Abstract
Background
In TAILOR‐PCI, genotype‐guided selection of P2Y12 inhibitors after percutaneous coronary intervention did not significantly reduce the risk of ischemic events at 12 months. The Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping (ABCD‐GENE) score identifies patients with high platelet reactivity on clopidogrel at increased risk of ischemic events. The aim of this study was to investigate the value of the ABCD‐GENE score for tailoring P2Y12 inhibitor selection after percutaneous coronary intervention.
Methods and Results
In a post hoc analysis of the TAILOR‐PCI, outcomes were analyzed by ABCD‐GENE score and allocation to genotype‐guided or conventional P2Y12 inhibitor selection. Primary (death, myocardial infarction, or stroke) and secondary (cardiovascular death, myocardial infarction, stroke, stent thrombosis, or severe recurrent ischemia) outcomes were assessed. Among 3883 patients discharged on clopidogrel in the genotype‐guided and conventional therapy groups, 15.8% and 84.2% had high (≥10 points) or low (<10) ABCD‐GENE scores, respectively. At 12 months, both the primary (5.2% versus 2.6%, P<0.001) and secondary outcomes (7.7% versus 4.6%, P=0.001) were significantly increased in patients with high ABCD‐GENE score. Among 4714 patients allocated to genotype‐guided or conventional therapy, the former did not significantly reduce the 12‐month risk of the primary and secondary outcomes in both the high and low ABCD‐GENE score groups (pinteraction=0.48 and 0.27, respectively).
Conclusions
Among patients with percutaneous coronary intervention on clopidogrel, the ABCD‐GENE score was helpful in identifying those at higher risk. The ABCD‐GENE score may potentially enhance the precision of tailored selection of P2Y12 inhibitors, which needs to be confirmed in prospective investigations.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique Identifier: NCT01742117.
Keywords: antiplatelet therapy, genetic testing, ischemia, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention, Pharmacology
Nonstandard Abbreviations and Acronyms
- ABCD‐GENE
Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping
- CYP
cytochrome P450
- DAPT
dual antiplatelet therapy
- HPR
high platelet reactivity
- LOF
loss of function
Clinical Perspective
What is New?
Among patients with percutaneous coronary intervention on clopidogrel in the TAILOR‐PCI trial, the ABCD‐GENE (Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping) score was helpful in identifying those at higher risk of cardiovascular events.
What are the Clinical Implications?
The ABCD‐GENE score may potentially enhance the precision of tailored selection of oral P2Y12 inhibitors, which needs to be confirmed in prospective investigations.
Dual antiplatelet therapy (DAPT), consisting of aspirin and an oral platelet adenosine diphosphate P2Y12 receptor inhibitor, is the standard of care after percutaneous coronary intervention (PCI). 1 Clopidogrel, the most widely prescribed oral P2Y12 inhibitor, requires 2 steps of hepatic biotransformation by the cytochrome P450 (CYP) system to generate its active metabolite. 2 The CYP2C19 enzyme plays a key role in this process as it is involved in both metabolic steps. Genetic polymorphisms regulate CYP2C19 enzyme activity and thus modulate the pharmacokinetic and pharmacodynamic effects of clopidogrel. 3 Carriers of CYP2C19 loss‐of‐function (LOF) genotypes are associated with impaired clopidogrel metabolism and reduced clopidogrel‐induced antiplatelet effects resulting in increased rates of high platelet reactivity (HPR) and thrombotic complications in patients undergoing PCI. 4 , 5 , 6 , 7
Ticagrelor, compared with clopidogrel, is a more potent oral P2Y12 inhibitor with reduced rates of HPR, a marker of thrombotic risk, and is associated with superior ischemic benefit. 8 , 9 , 10 , 11 Such benefit, however, comes at the expense of an increased risk of major bleeding. It has been suggested that reserving ticagrelor to patients with PCI in need of enhanced antithrombotic protection, such as those using clopidogrel‐HPR and CYP2C19 LOF carriers, and using clopidogrel in the remainder (ie, patients without HPR and CYP2C19 LOF non‐carriers), would minimize the risk of bleeding, without trade‐off in ischemic protection. 11 , 12 Knowledge of CYP2C19 genotypes that are associated with HPR could therefore allow for the individualized use of P2Y12 inhibiting therapy. 13 , 14
In TAILOR‐PCI, a large, randomized trial of PCI patients with LOF alleles, genotype‐guided selection of the P2Y12 inhibitor, compared with conventional clopidogrel therapy, demonstrated a non‐statistically significant reduction in the incidence of ischemic events at 12 months. Noteworthy was the hazard ratio (HR) of 0.66 (95% CI, 0.43–1.02) suggesting a treatment effect that the study was not powered to detect. 15 In addition, post‐hoc analyses suggested benefit at 3 months.
While the role of CYP2C19 LOF genotype on HPR is undisputed, a number of clinical factors also contribute to on clopidogrel‐HPR. 2 , 11 , 16 Thus, the ability to identify high risk patients who are more likely to benefit from a genotype‐guided approach to P2Y12 inhibitors could be enhanced by integrating both genetic and clinical variables associated with HPR. The Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping (ABCD‐GENE) score has been recently introduced as a simple tool integrating both genetic and clinical variables to identify patients using clopidogrel‐HPR who are at increased risk of adverse ischemic events. 17 This approach may augment the precision of a tailored strategy for using P2Y12 inhibitors.
In this post‐hoc analysis of the TAILOR‐PCI trial, we explored ischemic outcomes of PCI patients according to their ABCD‐GENE score (high versus low) and assignment to a genotype‐guided or conventional approach for the selection of P2Y12 receptor inhibition. We hypothesized that the ABCD‐GENE score is helpful in isolating a sizeable proportion of patients who may benefit from a genotype‐guided approach for the selection of DAPT.
METHODS
Data Source and Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study design and main results of the TAILOR‐PCI trial are published elsewhere. 15 , 18 In brief, TAILOR‐PCI was a multicenter trial that randomized 5302 patients undergoing PCI for acute coronary syndromes or stable coronary artery disease to rapid genotyping to guide post‐PCI selection of P2Y12 inhibiting therapy (ie, ticagrelor in carriers of CYP2C19 LOF genotypes and clopidogrel in non‐carriers) or conventional therapy with clopidogrel and with laboratory‐based genotyping performed at 12 months. All patients were on a background of aspirin therapy. The primary analysis in TAILOR‐PCI was performed in the 1849 patients with CYP2C19 LOF genotypes (CYP2C19*2 and *3). At 12 months, the HR for the primary end point (a composite of cardiovascular death, myocardial infarction [MI], stroke, stent thrombosis, and severe recurrent ischemia) did not statistically significantly differ between the genotype‐guided and conventional therapy groups. The trial was approved by the ethics boards of participating sites. and all participants provided written informed consent.
ABCD‐GENE Score
The ABCD‐GENE score integrates CYP2C19 LOF genotypes (6 points for 1 LOF allele, 24 points for 2 LOF alleles) with 4 clinical risk factors influencing clopidogrel response (age >75 years [4 points], body mass index >30 kg/m2 [4 points], chronic kidney disease [creatinine clearance <60 mL/min, 3 points], and diabetes [3 points]). 17 The score was built from the data set of the GRAVITAS (Gauging Responsiveness With a VerifyNow P2Y12 Assay: Impact on Thrombosis and Safety) trial and validated for the pharmacodynamic outcome of HPR and for clinical outcomes (death and the composite of death, MI, or stroke) in 2 external data sets. 17 The ABCD‐GENE score ranges between 0 and 38 points, with a high score (≥10 points) identifying patients treated with clopidogrel with a greater likelihood of having HPR and adverse ischemic events after PCI. 17
Study Outcomes
The primary outcome of interest was the composite of death, MI, or stroke at 12 months after index PCI. The secondary outcome of interest was the composite of cardiovascular death, MI, stroke, definite or probable stent thrombosis, or severe recurrent ischemia at 12 months after index PCI. These outcomes were chosen as they were the primary clinical end points in the ABCD‐GENE score validation study and the TAILOR‐PCI trial, respectively. 15 , 17 , 18 All‐cause death and the composite of major or minor bleeding (as defined by the Thrombolysis in Myocardial Infarction criteria) were also analyzed.
Statistical Analysis
Continuous data are presented as mean (SD) unless otherwise noted and were compared using the Wilcoxon rank sum test. Categorical variables are presented as counts and proportions and were compared using the Chi‐square test. The cumulative incidences of the primary and secondary outcomes at 12 months according to ABCD‐GENE score and/or treatment group (ie, genotype‐guided, or conventional therapy) were calculated using the Kaplan‒Meier method (time‐to‐first event) and compared using a permutation‐based log‐rank test. An exploratory analysis at 3 months was also conducted because of the strong effect for genotyping observed in the first 3 months of the main trial. Patients who withdrew or who were lost to follow‐up were treated as censored at the date of their last contact. Treatment effect estimates are presented as HRs with corresponding 95% CIs, using Firth correction in Cox regression models. The interaction between the treatment arm and ABCD‐GENE score group was analyzed for each study outcome. A sensitivity analysis was conducted considering recurrent events over the study time course.
Only subjects with complete data for the calculation of the ABCD‐GENE score were eligible for analysis. Since the score was designed to indicate the potential for HPR on clopidogrel, only subjects discharged on clopidogrel were analyzed for the comparison of outcomes in high (≥10) versus low (<10) ABCD‐GENE score groups. However, all eligible subjects regardless of medication at discharge were included in the analysis of genotype‐guided versus conventional therapy, assessed within the ABCD‐GENE score groups.
All hypotheses tests were 2‐sided with a 0.05 type I error rate. All statistical analyses were conducted with the SAS software, version 9.4 (SAS Institute Inc).
RESULTS
Baseline Characteristics
Complete data for the calculation of the ABCD‐GENE score were available in 4714 subjects. Among those discharged on clopidogrel (n=3883, Figure 1), the ABCD‐GENE score ranged between 0 and 35 points (mean 5.2±5.5; median 4, interquartile range, 0–7). A total of 613 (15.8%) patients had a high ABCD‐GENE score and 3270 (84.2%) had a low ABCD‐GENE score. Self‐reported adherence rates per follow‐up days were 96.8% and 97.0% in patients with high and low ABCD‐GENE score, respectively.
Figure 1. Study flowchart.
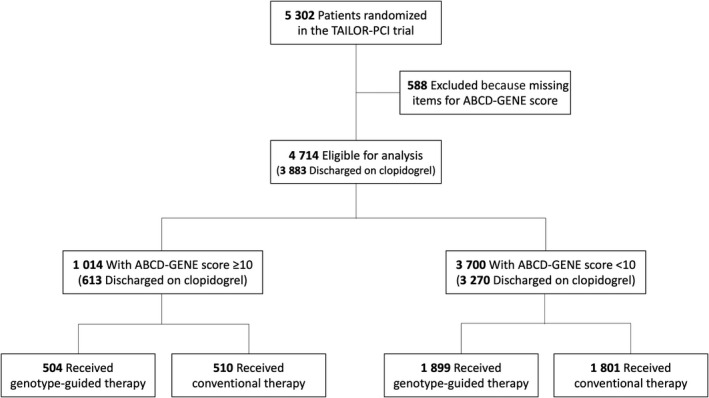
A total of 4714 patients randomized in the TAILOR‐PCI trial had complete data for the calculation of the ABCD‐GENE score. Of them 1014 had a high (≥10) ABCD‐GENE score and 3700 (<10) had a low ABCD‐GENE score. A total of 3883 patients were discharged on clopidogrel (613 and 3270 with high and low ABCD‐GENE scores, respectively). ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping.
Table 1 summarizes baseline characteristics of such study patients according to ABCD‐GENE score groups. In addition to expected differences in variables included in the ABCD‐GENE score, patients with a high versus low score were more frequently women and East Asians, and more likely presented with hypertension and history of heart failure or revascularization. Current smokers were more frequent among patients with a low ABCD‐GENE score. Other clinical and angiographic variables were generally similar between patients with high and low ABCD‐GENE scores.
Table 1.
Baseline Characteristics of Study Patients Discharged on Clopidogrel According to ABCD‐GENE Score Groups
| Variable |
High (≥10) ABCD‐GENE score (n=613) |
Low (<10) ABCD‐GENE score (n=3270) |
P value |
|---|---|---|---|
| Age at PCI, y (SD) | 65.9 (12.4) | 61.9 (10.5) | <0.0001 |
| Sex | |||
| Men | 410 (66.9%) | 2506 (76.6%) | <0.0001 |
| Women | 203 (33.1%) | 764 (23.4%) | |
| Body mass index, n (SD) | 31.2 (6.7) | 28.8 (6.1) | <0.0001 |
| Race or ethnicity | |||
| White | 369 (60.2%) | 2173 (66.5%) | 0.0002 |
| East Asian | 167 (27.2%) | 644 (19.7%) | |
| South Asian | 30 (4.9%) | 126 (3.9%) | |
| Black | 17 (2.8%) | 77 (2.4%) | |
| Hispanic or Latino | 14 (2.3%) | 115 (3.5%) | |
| Other/Unknown | 16 (2.6%) | 135 (4.1%) | |
| Cardiovascular risk factors | |||
| Diabetes | 285 (46.5%) | 776 (23.7%) | <0.0001 |
| Hypertension | 442 (72.1%) | 2035 (62.2%) | <0.0001 |
| Dyslipidemia | 341 (55.6%) | 1715 (52.4%) | 0.15 |
| Smoking status | |||
| Current smoker | 107 (18.0%) | 833 (25.9%) | 0.0002 |
| Former/recent | 217 (36.5%) | 1056 (32.8%) | |
| Never smoked | 271 (45.5%) | 1326 (41.2%) | |
| Prior history | |||
| Heart failure | 86 (14.0%) | 240 (7.3%) | <0.0001 |
| Myocardial infarction | 100 (16.3%) | 478 (14.6%) | 0.28 |
| PAD | 22 (3.6%) | 88 (2.7%) | 0.22 |
| PCI | 170 (27.7%) | 757 (23.1%) | 0.01 |
| CABG | 61 (10.0%) | 237 (7.2%) | 0.02 |
| Stroke or TIA | 22 (3.6%) | 88 (2.7%) | 0.22 |
| COPD | 30 (4.9%) | 126 (3.9%) | 0.23 |
| Creatinine clearance <60 mL/min | 200 (32.6%) | 222 (6.8%) | <0.001 |
| Multivessel disease | 265 (43.2%) | 1361 (41.6%) | 0.46 |
| Hospital presentation | |||
| Stable CAD | 123 (20.1%) | 585 (17.9%) | 0.05 |
| Unstable Angina/NSTEMI | 380 (62.0%) | 1990 (60.9%) | |
| STEMI | 110 (17.9%) | 695 (21.3%) | |
| CYP2C19 status (no. of *2 alleles) | |||
| 0 | 166 (27.4%) | 2804 (87.1%) | <0.0001 |
| 1 | 356 (58.7%) | 416 (12.9%) | |
| 2 | 84 (13.9%) | 0 (0.0%) | |
Data are shown as n (%) if not otherwise indicated.
ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CYP, cytochrome P450; NSTEMI, non–ST‐segment–elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and TIA, transient ischemic attack.
Clinical Outcomes of Patients With High or Low ABCD‐GENE Scores Treated With Clopidogrel
At 12 months, the risk of the primary composite outcome of death, MI, or stroke was significantly increased in patients with high ABCD‐GENE score (5.2% versus 2.6%, HR, 2.04; 95% CI, 1.35–3.07; P<0.001) compared with those with low ABCD‐GENE score (Figure 2 and Table 2). The magnitude of the point estimate was similar for patients with (HR, 2.08; 95% CI, 1.34–3.23) and without (HR, 2.06; 95% CI, 0.68–6.28) an acute coronary syndrome (P for interaction=0.99). The effect estimate remained significant and was larger in magnitude in the sensitivity analysis that took the risk of recurrent events into account (HR, 2.32; 95% CI, 1.61–3.37; P<0.001). Compared to CYP2C19 LOF non‐carriers, the presence of 1 (HR, 0.99; 95% CI, 0.63–1.55) or 2 (HR, 1.01; 95% CI, 0.42–2.86) LOF alleles in isolation did not predict the risk of future events (Figure 3).
Figure 2. Incidence of death, myocardial infarction, or stroke in patients discharged on clopidogrel with high ABCD‐GENE score ≥10 or <10.
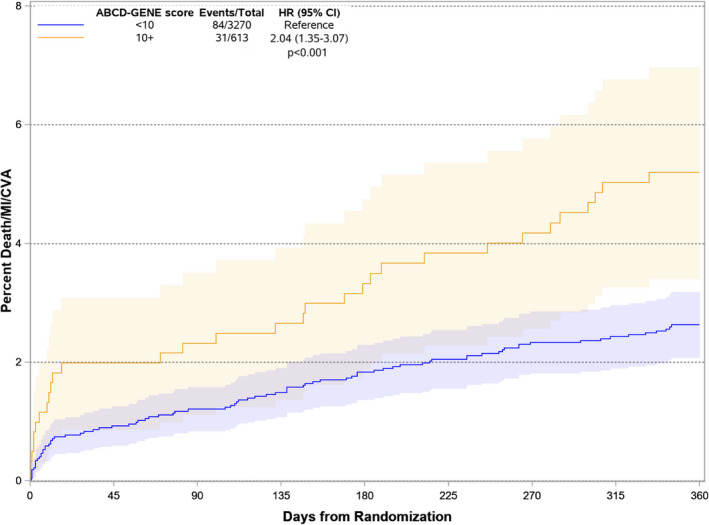
ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; CVA, cerebrovascular accidents; HR, hazard ratio; and MI, myocardial infarction. The shaded areas represent 95% CIs for the survival curves.
Table 2.
Clinical Outcomes in Patients With High or Low ABCD‐GENE‐Score
| Variable |
High (≥10) ABCD‐GENE score (n=613) |
Low (<10) ABCD‐GENE score (n=3270) |
HR (95% CI) | P value |
|---|---|---|---|---|
| Time to first event analysis | ||||
| All‐cause death, MI, or stroke | 31 (5.2%) | 84 (2.6%) | 2.04 (1.35–3.07) | <0.001 |
| Cardiovascular death, MI, stroke, definite or probable ST, or severe recurrent ischemia | 46 (7.7%) | 146 (4.6%) | 1.74 (1.25–2.43) | 0.001 |
| All‐cause death | 11 (1.8%) | 32 (1.0%) | 1.92 (0.97–3.79) | 0.060 |
| Major/minor bleeding | 12 (2.0%) | 33 (1.0%) | 2.04 (1.06, 3.94) | 0.033 |
| Recurrent events analysis | ||||
| All‐cause death, MI, or stroke | 38 (6.5%) | 90 (2.9%) | 2.32 (1.61–3.37) | <0.001 |
| Cardiovascular death, MI, stroke, definite or probable ST, or severe recurrent ischemia | 57 (9.7%) | 162 (5.1%) | 1.90 (1.43–2.52) | <0.001 |
Estimates are calculated by the Kaplan‒Meier method.
ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; HR, hazard ratio; MI, myocardial infarction; and ST, stent thrombosis.
Figure 3. Summary of results.
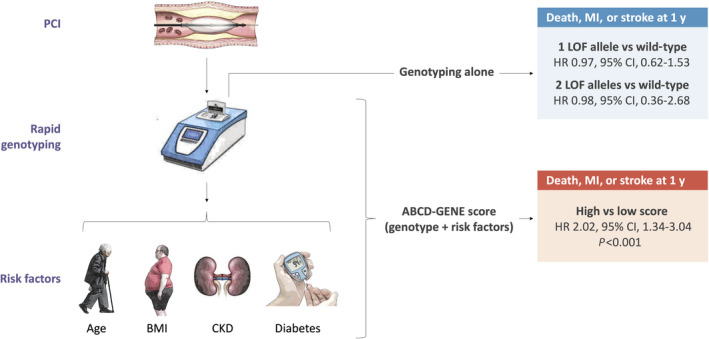
At 1 year, the risk of death, myocardial infarction, or stroke was significantly predicted by the ABCD‐GENE score (high versus low) but not by the presence of 1 or 2 loss of function alleles. ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; BMI, body mass index; CKD, chronic kidney disease; HR, hazard ratio, LOF, loss of function; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
The results of the secondary outcomes for patients with high or low ABCD‐GENE‐score are shown in Table 2. The risk of the secondary composite outcome was significantly increased in patients with high ABCD‐GENE score in both the analyses of time to first event and recurrent events. Patients with high ABCD‐GENE score had an increased risk of major or minor bleeding (HR, 2.04; 95% CI, 1.06‒3.94; P=0.033). At 3 months, the risk of the primary composite outcome was significantly increased in patients with a high ABCD‐GENE score (HR, 1.98; 95% CI, 1.08–3.65; P=0.027), while the risk of the secondary composite outcome was not (HR, 1.57; 95% CI, 0.94–2.66; P=0.086).
Clinical Outcomes by Treatment Randomization in ABCD‐GENE Score Subgroups
Of 4714 patients randomized in the TAILOR‐PCI trial with complete data for the calculation of the ABCD‐GENE score, 1014 had a high ABCD‐GENE score, and 3700 had a low ABCD‐GENE score (Figure 1). At 12 months, the risk of the primary composite outcome was not significantly reduced in the genotype‐guided therapy arm compared with the conventional therapy arm in both the high (3.7% versus 5.2%; HR, 0.70; 95% CI, 0.38–1.28) and low (2.4% versus 2.7%; HR, 0.91; 95% CI, 0.60–1.37; P value for interaction=0.49) ABCD‐GENE score subgroups (Table 3 and Figure 4), although both the absolute and relative treatment effects were nominally larger in patients with high ABCD‐GENE score. Similarly, there was no significant treatment‐by‐group interaction for the secondary composite outcome (p for interaction=0.27, Table 3), but both treatment estimates were directionally more in favor of genotype‐guided therapy in patients with high ABCD‐GENE score. All‐cause death and major or minor bleeding were also not significantly associated with a significant treatment interaction (Table 3). No statistically significant treatment interactions were also noted at 12 months in sensitivity analyses restricted to CYP2C19 LOF allele carriers, where the treatment estimates for the primary and secondary composite end points in the high and low ACBD‐GENE score groups were similar (Table 4).
Table 3.
Clinical Outcomes With Genotype‐Guided Therapy or Conventional Therapy in Patients With High and Low ABCD‐GENE‐Score
| Variable |
High (≥10) ABCD‐GENE score (n=1014) |
Low (<10) ABCD‐GENE score (n=3700) |
Pint | ||||
|---|---|---|---|---|---|---|---|
| GGT | CT | HR (95% CI) | GGT | CT | HR (95% CI) | ||
| Time to first event analysis | |||||||
| All‐cause death, MI, or stroke | 3.7% | 5.2% | 0.70 (0.38–1.28) | 2.4% | 2.7% | 0.91 (0.60–1.37) | 0.49 |
| Cardiovascular death, MI, stroke, definite or probable ST, or severe recurrent ischemia | 5.8% | 8.1% | 0.70 (0.44–1.14) | 4.4% | 4.5% | 0.97 (0.71–1.33) | 0.27 |
| All‐cause death | 1.0% | 2.0% | 0.53 (0.19–1.52) | 1.0% | 1.0% | 1.06 (0.55–2.05) | 0.27 |
| Major/minor bleeding | 1.9% | 1.6% | 1.13 (0.44–2.91) | 1.1% | 1.0% | 1.10 (0.59–2.08) | 0.97 |
Estimates are calculated by the Kaplan‒Meier method.
ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; CT, conventional therapy; GGT, genotype‐guided therapy; HR, hazard ratio; MI, myocardial infarction; and ST, stent thrombosis.
Figure 4. Incidence of death, myocardial infarction, or stroke in patients who received genotype‐guided or conventional therapy by ABCD‐GENE score groups.
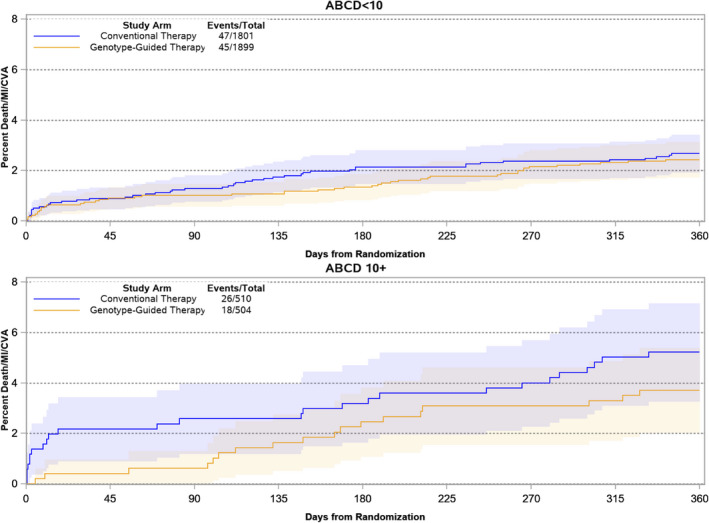
At 1 year, the risk of death, myocardial infarction, or stroke was not significantly reduced in the genotype‐guided therapy arm compared with the conventional therapy in both the high and low ABCD‐GENE score g subgroups. ABCG‐GENE denotes Age, Body Mass Index, Chronic Kidenye Disease, Diabetes, and Genotyping; CVA, cerebrovascular accidents; HR, hazard ratio; and MI, myocardial infarction.
Table 4.
Clinical Outcomes With Genotype‐guided Therapy or Conventional Therapy in Patients With High and Low ABCD‐GENE‐Score and Carriers of CYP2C19 Loss‐of‐Function Mutation
| Variable |
High (≥10) ABCD‐GENE scores (n=856) |
Low (<10) ABCD‐GENE score (n=839) |
Pint | ||||
|---|---|---|---|---|---|---|---|
| GGT | CT | HR (95% CI) | GGT | CT | HR (95% CI) | ||
| Time to first event analysis | |||||||
| All‐cause death, MI, or stroke | 3.2% | 4.1% | 0.76 (0.37–1.57) | 1.5% | 1.9% | 0.81 (0.28–2.32) | 0.93 |
| Cardiovascular death, MI, stroke, definite or probable ST, or severe recurrent ischemia | 5.4% | 7.2% | 0.73 (0.42–1.26) | 3.1% | 4.7% | 0.64 (0.31–1.31) | 0.78 |
| All‐cause death | 1.0% | 1.4% | 0.67 (0.19–2.36) | 0.5% | 1.0% | 0.54 (0.10–2.94) | 0.84 |
| Major/minor bleeding | 1.3% | 1.7% | 0.72 (0.23–2.25) | 2.3% | 1.2% | 1.95 (0.65–5.82) | 0.21 |
Estimates are calculated by the Kaplan‒Meier method.
ABCD‐GENE indicates Age, Body Mass Index, Chronic Kidney Disease, Diabetes, and Genotyping; CT, conventional therapy; GGT, genotype‐guided therapy; HR, hazard ratio; MI, myocardial infarction; and ST, stent thrombosis.
At 3 months, the risk of the primary composite outcome in the primary cohort was not significantly reduced in the genotype‐guided therapy arm compared with the conventional therapy arm in patients with low ABCD‐GENE score (1.0% versus 1.3%; HR, 0.79; 95% CI, 0.43–1.45), while it was significantly reduced in patients with a high ABCD‐GENE score (0.6% versus 2.6%; HR, 0.26; 95% CI, 0.08–0.86), although the statistical interaction was formally not significant (P value for interaction=0.11). Similarly, the risk of the secondary composite outcome was significantly reduced in the genotype‐guided therapy arm compared with the conventional therapy arm in patients with high ABCD‐GENE score (0.8% versus 3.4%; HR, 0.26; 95% CI, 0.09–0.74) but not in patients with low ABCD‐GENE score (1.8% versus 2.0%; HR, 0.90; 95% CI, 0.56–1.45; P value for interaction=0.034).
DISCUSSION
The key findings of this post hoc analysis of the TAILOR‐PCI trial can be summarized as follows. First, in a large sample of patients discharged on clopidogrel from a trial of genetic testing to guide the selection of P2Y12 inhibitors, the ABCD‐GENE score identified a sizeable proportion of individuals (ie, 1 out of 6) with a higher risk of death, MI, or stroke at 12 months. Secondly, the absolute and relative treatment effects at 12 months of genotype‐guided therapy versus conventional therapy appeared nominally larger in patients with a high ABCD‐GENE score, but these results were not statistically significant. Therefore, these findings must be considered exploratory in nature and only generate the hypothesis that the ABCD‐GENE score might help in isolating a subset of patients at increased risk of clinical events after PCI, who may benefit from escalation of DAPT because of increased baseline risk that is not only attributable to their genetic make‐up, but also attributable to the presence of specific demographic and clinical factors.
The ABCD‐GENE score enhances the predictive ability of genotype testing for on clopidogrel‐HPR by adding clinical variables (ie, age, body mass index, chronic kidney disease, and diabetes) that are also associated with clopidogrel response. 17 In particular, using a cutoff value of 10 points, the score identifies with high specificity and positive predictive value the probability of on clopidogrel‐HPR. Given the established association of its components with clinical outcomes, the score was also found to predict recurrent atherothrombotic events in patients with acute coronary syndromes. 17 This predictive ability was confirmed in the present study, including a broad cohort of patients undergoing PCI, where patients with a high ABCD‐GENE score had a 2‐fold higher risk of hard clinical events, with no interaction based on clinical presentation (acute or chronic coronary artery disease). The score also predicted bleeding, which is not surprising given the inclusion of clinical variables that are linked with bleeding (ie, age, chronic kidney disease) and also emerged as univariate predictors in exploratory analyses of our data set (data not shown). Of interest, genotyping alone did not predict the occurrence of adverse clinical outcomes in our study. Such finding was unexpected in light of the well‐established association between CYP2C19 LOF alleles, on clopidogrel‐HPR, and thrombotic complications in prior studies. 3 However, it may suggest that a consistent link with the low incidence of clinical outcomes in the modern coronary artery stenting era may be more elusive without consideration of other factors that contribute to ischemic risk. Hence, the addition of age, body mass index, renal function and diabetes increases the predictivity of genotype testing for on clopidogrel‐HPR and improves the ability to predict ischemic events. 17 Of note, although the ABCD‐GENE score was originally derived and validated for the pharmacodynamic outcome of HPR and clinical outcomes in populations mostly composed of Whites, the predictive value of this score to identify HPR status was recently validated among Asians who were significantly represented in TAILOR PCI. 19 These observations support the validity of the ABCD‐GENE score across ethnicities.
The high predictive value of the ABCD‐GENE score might be useful in selecting a proportion of patients, particularly those with acute coronary syndromes, for de‐escalation from prasugrel or ticagrelor to clopidogrel, which is a strategy suggested to reduce the risk of bleeding with no trade‐off in ischemic events. 4 , 14 , 20 Similar to other standard‐of‐care laboratory assessments, the use of rapid genetic testing assays allows obtaining results in a timely fashion for decision making on the choice of P2Y12 inhibitor at the time of PCI. 21 Although according to guidelines prasugrel and ticagrelor remain the preferential options in patients with acute coronary syndromes, this means offering clopidogrel rather than prasugrel or ticagrelor to patients with high bleeding but low ischemic risk, or in other clinical scenarios such as drug intolerance or cost issues. The objective of the present study was to test the hypothesis that the ABCD‐GENE score might be useful in selecting candidates to the opposite and complementary strategy, ie, escalating from clopidogrel to a more potent P2Y12 inhibitor to reduce the incidence of ischemic events. In a recent meta‐analysis, guided escalation of antiplatelet therapy (ie, by means of genotype or platelet‐function testing) was found to reduce the incidence of ischemic events with no increase in bleeding events. 14 In studies from that meta‐analysis, escalation was achieved by a switch from clopidogrel alone to ticagrelor, prasugrel, double‐dose clopidogrel, or addition of cilostazol; the results of our analysis are specific to escalation to ticagrelor.
Although the escalation strategy was not significantly effective in TAILOR‐PCI, benefit was suggested in the treatment effect (HR, 0.21) at 3 months, and the statistically neutral results of the study may be explained by a smaller than anticipated treatment effect (ie, 34% observed versus 50% predicted relative risk reduction with the initial genotyping strategy) which the study was not sufficiently powered to detect. 15 , 22 We hypothesized that restricting the analysis to patients with a high ABCD‐GENE score could identify a high‐risk subgroup for future studies of guided escalation of DAPT, where the treatment effect of ticagrelor escalation would be presumably even more pronounced. In our study, the CIs around the point estimate for each comparison of genotype‐guided and conventional therapy in ABCD‐GENE score subgroups were large. Still, the nominal relative risk reduction of all‐cause death, MI, or stroke at 12 months with the genotype‐guided therapy was 30% in patients with high ABCD‐GENE score and 9% in those with low ABCD‐GENE score, and the nominal relative risk reductions for the secondary composite outcome were 30% and 3%, respectively, suggesting the potential to identify those patients who could derive a benefit. Consistent with the results of TAILOR PCI, the relative risk reduction of the primary end point in patients with high ABCD‐GENE score was magnified in the analysis at 3 months (ie, 77%), suggesting potential clinical implications in patients in whom short DAPT duration is being considered. 1 However, the exploratory nature of these observations makes these findings hypothesis generating and warrants prospective investigation.
Study Limitations
Our findings must be interpreted with caution because the outcomes of subgroups from a neutral trial are prone to type I error (ie, false positives). Also, the large confidence intervals around treatment estimates may reflect a type II error (ie, false negatives). Even so, it remains plausible that patients with a higher ABCD‐GENE score, a marker of adverse events, could be expected to benefit the most from a targeted escalation strategy; this hypothesis warrants further investigation in a dedicated trial. Our analysis at 3 months needs to be considered exploratory in nature and interpreted with caution as the TAILOR PCI trial was designed for 12 months of DAPT. The majority of patients in this PCI cohort presented with acute coronary syndromes, which may limit the generalizability of our findings to patients undergoing PCI in the context of stable coronary artery disease; however, our findings that the ABCD‐GENE score isolates patients at higher risk were consistent irrespective of clinical presentation.
CONCLUSIONS
In a large population of patients undergoing PCI and discharged on clopidogrel, the ABCD‐GENE score identified a subset at increased risk of ischemic events. Escalating to more potent P2Y12 inhibitors (prasugrel or ticagrelor) in patients with a high ABCD‐GENE score and using clopidogrel in the remainder is a plausible working hypothesis for future investigation.
Sources of Funding
Funding for the TAILOR PCI trial was provided by the National Institutes of Health (grants U01HL128606 and U01HL128626).
Disclosures
D.C. declares that he has received consulting and speaking fees from Amgen, Boehringer Ingelheim, Biotronik, Daiichi Sankyo, and Sanofi Aventis outside the present work.
D.J.A. declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical, outside the present work. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and Scott R. MacKenzie Foundation. S.G.G. reports receiving research grant support (eg, steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (eg, advisory boards) from: Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi‐Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, JAMP Pharma, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharma; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Centre for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, and PERFUSE Research Institute. D.Y.S. is supported by a Mid‐Career Investigator Award of the Heart and Stroke Foundation of Ontario. He has received unrestricted grant support (physician‐initiated grants) from Eli Lilly Canada, Spartan Biosciences, Aggredyne, Diapharma /Roche Diagnostics, Fujimori Kogyo; is a member of the advisory board and has received honoraria from AstraZeneca Canada, Bayer Canada, Servier Canada. All other authors declare no conflicts of interest.
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy. J Am Coll Cardiol. 2018;72:2915–2931. doi: 10.1016/j.jacc.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 2. Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel. J Am Coll Cardiol. 2007;49:1505–1516. 10.1016/j.jacc.2006.11.044 [DOI] [PubMed] [Google Scholar]
- 3. Marín F, González‐Conejero R, Capranzano P, Bass TA, Roldán V, Angiolillo DJ. Pharmacogenetics in cardiovascular antithrombotic therapy. J Am Coll Cardiol. 2009;54:1041–1057. 10.1016/j.jacc.2009.04.084 [DOI] [PubMed] [Google Scholar]
- 4. Galli M, Franchi F, Rollini F, Cavallari LH, Capodanno D, Crea F, Angiolillo DJ. Genetic testing in patients undergoing percutaneous coronary intervention: rationale, evidence and practical recommendations. Expert Rev Clin Pharmacol. 2021;14(8):1–16. 10.1080/17512433.2021.1927709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. 10.1001/jama.2009.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Price MJ, Murray SS, Angiolillo DJ, Lillie E, Smith EN, Tisch RL, Schork NJ, Teirstein PS, Topol EJ. Influence of genetic polymorphisms on the effect of high‐ and standard‐dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J Am Coll Cardiol. 2012;59:1928–1937. 10.1016/j.jacc.2011.11.068 [DOI] [PubMed] [Google Scholar]
- 7. Mega JL, Simon T, Collet J‐P, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, et al. Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA. 2010;304:1821–1830. 10.1001/jama.2010.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, et al. Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease. Circulation. 2009;120:2577–2585. 10.1161/CIRCULATIONAHA.109.912550 [DOI] [PubMed] [Google Scholar]
- 9. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 10. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. 10.1038/nrcardio.2014.156 [DOI] [PubMed] [Google Scholar]
- 11. Sibbing D, Aradi D, Alexopoulos D, ten Berg J, Bhatt DL, Bonello L, Collet J‐P, Cuisset T, Franchi F, Gross L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. doi: 10.1016/j.jcin.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 12. Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, So D, Geller N, Goodman SG, Hasan A, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta‐analysis. JACC Cardiovasc Interv. 2021;14:739–750. doi: 10.1016/j.jcin.2021.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ’t Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, et al. A genotype‐guided strategy for Oral P2Y 12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621–1631. doi: 10.1056/NEJMoa1907096 [DOI] [PubMed] [Google Scholar]
- 14. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D, Porto I, Angiolillo DJ. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. Lancet. 2021;397:1470–1483. 10.1016/S0140-6736(21)00533-X [DOI] [PubMed] [Google Scholar]
- 15. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae J‐H, Jeong MH, Chavez I, et al. Effect of genotype‐guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention. JAMA. 2020;324:761. 10.1001/jama.2020.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn H‐P, Büttner HJ, Neumann F‐J. Impact of cytochrome P450 2C19 loss‐of‐function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55:2427–2434. 10.1016/j.jacc.2010.02.031 [DOI] [PubMed] [Google Scholar]
- 17. Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, ten Berg JM, Sibbing D, Price MJ. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel. JACC Cardiovasc Interv. 2020;13:606–617. doi: 10.1016/j.jcin.2020.01.226 [DOI] [PubMed] [Google Scholar]
- 18. Pereira NL, Rihal CS, So DYF, Rosenberg Y, Lennon RJ, Mathew V, Goodman SG, Weinshilboum RM, Wang L, Baudhuin LM, et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv. 2019;12(4). doi: 10.1161/CIRCINTERVENTIONS.119.007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saito Y, Nishi T, Wakabayashi S, Ohno Y, Kitahara H, Ariyoshi N, Kobayashi Y. Validation of the ABCD‐GENE score to identify high platelet reactivity in east Asian patients undergoing percutaneous coronary intervention. Int J Cardiol. 2021;327:15–18. 10.1016/j.ijcard.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 20. Capodanno D, Morice M‐C, Angiolillo DJ, Bhatt DL, Byrne RA, Colleran R, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Trial design principles for patients at high bleeding risk undergoing PCI. J Am Coll Cardiol. 2020;76:1468–1483. doi: 10.1016/j.jacc.2020.06.085 [DOI] [PubMed] [Google Scholar]
- 21. Franchi F, Rollini F, Rivas J, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, Shaikh Z, et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss‐of‐function genotypes. JACC Basic to Transl Sci. 2020;5:419–428. 10.1016/j.jacbts.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moliterno DJ, Smyth SS, Abdel‐Latif A. CYP2C19 genotyping to guide antiplatelet therapy after percutaneous coronary interventions: one size rarely fits all. JAMA. 2020;324:747–749. doi: 10.1001/jama.2020.13094 [DOI] [PubMed] [Google Scholar]


