Abstract
Background
Our objective was to investigate stroke severity and subsequent rate of mortality among patients with and without atrial fibrillation (AF). Contemporary data on stroke severity and prognosis in patients with AF are lacking.
Methods and Results
First‐time ischemic stroke patients from the Danish Stroke Registry (January 2005–December 2016) were included in an observational study. Patients with AF were matched 1:1 by sex, age, calendar year, and CHA2DS2‐VASc score with patients without AF. Stroke severity was determined by the Scandinavian Stroke Scale (0–58 points). The rate of death was estimated by Kaplan‐Meier plots and multivariable Cox regression. Among 86 458 identified patients with stroke, 17 205 had AF. After matching, 14 662 patients with AF and 14 662 patients without AF were included (51.8% women; median age, 79.6 years [25th–75th percentile, 71.8–86.0]). More patients with AF had very severe stroke (0–14 points) than patients without AF (13.7% versus 7.9%, P<0.01). The absolute rates of 30‐day and 1‐year mortality were significantly higher for patients with AF (12.1% and 28.4%, respectively) versus patients without AF (8.7% and 21.8%, respectively). This held true in adjusted models for 30‐day mortality (hazard ratio [HR], 1.40 [95% CI, 1.30–1.51]). However, this association became nonsignificant when additionally adjusting for stroke severity (HR, 1.10 [95% CI, 1.00–1.23]). AF was associated with a higher rate of 1‐year mortality (HR, 1.39 [95% CI, 1.32–1.46]), although it was mediated by stroke severity (HR, 1.15 [95% CI, 1.09–1.23], model including stroke severity).
Conclusions
In a contemporary nationwide cohort of patients with ischemic stroke, patients with AF had more severe strokes and higher mortality than patients without AF. The difference in mortality was mainly driven by stroke severity.
Keywords: atrial fibrillation, epidemiology, ischemic stroke, severity, stroke severity
Subject Categories: Epidemiology, Cardiovascular Disease, Ischemic Stroke, Cerebrovascular Disease/Stroke, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- OAC
oral anticoagulation
- SSS
Scandinavian Stroke Scale
- VKA
vitamin K antagonist
Clinical Perspective
What Is New?
Despite improvement of atrial fibrillation (AF) detection and stroke prevention during the past decades, AF is still associated with higher stroke severity and higher subsequent mortality.
However, the observed higher mortality was primarily mediated by the higher stroke severity.
What Are the Clinical Implications?
This study on real‐world data underlines that AF detection and stroke prevention remain important, and that a window of opportunity exists for improving outcomes.
Clinicians may focus more on underuse or inappropriate use of oral anticoagulation and engage more in a multidisciplinary approach to this patient group.
Future research in the effect of AF screening is needed as well as focus on identification of comorbidities in patients with AF affecting prognosis.
Ischemic stroke is a major risk for patients with atrial fibrillation (AF) and is the basis for the risk stratification of patients and the consideration of anticoagulation therapy. 1 For these considerations, not only is the risk of stroke important but also the stroke severity, which is one of the most important factors for recovery. 2 AF is estimated to account for 20% to 25% of all ischemic strokes. 1 Studies suggest that AF‐related strokes may be more severe than strokes from other causes, yet this knowledge is based on few studies, most of them with data from almost 30 years ago using different metrics for severity mostly based on rehabilitation goals and primarily on selected study populations. 3 , 4 , 5 , 6 , 7 , 8 The most recent studies were conducted on a Danish study population 8 and on patients in the Registry of the Canadian Stroke Network, 9 with follow‐up ending in 2007 and 2008, respectively. Treatment of AF has improved over the past decade, with a focus on thromboembolic risk stratification and oral anticoagulation (OAC) therapy as well as treatment for coexisting risk factors such as hypertension and hypercholesterolemia. Improved access to advanced imaging at dedicated stroke units and better acute treatment of stroke with thrombolysis and endovascular treatment have decreased morbidity significantly. 10 However, the studies above are still used as benchmarks for aligning our knowledge, research, and health care plans for patients with stroke today. There is a need for a well‐powered contemporary cohort study in the modern era. We aimed to determine stroke severity and subsequent short‐ and long‐term mortality in patients with atrial fibrillation compared with patients without atrial fibrillation.
METHODS
Data Sources
In Denmark, all citizens are given a unique identification number enabling the linkage of nationwide validated administrative registries. Information about vital status was drawn from the Danish Civil Registration System. 11 Prescription filling data were drawn from the Danish National Prescription Registry. 12 The diagnosis of AF and all comorbidities were obtained from the Danish National Patient Registry with data on admissions to all hospitals from January 1, 1977 to December 31, 2016. 13 The Danish Register of Causes of Death provided information on the date of death until December 31, 2017 (Figure 1). All International Classification of Diseases (ICD)‐8 and ‐10 codes used are shown in Table S1. No additional raw data are available. Further details of the analyses of the study are available from the corresponding author on request.
Figure 1 Data sources and flowchart.
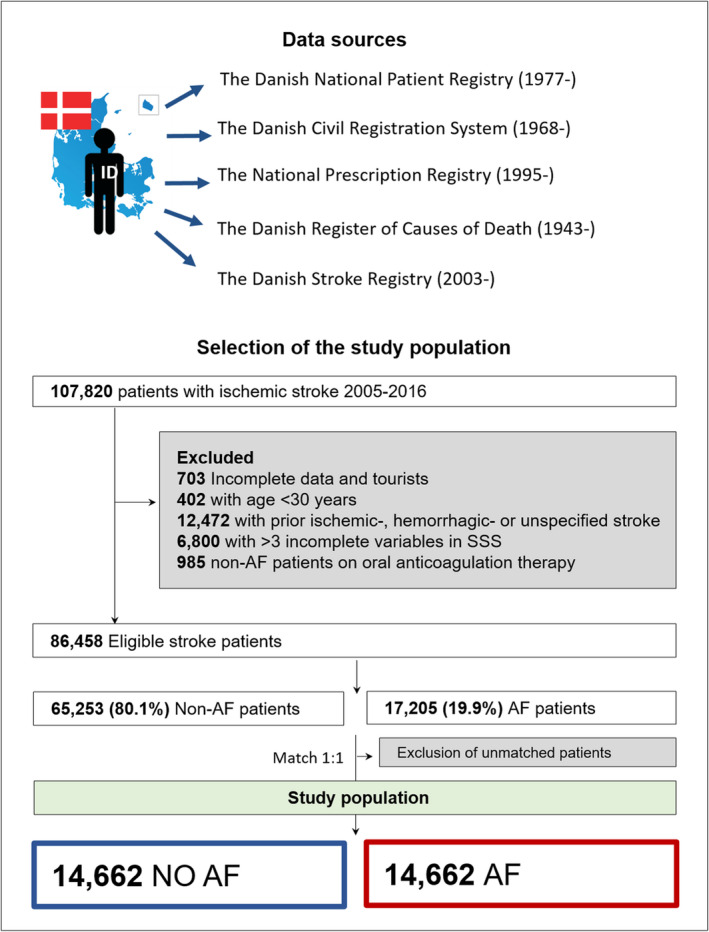
Matched 1:1 by age, sex, calendar year, and comorbidities (hypertension, heart failure, transient ischemic attack, thromboembolism, and diabetes). AF indicates atrial fibrillation; and SSS, Scandinavian Stroke Scale.
Danish Stroke Registry and the Scandinavian Stroke Scale
The diagnosis of stroke and Scandinavian Stroke Scale (SSS) score were collected from the Danish Stroke Registry. From 2003 onward, all strokes and transient ischemic attacks in patients ≥18 years old admitted to a Danish hospital have been registered in this nationwide clinical stroke registry. Registration is mandatory by law, and stroke data are collected prospectively during admission using a standardized registration form. 14 The registry captures 97% of all stroke episodes (sensitivity) and has a positive predictive value of the registered stroke episodes of 90% when the medical records are used as the gold standard. 15 The scoring by the SSS was based on the level of consciousness (6‐0 points), eye movement (4‐0 points), speech (10‐0 points), orientation (6‐0 points), facial palsy (2‐0 points), gait (12‐0 points), and hand, arm, and leg motor power (6‐0 points each). The SSS can be divided into 4 different categories: very severe stroke (0–14 points), severe stroke (15–29 points), moderate stroke (30–44 points), and mild stroke (45–58 points). 16 The ability of the SSS to identify mortality and disability after stroke and interobserver agreement have previously been validated and found equal to other acute stroke scales, such as the National Institutes of Health Stroke Scale. 17 , 18 , 19 , 20
Study Population and Outcomes
Patients ≥30 years old with a first‐time ischemic stroke or unspecified stroke were included between January 1, 2005 and December 31, 2016. A Danish study found that two‐thirds of unspecified strokes registered are ischemic strokes; therefore, these patients were included. 21 Stroke severity was determined on admission, and patients were followed for 1 year or until death, whichever occurred first. The outcomes were stroke severity, in‐hospital mortality, 30‐day mortality, and 1‐year mortality. The patients were categorized as having AF (or atrial flutter [DI48]; positive predictive value, 99%) 22 if they had a history of AF or were diagnosed with AF at discharge for index admission with stroke. Patients with prior ischemic, hemorrhagic, or unspecified stroke were excluded. Tourists with no Danish identification number, patients with incomplete data, and patients without AF treated with OAC were excluded. A maximum of 3 missing variables in the SSS were allowed. For 1706 patients with 1 to 3 missing variables, we imputed data by assigning the best possible score for the specific missing variable (ie, consciousness [6 points], eye movement [4 points], speech [10 points], orientation [6 points], facial palsy [2 points], gait [12 points], and hand, arm, and leg motor power [6 points each]). This was based on the assumption that doctors may leave out registration of those areas that are not affected in the patient (Figure 1).
Covariates
Information on comorbidities and prior diagnosis of AF were obtained from the Danish National Patient Registry using discharge diagnose codes. The modified CHA2DS2‐VASc score (congestive heart failure [1 point], hypertension [1 point], age ≥75 years [2 points], diabetes [1 point], history of stroke/transient ischemic attack/systemic arterial thromboembolism [2 points], vascular disease [1 point], age 65 to 74 years [1 point], and sex category [women, 1 point]) was calculated ranging from 0 to 9 points. Concomitant pharmacotherapy was assessed by prescription fillings 180 days before the stroke date.
Stroke Severity and Oral Anticoagulation Over Time
Trends in stroke severity were examined by calendar year. Additionally, trends in the status of antithrombotic therapy at the time of admission (no antithrombotics, antiplatelet therapy only, vitamin K antagonist [VKA], and direct oral anticoagulants [DOAC]) among patients with AF were analyzed.
Statistical Analysis
The baseline characteristics are presented as medians with quartiles and as numbers and percentages for categorical data. The differences between categorical variables were compared using the χ2 test, the Kruskal‐Wallis test for continuous data, and the Cochran‐Mantel‐Haenszel test for stratified groups.
Patients with AF were matched by risk‐set matching with patients without AF 1:1 by sex, age (up to 2 years difference), calendar year, and comorbidities (congestive heart failure, hypertension, diabetes, transient ischemic attack, thromboembolism, and vascular disease [ischemic heart disease or peripheral artery disease]) present in the CHA2DS2‐VASc score.
The association between AF and very severe stroke (0–14 points on the SSS) was analyzed in a univariable and a multivariable conditional logistic regression model (ie, comparing cases with their matched control subjects). The multivariable model was adjusted for clinically relevant confounders (chronic obstructive lung disease, chronic kidney disease, liver disease, cancer, alcohol abuse, prior bleeding, dementia, and prior use of statins).
Cumulative incidence curves were drawn for 30‐day and 1‐year mortality based on the Kaplan‐Meier estimate, including in‐hospital mortality. Tests for differences between the curves were performed by the log‐rank test.
Adjusted rates of 30‐day and 1‐year mortality were calculated by a Cox proportional hazards model comparing patients with AF with patients without AF, conditional on the matching and adjusted for chronic obstructive lung disease, chronic kidney disease, liver disease, cancer, alcohol abuse, prior bleeding, dementia, and prior use of statins. One Cox model was additionally adjusted for stroke severity (mild [reference], moderate, severe, and very severe stroke) to test whether the effect of AF on mortality was mediated by the initial stroke severity. The Cox proportional hazard assumption, linearity of continuous variables, and relevant interactions were tested, and assumptions underlying the model were found valid.
A P value of <0.05 and a 95% CI not including 1 were considered statistically significant. SAS software (version 9.4; SAS Institute, Cary, NC) was used for all analyses.
Sensitivity Analyses
Several sensitivity analyses were performed. Patients with AF were stratified according to antithrombotic therapy (antiplatelet therapy, VKA, or DOAC), with patients with AF on no antithrombotic therapy as a reference in a multivariable logistic regression model analyzing the association between AF and very severe strokes. Patients with new and prior AF were analyzed separately, and the outcome of stroke severity and risk of mortality were investigated. Patients with AF and patients without AF were stratified by sex and age. Stroke severity and mortality were investigated, stratified by sex. The analyses were repeated on the unmatched population. All analyses were repeated on a population with no missing variables in the SSS.
Ethical Approval
For anonymous registry‐based studies in Denmark, no ethical approval is required. The study was approved by the Danish Data Protection Agency (approval number: P‐2019‐191).
RESULTS
Baseline Characteristics
From January 1, 2005 to December 31, 2016, there were 86 458 eligible patients with first‐time ischemic stroke identified, of whom 17 205 (19.9%) had prior AF or AF diagnosed during stroke hospitalization. After matching, each group consisted of 14 662 patients with and without AF (Figure 1). In total, 90.9% had ischemic stroke, and 9.1% had unspecified stroke. Among patients with AF, 38.0% had new AF, and 62.0% had prior AF. The median age of the study population was 79.6 years (25th–75th percentile: 71.8–86.0), and 51.8% were women. After matching, the comorbidities were equally distributed between the 2 groups. Patients with AF had a higher concomitant use of β‐blockers and lower use of concomitant antiplatelet therapy than patients without AF, and 24.7% of patients with AF had prior OAC therapy (Table).
Table 1.
Baseline Characteristics of the Matched Study Population
| Variable | No AF | AF | P value |
|---|---|---|---|
| No. of patients (%) | 14 662 (50.0) | 14 662 (50.0) | NA |
| Patient age, y, median (25th–75th percentile) | 79.6 (71.8–86.0) | 79.6 (71.8–86.0) | NA |
| Women, n (%) | 7596 (51.8) | 7596 (51.8) | NA |
| Comorbidities, n (%) | |||
| Heart failure | 2143 (14.6) | 2143 (14.6) | >.99 |
| Hypertension | 7801 (53.2) | 7801 (53.1) | >.99 |
| Diabetes | 1623 (11.1) | 1623 (11.1) | >.99 |
| Peripheral artery disease | 1294 (8.8) | 1050 (7.1) | <0.01 |
| Alcohol abuse | 675 (4.6) | 725 (4.9) | 0.17 |
| Liver disease | 302 (2.1) | 347 (2.4) | 0.07 |
| Chronic kidney disease | 822 (5.6) | 889 (6.1) | 0.10 |
| Prior bleeding | 2248 (15.3) | 2782 (19.0) | <0.01 |
| Cancer | 2717 (18.5) | 2835 (19.3) | 0.08 |
| Ischemic heart disease | 4343 (29.6) | 4593 (31.3) | <0.01 |
| Chronic obstructive lung disease | 1609 (11.0) | 1743 (11.9) | 0.01 |
| Dementia | 764 (5.2) | 806 (5.5) | 0.28 |
| Transient ischemic attack | 902 (6.2) | 902 (6.2) | >.99 |
| Thromboembolism | 238 (1.6) | 238 (1.6) | >.99 |
| Concomitant therapy, n (%) | |||
| Digoxin | 231 (1.6) | 3208 (21.9) | <0.01 |
| Amiodarone | 35 (0.2) | 246 (1.7) | <0.01 |
| β‐blockers | 4491 (30.6) | 7002 (47.8) | <0.01 |
| Renin angiotensin system inhibitor | 6421 (43.8) | 5679 (38.7) | <0.01 |
| Loop diuretics | 2862 (19.5) | 3793 (25.9) | <0.01 |
| Thiazide | 3322 (22.7) | 2897 (19.8) | <0.01 |
| Spiron | 697 (4.8) | 778 (5.3) | 0.03 |
| Diuretics in combination* | 2058 (14.0) | 1655 (11.3) | <0.01 |
| Statins | 4067 (27.7) | 3830 (26.1) | <0.01 |
| Calcium channel blockers | 4113 (28.1) | 3818 (26.0) | <0.01 |
| Anticoagulation therapy † | |||
| No antithrombotic therapy | 8686 (59.2) | 5888 (40.2) | <0.01 |
| Antiplatelet therapy only | 5976 (40.8) | 5165 (35.2) | |
| Vitamin K antagonists | 0 (0.0) | 2951 (20.1) | |
| Direct oral anticoagulants | 0 (0.0) | 667 (4.6) | |
| CHA2DS2‐VASc score | |||
| 0 | 562 (3.8) | 564 (3.9) | 1.00 |
| 1 | 1263 (8.6) | 1265 (8.6) | |
| ≥2 | 12 837 (87.6) | 12 833 (87.5) | |
| SSS, median (25th–75th percentile) | 49.0 (37.0–55.0) | 45.0 (26.0–54.0) | <0.01 |
AF indicates atrial fibrillation; NA, not applicable; and SSS, Scandinavian Stroke Scale; and CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior TIA or thromboembolism, vascular disease, age 65–74 years, sex category).
Anatomical Therapeutic Chemical code C07C, C08G, C03B, C03X.
Prescription of oral anticoagulation therapy is based on the last prescription before the stroke of either vitamin K antagonists or direct oral anticoagulants; however, these patients can additionally be on antithrombotic treatment. Patients categorized under antithrombotic treatment are not treated with any of the oral anticoagulants.
Stroke Severity Among Patients With and Without AF
Patients with AF had a lower median SSS score than patients without AF (45.0 points [25th–75th percentile: 26.0–54.0] versus 49.0 points [25th–75th percentile: 37.0–55.0], P<0.01). Among patients with AF, 13.7% had very severe strokes, whereas 7.9% of patients without AF had very severe strokes (P<0.01) (Figure 2). In a univariate logistic regression model, AF was associated with very severe stroke (0–14 points) (odds ratio [OR], 1.87 [95% CI, 1.73–2.02]), and this association remained after adjustment (OR, 1.86 [95% CI, 1.72–2.01]). The characteristics of patients with very severe strokes compared with those with mild to severe strokes are shown in Table S2. Patients with very severe strokes were older, more often women, and had a higher comorbidity burden. Over time, the distribution of stroke severity remained stable in the AF and non‐AF groups; however, there was a small increase in mild strokes in both groups at the end of the study period (Figure S1).
Figure 2. Stroke severity based on Scandinavian Stroke Scale scoring (very severe to mild) among patients with and without atrial fibrillation (AF).
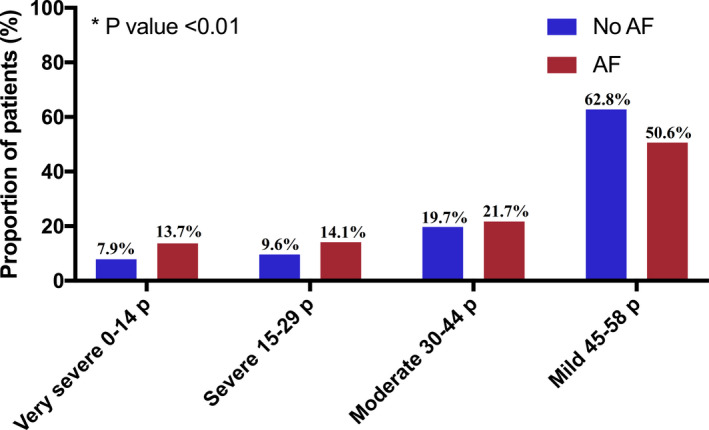
p indicates points. *P value: Chi square test between very severe stroke versus severe‐mild stroke among AF and patients with no AF.
Stroke Severity and Oral Anticoagulation Over Time
The trends in the status of antithrombotic therapy at the time of admission (no antithrombotic therapy, antiplatelet therapy only, VKA, and DOAC) among patients with AF are shown in Figure S2. The status of the different antithrombotic therapies was stable throughout the study period; however, in 2010, we observed an increase in the use of DOACs and a simultaneous decrease in the use of antiplatelet therapy.
Mortality
Among patients with AF, 10.8% died in‐hospital compared with 7.5% among patients with no AF (P<0.01). The crude 30‐day cumulative incidence of death was higher among patients with AF (12.1%; 95% CI, 11.6%–12.7%) than among patients without AF (8.7%; 95% CI, 8.35%–9.2%) (P<0.01) (Figure 3A). In a Cox model, AF was associated with a higher rate of 30‐day mortality (hazard ratio [HR], 1.40 [95% CI, 1.30–1.51]) than non‐AF (Figure 4A); however, when adding stroke severity to the model, the association became nonsignificant (HR, 1.10 [95% CI, 1.00–1.23]) (Figure 4A).
Figure 3. Unadjusted cumulative incidences of mortality.
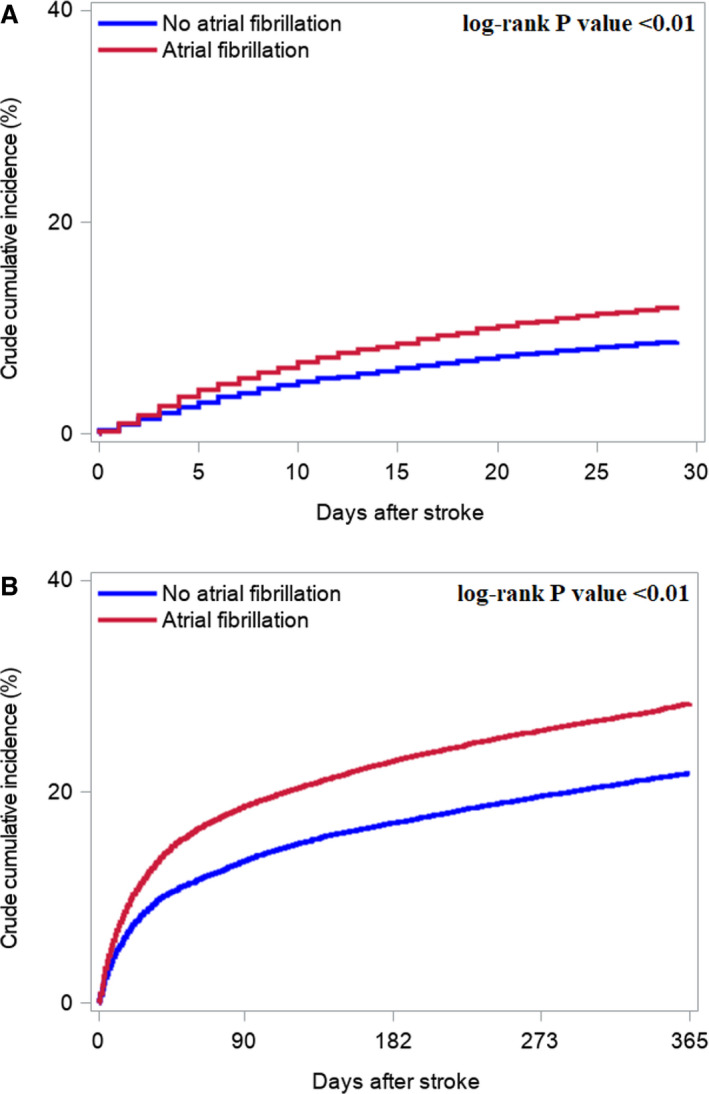
A, 30‐day mortality. B, 1‐year mortality.
Figure 4. Adjusted rates of mortality.
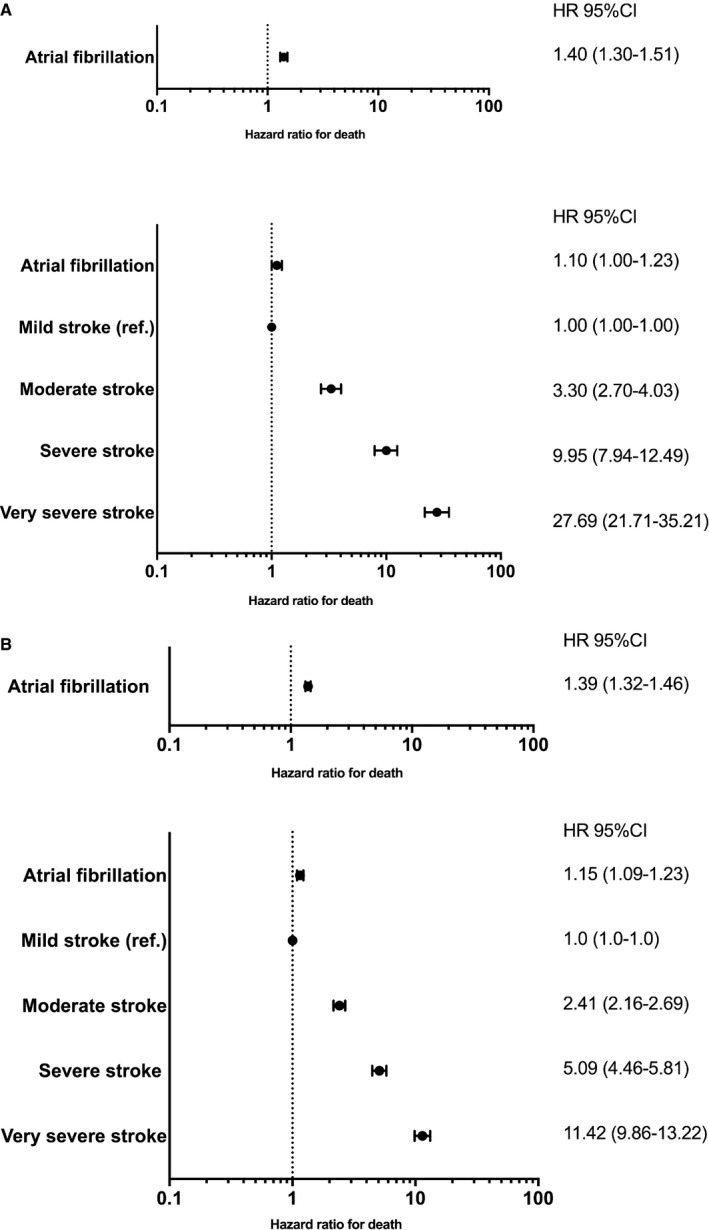
A, Adjusted rate of 30‐day mortality B, 1‐year mortality. All Cox models were adjusted for chronic obstructive lung disease, chronic kidney disease, liver disease, cancer, alcohol abuse, prior bleeding, dementia, and prior use of statins conditional on the matching. The lower forrest plots in Figure 4A and 4B depicts the models that are additionally adjusted for stroke severity. HR indicates hazard ratio; and ref, reference.
The 1‐year cumulative mortality incidence was higher among patients with AF (28.4%; 95% CI, 27.7%–29.1%) than among patients without AF (21.8%; 95% CI, 21.1%–22.4%) (P<0.01) (Figure 3B). In the adjusted analysis, AF was associated with a higher 1‐year mortality (HR, 1.39 [95% CI, 1.32–1.46]) than non‐AF (Figure 4B).
However, when stroke severity was included in the model, the rate was attenuated, indicating that mortality was mediated by stroke severity rather than AF (HR, 1.15 [95% CI, 1.09–1.23]) (Figure 4B). The change in magnitude of the association between AF and mortality was the same in the model for 30‐day mortality and 1‐year mortality when adjusting for stroke severity.
Sensitivity Analyses
By looking separately at patients with AF on OACs, it was found that the use of VKA was stable; however, patients with AF were gradually switched from antiplatelet therapy to DOACs from 2010 onward (Figure S2). Patients with AF on OACs were associated with a lower incidence of very severe stroke than patients with AF on antiplatelet therapy and no anticoagulation (Tables S3 and S4). In analysis stratified based on prior AF and new AF, patients with new AF had fewer comorbidities and lower median SSS than patients with prior AF (Table S5). However, the proportion of patients with very severe strokes was almost identical among patients with new AF and prior AF, not taking into account possible anticoagulation prophylaxis in patients with prior AF (Table S6). Absolute and relative risks of 30‐day and 1‐year mortality were higher for patients with prior AF compared with new AF (Figure S3).
In sex‐ and age‐stratified analysis, female patients were older than male patients overall. AF was associated with worse strokes in both sexes and across all age groups. A trend toward more very severe strokes was observed in women across age groups, and this trend seemed to be associated with sex and was not attenuated by age per se (Tables S7 through S10). AF was associated with higher 30‐day and 1‐year mortality across both sexes, and female patients had a higher risk of 30‐day and 1‐year mortality compared with male patients (Figure S4).
By analyzing the unmatched study cohort, we observed similar results as those found in the matched population (Table S11). In comparing patients with AF with patients without AF, 14.5% versus 5.4% had a very severe stroke, respectively (Figure S5). AF was associated with very severe stroke in unadjusted (OR, 2.96 [95% CI, 2.80–3.12]) as well as in the adjusted analysis (OR, 2.80 [95% CI, 2.65–2.96]). Patients with AF had higher in‐hospital mortality than patients without AF (11.9% versus 4.6%, P<0.01). The absolute 30‐day mortality and 1‐year mortality were higher for patients with AF than for patients without AF (30‐day mortality, 13.7% [95% CI, 13.2–14.2] versus 5.2% [95% CI, 5.0–5.3]; 1‐year mortality, 31.0% [95% CI, 30.3–31.7] versus 13.2% [95% CI, 12.9–13.4]) (Figure S6.1 and S6.2). AF was associated with a higher adjusted rate of 30‐day mortality than non‐AF; however, this association became nonsignificant when additionally adjusting for stroke severity (Figure S6.1.1). AF was associated with an adjusted rate of 1‐year mortality; however, this association was attenuated when stroke severity was added to the model (Figure S6.2.1). In analyses excluding all patients with missing variables in the SSS, the results were consistent with those of the main analyses.
DISCUSSION
We investigated the relationship between the presence of AF before or at admission for stroke and ischemic stroke severity and mortality in a nationwide cohort. The study yielded 2 main findings. (1) Patients with AF had more severe ischemic strokes than patients without AF. (2) Patients with AF had a higher rate of mortality after ischemic stroke than patients without AF; however, this AF‐related rate was primarily mediated by stroke severity rather than AF per se. These results were not confounded by differences in age, sex, and comorbidities between patients with AF and patients without AF, because these factors were controlled by matching.
Stroke Severity
In this study of ischemic stroke patients from 2005 to 2017, the proportion of patients with AF with very severe strokes was twice as high as that of patients without AF. This is in accordance with both prior imaging data 4 and pathological, 23 clinical, and observational 4 , 5 , 6 , 7 , 8 studies. However, the underlying pathophysiological mechanisms remain unclear. Worse stroke severity in patients with AF may be attributable to larger infarct size, greater likelihood of hemorrhagic transformation, AF‐related lower cardiac output, and less‐developed collateral circulation in the brain compared with chronic arterial atherosclerotic disease. 24
The absolute difference between the proportions of patients with severe strokes across studies is difficult to determine because of the use of heterogeneous stroke severity scales and different study setups. In this study, stroke severity was stable throughout the study period for both groups; however, more mild strokes were seen from 2010 onward. In the Copenhagen Stroke Study (1991–1993), 19% of the study population had a very severe stroke, compared with the average of our study population of ≈11%. 16 , 25 The Copenhagen Stroke Study was a single‐center study with 1197 patients, thus not directly comparable to our study. In an unmatched study cohort using the Danish Stroke Registry (2003–2007) including 3849 patients, Thygesen et al found a slightly higher proportion of patients with very severe stroke among both patients with AF and patients without AF than that in our study. 8 Overall, there seems to be a decrease in severe strokes in combination with an increase in mild strokes over the past 20 to 30 years in Denmark. This could be explained by the establishment of stroke units from 2008 to 2010 and improved quality and higher use of neuroimaging, hence the detection of more patients with mild stroke as well as improved stroke treatment. Also, higher use of anticoagulation therapy in patients with AF may have reduced stroke severity over time. 8 , 10 , 26 , 27 , 28 , 29 Better control of risk factors for stroke may also be the explanation; however, as life expectancy is increasing, the time to accumulate these risk factors is also increasing. Nevertheless, patients with AF still suffer from more severe strokes than patients without AF.
Our baseline data demonstrated that ≈40% of patients with AF (63% of patients with new AF and 26% of patients with prior AF) were not receiving any antithrombotic therapy, and a sensitivity analysis showed a decrease in the probability of severe strokes for patients with AF treated with either antiplatelet therapy or OACs. However, as hemorrhagic stroke and parameters such as compliance were not included in the analysis, the beneficial effectiveness of antithrombotic therapy cannot be fully elucidated in this study.
Mortality
Patients with AF more frequently have other heart diseases, 30 and studies have found that cardiovascular death in the acute phase of a stroke is more frequent in patients with AF than in patients without AF. 31 , 32 We found that compared with patients without AF, patients with AF had a higher in‐hospital mortality (10.8% versus 7.5%; unmatched population, 11.9% versus 4.6%), a higher absolute rate of 30‐day mortality (12.1% versus 8.7%; unmatched population, 13.7% versus 5.2%), and a higher 1‐year mortality rate (28.4% versus 21.8%; unmatched population, 31.0% versus 13.2%). Other studies have found in‐hospital mortality rates of 25% to 33% in patients with AF and 14% to 17% in patients without AF. 5 , 25 Thygesen et al found an absolute incidence of 30‐day mortality of 14.7% in patients with AF and 5.8% in patients without AF. For 1‐year mortality, the absolute incidence of mortality was 31.7% in patients with AF and 13.7% in patients without AF. 8 Other studies have found a 30‐day mortality ranging from 11.3% to 25.0% in patients with AF and from 3.4% to 14.0% in patients without AF. 3 , 6 , 7 Hence, prior studies have reported varying estimates of mortality across different study setups. We present results from a nationwide and valid in‐hospital stroke registry with the possibility of long‐term follow‐up.
It was observed in this study that stroke severity seems to mediate the relationship between AF and mortality. By adding stroke severity to the adjusted models, we observed that AF was no longer associated with a higher rate of 30‐day mortality than non‐AF, and the rate of 1‐year mortality became borderline significant. Most studies include stroke severity in the adjusted mortality analyses; however, some argue that it is questionable whether prestroke factors such as comorbidities and acute phase factors such as severity should be in the same model, because they reflect different phases in disease development. In line with our results, other studies found that the higher rate of mortality related to AF was neutralized after adjustment for stroke severity. 5 , 7 , 8 , 9 , 25 , 33 , 34 However, Kimura et al found that AF was still associated with higher odds of early mortality after adjustment for National Institutes of Health Stroke Scale with an OR of 1.27 (95% CI, 1.06–1.51). 6 Prediction models for mortality in patients with ischemic stroke may be useful in risk stratification in clinical care and rehabilitation. Smith et al used Get With The Guidelines–Stroke Program data to derive and validate a prediction model for a patient’s risk of in‐hospital ischemic stroke mortality and demonstrated higher C statistics when adding stroke severity (National Institutes of Health Stroke Scale) to the model. 33 However, they also found AF to be a predictor for in‐hospital mortality together with age, male sex, mode of arrival, previous myocardial infarction, and dyslipidemia.
We present contemporary data in a time period where stroke care and prevention have improved dramatically, and where better prevention mainly has been attributed to better hypertension, diabetes, and dyslipidemia control and smoking cessation. 10 This study suggests that screening for undiagnosed AF may be beneficial to reduce the risk of stroke by oral anticoagulation therapy.
There still seems to be an underuse of OACs. 1 We found an increase in use of DOACs from 2011 onward and a simultaneous decrease in use of antiplatelet therapy. VKA use was stable throughout the study period. In comparison, Gadsbøll et al found in an AF population free of stroke an increase in DOAC initiation from 2011 onward and a simultaneous decrease in VKA initiation.27 In patients treated with OACs, there might be unknown issues with compliance or bleeding complications, 35 reluctance to switch from a VKA to a DOAC when the time in the therapeutic international normalized ratio range is low, 36 or problems choosing a DOAC dose. 37 A multidisciplinary approach to managing patients with AF is necessary, because the future detection of AF, preventive OAC therapy, and more focus on comorbidities in patients with AF must be improved.
Limitations
The retrospective nature of the study is a limitation, and the Danish Stroke Registry was established as a quality improvement initiative. Some patients had missing variables, because data were collected during everyday clinical work. However, a structured audit process is executed every year to assess and maintain the quality of the database. 14 In total, 9.1% of the study population had an unspecified stroke, and based on Krarup et al, around one‐third of these may be hemorrhagic strokes. 21
Some patients may have died before arriving to the hospital and therefore absent in the registry; hence, the actual mortality may be higher than reported.
We do not know if the patients with AF suffered from a cardioembolic stroke, because we did not have data on stroke subtypes (ie, TOAST [Trial of ORG 10172 in Acute Stroke Treatment] classification). Additionally, we did not investigate interaction between thrombolysis or endovascular treatment and patients with and without AF that could affect mortality, which is examined by Hu et al and Smaal et al. 38 , 39
AF diagnosed after discharge was not included in the AF group, knowing that some might have had unknown AF status during hospitalization. Additionally, we cannot exclude reverse causality, because some studies have suggested that a stroke can trigger AF, 40 and we do not know if only severe strokes trigger AF. However, this is only relevant for patients with AF diagnosed during hospitalization. We did not have access to data on blood pressure, and imaging data were incomplete in the registry.
CONCLUSIONS
In a nationwide contemporary cohort of patients suffering ischemic stroke in the period 2005 to 2016, AF was associated with worse stroke severity than stroke without AF. AF was associated with a higher rate of mortality, which was primarily driven by worse stroke severity and not AF itself. This study underlines that stroke prevention with oral anticoagulation therapy and AF detection are still important in the modern era, and screening for AF in high‐risk patients may be beneficial. A window of opportunity exists for improving outcomes in this setting.
Sources of Funding
This work was supported by an internal grant from the Department of Cardiology, Copenhagen University Hospital, Denmark. The funding source did not have influence on any part of the submitted work including the study design; in the collection, analysis, and interpretation of data; in the writing of the article; and in the decision to submit the article for publication.
Disclosures
Dr Olesen receives speaker fees from Bristol‐Myers Squibb, Boehringer Ingelheim, Bayer, AstraZeneca, serves as a consultant for Bristol‐Myers Squibb, Boehringer Ingelheim, Novartis Healthcare, and Novo Nordisk, and receives funding for research from Bristol‐Myers Squibb and the Capital Region of Denmark (Foundation for Health Research). Dr Torp‐Pedersen has a research contract with Bayer and Novo Nordisk. Dr Gislason receives research grants from Boehringer Ingelheim, Bristol‐Myers Squibb, and Pfizer. Dr Køber serves as speaker for AstraZeneca and Novartis and reports no conflicts of interest to the presented article. Dr Kruuse serves as speaker for Novo Nordisk, Bristol‐Myers Squibb. Dr Johnsen serves as speaker for Bayer, Bristol‐Myers Squibb, and Pfizer, and as a consultant for Bayer, Bristol‐Myers Squibb, Sanofi, and Pfizer, and receives research grants from Bristol‐Myers Squibb and Pfizer. The remaining authors have no disclosures.
Supporting information
Acknowledgments
The authors thank the National Quality Improvement Program in Denmark for making it possible to work with the Danish Stroke Registry.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022638
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 2. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. doi: 10.1016/S1047-9651(18)30169-4 [DOI] [PubMed] [Google Scholar]
- 3. Lin HJ, Wolf PA, Kelly‐Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB. Stroke severity in atrial fibrillation: the Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.STR.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 4. Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology. 2003;22:118–123. doi: 10.1159/000068743 [DOI] [PubMed] [Google Scholar]
- 5. Steger C, Pratter A, Martinek‐Bregel M, Avanzini M, Valentin A, Slany J, Stöllberger C. Stroke patients with atrial fibrillation have a worse prognosis than patients without: data from the Austrian Stroke registry. Eur Heart J. 2004;25:1734–1740. doi: 10.1016/j.ehj.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 6. Kimura K, Minematsu K, Yamaguchi T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15 831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:679–683. doi: 10.1136/jnnp.2004.048827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, Spolveri S, Baruffi MC, Landini G, Ghetti A, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital‐based registry (The European Community Stroke Project). Stroke. 2001;32:392–398. doi: 10.1161/01.STR.32.2.392 [DOI] [PubMed] [Google Scholar]
- 8. Thygesen SK, Frost L, Eagle KA, Johnsen SP. Atrial fibrillation in patients with ischemic stroke: a population‐based study. Clin Epidemiol. 2009;1:55–65. doi: 10.2147/CLEP.S4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGrath ER, Kapral KK, Fang J, Eikelboom JW, O’Conghaile A, Canavan M, O’Donnell M. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology. 2013;81:825–832. doi: 10.1212/WNL.0b013e3182a2cc15 [DOI] [PubMed] [Google Scholar]
- 10. Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 12. Pottegård A, Schmidt SJA, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: The Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Toft SH. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnsen SP, Ingeman A, Hundborg HH, Schaarup SZ, Gyllenborg J. The Danish Stroke Registry. Clin Epidemiol. 2016;8:697–702. doi: 10.2147/CLEP.S103662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wildenschild C, Mehnert F, Thomsen RW, Iversen HK, Vestergaard K, Ingeman A, Johnsen SP. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2013;6:27–36. doi: 10.2147/CLEP.S50449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jørgensen HS, Nakayama H, Raaschou HO, Vive‐Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 17. Askim T, Bernhardt J, Churilov L, Indredavik B. The Scandinavian Stroke Scale is equally as good as the National Institutes of Health Stroke Scale in identifying 3‐month outcome. J Rehabil Med. 2016;48:909–912. doi: 10.2340/16501977-2155 [DOI] [PubMed] [Google Scholar]
- 18. Lindenstrøm E, Boysen G, Waage Christiansen L, á Rogvi Hansen B, Würtzen Nielsen P. Reliability of Scandinavian Neurological Stroke Scale. Cerebrovasc Dis. 1991;1:103–107. doi: 10.1159/000108825 [DOI] [Google Scholar]
- 19. Barber M, Fail M, Shields M, Stott DJ, Langhorne P. Validity and reliability of estimating the Scandinavian Stroke Scale score from medical records. Cerebrovasc Dis. 2004;17:224–227. doi: 10.1159/000075795 [DOI] [PubMed] [Google Scholar]
- 20. Scandinavian Stroke Study Group . Multicenter trial of hemodilution in ischemic stroke background and study protocol. Stroke. 1985;16:885–890. doi: 10.1161/01.str.16.5.885 [DOI] [PubMed] [Google Scholar]
- 21. Krarup L‐H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. doi: 10.1159/000102143 [DOI] [PubMed] [Google Scholar]
- 22. Frost L, Andersen LV, Vestergaard P, Husted S, Mortensen LS. Trend in mortality after stroke with atrial fibrillation. Am J Med. 2007;120:47–53. doi: 10.1016/j.amjmed.2005.12.027 [DOI] [PubMed] [Google Scholar]
- 23. Yamanouchi H, Tomonaga M, Shimada H, Matsushita S, Kuramoto K, Toyokura Y. Nonvalvular atrial fibrillation as a cause of fatal massive cerebral infarction in the elderly. Stroke. 1989;20:1653–1656. doi: 10.1161/01.STR.20.12.1653 [DOI] [PubMed] [Google Scholar]
- 24. Tu HTH, Campbell BCV, Christensen S, Collins M, De Silva DA, Butcher KS, Parsons MW, Desmond PM, Barber PA, Levi CR, et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc Dis. 2010;30:389–395. doi: 10.1159/000316886 [DOI] [PubMed] [Google Scholar]
- 25. Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27:1765–1769. doi: 10.1161/01.STR.27.10.1765 [DOI] [PubMed] [Google Scholar]
- 26. Fosbøl EL, Vinding NE, Lamberts M, Staerk L, Gundlund A, Gadsbøll K, Koeber L, Gislason GH, Olesen JB. Shifting to a non‐vitamin K antagonist oral anticoagulation agent from vitamin K antagonist in atrial fibrillation. Europace. 2018;20:e78–e86. doi: 10.1093/europace/eux193 [DOI] [PubMed] [Google Scholar]
- 27. Gadsbøll K, Staerk L, Fosbøl EL, Sindet‐Pedersen C, Gundlund A, Lip GYH, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38:899–906. doi: 10.1093/eurheartj/ehw658 [DOI] [PubMed] [Google Scholar]
- 28. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–1420. doi: 10.1093/europace/euq350 [DOI] [PubMed] [Google Scholar]
- 29. Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- 30. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham Study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703 [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson C, Britton M. Pathogenetic mechanism of stroke in non‐valvular atrial fibrillation: follow‐up of stroke patients with and without atrial fibrillation. J Intern Med. 1991;230:11–16. doi: 10.1111/j.1365-2796.1991.tb00400.x [DOI] [PubMed] [Google Scholar]
- 32. Kaarisalo MM, Immonen‐Räihä P, Marttila RJ, Salomaa V, Kaarsalo E, Salmi K, Sarti C, Sivenius J, Torppa J, Tuomilehto J. Atrial fibrillation and stroke. Mortality and causes of death after the first acute ischemic stroke. Stroke. 1997;28:311–315. doi: 10.1161/01.STR.28.2.311 [DOI] [PubMed] [Google Scholar]
- 33. Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, Hernandez AF, Peterson ED, Fonarow GC, Schwamm LH. Risk score for in‐hospital ischemic stroke mortality derived and validated within the Get With the Guidelines‐Stroke Program. Circulation. 2010;122:1496–1504. doi: 10.1161/CIRCULATIONAHA.109.932822 [DOI] [PubMed] [Google Scholar]
- 34. Appelros P, Nydevik I, Seiger A, Terént A. Predictors of severe stroke: Influence of preexisting dementia and cardiac disorders. Stroke. 2002;33:2357–2362. doi: 10.1161/01.STR.0000030318.99727.FA [DOI] [PubMed] [Google Scholar]
- 35. Grundtvig J, Ovesen C, Havsteen I, Christensen T, Gaist D, Iversen HK, Kruuse C, Lilja‐Cyron A, Ægidius K, Rosenbaum S, et al. Trends in incidence of oral anticoagulant‐related intracerebral hemorrhage and sales of oral anticoagulants in Capital Region of Denmark 2010–2017. Eur Stroke J. 2021;6:143–150. doi: 10.1177/23969873211008770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vinding NE, Bonde AN, Rørth R, Lamberts M, Olesen JB, Gislason GG, Torp‐Pedersen C, Køber L, Fosbøl EL. The importance of time in therapeutic range in switching from vitamin K antagonist to non‐vitamin K antagonist oral anticoagulants in atrial fibrillation. Europace. 2019;21:572–580. doi: 10.1093/europace/euy262 [DOI] [PubMed] [Google Scholar]
- 37. Vinding NE, Staerk L, Gislason GH, Torp‐Pedersen C, Bonde AN, Rørth R, Lee CJ, Olesen JB, Køber L, Fosbøl EL. Switching from vitamin K antagonist to dabigatran in atrial fibrillation: differences according to dose. Eur Heart J Cardiovasc Pharmacother. 2021;7:20–30. 10.1093/ehjcvp/pvz066 [DOI] [PubMed] [Google Scholar]
- 38. Hu Y, Chunmei JI. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: a meta‐analysis. BMC Neurol. 2021;21:66–. doi: 10.1186/s12883-021-02095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smaal JA, de Ridder IR, Heshmatollah A, van Zwam WH, Dippel D, Majoie CB, Brown S, Goyal M, Campbell B, Muir KW, et al. Effect of atrial fibrillation on endovascular thrombectomy for acute ischemic stroke. A meta‐analysis of individual patient data from six randomised trials: results from the HERMES collaboration. Eur Stroke J. 2020;5:245–251. doi: 10.1177/2396987320923447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seifert F, Kallmünzer B, Gutjahr I, Breuer L, Winder K, Kaschka I, Kloska S, Doerfler A, Hilz M‐J, Schwab S, et al. Neuroanatomical correlates of severe cardiac arrhythmias in acute ischemic stroke. J Neurol. 2015;262:1182–1190. doi: 10.1007/s00415-015-7684-9 [DOI] [PubMed] [Google Scholar]
- 41. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen A‐M S, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


