Abstract
Background
Studies have demonstrated increased risk of major atherothrombotic events in CYP2C19 loss‐of‐function (LOF) variant carriers versus non‐carriers treated with clopidogrel after percutaneous coronary intervention (PCI). We sought to evaluate real‐world outcomes with the clinical implementation of CYP2C19‐guided antiplatelet therapy after PCI.
Methods and Results
Data from 9 medical centers where genotyping was performed in the setting of PCI were included. Alternative therapy with prasugrel or ticagrelor was recommended for patients with a CYP2C19 LOF variant. The primary outcome was the composite of major atherothrombotic events (all‐cause death, myocardial infarction, ischemic stroke, stent thrombosis, or hospitalization for unstable angina) within 12 months following PCI. Moderate or severe/life‐threatening bleeding within 12 months was a secondary outcome. Among 3342 patients, 1032 (31%) were LOF carriers, of whom 571/1032 (55%) were treated with alternative therapy. In LOF carriers, the rate of major atherothrombotic events was lower in patients treated with alternative therapy versus clopidogrel (adjusted HR, 0.56; 95% CI 0.39–0.82). In those without a LOF allele, no difference was observed (adjusted HR, 1.07; 95% CI 0.71–1.60). There was no difference in bleeding with alternative therapy versus clopidogrel in either LOF carriers or those without a LOF allele.
Conclusions
Real‐world data demonstrate lower atherothrombotic risk in CYP2C19 LOF carriers treated with alternative therapy versus clopidogrel and similar risk in those without a LOF allele treated with clopidogrel or alternative therapy. These data suggest that PCI patients treated with clopidogrel should undergo genotyping so that CYP2C19 LOF carriers can be identified and treated with alternative therapy.
Keywords: clopidogrel, CYP2C19, pharmacogenetics
Subject Categories: Precision Medicine
Nonstandard Abbreviations and Acronyms
- CYP2C19
cytochrome P450 2C19
- LOF
loss‐of‐function
- TAILOR‐PCI
Tailored Antiplatelet Initiation to Lessen Outcomes due to Decreased Clopidogrel Response After Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
Results in a real‐world setting expand the generalizability of recent randomized trials and suggest patients treated with clopidogrel after percutaneous coronary intervention should be genotyped to ensure the absence of CYP2C19 LOF variants.
Although alternative P2Y12 inhibitors are preferred over clopidogrel in the setting of acute coronary syndrome, many patients remain poor candidates for alternative therapy.
What Are the Clinical Implications?
Implementation of CYP2C19 genotyping can help guide which patients derive the greatest clinical benefit from alternative agents and those in whom clopidogrel may be effective and safe.
Dual antiplatelet therapy with clopidogrel, prasugrel, or ticagrelor in combination with aspirin is recommended to reduce the risk of major adverse cardiovascular events (MACE) after percutaneous coronary intervention (PCI). 1 Clopidogrel is a prodrug dependent on the cytochrome P450 (CYP) 2C19 enzyme for bioactivation to its active metabolite, which inhibits platelet activation. Approximately 30% of individuals carry a loss‐of‐function (LOF; defined as the combination of no function and decreased function alleles 2 ) variant in CYP2C19 that results in reduced or absent enzyme activity, and diminished antiplatelet effects in clopidogrel‐treated patients. 3 As a consequence, clopidogrel is less effective at preventing adverse cardiovascular events after PCI in LOF allele carriers compared to non‐carriers, 4 whereas neither prasugrel nor ticagrelor are affected by CYP2C19 genotype. 5 , 6 , 7
Two randomized controlled trials recently examined outcomes after PCI with CYP2C19 genotype‐guided P2Y12 inhibitor therapy (ie, alternative therapy prescribed to LOF allele carriers and clopidogrel prescribed to those without a LOF allele). 8 , 9 The POPular‐Genetics trial found genotype‐guided treatment to be non‐inferior to universal use of ticagrelor or prasugrel with regard to net adverse atherothrombotic or major bleeding events while also significantly reducing the risk of bleeding events requiring clinical intervention. 8 In the tailored antiplatelet initiation to lessen outcomes due to decreased clopidogrel response after percutaneous coronary intervention (TAILOR‐PCI) trial, the reduction in major atherothrombotic events with use of alternative therapy versus clopidogrel in LOF allele carriers did not reach statistical significance (P=0.06), although there was a significant reduction in the prespecified analysis of cumulative major atherothrombotic events over 12 months and in the post hoc analysis of events at 90 days. 9 A recent meta‐analysis that included 15 949 genotyped patients from 7 randomized trials, including TAILOR‐PCI, reported that treatment with prasugrel or ticagrelor reduced major atherothrombotic events compared to clopidogrel in CYP2C19 LOF allele carriers (relative risk [RR], 0.70; 95% CI 0.59–0.83). 10 In contrast, no difference was observed in patients without a LOF allele (RR, 1.0; 95% CI 0.80–1.25) and a significant genotype*treatment interaction was reported (P=0.013), suggesting that the benefits conferred by using prasugrel or ticagrelor over clopidogrel was primarily evident in LOF allele carriers.
Multiple health care institutions offer clinical CYP2C19 genotyping to assist with antiplatelet selection after PCI. 11 , 12 On behalf of the NHGRI Implementing Genomics in Practice (IGNITE) Network Pharmacogenetics Working Group, we conducted an initial observational examination of outcomes in 1815 patients from 7 US institutions who received clinical CYP2C19 genotyping at the time of PCI. We reported a lower rate of atherothrombotic events among LOF allele carriers who were treated with prasugrel or ticagrelor versus clopidogrel. 13 We have continued to follow the original patients up to a year and expanded our cohort to 2 additional sites, and 1527 additional patients. Herein, we report outcomes with CYP2C19‐guided antiplatelet therapy in our expanded study population of patients who underwent PCI for either an acute coronary syndrome (ACS) or chronic coronary syndrome indication and received clinical CYP2C19 genotyping.
METHODS
Data and analytic codes that support findings from this manuscript are available from the authors upon reasonable request.
Study Population
Nine institutions (University of Florida, Gainesville; University of Florida, Jacksonville; University of North Carolina, Chapel Hill; University of Maryland, Baltimore; University of Alabama, Birmingham; University of Pennsylvania; University of Pittsburgh; University of Illinois, Chicago; and Indiana University) contributed data for 3342 patients. All patients were ≥18 years of age, underwent PCI for an ACS (n=2290) or a chronic coronary syndrome (n=1052) indication, were genotyped clinically for CYP2C19, and received a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor). Patients were included regardless of length of follow‐up.
The processes for performing genotype‐guided therapy across our sites have been previously described. 11 Data collection procedures were approved by the Institutional Review Board at each institution. Depending on the site, subjects gave informed consent or the requirement was waived. Briefly, genotyping was performed in a Clinical Laboratory Improvement Amendments (CLIA)‐licensed laboratory at each institution, with results returned via the electronic health record (EHR). All sites genotyped for the CYP2C19*2 and *3 LOF alleles, with additional rare LOF alleles detected at 5 of the 9 sites (Table S1). The *17 (gain‐of‐function) allele was also detected at all sites. The poor metabolizer (PM, 2 LOF alleles) and intermediate metabolizer (IM, one LOF allele) phenotypes were assigned in accordance with CPIC guidelines. 3 Alternative antiplatelet therapy with prasugrel or ticagrelor was recommended at each site for LOF carriers in the absence of contraindications, whereas no recommendations were made for those without a LOF allele. No treatment alterations were recommended based on the presence of a *17 allele according to Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. The ultimate prescribing decision was left to the clinician’s discretion.
Data Abstraction
Data were manually abstracted from the EHRs at each site using a common data collection form as previously described 13 but with slight modifications for this study. Data were collected through review of patient encounters, beginning with the hospitalization for the index PCI, defined as the PCI performed in association with CYP2C19 genotyping, and including subsequent outpatient encounters and hospitalizations. We followed patients until the outcome of interest, discontinuation of any P2Y12 inhibitor, up to 12 months post‐PCI, whichever was the earliest.
Outcomes were identified from provider‐reported diagnoses at each encounter or clinical notes in the event of death. One site also called patients to assess for the occurrence of events. Type of antiplatelet therapy was assigned to each patient at the time of each event or at the time of last follow‐up in which treatment with a P2Y12 inhibitor was documented.
Study End Points
The primary outcome was the composite of major atherothrombotic events, defined as the composite of all‐cause death or first occurrence of myocardial infarction, ischemic stroke, stent thrombosis, or hospitalization for unstable angina within 12 months following the index PCI, consistent with the TAILOR‐PCI trial. 9 Secondary outcomes were major atherothrombotic events within 90 days of the index PCI (consistent with the TAILOR‐PCI trial 9 ) and clinically significant bleeding events, defined as Global Use of Strategies to Open Occluded Arteries (GUSTO) moderate (requiring blood transfusion but not resulting in hemodynamic compromise) or severe/life‐threatening (intracerebral hemorrhage or bleeding resulting in hemodynamic compromise requiring treatment) 14 within 12 months of the index PCI.
Statistical Analysis
Data were curated and aggregated at the University of Florida, Gainesville. The primary analysis compared rates between LOF allele carriers treated with alternative therapy (LOF‐alternative group) versus clopidogrel (LOF‐clopidogrel group) and between patients without a LOF allele on clopidogrel (non‐LOF‐clopidogrel group) versus alternative therapy (non‐LOF‐alternative group). The interaction between LOF allele carrier status and P2Y12 inhibitor therapy at event or last follow‐up was also assessed. Pre‐specified subgroup analyses were completed in patients with an ACS indication (ST‐segment elevation or non‐ST‐segment elevation myocardial infarction or unstable angina) for PCI versus those with a non‐ACS/elective indication for PCI.
To adjust for differences between groups, we fit logistic regression models to estimate the probability (propensity score) of receiving clopidogrel versus alternative therapy conditional on baseline characteristics (socio‐demographics, BMI, smoking status, stent indication, stent type, medical history, and discharge medications). Propensity scores, estimated separately for each comparison (ie, LOF carriers, non‐LOF carriers and ACS‐specific strata), were then used to generate stabilized inverse probability of treatment weights (SIPTWs). 15 Non‐LOF propensity scores were further trimmed to remove non‐overlap between treatment groups. We evaluated differences in all covariates between treatment groups before and after weighting using appropriate test for univariate comparisons. We then used Cox proportional hazard models of the weighted comparison groups to estimate hazard ratios for atherothrombotic and bleeding events for LOF carriers and the non‐LOF groups. We also present Kaplan Meier Plots using the SIPTW weighted comparison groups. All statistical analyses were performed using R statistical software (version 4.0). 16
RESULTS
Patient Characteristics
Patient characteristics are shown in Table 1. Among the 3342 patients, the mean age was 63 years, 32% were female, 20% were of African ancestry, and 69% had an ACS indication for PCI. A total of 1032 (31%) patients were LOF allele carriers (28% IMs, 3% PMs), of whom 571 (55%) were treated with alternative therapy and 461 (45%) with clopidogrel 75 mg/day (Figure 1); 77% of PMs and 53% of IMs received alternative therapy (Figure S1). Among patients in the LOF group treated with alternative therapy, 337 received prasugrel, 219 received ticagrelor, and 15 received high‐dose clopidogrel (Figure 1). Among patients in the non‐LOF group (n=2310, 69%), 378 (16%) were treated with alternative therapy and 1932 (84%) with standard‐dose clopidogrel.
Table 1.
Patient Characteristics at the Time of Index PCI
| Characteristic | All patients (n=3342) | LOF‐alternative (n=571) | LOF‐clopidogrel (n=461) | Standardized differences for comparison of LOF‐alternative vs LOF‐clopidogrel | Non‐LOF‐alternative (n=378) | Non‐LOF‐clopidogrel (n=1932) | Standardized differences for comparison of non‐LOF alternative vs non‐LOF‐clopidogrel |
|---|---|---|---|---|---|---|---|
| Age, y | 63±12 | 60±11 | 64±12 | 0.336 | 60±10 | 64±12 | 0.349 |
| Female sex | 1075 (32) | 158 (28) | 161 (35) | 0.157 | 99 (26) | 657 (34) | 0.171 |
| Race | |||||||
| White | 2448 (73) | 413 (72) | 315 (68) | 0.088 | 287 (76) | 1433 (74) | 0.08 |
| Black | 659 (20) | 117 (21) | 109 (24) | 62 (16) | 371 (19) | ||
| Other † | 235 (7) | 41 (7) | 37 (8) | 29 (8) | 128 (7) | ||
| BMI, kg/m2 | 30±6 | 30±7 | 30±7 | 0.054 | 31±6 | 30±6 | 0.14 |
| Current smoker | 969 (29) | 178 (31) | 138 (30) | 0.027 | 114 (30) | 539 (28) | 0.05 |
| PCI indication | |||||||
| ACS | 2290 (69) | 412 (72) | 306 (66) | 0.125* | 290 (77) | 1282 (66) | 0.231* |
| STEMI | 628 (19) | 148 (26) | 53 (12) | 106 (28) | 321 (17) | ||
| NSTEMI | 939 (28) | 161 (28) | 134 (29) | 117 (31) | 527 (27) | ||
| Unstable angina | 723 (22) | 103 (18) | 119 (26) | 67 (18) | 434 (23) | ||
| Stable CAD | 936 (28) | 143 (25) | 137 (30) | 80 (21) | 576 (30) | ||
| Other/unknown | 116 (4) | 16 (3) | 18 (4) | 8 (2) | 74 (4) | ||
| PCI type | |||||||
| Drug‐eluting stent | 2868 (86) | 485 (85) | 388 (84) | 0.021 | 348 (92) | 1647 (85) | 0.216 |
| Bare metal stent | 371 (11) | 65 (11) | 48 (10) | 0.031 | 25 (7) | 233 (12) | 0.188 |
| PTCA | 85 (3) | 16 (3) | 20 (4) | 0.083 | 4 (1) | 45 (2) | 0.099 |
| Medical history | |||||||
| Diabetes | 1354 (41) | 190 (33) | 206 (45) | 0.236 | 130 (34) | 828 (43) | 0.175 |
| Hypertension | 2704 (81) | 428 (75) | 389 (84) | 0.236 | 286 (76) | 1601 (83) | 0.178 |
| Dyslipidemia | 2317 (69) | 394 (69) | 329 (71) | 0.052 | 244 (65) | 1350 (70) | 0.114 |
| CKD | 963 (29) | 161 (28) | 165 (36) | 0.163 | 59 (16) | 578 (30) | 0.346 |
| MI | 866 (26) | 142 (25) | 136 (30) | 0.104 | 97 (26) | 491 (25) | 0.006 |
| Coronary stent | 770 (23) | 113 (20) | 117 (25) | 0.134 | 83 (22) | 457 (24) | 0.04 |
| Stroke/TIA | 348 (10) | 41 (7) | 70 (15) | 0.256 | 16 (4) | 221 (11) | 0.271 |
| PVD | 335 (10) | 49 (9) | 66 (14) | 0.181 | 19 (5) | 201 (10) | 0.203 |
| Heart failure | 533 (16) | 78 (14) | 89 (19) | 0.153 | 38 (10) | 328 (17) | 0.204 |
| Atrial fibrillation | 316 (10) | 40 (7) | 55 (12) | 0.169 | 15 (4) | 206 (11) | 0.259 |
| Gastrointestinal or intracranial hemorrhage | 111 (3) | 20 (4) | 26 (6) | 0.102 | 7 (2) | 58 (3) | 0.075 |
| Cancer | 184 (6) | 26 (5) | 22 (5) | 0.01 | 14 (4) | 122 (6) | 0.12 |
| Discharge medication | |||||||
| Aspirin | 3269 (98) | 563 (99) | 449 (97) | 0.086 | 371 (98) | 1886 (98) | 0.037 |
| Statin | 3143 (94) | 548 (96) | 434 (94) | 0.084 | 365 (97) | 1796 (93) | 0.162 |
| ACE inhibitor or ARB | 2245 (67) | 400 (70) | 306 (66) | 0.079 | 251 (66) | 1288 (67) | 0.006 |
| β‐blocker | 2852 (85) | 483 (85) | 378 (82) | 0.07 | 325 (86) | 1666 (86) | 0.007 |
| Anticoagulant | 321 (10) | 36 (6) | 55 (12) | 0.196 | 21 (6) | 209 (11) | 0.193 |
Data are presented as number (%) or mean±SD. LOF‐clopidogrel patients were those with at least 1 loss‐of‐function allele (ie, *2, *3…) treated with clopidogrel. LOF‐alternative patients were those with at least 1 loss‐of‐function allele (ie, *2, *3…) treated with prasugrel, ticagrelor, or high dose clopidogrel. Non‐LOF patients were those with no loss‐of‐function allele: *1/*1, *1/*17, or *17/*17 genotype. ACE indicates angiotensin‐converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease defined as an estimated glomerular filtration rate of <60 mL/min per 1.73 m2; MI, myocardial infarction; LOF, loss‐of‐function; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; and TIA, transient ischemic attack.
Standardized difference for comparison of primary PCI indication categories (ie, ACS vs stable CAD or other) between groups.
Indicates patients who self‐identify as American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or more than one race, and patients with unknown race in the electronic health record.
Figure 1. Study population by CYP2C19 group and antiplatelet therapy.
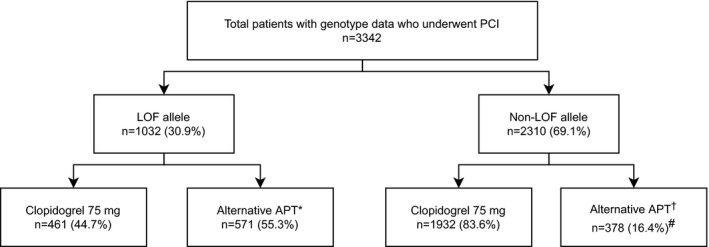
A total of 22/461 (5%) of patients in the LOF‐clopidogrel group were CYP2C19 PMs. A total of 76/571 (13%) of patients in the LOF‐alternative group were CYP2C19 PMs. APT indicates antiplatelet therapy; LOF, loss‐of‐functio; and PCI, percutaneous coronary intervention. *Alternative therapy in patients with 1 or 2 LOF alleles consisted of prasugrel (n=337), ticagrelor (n=219), or high‐dose clopidogrel (150 mg/day, n=4; 225 mg/day, n=11). †Alternative therapy in the non‐LOF group consisted of prasugrel (n=231), ticagrelor (n=146), or high‐dose clopidogrel (150 mg/day, n=1). #P<0.001 for use of alternative therapy in the non‐LOF group compared with the LOF group.
There were differences in age, sex, medical history, and anticoagulant use between LOF allele carriers prescribed alternative therapy versus clopidogrel (Table 1), pointing to an older population with more comorbidities receiving clopidogrel. These characteristics along with body mass index, stent type, and statin use also differed between the non‐LOF‐alternative and non‐LOF‐clopidogrel groups with a similar trend toward older patients with more comorbidities receiving clopidogrel. These imbalances were negligible after propensity score adjustment. All standardized differences were <10% in LOF allele carriers, suggesting adequate covariate balance between groups (Table S2). Among patients in the non‐LOF group, the standardized difference between treatments for history of gastrointestinal or intracranial hemorrhage was 10.5%. Adjusting for this variable in the Cox proportional hazard model of the weighted comparison groups did not affect results (Table 2).
Table 2.
Primary and Secondary Outcomes by CYP2C19 and Antiplatelet Therapy
| Clinical outcome by CYP2C19‐P2Y12 inhibitor | Event no.* | Event rate (per 100 patient years) † | Unadjusted HR (95% CI) | P value | Adjusted HR ‡ (95% CI) | P value |
|---|---|---|---|---|---|---|
| Primary outcome | ||||||
| Major atherothrombotic events | ||||||
| LOF‐clopidogrel | 71 | 34.4 | Reference | Reference | ||
| LOF‐alternative | 51 | 17.1 | 0.50 (0.35–0.72) | <0.001 | 0.56 (0.39–0.82) | 0.002 |
| Non‐LOF‐clopidogrel | 197 | 19.9 | Reference | Reference | ||
| Non‐LOF‐alternative | 34 | 18.1 | 0.91 (0.63–1.30) | 0.591 | 1.08 (0.72–1.62) § | 0.715 |
| Secondary outcomes | ||||||
| Major atherothrombotic events at 90 d | ||||||
| LOF‐clopidogrel | 40 | 52.5 | Reference | Reference | ||
| LOF‐alternative | 20 | 18.9 | 0.37 (0.22–0.63) | <0.001 | 0.40 (0.23–0.71) | 0.002 |
| Non‐LOF‐clopidogrel | 104 | 30.8 | Reference | Reference | ||
| Non‐LOF‐alternative | 18 | 26.1 | 0.84 (0.51–1.39) | 0.502 | 1.09 (0.64–1.86) | 0.752 |
| Clinically significant bleeding events | ||||||
| LOF‐clopidogrel | 15 | 7.1 | Reference | Reference | ||
| LOF‐alternative | 25 | 7.9 | 1.13 (0.60–2.15) | 0.705 | 1.15 (0.60–2.20) | 0.685 |
| Non‐LOF‐clopidogrel | 69 | 6.8 | Reference | Reference | ||
| Non‐LOF‐alternative | 15 | 7.4 | 1.10 (0.63–1.92) | 0.745 | 1.30 (0.71–2.38) | 0.397 |
GIB indicates gastrointestinal bleed; HR, hazard ratio; ICH, intracerebral brain hemorrhage; LOF, loss‐of function; and PCI, percutaneous coronary intervention.
Data are presented as the number of patients in each group who experienced the event over 12 months of follow‐up after the index PCI.
The event rate was calculated as the number of events per 100 patient‐years of follow‐up.
The hazard ratio was adjusted with stabilized inverse probability weights derived from exposure propensity scores.
The HR after adjusting for history of GIB or ICH: adjusted HR 1.08; 95% CI, 0.72–1.62; P=0.726.
Clinical Outcomes
The median follow‐up after PCI was 6.3 months (interquartile range: 1.0–11.0 months), during which 353 patients (10.6%) had a major atherothrombotic event (event rate 21 per 100 patient‐years). For the primary analysis (Table 2 and Figure 2A), the rate of major atherothrombotic events was lower among LOF allele carriers receiving alternative therapy versus clopidogrel (17.1 versus 34.4 per 100 patient‐years, unadjusted HR, 0.50; 95% CI: 0.35–0.72; P<0.001) and remained lower after propensity score adjustment (adjusted HR, 0.56; 95% CI: 0.39–0.82; P=0.002). In contrast, major atherothrombotic event rates in patients without a LOF allele did not significantly differ between the alternative therapy and clopidogrel‐treated groups (18.1 versus 19.9 per 100 patient‐years, respectively; adjusted HR, 1.08; 95% CI, 0.72–1.62; P=0.715; Table 2) (P=0.030 for interaction between LOF carrier status and P2Y12 inhibitor therapy at event or last follow‐up). Consistent with previous reports, 3 clopidogrel‐treated patients with a LOF allele had a higher rate of atherothrombotic events compared with clopidogrel‐treated patients without a LOF allele (34.4 versus 19.9 per 100 patient‐years, HR, 1.67; 95% CI, 1.27–2.19; P<0.001).
Figure 2. Major atherothrombotic events with clinical implementation of CYP2C19‐guided antiplatelet therapy after PCI.
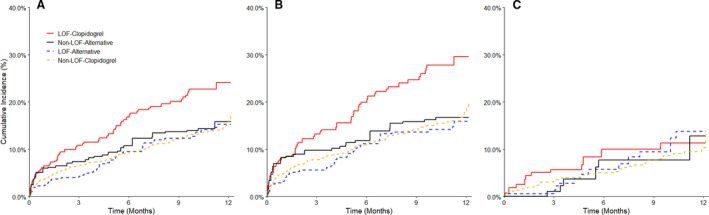
A, All patients. B, Patients with an ACS indication for PCI. C, Patients with a non‐ACS/elective indication for PCI. ACS indicates acute coronary syndrome; LOF, loss‐of‐function; and PCI, percutaneous coronary intervention. Weighted data are shown for patients with a CYP2C19 loss‐of‐function (LOF) allele treated with clopidogrel (LOF‐clopidogrel) or alternative therapy (LOF‐alternative) and for patients without a LOF allele treated with clopidogrel (non‐LOF‐clopidogrel) or alternative therapy (non‐LOF‐alternative). Note that patients were standardized to 2 different populations based on LOF allele status.
The difference in risk of major atherothrombotic events between the LOF‐alternative and LOF‐clopidogrel groups was more pronounced at 90 days (18.9 versus 52.5 per 100 patient‐years, adjusted HR, 0.40; 95% CI 0.23–0.71; P=0.002), while no difference was observed at 90 days between non‐LOF treatment groups (adjusted HR, 1.09; 95% CI 0.64–1.86; P=0.752). In addition, CYP2C19 IMs receiving alternative therapy had lower major atherothrombotic event rates when compared with IMs receiving clopidogrel (16.0 versus 35.1 per 100 patient‐years, HR, 0.46; 95% CI, 0.31–0.68; P<0.001).
The majority of atherothrombotic events (280 of 353 total events, 79%) occurred in patients presenting with an ACS at the time of PCI (Table 3 and Figure 2B). Among ACS patients, the rate of atherothrombotic events was lower in the LOF‐alternative group (17.8 per 100 patient‐years) compared to the LOF‐clopidogrel group (46.3 per 100 patient‐years; adjusted HR, 0.49; 95% CI 0.32–0.76, P=0.001). However, rates were similar in the non‐LOF‐alternative and non‐LOF‐clopidogrel groups (19.6 and 23.8 per 100 patient‐years, respectively; adjusted HR, 1.05; 95% CI 0.67–1.66, P=0.834). Atherothrombotic event rates were lower overall in patients without an ACS indication for PCI, ranging from 12.5 to 15.2 per 100 person‐years, and did not differ between the alternative therapy and clopidogrel groups in either LOF carriers or non‐LOF patients (Table 3 and Figure 2C).
Table 3.
Major Atherothrombotic Events in acute coronary syndrome and Chronic Coronary Syndrome (Non‐ACS) Subgroups by CYP2C19 and Antiplatelet Therapy
| Major atherothrombotic events by CYP2C19‐P2Y12 inhibitor | Event no. (%)* | Event rate (per 100 ) † | Unadjusted HR (95% CI) | P value | Adjusted HR ‡ (95% CI) | P value |
|---|---|---|---|---|---|---|
| ACS | ||||||
| LOF‐clopidogrel | 60 (19.6) | 46.3 | Reference | Reference | ||
| LOF‐alternative | 38 (9.2) | 17.8 | 0.39 (0.26–0.59) | <0.001 | 0.49 (0.32–0.76) | 0.001 |
| Non‐LOF‐clopidogrel | 154 (12.0) | 23.8 | Reference | Reference | ||
| Non‐LOF‐alternative | 28 (9.7) | 19.6 | 0.82 (0.55–1.23) | 0.346 | 1.05 (0.67–1.66) | 0.834 |
| Non‐ACS | ||||||
| LOF‐clopidogrel | 11 (7.1) | 14.3 | Reference | Reference | ||
| LOF‐alternative | 13 (8.2) | 15.2 | 1.06 (0.48–2.37) | 0.883 | 1.02 (0.44–2.36) | 0.957 |
| Non‐LOF‐clopidogrel | 43 (6.6) | 12.5 | Reference | Reference | ||
| Non‐LOF‐alternative | 6 (6.8) | 13.3 | 1.03 (0.44–2.42) | 0.946 | 0.74 (0.30–1.84) | 0.522 |
ACS indicates acute coronary syndrome; and LOF, loss‐of‐function.
Data are presented as the number (%) of patients in each group who experienced the event over 12 months of follow‐up after the index PCI.
The event rate was calculated as the number of events per 100 patient‐years of follow‐up.
The HR was adjusted with inverse probability weights derived from exposure propensity scores.
A clinically significant bleeding event occurred in 124 (3.7%) patients (Table 2 and Figure 3). Bleeding rates were similar in the LOF‐alternative and LOF‐clopidogrel groups (7.9 and 7.1 per 100 patient‐years, respectively; adjusted HR, 1.15; 95% CI 0.60–2.20; P=0.685), and in the non‐LOF‐alternative and non‐LOF‐clopidogrel groups (7.4 and 6.8 per 100 patient‐years, respectively; adjusted HR, 1.30; 95% CI 0.71–2.38; P=0.397).
Figure 3. Clinically significant bleeding events with clinical implementation of CYP2C19‐guided antiplatelet therapy after PCI.
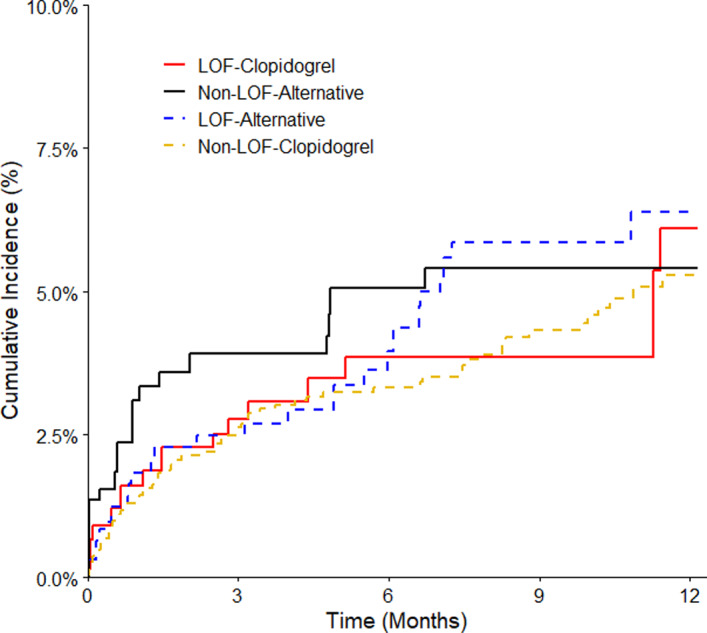
Weighted data are shown for patients with a CYP2C19 LOF allele treated with clopidogrel (LOF‐clopidogrel) or alternative therapy (LOF‐alternative) and patients without a LOF allele treated with either clopidogrel (non‐LOF‐clopidogrel) or alternative therapy (non‐LOF‐alternative). Note that patients were standardized to 2 different populations based on LOF allele status. The number of patients in each group was slightly different than for the atherothrombotic end point as patients were stratified based on antiplatelet therapy prescribed at the time of the bleeding event or last follow‐up. LOF indicates loss‐of‐function; and PCI, percutaneous coronary intervention.
DISCUSSION
To date, this is the largest real‐world investigation of clinical outcomes from clinical implementation of CYP2C19 testing to guide antiplatelet therapy prescribing for patients undergoing PCI. We demonstrate the feasibility of a genotype‐guided strategy across multiple institutions, as evidenced by frequent use of prasugrel or ticagrelor in CYP2C19 LOF allele carriers. We also observed lower atherothrombotic event rates in LOF allele carriers treated with alternative therapy compared with clopidogrel, particularly among patients with an ACS indication for PCI. In contrast, no difference in atherothrombotic event risk was observed in those without a LOF allele treated with clopidogrel compared to alternative therapy. There were also no significant differences in bleeding event rates between clopidogrel and alternative therapy regardless of LOF carrier status. These findings validate results from prior nonrandomized studies, small randomized trials, and meta‐analyses demonstrating that a genotype‐guided strategy, with use of prasugrel or ticagrelor in CYP2C19 LOF allele carriers, lowers atherothrombotic event risk compared with conventional treatment strategies without significantly increasing major bleeding risk. 10 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24
Our findings also extend those from recent large randomized clinical trials to a real‐world clinical setting, providing further evidence of the safety and effectiveness of CYP2C19 genotype‐guided antiplatelet therapy following PCI. The TAILOR‐PCI trial was conducted in patients with either an ACS or chronic coronary syndrome indication for PCI and compared the efficacy of ticagrelor versus clopidogrel in the subset of patients with a LOF allele. While the reduction in major atherothrombotic events between groups at 12 months did not reach statistical significance (HR, 0.66; 95% CI 0.43–1.02, P=0.06), post hoc analysis showed a significant reduction in events at 90 days (HR, 0.21; 95% CI 0.08–0.54, P=0.001). 9 We similarly observed lower atherothrombotic event rates in LOF allele carriers treated with alternative therapy versus clopidogrel at 90 days (adjusted HR, 0.40; 95% CI 0.23–0.71, P=0.002), and unlike TAILOR‐PCI, the difference was also evident at 12 months (adjusted HR, 0.56; 95% CI 0.39–0.82, P=0.003). The observed effect sizes in our study were similar to TAILOR‐PCI. Additionally, there was no difference in clinically significant bleeding events between LOF carriers treated with alternative therapy compared to clopidogrel in either our real‐world study (HR, 1.15; 95% CI 0.60–2.21) or TAILOR‐PCI (HR, 1.22; 95% CI 0.60–2.51). 9
Most atherothrombotic events in our study population occurred in those presenting with an ACS indication for PCI, and subgroup analyses were in line with previous analyses suggesting that the strongest associations between CYP2C19 LOF allele status and clopidogrel treatment outcomes occur in ACS patients. 25 , 26 It is worth noting that, we were likely underpowered to confidently determine whether treatment of LOF allele carriers with prasugrel or ticagrelor significantly lowers atherothrombotic event risk compared to clopidogrel in patients without an ACS indication for PCI due to the relatively small subgroup sample size and lower event rates. This is evidenced by the differences in the major atherothrombotic events rates between LOF allele carriers receiving clopidogrel for ACS and stable CAD (46.3 versus 14.3 event per 100 patient‐years). Also, given our pragmatic study design and high frequency of alternative therapy use in CYP2C19 PMs, the overwhelming majority of LOF allele carriers prescribed clopidogrel in our study population were CYP2C19 IMs. Thus, while we observed no difference in atherothrombotic event risk across groups in elective PCI patients, we cannot rule out a higher risk of atherothrombotic events in PMs prescribed clopidogrel. Given the lower magnitude of clinical benefit in elective PCI, a cost‐effectiveness analysis of genotype‐guided antiplatelet therapy in ACS relative to non‐ACS patients is warranted.
The POPular Genetics trial focused on patients undergoing PCI following ST‐segment elevation MI and demonstrated that a genotype‐guided approach, with CYP2C19 LOF allele carriers treated with ticagrelor or prasugrel and non‐LOF patients treated with clopidogrel, was non‐inferior to universal ticagrelor or prasugrel use for risk of developing major cardiovascular or bleeding events after primary PCI. 8 Consistent with these results and a recent meta‐analysis, 10 we observed no significant difference in atherothrombotic event rates in patients without a LOF allele treated with clopidogrel compared to those treated with prasugrel or ticagrelor. Notably, there remained no significant difference in event rate when focusing on patients with an ACS indication for PCI. Together, these data suggest that in patients without a LOF allele, clopidogrel is similarly effective as prasugrel or ticagrelor. The POPular Genetics trial also reported lower bleeding rates with genotype‐guided therapy compared with alternative therapy, which we did not observe in our study. Lower bleeding rates in the POPular Genetics trial were driven by a reduction of minor bleeding, defined as bleeding requiring clinical intervention. Minor bleeding is more difficult to detect from electronic health records, and was not comprehensively captured in our study. 27
The 2020 European Society of Cardiology guidelines for the management of ACS considers CYP2C19‐guided antiplatelet therapy an alternative to 12 months of dual antiplatelet therapy with prasugrel or ticagrelor based on results from POPular Genetics. 8 , 28 These recommendations are consistent with those from expert consensus on the use of genetic testing in patients undergoing PCI. 29 , 30 Our real‐world data support these recommendations, and demonstrate the safety and effectiveness of using CYP2C19 testing to guide an antiplatelet therapy after an ACS. Given the emergence of important questions regarding the relative clinical effectiveness of prasugrel versus ticagrelor in ACS patients, 31 it is important to point out that alternative therapy in both TAILOR‐PCI and POPular Genetics was comprised almost exclusively ticagrelor (prasugrel was not used in TAILOR‐PCI, and was only used in ≈2% of patients in POPular Genetics). 9 , 32 Thus, our real‐world data in which either prasugrel or ticagrelor was used as alternative therapy add to the body of evidence on outcomes with genotype‐guided antiplatelet therapy after PCI, and specifically suggest that treatment of LOF carriers with either ticagrelor or prasugrel is associated with improved outcomes.
The real‐world nature of our data allowed for evaluation of higher‐risk patients than those included in other clinical trials. For example, ≈40% of our study population had diabetes, 80% had hypertension, 30% had chronic kidney disease, 25% had history of myocardial infarction, and 10% were treated with an oral anticoagulant. In TAILOR‐PCI and POPular Genetics, respectively, ≈28% and 12% had diabetes, 62% and 40% had hypertension, 10% and 10% had chronic kidney disease, 15% and 8% had history of myocardial infarction, and 0% (excluded) and 4% were treated with an oral anticoagulant. This likely contributed to higher atherothrombotic and bleeding event rates in our real‐world study population than reported in the randomized clinical trials of CYP2C19 genotype‐guided antiplatelet therapy. Importantly, the results from our study, together with the TAILOR‐PCI and POPular Genetics randomized trials and recent meta‐analyses, 8 , 9 , 10 , 24 provide compelling data supporting the effectiveness of a genotype‐guided strategy. We also observed higher atherothrombotic event rates in clopidogrel‐treated patients with a LOF allele compared with those without a LOF allele, as has been reported in multiple studies. 33 , 34 , 35 , 36 , 37 In addition, prior studies have demonstrated that the increased function CYP2C19*17 allele does not impact atherothrombotic or bleeding outcomes in clopidogrel‐treated patients without a LOF allele. 32 , 38 Collectively, these data lend additional evidence supporting current CPIC guideline recommendations that recommend the use of prasugrel or ticagrelor in patients found to carry a CYP2C19 LOF allele (both IMs and PMs) 3 and also suggest that clopidogrel should not be prescribed following PCI in the absence of CYP2C19 genotyping to reduce the risk of adverse clinical consequences of clopidogrel use in LOF allele carriers.
Study Limitations
We recognize several limitations of our study. The real‐world nature of our study meant that prescribers could choose to follow the genotype recommendations or not. Together with the non‐randomized design, this resulted in imbalances between groups, which could potentially confound results. While balance was achieved across comparison groups after weighting, we cannot rule out residual confounding around the selection of antiplatelet therapy, especially since we did observe some channeling of clopidogrel toward older more multi‐morbid patients with conceivably higher risk for atherothrombotic events. The minor differences in HRs before and after adjustment might alleviate some of these concerns. Another limitation was that there was not a control group who did not receive genetic testing, thus this study cannot evaluate the effectiveness of genetic testing against a genotype agnostic treatment approach. Further, outcomes were determined based on EHR data, without event adjudication and variable length of follow‐up. As such, clinical events that were treated in other health systems and deaths may have been missed. Our focus on clinically actionable bleeding (GUSTO moderate or severe/life‐threatening bleeding) yielded a low number of bleeding events, which limited power to detect differences between drug‐phenotype groups. Finally, while there is interest in prasugrel versus ticagrelor treatment outcomes, 31 and it would be of interest to evaluate outcomes by LOF carrier status across clopidogrel, prasugrel, ticagrelor treated patients, the numbers in the subgroups are too small in our study to compare outcomes with appropriate propensity score matching. Future studies in larger populations are needed to evaluate effectiveness and safety of using prasugrel versus ticagrelor in a real‐world setting of genotype‐guided antiplatelet therapy after PCI.
CONCLUSIONS
In summary, our prospective, real‐world outcomes data expand the generalizability of what has been reported in recent prospective randomized controlled trials and demonstrate the clinical effectiveness of incorporating CYP2C19 genotyping into antiplatelet therapy prescribing decisions after PCI. Furthermore, these data suggest patients with ACS undergoing PCI especially benefit from a genotype‐guided strategy and support multiple recent economic analyses demonstrating that CYP2C19‐guided antiplatelet therapy is a cost‐effective strategy for ACS patients undergoing PCI. 39 , 40 Although the use of prasugrel and ticagrelor has been increasing in the post‐ACS/PCI setting in recent years, a significant portion of patients remain poor candidates for these alternative antiplatelet agents due to higher bleeding risk, lower tolerability, difficulty with adherence and access, as well as higher copay costs compared with clopidogrel. 41 The cumulative data suggest that when clopidogrel is prescribed as part of dual antiplatelet therapy post‐PCI in ACS, CYP2C19 genotype should be assessed to avoid clopidogrel use in patients carrying a LOF allele due to the increased risk of major atherothrombotic events.
Sources of Funding
This work was supported by grants from the National Institutes of Health (NIH, U01 HG007269 [Johnson, Cavallari, Duarte, McDonough and Winterstein], U01 HG007775 [Beitelshees], and U01 HG007762 and U01HG010245 [Skaar and Kreutz]), and by the NIH IGNITE Network (https://gmkb.org/ignite/). Additional support provided by NIH grants R01 HL149752 (Cavallari, C. R. Lee), T32 HG008958 (Thomas), and K01 HL141690 (McDonough); NIH grants U01 GM074492 and U01 HL105198 (both part of the NIH Pharmacogenomics Research Network); and by substantial institutional support from the University of Florida and its Clinical Translational Science Institute (NIH grants UL1 TR000064 and UL1 TR001427) (Cavallari, Johnson, Duarte, McDonough); NIH UL1 TR002489 (C. R. Lee, Stouffer); Penn Center for Precision Medicine at the Perelman School of Medicine at the University of Pennsylvania (Tuteja); NIH R01 HL092173, K24 HL133373, UL1 TR000165 and Hugh Kaul Precision Medicine Institute at the University of Alabama, Birmingham (Limdi); NIH UL1 TR0000005, the University of Pittsburgh/UPMC Institute for Precision Medicine, American Society of Health System Pharmacists, American Heart Association grant 17MCPRP33400175, and by an Anonymous Donor (Empey, Coons, Stevenson); NIH U54 MD010723 (J. C. Lee). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
Franchi receives honoraria from AstraZeneca and Sanofi. Kreutz receives research funding from Idorsia and consulting fees from Haemonetics. Skaar receives consulting fees from Indiana University Health. Giri receives honoraria from Astra Zeneca and Inari Medical. Angiolillo has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St. Jude Medical. Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. Johnson is a consultant for United Health Group. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S2
Figure S1
For Sources of Funding and Disclosures, see page 11.
References
- 1. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–2931. doi: 10.1016/j.jacc.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 2. Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl‐Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TE, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19:215–223. doi: 10.1038/gim.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mega JL, Simon T, Collet J‐P, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, et al. Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949 [DOI] [PubMed] [Google Scholar]
- 6. Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3 [DOI] [PubMed] [Google Scholar]
- 7. Franchi F, Rollini F, Rivas J, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, Shaikh Z, et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss‐of‐function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC Basic Transl Sci. 2020;5:419–428. doi: 10.1016/j.jacbts.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van 't Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, et al. A genotype‐guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621–1631. [DOI] [PubMed] [Google Scholar]
- 9. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae J‐H, Jeong MH, Chavez I, et al. Effect of genotype‐guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR‐PCI randomized clinical trial. JAMA. 2020;324:761–771. doi: 10.1001/jama.2020.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, So D, Geller N, Goodman SG, Hasan A, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y. JACC Cardiovasc Interv. 2021;14:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Empey PE, Stevenson JM, Tuteja S, Weitzel KW, Angiolillo DJ, Beitelshees AL, Coons JC, Duarte JD, Franchi F, Jeng LJB, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype‐guided antiplatelet therapy. Clin Pharmacol Ther. 2018;104:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luzum JA, Pakyz RE, Elsey AR, Haidar CE, Peterson JF, Whirl‐Carrillo M, Handelman SK, Palmer K, Pulley JM, Beller M, et al. The pharmacogenomics research network translational pharmacogenetics program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102:502–510. doi: 10.1002/cpt.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavallari LH, Lee CR, Beitelshees AL, Cooper‐DeHoff RM, Duarte JD, Voora D, Kimmel SE, McDonough CW, Gong Y, Dave CV, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype‐guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 15. Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in‐hospital smoking cessation counseling on mortality. Multivariate Behav Res. 2011;46:119–151. doi: 10.1080/00273171.2011.540480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. Available at: https://www.R‐project.Org/. Accessed January 28, 2022. [Google Scholar]
- 17. Hulot JS, Chevalier B, Belle L, Cayla G, Khalife K, Funck F, Berthier R, Piot C, Tafflet M, Montalescot G, et al. Routine CYP2C19 genotyping to adjust thienopyridine treatment after primary PCI for STEMI: results of the GIANT study. JACC Cardiovasc Interv. 2020;13:621–630. [DOI] [PubMed] [Google Scholar]
- 18. Martin J, Williams AK, Klein MD, Sriramoju VB, Madan S, Rossi JS, Clarke M, Cicci JD, Cavallari LH, Weck KE, et al. Frequency and clinical outcomes of CYP2C19 genotype‐guided escalation and de‐escalation of antiplatelet therapy in a real‐world clinical setting. Genet Med. 2020;22:160–169. doi: 10.1038/s41436-019-0611-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, Moruzzi P, Patrizi G, Tagliazucchi GM, Crocamo A, et al. Pharmacogenomic approach to selecting antiplatelet therapy in acute coronary syndromes: PHARMCLO trial. J Am Coll Cardiol. 2018;71:1869–1877. [DOI] [PubMed] [Google Scholar]
- 20. Sánchez‐Ramos J, Dávila‐Fajardo CL, Toledo Frías P, Díaz Villamarín X, Martínez‐González LJ, Martínez Huertas S, Burillo Gómez F, Caballero Borrego J, Bautista Pavés A, Marín Guzmán MC, et al. Results of genotype‐guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289–295. doi: 10.1016/j.ijcard.2016.09.088 [DOI] [PubMed] [Google Scholar]
- 21. Shen DL, Wang B, Bai J, Han Q, Liu C, Huang XH, Zhang JY. Clinical value of CYP2C19 genetic testing for guiding the antiplatelet therapy in a Chinese population. J Cardiovasc Pharmacol. 2016;67:232–236. doi: 10.1097/FJC.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 22. Gower MN, Ratner LR, Williams AK, Rossi JS, Stouffer GA, Lee CR. Clinical utility of CYP2C19 genotype‐guided antiplatelet therapy in patients at risk of adverse cardiovascular and cerebrovascular events: a review of emerging evidence. Pharmgenomics Pers Med. 2020;13:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss‐of‐function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052 [DOI] [PubMed] [Google Scholar]
- 24. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, Porto I, Angiolillo DJ. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. Lancet. 2021;397:1470–1483. doi: 10.1016/S0140-6736(21)00533-X [DOI] [PubMed] [Google Scholar]
- 25. Johnson JA, Roden DM, Lesko LJ, Ashley E, Klein TE, Shuldiner AR. Clopidogrel: a case for indication‐specific pharmacogenetics. Clin Pharmacol Ther. 2012;91:774–776. doi: 10.1038/clpt.2012.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim H‐S, Chang K, Koh Y‐S, Park M‐W, Choi Y‐S, Park C‐S, Oh M, Kim E‐Y, Shon J‐H, Shin J‐G, et al. CYP2C19 poor metabolizer is associated with clinical outcome of clopidogrel therapy in acute myocardial infarction but not stable angina. Circ Cardiovasc Genet. 2013;6:514–521. doi: 10.1161/CIRCGENETICS.113.000109 [DOI] [PubMed] [Google Scholar]
- 27. Serebruany VL, Atar D. Assessment of bleeding events in clinical trials–proposal of a new classification. Am J Cardiol. 2007;99:288–290. doi: 10.1016/j.amjcard.2006.07.091 [DOI] [PubMed] [Google Scholar]
- 28. Collet J‐P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.15829/1560-4071-2021-4418 [DOI] [PubMed] [Google Scholar]
- 29. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 30. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, et al. International expert consensus on switching platelet P2Y12 receptor‐inhibiting therapies. Circulation. 2017;136:1955–1975. [DOI] [PubMed] [Google Scholar]
- 31. Schüpke S, Neumann F‐J, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, Richardt G, Liebetrau C, Witzenbichler B, Antoniucci D, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973 [DOI] [PubMed] [Google Scholar]
- 32. Claassens DMF, Bergmeijer TO, Vos GJA, Hermanides RS, van ’t Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, et al. Clopidogrel versus ticagrelor or prasugrel after primary percutaneous coronary intervention according to CYP2C19 genotype: a popular genetics subanalysis. Circ Cardiovasc Interv. 2021;14:e009434. doi: 10.1161/CIRCINTERVENTIONS.120.009434 [DOI] [PubMed] [Google Scholar]
- 33. Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Buttner HJ, et al. Cytochrome P450 2C19 681G>A polymorphism and high on‐clopidogrel platelet reactivity associated with adverse 1‐year clinical outcome of elective percutaneous coronary intervention with drug‐eluting or bare‐metal stents. J Am Coll Cardiol. 2008;51:1925–1934. [DOI] [PubMed] [Google Scholar]
- 34. Collet J‐P, Hulot J‐S, Pena A, Villard E, Esteve J‐B, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0 [DOI] [PubMed] [Google Scholar]
- 35. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, et al. Cytochrome P‐450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171 [DOI] [PubMed] [Google Scholar]
- 36. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dorrler K, Morath T, Schomig A, Kastrati A, von Beckerath N. Cytochrome P450 2C19 loss‐of‐function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041 [DOI] [PubMed] [Google Scholar]
- 38. Lee CR, Thomas CD, Beitelshees AL, Tuteja S, Empey PE, Lee JC, Limdi NA, Duarte JD, Skaar TC, Chen Y, et al. Impact of the CYP2C19*17 allele on outcomes in patients receiving genotype‐guided antiplatelet therapy after percutaneous coronary intervention. Clin Pharmacol Ther. 2021;109:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Limdi NA, Cavallari LH, Lee CR, Hillegass WB, Holmes AM, Skaar TC, Pisu M, Dillon C, Beitelshees AL, Empey PE, et al. Cost‐effectiveness of CYP2C19‐guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real‐world data. Pharmacogenomics J. 2020;20:724–735. doi: 10.1038/s41397-020-0162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu YE, Swanson KM, Rojas RL, Wang Z, St. Sauver JL, Visscher SL, Prokop LJ, Bielinski SJ, Wang L, Weinshilboum R, et al. Systematic review of the evidence on the cost‐effectiveness of pharmacogenomics‐guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–486. doi: 10.1038/s41436-019-0667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, Heestermans T, Tjon Joe Gin M, Waalewijn R, Hofma S, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non‐ST‐elevation acute coronary syndrome (POPular AGE): the randomised, open‐label, non‐inferiority trial. Lancet. 2020;395:1374–1381. doi: 10.1016/S0140-6736(20)30325-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2
Figure S1


