181 (46) | 85 (55) | 53 (45) | 43 (36) | 0.005 |
| Chronic kidney disease | 393 (154, 118, 121) | 145 (37) | 71 (46) | 39 (33) | 35 (29) | 0.008 |
| Myocardial infarction | 393 (154, 118, 121) | 136 (35) | 56 (36) | 40 (34) | 40 (33) | 0.83 |
| Pulmonary hypertension | 393 (154, 118, 121) | 79 (20) | 35 (23) | 28 (24) | 16 (13) | 0.08 |
BMI indicates body mass index; HT, heart transplantation; ICD, implantable cardioverter defibrillator; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; NA, not applicable; NYHA, New York Heart Association; UNOS, United Network for Organ Sharing; VAD, ventricular assist device.
Data are expressed as total number (total number of HT candidates with MCS, total number of HT candidates without MCS, total number of candidates ineligible for HT and scheduled for long‐term MCS).
Data are expressed as mean±SD, number (percentage), or median (quartile 1, quartile 3).
Rates of Assessment Completion and Psychometrics
Baseline completion of assessments varied based on type of assessment and group. Rates of patient completion of self‐report assessments and the interview were excellent (ranges, 92%–99% and 91%–92%, respectively), whereas completion rates for the performance‐based measures (6MWT and 5‐m gait speed) were lower (range, 24%–66%) and lowest for patients awaiting long‐term MCS (Table S2). The most frequently reported reason for incomplete performance‐based measures was that the participant was too sick, which varied by site (1%–22%; Table S3). Sensitivity analyses based on observed (unimputed) 6MWT data show that, for each outcome, model coefficients are not impacted substantially; their directionality and statistical significance remaining unchanged. Cronbach’s α were acceptable for the outcome variables (EQ‐5D‐3L, 0.72; KCCQ‐12 OSS, 0.90) in our SUSTAIN‐IT study cohort.
Baseline Comparisons of HRQOL Among Groups
Baseline overall HRQOL for the entire sample was poor to fair (EQ‐5D‐3L mean±SD VAS score, 55±23; KCCQ‐12 mean±SD OSS, 47±23; Table 2). Significant differences were found among groups. Long‐term MCS candidates had worse HRQOL than HT candidates with and without MCS (EQ‐5D‐3L mean±SD VAS score, 46±23 versus 68±18 versus 54±23 [P<0.001]; KCCQ‐12 mean±SD OSS, 35±21 versus 60±21 versus 49±22 [P<0.001], respectively), whereas HT candidates with MCS had the highest scores. Regarding EQ‐5D‐3L dimensions, >50% of the total sample reported problems with mobility, usual activities, and pain/discomfort. Long‐term MCS candidates had significantly more problems with mobility, self‐care, usual activities, and anxiety/depression than HT candidates with and without MCS (Table 2). No group differences were detected for pain/discomfort. Although reports of “extreme problems” were generally low for the aforementioned dimensions, long‐term MCS candidates reported more extreme problems regarding mobility, self‐care, and usual activities than both groups of HT candidates (Table S4). KCCQ‐12 domain scores also differed significantly among groups. Long‐term MCS candidates reported more physical and social limitations, higher symptom frequency, and worse quality of life than the 2 HT candidate groups, whereas HT candidates with MCS had the highest domain scores among the 3 groups (Table 2).
Table 2.
Patient Questionnaires, Interview, and Performance‐Based Assessments Completed at Baseline
| Variable | Available sample total (by group)* | Total sample, n=393 † | Long‐term MCS candidates, n=154 † | HT candidates with MCS, n=118 † | HT candidates without MCS, n=121 † | P value |
|---|---|---|---|---|---|---|
| EQ‐5D‐3L VAS score | 382 (144, 118, 120) | 55±23 | 46±23 | 68±18 | 54±23 | <0.001 |
| EQ‐5D‐3L VAS score, by 25‐point ranges | 382 (144, 118, 120) | <0.001 | ||||
| 0 to 24 | 35 (9) | 22 (15) | 2 (2) | 11 (9) | ||
| 25 to 49 | 99 (26) | 52 (36) | 9 (8) | 38 (32) | ||
| 50 to 74 | 150 (39) | 51 (35) | 57 (48) | 42 (35) | ||
| 75 to 100 | 98 (26) | 19 (13) | 50 (42) | 29 (24) | ||
| EQ‐5D‐3L mobility, percent with problems | 380 (143, 118, 119) | 210 (55) | 92 (64) | 54 (46) | 64 (54) | 0.010 |
| EQ‐5D‐3L self‐care, percent with problems | 380 (143, 118, 119) | 124 (33) | 67 (47) | 32 (27) | 25 (21) | <0.001 |
| EQ‐5D‐3L usual activities, percent with problems | 381 (143, 118, 120) | 262 (69) | 113 (79) | 71 (60) | 78 (65) | 0.003 |
| EQ‐5D‐3L pain/discomfort, percent with problems | 380 (143, 118, 119) | 194 (51) | 74 (52) | 54 (46) | 66 (55) | 0.32 |
| EQ‐5D‐3L anxiety/depression, percent with problems | 380 (143, 118, 119) | 135 (36) | 62 (43) | 41 (35) | 32 (27) | 0.021 |
| KCCQ‐12 physical limitation | 370 (137, 117, 116) | 49±28 | 38±27 | 59±26 | 53±26 | <0.001 |
| KCCQ‐12 symptom frequency | 383 (145, 118, 120) | 58±26 | 46±24 | 71±22 | 61±26 | <0.001 |
| KCCQ‐12 quality of life | 383 (145, 118, 120) | 35±27 | 23±23 | 51±27 | 35±25 | <0.001 |
| KCCQ‐12 social limitation | 364 (132, 115, 117) | 46±30 | 34±28 | 60±26 | 47±30 | <0.001 |
| KCCQ‐12 overall summary score | 383 (145, 118, 120) | 47±23 | 35±21 | 60±21 | 49±22 | <0.001 |
| KCCQ‐12 overall summary score, by 25‐point ranges | 383 (145, 118, 120) | <0.001 | ||||
| 0 to 24 | 81 (21) | 58 (40) | 7 (6) | 16 (13) | ||
| 25 to 49 | 128 (33) | 49 (34) | 30 (25) | 49 (41) | ||
| 50 to 74 | 123 (32) | 34 (23) | 53 (45) | 36 (30) | ||
| 75 to 100 | 51 (13) | 4 (3) | 28 (24) | 19 (16) | ||
| PHQ‐8≥10 | 382 (147, 115, 120) | 88 (23) | 49 (33) | 16 (14) | 23 (19) | <0.001 |
| PHQ‐8 total score | 382 (147, 115, 120) | 6.4±5.2 | 8.2±5.9 | 4.7±4.5 | 5.9±4.1 | <0.001 |
| STAI‐state total score | 382 (147, 115, 120) | 37±11 | 39±11 | 35±12 | 34±10 | <0.001 |
| MoCA total score | 359 (141, 108, 110) | 25.0±3.3 | 23.8±3.6 | 25.4±2.9 | 26.3±2.7 | <0.001 |
| Six‐min walk, m | 209 (51, 89, 69) | 309±121 | 204±115 | 348±106 | 336±96 | <0.001 |
| Gait speed, m/s | 183 (37, 78, 68) | 1.1±0.3 | 0.9±0.3 | 1.1±0.3 | 1.1±0.3 | <0.001 |
EQ‐5D‐3L VAS score indicates EuroQol‐5 Dimension Questionnaire Visual Analog Scale (score range 0=worst to 100=best imaginable health state); HT, heart transplantation; KCCQ‐12, Kansas City Cardiomyopathy Questionnaire–12 (score range: 0=worst to 100=best health status); MCS, mechanical circulatory support; MoCA, Montreal Cognitive Assessment (score range: 0=worst to 30=best score; cognitive dysfunction is defined as a MoCA score<26); PHQ‐8, Personal Health Questionnaire–8 (depression screen; score range 0=less to 24=worse depression); and STAI‐state, State‐Trait Anxiety Inventory–state form (score range 20=less to 80=worse anxiety).
Data are expressed as total number (total number of HT candidates with MCS, total number of HT candidates without MCS, total number of candidates ineligible for HT and scheduled for long‐term MCS).
Data are expressed as mean±SD or number (percentage).
Factors Associated With HRQOL in Patients Awaiting HT and Long‐Term MCS
We identified minimal variability among centers and therefore chose not to control for center effects. Multicollinearity among independent variables was minimal. Significant variables from the univariable models (Table S5) were included in the multivariable models. In the multivariable model for the EQ‐5D‐3L VAS score, being an HT candidate with MCS was associated with an 8.6‐point increase (95% CI, 2.7–14.4; P=0.004) in the VAS score compared with HT candidates without MCS. Decreased 6MWT distance and more depressive symptoms were associated with a decreased EQ‐5D‐3L VAS score and along with patient group explained 33% of the variance (Table 3). Patient group was not significantly associated with the KCCQ‐12 OSS in the multivariable model. Increased depressive symptoms, decreased 6MWT distance, and higher NHYA class were associated with decreased HRQOL using the KCCQ‐12 OSS, explaining 58% of variance (Table 3).
Table 3.
Factors Associated With Health‐Related Quality of Life in Patients Awaiting HT and Long‐Term MCS Using Multivariable Linear Regression Models
| Effect | Effect size | 95% Confidence limits | P value | |
|---|---|---|---|---|
| EQ‐5D VAS score (R 2=0.33) | ||||
| Intercept | 63.7 | 27.8 | 99.7 | <0.001 |
| Patient group | ||||
| Long‐term MCS candidates | −1.3 | −8.0 | 5.4 | 0.71 |
| HT candidates with MCS | 8.6 | 2.7 | 14.4 | 0.004 |
| HT candidates without MCS | Reference | Reference | Reference | Reference |
| Patient White race | −2.2 | −8.1 | 3.7 | 0.50 |
| Patient PHQ‐8 total score | −1.4 | −1.9 | −0.8 | <0.001 |
| Patient 6‐min walk, m | ||||
| 0 | −9.8 | −17.7 | −2.0 | 0.015 |
| 1 to 183 | −6.9 | −15.0 | 1.1 | 0.09 |
| 184 to 300 | −2.2 | −9.9 | 5.6 | 0.58 |
| 301 to 378 | 0.9 | −6.3 | 8.1 | 0.81 |
| >378 | Reference | Reference | Reference | Reference |
| Patient history of arrhythmia | −3.4 | −7.6 | 0.8 | 0.11 |
| Patient history of chronic kidney disease | −3.3 | −7.7 | 1.2 | 0.15 |
| Patient history of diabetes | −2.6 | −6.8 | 1.7 | 0.23 |
| Patient history of hypertension | −1.6 | −5.9 | 2.8 | 0.48 |
| Patient NYHA class at enrollment | ||||
| 1 | Reference | Reference | Reference | Reference |
| 2 | −2.6 | −13.4 | 8.2 | 0.63 |
| 3 | −8.0 | −18.7 | 2.7 | 0.14 |
| 4 | −6.2 | −17.8 | 5.3 | 0.29 |
| Patient history of smoking | 2.9 | −1.8 | 7.6 | 0.23 |
| Patient STAI‐state total score | −0.03 | −0.3 | 0.2 | 0.82 |
| Patient male sex | −3.3 | −8.8 | 2.2 | 0.24 |
| Patient age | 0.3 | −0.2 | 0.8 | 0.30 |
| KCCQ‐12 overall summary score (R 2=0.58) | ||||
| Intercept | 65.4 | 36.4 | 94.3 | <0.001 |
| Patient group | ||||
| Long‐term MCS candidates | −0.5 | −5.8 | 4.7 | 0.84 |
| HT candidates with MCS | 3.7 | −0.9 | 8.3 | 0.11 |
| HT candidates without MCS | Reference | Reference | Reference | Reference |
| Patient PHQ‐8 total score | −2.2 | −2.6 | −1.8 | <0.001 |
| Patient 6‐min walk, m | ||||
| 0 | −15.9 | −22.1 | −9.7 | <0.001 |
| 1 to 183 | −16.3 | −22.6 | −10.0 | <0.001 |
| 184 to 300 | −7.3 | −13.4 | −1.2 | 0.019 |
| 301 to 378 | −3.8 | −9.5 | 1.9 | 0.19 |
| >378 | Reference | Reference | Reference | Reference |
| Patient insurance type | ||||
| Medicare/Medicaid | −1.8 | −5.4 | 1.8 | 0.32 |
| Private insurance | Reference | Reference | Reference | Reference |
| Patient history of diabetes | −1.4 | −4.7 | 1.9 | 0.41 |
| Patient NYHA class at enrollment | ||||
| 1 | Reference | Reference | Reference | Reference |
| 2 | −2.7 | −11.2 | 5.8 | 0.53 |
| 3 | −8.9 | −17.2 | −0.5 | 0.039 |
| 4 | −12.1 | −21.2 | −3.1 | 0.009 |
| Patient history of smoking | 1.4 | −2.2 | 5.1 | 0.45 |
| Patient STAI‐state total score | −0.03 | −0.2 | 0.2 | 0.75 |
| Patient age | 0.3 | −0.2 | 0.7 | 0.26 |
EQ‐5D VAS indicates EuroQol Visual Analog Scale; HT, heart transplantation; KCCQ‐12, Kansas City Cardiomyopathy Questionnaire–12; MCS, mechanical circulatory support; NYHA, New York Heart Association; PHQ‐8, Personal Health Questionnaire–8; and STAI, State‐Trait Anxiety Inventory.
Discussion
Guided by our theoretical framework, we have extended knowledge of HRQOL in adult patients to those who are 60 to 80 years of age before advanced surgical therapies. In addition, we have expanded on baseline findings from studies regarding intended goal of therapy (ie, INTERMACS, MOMENTUM 3 trial, and the European study by Emin et al) 8 , 12 , 15 by comparing HRQOL among older patients who were awaiting HT with or without MCS or long‐term MCS if ineligible for HT. Key baseline findings in this older cohort of patients with HF from the SUSTAIN‐IT study were that overall HRQOL was poor to fair, and along with domains of HRQOL, important differences were detected among groups (ie, worse before long‐term MCS), thus supporting our first hypothesis that baseline overall and domain‐specific HRQOL of long‐term MCS candidates would be different from baseline HRQOL of the 2 HT candidate groups. Furthermore, we identified factors (ie, patient group, indicators of HF severity, and psychological factors) associated with worse baseline HRQOL for older patients with advanced HF, which supported our second hypothesis.
Notably, long‐term MCS candidates who had worse HRQOL, compared with both HT candidate groups, were older and had more comorbidities (including diabetes and chronic kidney disease) and also had a higher NYHA class, lower left ventricular ejection fraction, and lower 6MWT and gait speed, which suggest a higher severity of HF. Thus, it is perhaps not unexpected that compared with older HT candidates, older long‐term MCS candidates had significantly worse overall generic and HF‐specific HRQOL, which are clinically important differences. 17 , 20 , 21 Our findings are partially supported by findings from the observational ROADMAP (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management) study (overall mean±SD age, 63±13 years), which enrolled 2 groups of patients with advanced HF who were ambulatory: those scheduled for long‐term MCS and those who remained on optimal medical management. ROADMAP investigators reported that HRQOL was lower at baseline in patients with long‐term MCS compared with those on optimal medical management (45±20 versus 58±20, respectively). 11 In contradistinction, INTERMACS investigators and MOMENTUM 3 trial investigators detected no clinically important differences in baseline overall HRQOL between adult HT candidates with MCS (which included patients with BTC MCS in the MOMENTUM 3 trial) and those awaiting long‐term MCS. 12 , 15 Differences in findings between these 2 studies and our study are unclear but may be attributed to age differences in groups among studies. For example, the mean±SD ages of candidates for DT MCS and candidates for BTT/BTC MCS in the MOMENTUM 3 trial were 63±12 years of age and 55±12 years of age, respectively, whereas the mean±SD ages for long‐term MCS (ie, DT) candidates and HT candidates with MCS (BTT) in the SUSTAIN‐IT study were 69±5 years of age and 64±3 years of age, respectively. Also, MOMENTUM 3 trial investigators combined patients with BTT and BTC MCS (including those who were likely, moderately likely, and unlikely to become transplant eligible) into 1 group, 12 which may have influenced their findings, as moderately likely and unlikely patients with BTC MCS may have been more similar to DT candidates than HT candidates.
In addition, older patients awaiting HT with MCS had the highest HRQOL scores, which most likely represents the positive impact of MCS on reducing HF symptoms and improving functional capacity. Emin et al 8 also reported clinically important differences in overall HRQOL in their younger cohort of patients, favoring HT candidates with MCS compared with those on medical therapy (KCCQ‐12 OSS, 52.6±22.0 versus 33.3±21.1, respectively).
Few studies have identified factors associated with HRQOL in older patients with HF awaiting advanced surgical therapies. The association between being an HT candidate with MCS and better HRQOL may be attributed to improved health on MCS and the anticipation of HT. We previously reported that patients awaiting HT with MCS commented that the device reduced HF symptoms and improved participation in usual activities, yet patients were also anxious to undergo HT and “get on with life.” 28 Less distance walked on the 6MWT and its association with worse HRQOL is also logical, as it reflects impaired functional capacity, which was also found by Stehlik and colleagues 5 in their report from the REVIVAL study. Similar to our findings, Stehlik et al 5 also reported that a higher NYHA class was associated with worse HRQOL at baseline. Notably, significantly more long‐term MCS candidates in our study were NYHA class IV than both HT candidate groups. Given the strength of the association of a higher NYHA class with a decrease in HRQOL, monitoring NYHA class in long‐term MCS candidates is especially warranted. The association between depressive symptoms and HRQOL in patients with HF is well supported in the literature in, on average, middle‐aged and older‐aged cohorts. 11 , 29 , 30 Rutledge and colleagues 31 reported that depression was more prevalent in patients with HF with higher NYHA class, also providing support for our findings.
This baseline report from the SUSTAIN‐IT study provides valuable information for shared decision‐making discussions with older patients with advanced HF when considering surgical treatment options and regarding targets for HRQOL‐related treatment. It is well known that HRQOL influences preferences for treatment. 32 A critical question is the following: Will older patients with advanced HF incur more HRQOL‐related benefit from HT or long‐term MCS? The answer to this question is complex, as symptom burden, functional capacity, comorbidities, other outcomes (eg, survival), and postoperative risks and benefits must be considered. Our “head‐to‐head” comparison of baseline HRQOL among older patients before these surgical therapies partially addresses this question. Understanding differences in baseline HRQOL in our 3 groups of older patients provides critical information that may be useful to gauge the amount of improvement in HRQOL after HT or long‐term MCS that may ultimately contribute to identifying which therapy conveys more HRQOL‐related benefit. In support of this notion, findings from our INTERMACS study of HRQOL by severity of HF from before through 1 year after MCS implant (patient mean±SD age, 53±12 years of age) revealed greater gains in HRQOL by patients with lower INTERMACS profiles (ie, more‐severe HF) than patients with higher INTERMACS profiles (ie, less‐severe HF) despite postimplant adverse events. 10 Thus, sharing baseline HRQOL with older patients regarding potential surgical treatment options, that in the opinion of the clinician may provide benefit, contributes to a more informed shared decision‐making process, which has been well described in the literature, 33 and importantly is iterative, as patient preferences for survival versus HRQOL can change over time, often based on symptom burden and functional capacity. 34
Our study also contributes to a better understanding of differences in HRQOL in older patients before HT and long‐term MCS, especially domain‐specific HRQOL (eg, problems with mobility, usual activities, social functioning, anxiety/depression, and pain/discomfort and potentially worsening HF symptoms), which provides important individualized targets for treatment. We and others recommend assessment of baseline HRQOL by self‐report, rather than proxy (eg, physicians and family members), which can be discrepant, either overestimating or underestimating HRQOL. 18 , 35 These data can be captured and scored electronically in real time. Careful and frequent monitoring of older patients’ HF trajectory, NYHA class, symptoms, HRQOL (both overall and domains), functional capacity, 33 , 36 and subsequent development of a treatment plan, including consultation with the palliative care team and allied health team members, especially physical therapists, psychologists, and social workers, may contribute to enhanced HRQOL and improved health while awaiting HT or long‐term MCS. Notably, older long‐term MCS candidates in our study reported more problems with physical function (ie, mobility, self‐care, and usual activities) and mood (ie, anxiety and depression) than both groups of older HT candidates. Assessment of these HRQOL domains in this group of patients with advanced HF and the development of a treatment plan is especially warranted. Our recommendations are partially supported by Dew et al, 37 who identified key domains for evaluation of candidacy for cardiothoracic transplantation and MCS, including evaluations of patients’ current mental and social histories and understanding of their current illness, impact on daily functioning, symptoms, and treatment. Ultimately, understanding and subsequently treating HRQOL‐focused problems while older patients with advanced HF await HT or long‐term MCS may contribute to better outcomes after these surgical therapies.
Subsequent analyses from the SUSTAIN‐IT study will compare changes over time in overall and domain‐specific HRQOL early (baseline to 6 months) and later (baseline to 2 years) after HT (with or without MCS as a pretransplant management strategy) versus long‐term MCS and identify factors related to change in HRQOL. Future findings from our study may increase knowledge of this patient‐centric outcome so that clinicians may better inform older patients with advanced HF considering these treatment options as to which option may convey more HRQOL‐related benefit and initiate postoperative HRQOL‐focused treatment, especially regarding domain‐specific findings.
A limitation of our study is that older patients enrolled in the SUSTAIN‐IT study were prescreened and deemed eligible/awaiting advanced surgical therapies; thus, their HRQOL may not be reflective of the broader population with advanced HF. Also, a substantial number of patients in our total patient pool were not approached as a result of being too sick or were approached and refused participation for similar reasons, which may have resulted in an overestimation of HRQOL. In addition, our sample was fairly homogeneous, and more patients had more than a high school education than samples from other studies, which may limit generalizability. Lastly, lower completion rates of performance‐based measures, which varied by site, may also have influenced our findings, although we used a robust method to impute data for the 6MWT.
Conclusions
These findings demonstrate important differences in overall and domain‐specific HRQOL of older patients with advanced HF before HT or long‐term MCS. Understanding differences in HRQOL may guide decisions toward more personalized, advanced HF therapies. Older patients before long‐term MCS are an especially appropriate target for these therapies given their worse HRQOL compared with both HT candidate groups.
Sources of Funding
This work was sponsored by the National Institutes of Health, National Institute on Aging; SUSTAIN‐IT (Sustaining Quality of Life of the Aged: Heart Transplant or Mechanical Circulatory Support; R01AG047416; Dr Grady [principal investigator]; ClinicalTrials.gov: NCT02568930).
Disclosures
Dr Grady reports the following: consultant for Amgen, Inc., paid lecturer with the American Heart Association, and grant support from the National Institutes of Health (National Institute on Aging and National Heart Lung and Blood Institute). Dr Spertus reports the following: consultant for Amgen, Inc., Novartis, Bayer, Myokardia, and Janssen; scientific advisory board of United HealthCare; board of directors of Blue Cross Blue Shield, Kansas City; and copyright holder of the Kansas City Cardiomyopathy Questionnaire. Dr Petty reports speaking honorarium from Abbott Inc. M. Murray reports consulting on a research project for GE Healthcare. Dr Silvestry reports consulting for Abiomed, Medtronic, Syncardia, and Abbott. Dr Kirklin is the Director of the Data Coordinating Center for Society of Thoracic Surgeons INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) and reports grant support from the National Institutes of Health/National Institute on Aging. Dr Yancy reports spousal employment with Abbott Labs, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1–S5
Figure S1
Supplemental Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024385
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Virani S, Alonso A, Benjamin E, Bittencourt M, Callaway C, Carson A, Chamberlain A, Chang A, Cheng S, Delling F, et al. Heart disease and stroke statistics – 2020 update. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Normand S‐LT, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998‐2008. J Am Med Assoc. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, et al. Advanced (Stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Cardiac Fail. 2015;21:519–534. doi: 10.1016/j.cardfail.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 4. Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde‐McLean RC, DeVore AD, Pamboukian SV, Thibodeau JT, Teuteberg JJ, Cadaret L, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MEDaMACS) Registry. J Heart Lung Transplant. 2019;38:408–417. doi: 10.1016/j.healun.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stehlik J, Mountis M, Haas D, Palardy M, Ambardekar A, Estep J, Ewald G, Russell S, Robinson S, Jorde U, et al. Quality of life and treatment for ventricular assist device therapy in ambulatory advanced heart failure: a report from the REVIVAL study. J Heart Lung Transplant. 2020;39:27–36. doi: 10.1016/j.healun.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grady K, Jalowiec A, White‐Williams C, Pifarre R, Kirklin J, Bourge R, Costanzo MR. Predictors of quality of life in patients with advanced heart failure awaiting transplantation. J Heart Lung Transplant. 1995;14:2–10. [PubMed] [Google Scholar]
- 7. Grady KL, Wissman S, Naftel D, Myers S, Gelijins A, Moskowitz A, Pagani F, Young J, Spertus J, Kirklin J. Age and gender differences in HRQOL and factors related to change in HRQOL from before to 6 months after LVAD implantation: findings from INTERMACS. J Heart Lung Transplant. 2016;35:777–788. doi: 10.1016/j.healun.2016.01.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emin A, Rogers C, Banner N. Quality of life of advanced chronic heart failure: medical care, mechanical circulatory support and transplantation. Euro J Cardio Thorac Surg. 2016;50:269–273. doi: 10.1093/ejcts/ezw054 [DOI] [PubMed] [Google Scholar]
- 9. Cowger J, Naka Y, Aaronson K, Horstmanshof D, Gulati S, Rinde‐Hoffman D, Pinney S, Adatya S, Farrar D, Jorde U. Quality of life and functional capacity outcomes in the MOMENTUM 3 trial at 6 months: a call for new metrics for left ventricular assist device patients. J Heart Lung Transplant. 2018;37:15–24. doi: 10.1016/j.healun.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 10. Grady KL, Naftel D, Stevenson L, Dew MA, Weidner G, Pagani F, Kirklin JK, Myers S, Baldwin T, Young J. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2014;33:412–421. doi: 10.1016/j.healun.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stehlik J, Estep J, Selzman C, Rogers J, Spertus J, Shah K, Chuang J, Farrar D, Starling R. Patient‐reported health‐related quality of life is a predictor of outcomes in ambulatory heart failure patients treated with left ventricular assist device compared with medical management results from the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ Heart Fail. 2017;10:e003910. doi: 10.1161/CIRCHEARTFAILURE.116.003910 [DOI] [PubMed] [Google Scholar]
- 12. Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, Itoh A, Uriel N, Cleveland JC, Raval NY, et al. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the Multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. J Am Med Assoc Cardiol. 2020;5:411–419. doi: 10.1001/jamacardio.2019.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty‐sixth adult heart transplantation report ‐ 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1056–1066. doi: 10.1016/j.healun.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, et al. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38:114–126. doi: 10.1016/j.healun.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 15. White‐Williams C, Fazeli P, Kirklin J, Pamboukian S, Grady KL. Differences in health‐related quality of life by implant strategy: analyses from the Interagency for Mechanically Assisted Circulatory Support. J Heart Lung Transplant. 2020;39:62–73. doi: 10.1016/j.healun.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 16. Shamaskin A, Rybarczyk B, Wang E, White‐Williams C, Cotts W, McGee E, Grady K. Older patients have better quality of life, adjustment and adherence than younger patients 5 years post heart transplantation. J Heart Lung Transplant. 2012;31:478–484. doi: 10.1016/j.healun.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 17. Grady KL, Naftel DC, Myers S, Dew MA, Weidner G, Spertus JA, Idrissi K, Lee HB, McGee EC, Kirklin JK. Change in health‐related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: analyses from INTERMACS. J Heart Lung Transplant. 2015;34:213–221. doi: 10.1016/j.healun.2014.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. New York: Lippincott Williams & Williams; 1996. [Google Scholar]
- 19. Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF‐36 Health Survey questionnaire. Qual Life Res. 1993;2:169–180. doi: 10.1007/BF00435221 [DOI] [PubMed] [Google Scholar]
- 20. Spertus JA, Jones PG. Development and validation of a short version of the Kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer R. The PHQ‐9: a new depression diagnostic and severity measure. Psychiatric Ann. 2002;32:509–515. doi: 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 23. Spielberger C, Gorsuch R, Lushene R. STAI Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc.; 1970. [Google Scholar]
- 24. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 25. Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six‐minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325 [DOI] [PubMed] [Google Scholar]
- 26. Afilalo J, Eisenberg MJ, Morin J‐F, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039 [DOI] [PubMed] [Google Scholar]
- 27. Little RJ, Rubin DB. Statistical Analysis With Missing Data. Hoboken, NJ: John Wiley & Sons; 2019. [Google Scholar]
- 28. Grady KL, Magasi S, Hahn EA, Buono S, McGee EC, Yancy C. Health‐related quality of life in mechanical circulatory support: development of a new conceptual model and items for self‐administration. J Heart Lung Transplant. 2015;34:1292–1304. doi: 10.1016/j.healun.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 29. Hallas C, Wray J, Andreou P, Banner N. Depression and perceptions about heart failure predict quality of life in patients with advanced heart failure. Heart Lung. 2011;40:111–121. doi: 10.1016/j.hrtlng.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 30. Jaarsma T, Johansson P, Agre S, Stromberg A. Quality of life and symptoms of depression in advanced heart failure patients and their partners. Curr Opin Support Pall Care. 2010;4:233–237. doi: 10.1097/SPC.0b013e328340744d [DOI] [PubMed] [Google Scholar]
- 31. Rutledge T, Reis V, Linke S, Greenberg B, Mills P. Depression in heart failure. A meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055 [DOI] [PubMed] [Google Scholar]
- 32. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preference for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4 [DOI] [PubMed] [Google Scholar]
- 33. Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, Cook NR, Felker GM, Francis GS, Hauptman PJ, et al. Decision‐ making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, Kosecoff J, Young RT, Brook RH, Delbanco TI. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med. 1991;114:451–454. doi: 10.7326/0003-4819-114-6-451 [DOI] [PubMed] [Google Scholar]
- 36. Grady KL, Stevenson L, Pagani F, Teuteberg J, Pamboukian S, Birks E, Moore S, Kirklin J. Beyond survival: recommendations from INTERMACS for assessing function and quality of life with mechanical circulatory support. J Heart Lung Transplant. 2012;31:1158–1164. doi: 10.1016/j.healun.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 37. Dew MA, DiMartini AF, Dobbels F, Grady KL, Jowsey‐Gregoire SG, Kaan A, Kendall K, Young Q‐R, Abbey SE, Butt Z, et al. The 2018 ISHLT/APM/AST/ICCAC/STSW recommendations for the psychosocial evaluation of adult cardiothoracic transplant candidates and candidates for long‐term mechanical circulatory support. J Heart Lung Transplant. 2018;37:803–823. doi: 10.1016/j.healun.2018.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S5
Figure S1


