Abstract
Background
Treatment with an automated external defibrillator (AED) improves outcome in out‐of‐hospital cardiac arrest (OHCA). Audiovisual feedback from an AED may assist bystanders achieve higher quality cardiopulmonary resuscitation. However, the association between audiovisual feedback and clinical outcomes is not well assessed in real‐life OHCA. The aim of this study was to assess the association between audiovisual feedback from an AED used in bystander resuscitation with rates of return of spontaneous circulation (ROSC) and 30‐day survival in a real‐life cohort of patients with OHCA.
Methods and Results
We included 325 patients treated with bystander AED use before arrival of emergency medical services during 2016 to 2019 from the Capital Region of Denmark. Patients were divided into a “feedback” and a “nonfeedback” group, depending on presence of audiovisual feedback from the AED. Audiovisual feedback was defined as voice prompts with continuous feedback to ongoing resuscitation. Rates of ROSC upon hospital admission and 30‐day survival were assessed, and univariate and multivariable models were applied to decide the association to audiovisual feedback. Multivariable models were adjusted for sex, age, primary heart rhythm, and location of OHCA. A total of 155 (48%) patients had a bystander AED applied with audiovisual feedback and 170 (52%) without audiovisual feedback. A lower rate of ROSC was found in the feedback group compared with the nonfeedback group (33% [n=51] versus 45% [n=76]; P=0.03). No association was observed between AV feedback and 30‐day survival (feedback=27% [n=42] and nonfeedback=31% [n=53]; P=0.49). In the unadjusted logistic regression model, audiovisual feedback was associated with a decreased chance of ROSC (odds ratio, 0.61; 95% CI, 0.38–0.95; P=0.03), which remained significant after adjusted analysis (odds ratio, 0.53; 95% CI, 0.29–0.97; P=0.04), whereas we found no significant association between audiovisual feedback and 30‐day survival in the unadjusted and adjusted analyses.
Conclusions
Audiovisual feedback from an AED used by bystanders was associated with a lower chance of ROSC at hospital admission, but we found no significant difference in 30‐day survival. Focus on early and correct bystander cardiopulmonary resuscitation and AED use remain key for OHCA survival.
Keywords: acute cardiac care, cardiopulmonary resuscitation, defibrillators, emergency medical services, feedback, out‐of‐hospital cardiac arrest, return of spontaneous circulation
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- OHCA
out‐of‐hospital cardiac arrest
- ROSC
return of spontaneous circulation
Clinical Perspective
What Is New?
Audiovisual feedback (compression depth and rate) is used in some automated external defibrillators for bystander resuscitation, but the effect on clinical outcomes in real‐life out‐of‐hospital cardiac arrest settings remains unknown.
This study showed an association between the use of automated external defibrillators with audiovisual feedback and lower rates of return of spontaneous circulation at hospital admission but found no difference in 30‐day survival.
What Are the Clinical Implications?
Since audiovisual feedback from an automated external defibrillator was developed to improve compression depth and rate in cardiopulmonary resuscitation, we need to investigate possible explanations to our findings: Does audiovisual feedback prolong hands‐off time? Do bystanders get confused by excessive information, or should audiovisual feedback be used only in certain environments and settings?
Each year up to 700 000 individuals suffer from out‐of‐hospital cardiac arrest (OHCA) in Europe, and despite improved treatment options, survival remains low. 1 , 2 Bystander interventions including bystander cardiopulmonary resuscitation (CPR) and the use of an automated external defibrillator (AED) are very important to optimize OHCA survival and have been emphasized in international resuscitation guidelines. 1 , 3 , 4 , 5 , 6 , 7 In addition, the quality of CPR, including compression rate and depth, has shown to be associated with improved survival following OHCA. 8 , 9 Accordingly, in an effort to improve bystander CPR quality, AEDs providing audiovisual feedback during ongoing CPR have been marketed. Although studies have shown improvements of compression rate and depth when applied by professional emergency medical personnel, no improvements have yet been found in clinical outcomes. 10 , 11 , 12 , 13
The extended usage of public AEDs has provided greater knowledge regarding bystander CPR, which is often found to be inconsistent and outside guideline recommendations. 14 , 15 Laypersons performing CPR are often untrained, and audiovisual feedback may therefore have a greater impact on the quality of bystander CPR. The impact of audiovisual feedback on bystander CPR has previously been investigated in a Danish pilot study, which did not find any association between audiovisual feedback and return of spontaneous circulation (ROSC) or other clinical outcomes. 16 However, the study used data from 2011 to 2014, where bystander CPR, AED usage, and AED deployment in the society was markedly lower compared with 2016 to 2019. 17 In addition, recommendations regarding bystander usage of AEDs with audiovisual feedback are still unclear in international guidelines, and questions remain unanswered. 18 , 19
Based on the above, we aimed to examine the effects of audiovisual feedback in bystander resuscitation on ROSC rates in a large contemporary sample.
Methods
The data, analytic methods, and study materials are available from the corresponding author upon reasonable request and adherence to Danish General Data Protection Regulation rules.
The study was designed as a retrospective, observational study including AED data from the Capital Region of Denmark from the period 2016 to 2019. In Denmark, a nationwide volunteer‐based AED network was established in 2007 (https://www.hjertestarter.dk/english) and further linked to the emergency medical services (EMS) dispatch centers in 2011. 20 , 21 This linkage supports bystanders to suspected OHCA with guidance through the emergency telephone (1‐1‐2) to deliver dispatch‐assisted CPR and, if possible, to retrieve the nearest available AED. Further, in October 2017, a volunteer citizen responder system was implemented in the Capital Region of Denmark, alerting nearby citizen responders through a mobile app to start CPR and retrieve an AED before EMS arrival. 22 If a public‐access AED is applied before the arrival of EMS, the AED is always substituted with an EMS defibrillator to continue resuscitation if ROSC is not already achieved. In the Capital Region of Denmark, AED data are systematically extracted following public‐access AED use. At EMS evacuation from the scene, the public‐access AED is brought to the EMS dispatch center where data are extracted and sent to the receiving hospital; thereafter, the AED is returned to its owner. Data from EMS defibrillators are not routinely extracted following use. OHCA characteristics are obtained by the EMS and included in the Danish Cardiac Arrest Registry, in accordance with Utstein Guidelines. 23
Ethics
Data were handled and approved according to the data protection law (journal number VD‐2018‐28, I‐Suite number 6222), and approval from the Danish Health Authority was also obtained (journal number: 20051145). No ethical approval is needed for retrospective registry studies in Denmark.
Study Population
All OHCA cases treated with an AED by a layperson in the Capital Region of Copenhagen from the period 2016 to 2019 were obtained, and 438 OHCAs were identified where an AED had been applied and used by a bystander before EMS arrival. Patients were admitted at a tertiary heart center if OHCA etiology were considered cardiac, and post–cardiac arrest care was based on identical guidelines throughout the Capital Region of Copenhagen. The ECG obtained from the AED was analyzed by the author (L.O.) and by a cardiologist (F.F.). Analysis included registration of date, social security number, AED manufacturer, primary heart rhythm, time points for “power on” and “power off,” and time points for “pads on” and “pads off.” Cases were excluded because of fault or lack of social security number, serial number, or other possible markers for identification on the AED record. Further, cases with missing data in the Danish Cardiac Arrest Registry were excluded.
Patients were divided into a “feedback” group and a “nonfeedback” group according to the feedback mechanism provided by the AED (Figure). The feedback group included patients for whom the AED continuously delivered audiovisual feedback and a metronome for compression rate during resuscitation, whereas the nonfeedback group involved patients for whom the AED delivered only a metronome or no feedback at all. The latter was therefore further subdivided into a “metronome” and a “no feedback” group for secondary analyses.
Figure 1. Flowchart of inclusion.
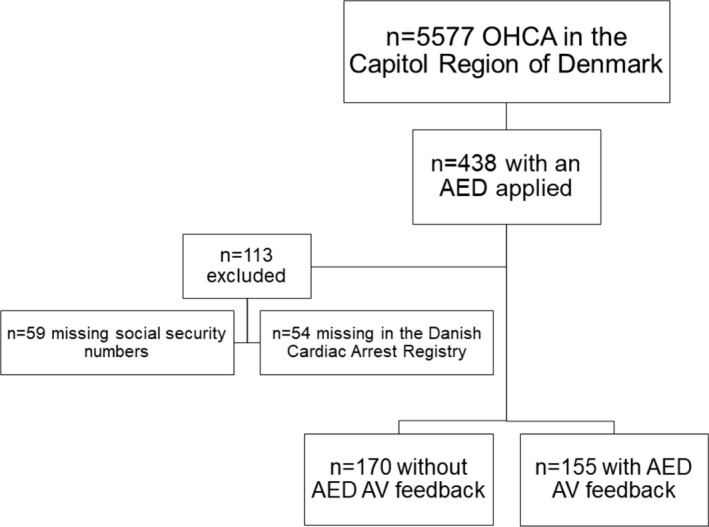
AED indicates automated external defibrillator; AV, audiovisual; and OHCA, out‐of‐hospital cardiac arrest.
AED Models and Audiovisual Feedback
In total, 13 different AED models were included in the study with two ZOLL AED models in the feedback group and 11 AED models in the nonfeedback group. The two ZOLL AED models (48% of all included AEDs) provided audiovisual feedback and metronome guiding, 8 AED models (23% of all included AEDs) provided only a metronome, and 3 AED models provided no feedback or metronome (29% of all included AEDs).
Audiovisual feedback included graphic icons on the AED with adhering voice prompts, providing rescuer feedback step by step through the resuscitation process with consistent and compliant CPR. Thus, the audiovisual feedback seeks to optimize CPR when either the compression rate or depth is not ideal by correcting the bystander performing CPR with audio prompts and further illustrating the CPR step graphically on the AED display. All AEDs in the feedback group were from the manufacturer ZOLL (ZOLL AED Plus and ZOLL AED Pro, ZOLL Medical Corporation), while AEDs in the nonfeedback group were from several other manufacturers (Table S1 lists all AEDs included).
Data Collection
We acquired OHCA data from the Capitol Region of Denmark (included in the nationwide Danish Cardiac Arrest Registry) with information regarding the OHCA incident such as date, time, age, sex, location of OHCA, witnessed OHCA by EMS, witnessed OHCA by bystander, bystander CPR, bystander usage of AED, primary ECG rhythm, EMS response time, ROSC at admission, condition at arrival at the hospital and 30‐day survival. Variables collected from AED extraction included: type of AED (company and model), time points for AED turned on and off, and primary rhythm detected. A small part of the AED ECGs was extracted solely with text information, and a primary rhythm could not be analyzed in these extractions (n=10 in the audiovisual feedback group, n=22 in the nonfeedback group). Additionally, EMS response time was missing in a few cases (n=8).
Outcomes of Interest
ROSC and 30‐day survival were the 2 primary outcomes of interest in this study. ROSC was defined as the resumption of cardiac rhythm with a perceptible pulse and was determined by professional EMS personnel. Thirty‐day survival was defined as survival 30 days from the time of OHCA, with death being all‐cause mortality. The Danish Cardiac Arrest Registry was the source of information for both outcomes of interest.
Statistical Analysis
Categorical variables are presented as numbers (n) and percentages (%), and comparisons between groups were made using the chi‐square test. Continuous variables were presented as means with SDs or medians with 25th to 75th percentiles if a skewed distribution was present. Normality was assessed visually by application of QQ plots. For comparisons of continuous variables between the groups we performed the Student’s t‐test and the Wilcoxon rank‐sum test, as appropriate. Univariate logistic regression analyses were performed to assess the associations between feedback, age, and sex with binary outcomes, including ROSC upon admission and 30‐day survival. We applied multivariable logistic regression to assess the association between feedback and binary outcomes after adjustment for sex, age, primary ECG rhythm, location of OHCA, and EMS response time. The included covariates were selected a priori based on previous knowledge on predictors for ROSC and survival following OHCA. 3 , 4 Further, secondary analyses of 2 subgroups of nonfeedback devices (metronome and no feedback at all) were made, and univariate logistic regression analyses were applied, stratified by primary ECG rhythm since this is an important predictor of ROSC. The multivariable logistic regression analyses were tested for interaction between feedback and all the included covariates. We prespecified to only include interaction links with a P value <0.05 in the final model. A P value <0.05 was considered statistically significant. R Studio, version 1.2.5001, was used for all analyses (RStudio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA; URL: http://www.rstudio.com/).
Results
Of 438 OHCA cases with a bystander AED applied, 325 cases (73%) had data available and included for further analyses (Figure). Of these, 155 (48%) patients had an AED applied with audiovisual feedback and 170 (52%) without audiovisual feedback. No differences in Utstein characteristics were found between the 2 groups (Table 1).
Table 1.
Patient Baseline Characteristics According to AED Feedback
| Audiovisual feedback mechanism | |||||
|---|---|---|---|---|---|
| Variable | N | Overall, N=325*, † | 0, N=170*, † | 1, N=155*, † | P value * , † |
| Age, y, mean (SD) | 325 | 69 (17) | 71 (16) | 68 (17) | 0.11 |
| Sex, male, n (%) | 325 | 227 (70) | 119 (70) | 108 (70) | 0.95 |
| Bystander witnessed, n (%) | 325 | 196 (60) | 107 (63) | 89 (57) | 0.31 |
| Bystander CPR, n (%) | 325 | 314 (97) | 162 (95) | 152 (98) | 0.17 |
| Shockable primary AED rhythm, n (%) | 293 | 125 (43) | 66 (45) | 59 (41) | 0.50 |
| Public place of arrest, n (%) | 325 | 166 (51) | 90 (53) | 76 (49) | 0.48 |
| AED shock before EMS arrival, n (%) | 325 | 134 (41) | 70 (41) | 64 (41) | 0.98 |
| EMS response time, min, median (IQR) | 317 | 6.48 (4.88–8.57) | 6.30 (4.53–7.78) | 7.02 (5.16–9.32) | 0.02 |
| Admission status, n (%) | 325 | 0.12 | |||
| Deceased | 182 (56) | 87 (51) | 95 (61) | ||
| Continuous CPR | 16 (4.9) | 7 (4.1) | 9 (5.8) | ||
| ROSC and comatose | 69 (21) | 44 (26) | 25 (16) | ||
| ROSC and awake | 58 (18) | 32 (19) | 26 (17) | ||
| ROSC upon hospital admission, n (%) | 325 | 127 (39) | 76 (45) | 51 (33) | 0.03 |
| 30‐day survival, n (%) | 325 | 95 (29) | 53 (31) | 42 (27) | 0.42 |
AED indicates automated external defibrillator; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; IQR, interquartile range; and ROSC, return of spontaneous circulation.
Mean (SD); n (%); median (IQR).
Wilcoxon rank‐sum test; Pearson’s chi‐squared test.
ROSC at Admission
Information about ROSC upon arrival at the hospital was available in 325 (100%) of the cases, with 127 (39%) patients achieving ROSC upon hospital arrival.
A total of 51 (33%) patients achieved ROSC at hospital arrival in the feedback group compared with 76 (45%) in the nonfeedback group (P=0.03).
In the unadjusted logistic regression model, we found an association between feedback and ROSC upon admission (odds ratio [OR], 0.61; 95% CI, 0.38–0.95; P=0.03); and after adjustment for age, sex, primary ECG rhythm, location of OHCA, and EMS response time, feedback remained significantly associated with a decreased chance of ROSC upon admission (OR, 0.53; 95% CI, 0.29–0.97; P=0.04; Table 2). We found no significant interaction between feedback and age (P=0.22), sex (P=0.41), rhythm (P=0.17), location (P=0.46), or EMS response time (P=0.46) in multivariable models.
Table 2.
Univariate and Multivariable Cox Regression Analyses of Association to ROSC
| Univariate | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | OR | 95% CI | P value | OR | 95% CI | P value |
| Audiovisual feedback | 325 | 0.61 | 0.38–0.95 | 0.03 | 0.53 | 0.29–0.97 | 0.04 |
| Age, y | 325 | 0.97 | 0.96–0.99 | <0.001 | 0.98 | 0.96–0.99 | 0.009 |
| Sex, male | 325 | 2.76 | 1.64–4.79 | <0.001 | 1.55 | 0.78–3.15 | 0.2 |
| Primary AED rhythm, shockable | 293 | 9.02 | 5.33–15.6 | <0.001 | 8.11 | 4.50–15.1 | <0.001 |
| Place of arrest, public | 325 | 4.98 | 3.08–8.23 | <0.001 | 3.38 | 1.82–6.37 | <0.001 |
| EMS response time, min | 317 | 0.96 | 0.89–1.03 | 0.3 | 1.01 | 0.92–1.10 | 0.9 |
The multivariable model was adjusted for all variables included in the table.
AED indicates automated external defibrillator; EMS, emergency medical services; OR, odds ratio, and ROSC, return of spontaneous circulation.
Thirty‐Day Survival
Information on 30‐day survival was available in 325 (100%) patients and 95 (29%) patients achieved 30‐day survival.
In the feedback group, 42 (27%) patients achieved 30‐day survival compared with 53 (31%) in the nonfeedback group (P=0.42).
We found no association between feedback and 30‐day survival in the unadjusted logistic regression model (OR, 0.82; 95% CI, 0.51–1.32; P=0.42) or in the multivariable model adjusted for age, sex, primary ECG rhythm, location of OHCA, and EMS response time (OR, 0.57; 95% CI, 0.42–1.61; P=0.57). There was no significant interaction between feedback and age (P=0.99), sex (P=0.20), rhythm (P=0.0.67), location (P=0.69), or EMS response time (P=0.21) in multivariable models.
Subgroup Analyses
When dividing AEDs without audiovisual feedback into subgroups, 77 AEDs (45%) supplied a metronome, and 93 (55%) supplied no feedback at all. ROSC upon hospital admission was achieved in 38 (49% and 41%) patients in the metronome and no feedback group, accordingly.
Rates of ROSC were lower in the audiovisual feedback group compared with the metronome group (P=0.02). Further, in the unadjusted univariate model, we found a decreased chance of ROSC in the feedback group (OR, 0.50; 95% CI, 0.29–0.88; P=0.02), but after adjustment for age, sex, primary ECG rhythm, location of OHCA, and EMS response time, feedback was not significantly associated with a decreased chance of ROSC upon hospital admission (OR, 0.65; 95% CI, 0.30–1.42; P=0.28).
Rates of ROSC were not different between the audiovisual feedback group and AEDs with no feedback at all (P=0.21). We found no association between feedback and ROSC in the unadjusted univariate model (OR, 0.71; 95% CI, 0.42–1.21; P=0.21), but after adjustment for age, sex, primary ECG rhythm, location of OHCA, and EMS response time, feedback was associated with a decreased chance of ROSC upon hospital admission (OR, 0.45; 95% CI, 0.21–0.91; P=0.03).
There was no difference in rates of ROSC between the metronome group and AEDs with no feedback at all (P=0.27), and no association with ROSC was found in either the unadjusted univariate model (OR, 1.41; 95% CI, 0.77–2.60; P=0.27) or the adjusted multivariable model (OR, 0.70; 95% CI, 0.28–1.66; P=0.43).
We further divided patients into “shockable” (ventricular fibrillation/ventricular tachycardia, n=125) and “nonshockable” rhythm (pulseless electric activity/asystole, n=168), according to primary rhythm detection on the AED ECG. When comparing these 2 groups stratified by audiovisual feedback, we found no difference in proportions of ROSC and 30‐day survival (ROSC, shockable, P=0.32; nonshockable, P=0.06; and 30‐day survival, shockable, P=0.28; nonshockable, P=0.37).
Further, we found no difference between the 2 audiovisual feedback groups in regard to conversion to a shockable rhythm for patients in the nonshockable group (P=0.64) and time from AED onset to the attachment of AED pads (P=0.54).
Discussion
This study sought to investigate whether bystander usage of AEDs providing audiovisual feedback for bystander CPR in patients with OHCA was associated with a higher rate of ROSC at hospital admission as well as increased 30‐day survival. We found that AEDs with audiovisual feedback had a decreased chance of ROSC at hospital admission, but no difference in 30‐day survival, compared with nonfeedback AEDs.
In resuscitation, both the bystander use of an AED and early bystander CPR are associated with improved clinical outcome. 21 , 24 , 25 CPR in cardiac arrest can, if performed correctly, maintain a sufficient degree of arterial perfusion to crucial organs such as the brain and heart. 26 In an effort to improve bystander CPR quality, audiovisual feedback has been integrated into several AEDs marketed in recent years (ZOLL AED Plus and Pro, Powerheart AED G5, and Samaritan PAD 500P). This subsequent development is attributable to studies reporting that EMS personnel provide better‐quality CPR with the addition of audiovisual feedback, although there has been no evidence of improved patient outcomes. 10 , 11 Unlike a previous smaller Danish pilot study, 16 we found that audiovisual feedback from an AED used in bystander resuscitation was associated with lower rates of ROSC, though with no impact on 30‐day survival. Guidelines from the European Resuscitation Council from 2010 and 2015 both suggested the beneficial use of audiovisual feedback in basic life support resuscitation performed by bystanders, but recent European Resuscitation Council Guidelines from 2021 are more cautious and indicate that audiovisual feedback may not be beneficial for bystanders. 1 , 27 , 28 Further, updated recommendations from the International Consensus on Cardiopulmonary Resuscitation suggest the use of audiovisual feedback during CPR in clinical practice but against the use of audiovisual feedback in CPR in isolation. 18 The American Heart Association 2020 Guidelines states that audiovisual feedback in resuscitation may be reasonable for improving CPR quality and that no significant harm has been shown. 19 Based on these latest differing recommendations and an increasing bystander assistance with the use of AEDs in prehospital resuscitation the past 10 years, we believe that renewed investigation regarding the effect of audiovisual feedback from AEDs on clinical outcomes in bystander resuscitation is of great relevance. Further, although the use of bystander AEDs has increased, there is still a great need for further attention and promotion, since the intervention is significantly associated with good outcome in OHCA.
In a study by Nishi et al, 15 CPR delivered by a health care worker or trained layperson was compared with nontrained laypersons, with pauses being further prolonged in laypersons without CPR training. This implies that laypersons without sufficient training in CPR may need additional attention and support in CPR during resuscitation, which could be partly remedied by audiovisual feedback from an AED in addition to dispatch‐assisted CPR. Since this study did not contain data on CPR metrics to analyze the quality of CPR, there are 2 possible explanations of the results, one being that audiovisual feedback improves bystander CPR quality without improving outcomes, and the other being that audiovisual feedback does not improve CPR quality sufficiently in untrained bystanders to improve outcome.
Further, time to treatment is extremely important in resuscitation, and one may argue that audiovisual feedback is a distracting and time‐limiting factor for bystanders providing CPR. According to the latest guidelines in basic life support, the focus should be on minimizing both no and low flow time to provide a critical amount of cerebral circulation until the arrival of EMS and the possible start of advanced life supportive treatment. 28 The distraction of extraneous audiovisual feedback and prompts may preclude the responder’s capability to improve delivery of CPR and rapid defibrillation in a critical situation. Furthermore, the specially designed AED feedback pads could be more complicated to apply for laypersons and crucial “hands‐off” time with delaying CPR, which is not the case for regular AED pads. Hands‐off time is known to be an important factor for clinical outcome, 29 but a study by the Resuscitation Outcomes Consortium Investigators found that in EMS resuscitation one‐third of the time was hands‐off and that less than one‐third of the patients received CPR with an optimal compression rate and depth. 30 Further, Cheskes et al 31 reported that minimizing pre‐ and perishock pauses was associated with higher odds of survival. To minimize pauses, next generation AEDs with artifact filtering technology to visualize underlying ECG rhythms could allow ongoing CPR and optimization of resuscitation.
In our study, we did not find any differences in rates of ROSC or 30‐day survival in victims presenting with a shockable versus nonshockable primary rhythm, a finding in accordance with previous studies. 32 , 33 Furthermore, we did not find an association between rhythm conversion from nonshockable to shockable rhythm during resuscitation. Rhythm conversion is not well understood, but the mechanism is suggested to be generated through an increased myocardial perfusion and excitability attributable to CPR. 34 However, rhythm conversion—and subsequently delivery of shock—from an initial nonshockable rhythm may be associated with improved prognosis according to some studies. 35 , 36 A study by Goto et al 37 reported benefits of rhythm conversion only being within the initial 20 minutes of cardiac arrest; and, further, a study by Zheng et al 38 suggested that a conversion from an initial nonshockable rhythm during CPR was associated with only improved outcomes in patients with asystole as the initial rhythm. Nevertheless, rhythm conversion during resuscitation may depend on the quality of early CPR provided, and therefore an improvement of bystander CPR by audiovisual feedback from an AED could, theoretically, lead to a larger number of patients obtaining shockable rhythms and improved prognosis.
Limitations
An important limitation to our study was the lack of information on bystander training in CPR. A recent study found a correlation between higher levels of CPR training and CPR quality in bystander resuscitation. 39 This association seems obvious, but quality of bystander CPR is extremely difficult to investigate in real‐life studies. Development and monitoring with audiovisual feedback could be part of the solution, and possible benefits should be further investigated, but in this study, we had no information on the quality of CPR provided by bystanders or EMS personnel. In addition, no data were available on the treatment delivered by EMS or post–cardiac arrest care following admission. Further, AEDs included in the audiovisual feedback group consisted solely of ZOLL devices, which could be an explanatory factor of the study results rather than the presence of audiovisual feedback.
In our study, CPR metrics were not included because a large sample of the AEDs applied did not record CPR metrics of sufficient quality for analysis. Therefore, a comparison of CPR metrics between different AED feedback mechanisms could not be made.
Another limitation of our study was the lack of information on geographic location of the AEDs deployed. Hence, only location of OHCA was assessable and not location of the bystander AED used in resuscitation. If AEDs with feedback mechanisms are more often deployed at locations associated with poor outcome following OHCA (eg, in private homes and residential areas), this could affect the observed outcomes. Currently in Denmark, AEDs deliver shock before EMS arrival in ≈23% of all OHCA events in public locations, such as train stations and shops, compared with 6.7% in private homes. 17 Unfortunately, we did not have information on AED location by type of AED feedback in our study.
Conclusions
In this observational study, we found that bystander usage of AEDs with audiovisual feedback in resuscitation of patients with OHCA was associated with a decreased chance of ROSC at hospital admission but no difference in 30‐day survival, compared with patients with a nonfeedback AED involved in bystander resuscitation.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1
Presented in part at ESC: Acute CardioVascular Care (ACVC) Congress, Prague (E‐congress), March 12–14, 2021 and published in abstract form (Eur Heart J Acute Cardiovasc Care. 2021; Volume 10, Issue Supplement_1. https://doi.org/10.1093/ehjacc/zuab020.145).
For Sources of Funding and Disclosures, see page 7.
References
- 1. Perkins GD, Handley AJ, Koster RW, Castrén M, Smyth MA, Olasveengen T, Monsieurs KG, Raffay V, Gräsner JT, Wenzel V, et al. European Resuscitation Council Guidelines for Resuscitation 2015. Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015;95:81–99. doi: 10.1016/j.resuscitation.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 2. Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS‐treated out‐of‐hospital cardiac arrest in Europe. Resuscitation. 2005;67:75–80. doi: 10.1016/j.resuscitation.2005.03.021 [DOI] [PubMed] [Google Scholar]
- 3. Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang‐Jensen T, Olesen JB, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out‐of‐hospital cardiac arrest. JAMA. 2013;310:1377–1384. doi: 10.1001/jama.2013.278483 [DOI] [PubMed] [Google Scholar]
- 4. Kragholm K, Wissenberg M, Mortensen RN, Hansen SM, Malta Hansen C, Thorsteinsson K, Rajan S, Lippert F, Folke F, Gislason G, et al. Bystander efforts and 1‐year outcomes in out‐of‐hospital cardiac arrest. N Engl J Med. 2017;376:1737–1747. doi: 10.1056/NEJMoa1601891 [DOI] [PubMed] [Google Scholar]
- 5. Buick JE, Drennan IR, Scales DC, Brooks SC, Byers A, Cheskes S, Dainty KN, Feldman M, Verbeek PR, Zhan C, et al. Improving temporal trends in survival and neurological outcomes after out‐of‐hospital cardiac arrest. Circ ‐Cardiovasc Qual Outcomes. 2018;11:1–11. doi: 10.1161/CIRCOUTCOMES.117.003561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holmberg MJ, Vognsen M, Andersen MS, Donnino MW, Andersen LW. Bystander automated external defibrillator use and clinical outcomes after out‐of‐hospital cardiac arrest: a systematic review and meta‐analysis. Resuscitation. 2017;120:77–87. doi: 10.1016/j.resuscitation.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 7. Weisfeldt ML, Sitlani CM, Ornato JP, Rea T, Aufderheide TP, Davis D, Dreyer J, Hess EP, Jui J, Maloney J, et al. Survival after application of automatic external defibrillators before arrival of the emergency medical system. Evaluation in the resuscitation outcomes consortium population of 21 million. J Am Coll Cardiol. 2010;55:1713–1720. doi: 10.1016/j.jacc.2009.11.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pepe PE, Duval S, Aufderheide TP, Goodloe JM, Debaty G, Labarère J, Sugiyama A, Yannopoulos D. Optimal combination of compression rate and depth during cardiopulmonary resuscitation for functionally favorable survival. JAMA Cardiol. 2019;4:900–908. doi: 10.1001/jamacardio.2019.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Idris AH, Guffey D, Pepe PE, Brown SP, Brooks SC, Callaway CW, Christenson J, Davis DP, Daya MR, Gray R, et al. Chest compression rates and survival following out‐of‐hospital cardiac arrest. Crit Care Med. 2015;43:840–848. doi: 10.1097/CCM.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 10. Kirkbright S, Finn J, Tohira H, Bremner A, Jacobs I, Celenza A. Audiovisual feedback device use by health care professionals during CPR: a systematic review and meta‐analysis of randomised and non‐randomised trials. Resuscitation. 2014;85:460–471. doi: 10.1016/j.resuscitation.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 11. Hostler D, Everson‐Stewart S, Rea TD, Stiell IG, Callaway CW, Kudenchuk PJ, Sears GK, Emerson SS, Nichol G. Effect of real‐time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster‐randomised trial. BMJ. 2011;342:371. doi: 10.1136/bmj.d512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeung J, Meeks R, Edelson D, Gao F, Soar J, Perkins GD. The use of CPR feedback/prompt devices during training and CPR performance: a systematic review. Resuscitation. 2009;80:743–751. doi: 10.1016/j.resuscitation.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 13. Bohn A, Weber TP, Wecker S, Harding U, Osada N, Van Aken H, Lukas RP. The addition of voice prompts to audiovisual feedback and debriefing does not modify CPR quality or outcomes in out of hospital cardiac arrest–a prospective, randomized trial. Resuscitation. 2011;82:257–262. doi: 10.1016/j.resuscitation.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 14. Gyllenborg T, Granfeldt A, Lippert F, Riddervold IS, Folke F. Quality of bystander cardiopulmonary resuscitation during real‐life out‐of‐hospital cardiac arrest. Resuscitation. 2017;120:63–70. doi: 10.1016/j.resuscitation.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 15. Nishi T, Takei Y, Kamikura T, Ohta K, Hashimoto M, Inaba H. Improper bystander‐performed basic life support in cardiac arrests managed with public automated external defibrillators. Am J Emerg Med. 2015;33:43–49. doi: 10.1016/j.ajem.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 16. Agerskov M, Hansen MB, Nielsen AM, Møller TP, Wissenberg M, Rasmussen LS. Return of spontaneous circulation and long‐term survival according to feedback provided by automated external defibrillators. Acta Anaesthesiol Scand. 2017;61:1345–1353. doi: 10.1111/aas.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danish Cardiac Arrest Registry ‐ Annual Report . 2020. Available at: https://hjertestopregister.dk/?page_id=428. Accessed January 30, 2021
- 18. Olasveengen TM, Mancini ME, Perkins GD, Avis S, Brooks S, Castrén M, Chung SP, Considine J, Couper K, Escalante R, et al. Adult basic life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:41–91. doi: 10.1161/CIR.0000000000000892 [DOI] [PubMed] [Google Scholar]
- 19. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:366–468. doi: 10.1161/CIR.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 20. Hansen CM, Lippert FK, Wissenberg M, Weeke P, Zinckernagel L, Ruwald MH, Karlsson L, Gislason GH, Nielsen SL, Køber L, et al. Temporal trends in coverage of historical cardiac arrests using a volunteer‐based network of automated external defibrillators accessible to laypersons and emergency dispatch centers. Circulation. 2014;130:1859–1867. doi: 10.1161/CIRCULATIONAHA.114.008850 [DOI] [PubMed] [Google Scholar]
- 21. Hansen SM, Hansen CM, Folke F, Rajan S, Kragholm K, Ejlskov L, Gislason G, Køber L, Gerds TA, Hjortshøj S, et al. Bystander defibrillation for out‐of‐hospital cardiac arrest in public vs residential locations. JAMA Cardiology. 2017;2:507–514. doi: 10.1001/jamacardio.2017.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andelius L, Malta Hansen C, Lippert FK, Karlsson L, Torp‐Pedersen C, Kjær Ersbøll A, Køber L, Collatz Christensen H, Blomberg SN, Gislason GH, et al. Smartphone activation of citizen responders to facilitate defibrillation in out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2020;76:43–53. doi: 10.1016/j.jacc.2020.04.073 [DOI] [PubMed] [Google Scholar]
- 23. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out‐of‐hospital cardiac arrest: a statement for healthcare professionals from a task force of the International Liaison Committee. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 24. Blom MT, Beesems SG, Homma PCM, Zijlstra JA, Hulleman M, Van Hoeijen DA, Bardai A, Tijssen JGP, Tan HL, Koster RW. Improved survival after out‐of‐hospital cardiac arrest and use of automated external defibrillators. Circulation. 2014;130:1868–1875. doi: 10.1161/CIRCULATIONAHA.114.010905 [DOI] [PubMed] [Google Scholar]
- 25. Wellens HJ, Lindemans FW, Houben RP, Gorgels AP, Volders PG, Ter Bekke RMA, Crijns HJ. Improving survival after out‐of‐hospital cardiac arrest requires new tools. Eur Heart J. 2016;37:1499–1503. doi: 10.1093/eurheartj/ehv485 [DOI] [PubMed] [Google Scholar]
- 26. Lurie KG, Nemergut EC, Yannopoulos D, Sweeney M. The physiology of cardiopulmonary resuscitation. Anest Analg. 2016;122:767–783. doi: 10.1213/ANE.0000000000000926 [DOI] [PubMed] [Google Scholar]
- 27. Koster RW, Baubin MA, Bossaert LL, Caballero A, Cassan P, Castrén M, Granja C, Handley AJ, Monsieurs KG, Perkins GD, et al. European Resuscitation Council Guidelines for Resuscitation 2010 section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81:1277–1292. doi: 10.1016/j.resuscitation.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olasveengen TM, Semeraro F, Ristagno G, Castren M, Handley A, Kuzovlev A, Monsieurs KG, Raffay V, Smyth M, Soar J, et al. European Resuscitation Council Guidelines 2021: basic life support. Resuscitation. 2021;161:98–114. doi: 10.1016/j.resuscitation.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 29. Cunningham LM, Mattu A, O’Connor RE, Brady WJ. Cardiopulmonary resuscitation for cardiac arrest: the importance of uninterrupted chest compressions in cardiac arrest resuscitation. Am J Emerg Med. 2012;30:1630–1638. doi: 10.1016/j.ajem.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 30. Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, Kudenchuk PJ, Christenson J, Daya MR, Dorian P, et al. A trial of an impedance threshold device in out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365:798–806. doi: 10.1056/NEJMoa1010821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheskes S, Schmicker RH, Verbeek PR, Salcido DD, Brown SP, Brooks S, Menegazzi JJ, Vaillancourt C, Powell J, May S, et al. The impact of peri‐shock pause on survival from out‐of‐hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Resuscitation. 2014;85:336–342. doi: 10.1016/j.resuscitation.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herlitz J, Svensson L, Engdahl J, Silfverstolpe J. Characteristics and outcome in out‐of‐hospital cardiac arrest when patients are found in a non‐shockable rhythm. Resuscitation. 2008;76:31–36. doi: 10.1016/j.resuscitation.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 33. Myat A, Song K, Rea T. Out‐of‐hospital cardiac arrest: current concepts. Lancet. 2018;391:970–979. doi: 10.1016/S0140-6736(18)30472-0 [DOI] [PubMed] [Google Scholar]
- 34. Wah W, Wai KL, Pek PP, Ho AFW, Alsakaf O, Chia MYC, Noor JM, Kajino K, De Souza NNA, Ong MEH, et al. Conversion to shockable rhythms during resuscitation and survival for out‐of hospital cardiac arrest. Am J Emerg Med. 2017;35:206–213. doi: 10.1016/j.ajem.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 35. Luo S, Zhang Y, Zhang W, Zheng R, Tao J, Xiong Y. Prognostic significance of spontaneous shockable rhythm conversion in adult out‐of‐hospital cardiac arrest patients with initial non‐shockable heart rhythms: a systematic review and meta‐analysis. Resuscitation. 2020;121:1–8. doi: 10.1016/j.resuscitation.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 36. Olasveengen TM, Samdal M, Andreas P, Wik L, Sunde K. Progressing from initial non‐shockable rhythms to a shockable rhythm is associated with improved outcome after out‐of‐hospital cardiac arrest. Resuscitation. 2020;80:24–29. doi: 10.1016/j.resuscitation.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 37. Goto Y, Maeda T, Nakatsu‐Goto Y. Prognostic implications of conversion from nonshockable to shockable rhythms in out‐of‐hospital cardiac arrest. Crit Care. 2014;18:1–9. doi: 10.1186/s13054-014-0528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng R, Luo S, Liao J, Liu Z, Xu J, Zhan H, Liao X, Xiong Y, Idris A. Conversion to shockable rhythms is associated with better outcomes in out‐of‐hospital cardiac arrest patients with initial asystole but not in those with pulseless electrical activity. Resuscitation. 2016;107:88–93. doi: 10.1016/j.resuscitation.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 39. Lund‐Kordahl I, Mathiassen M, Melau J, Olasveengen TM, Sunde K, Fredriksen K. Relationship between level of CPR training, self‐reported skills, and actual manikin test performance–an observational study. Int J Emerg Med. 2019;12:2. doi: 10.1186/s12245-018-0220-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


