Angiotensin II (Ang II) type 1 receptors (AT1R) in the kidneys are the primary mediators of Ang II‐induced hypertension. 1 The effects of Ang II have been attributed mainly to the Gq/11‐coupled AT1a receptor (AT1aR) subtype, although AT1b receptors (AT1bR) are also expressed in blood vessel walls. 2 Recent studies support the concept that AT1aR is a mechanosensitive receptor in smooth muscle cells (SMCs) and plays a vital role in intraluminal pressure‐induced (myogenic) vasoconstriction. 3 However, the relative contributions of Gq/11 protein signaling and noncanonical β‐arrestin signaling in AT1aR regulation of myogenic vasoconstriction were unknown. A study published by Cui and colleagues in this issue of the Journal of the American Heart Association (JAHA) proposes that G proteins q/11 subunits (Gq/11)‐dependent signaling pathways, but not β‐arrestin‐dependent signaling, play a vital role in AT1aR‐induced development of myogenic vasoconstriction (Figure). 4 Although AT1bR has also been implicated in myogenic vasoconstriction, the authors present data that AT1bR deletion does not affect vasoconstriction in mouse renal arterioles. 4 Notably, the use of tamoxifen‐inducible, SMC‐specific, AT1aR knockout (SMMHC‐Cre+Agtr1a−/−) mice in this study has resulted in definitive evidence that AT1aR is essential for myogenic constriction of cerebral, mesenteric, and renal arteries. While pressure myography is a well‐established standard for studying the effect of intraluminal pressure on arterial contraction, the studies in isolated perfused kidneys are physiologically more relevant. Collectively, the findings in the article by Cui et al support the idea that SMC AT1aRs play a critical role in pressure‐induced vasoconstriction but do not influence cardiac function. 4 Understanding the signaling linkages of AT1aRs in myogenic vasoconstriction will be a crucial next step in the process of developing therapeutic strategies against hypertension.
Figure 1. Schematic diagram showing AT1aR‐dependent signaling activated by intraluminal pressure.
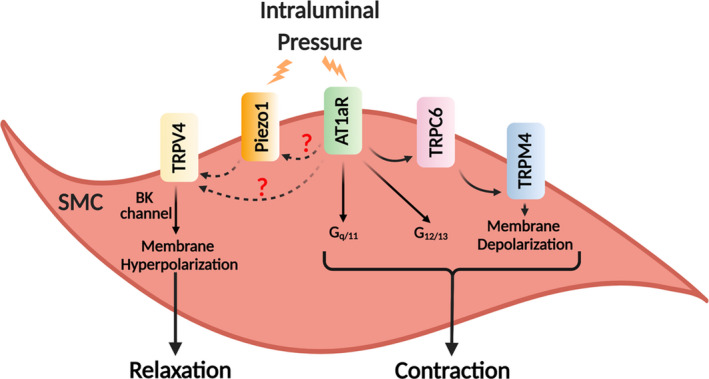
Intraluminal pressure induces vascular smooth muscle cells (SMC) contraction via AT1aR activation and Gq/11 signaling. Activation of AT1aRs elicits TRPC6 channel−TRPM4 channel signaling and membrane depolarization. The figure also shows potential signaling linkages of AT1aRs with TRPV4 and Piezo1 channels on the SMC membrane. AT1aR indicates angiotensin II 1a receptor subtype; BK channel, Ca2+‐activated large‐conductance K+ channel; G12/13, G proteins 12/13 subunits; Gq/11, G proteins q/11 subunits; TRPC6, transient receptor potential canonical 6; TRPM4, transient receptor potential melastatin 4; and TRPV4, transient receptor potential vanilloid 4.
More than a century ago, Bayliss reported intraluminal pressure‐induced vasoconstriction as an autoregulatory mechanism in small arteries. 5 Several signaling mechanisms have been proposed as mediators of myogenic vasoconstriction. Two events appear to be absolutely crucial for the development of myogenic vasoconstriction: SMC membrane depolarization and subsequent activation of voltage‐gated Ca2+ channels. Over the past 2 decades, research efforts have focused on deciphering the mechanisms for intraluminal pressure‐induced depolarization of SMC membranes. The activation of Piezo1, transient receptor potential melastatin 4 (TRPM4), and TRP canonical 6 (TRPC6) channels on SMC membranes has emerged as key events in pressure‐induced SMC membrane depolarization. More recent studies show that mechanosensitive TRPM4 and Piezo1 channels could be critical players in pressure‐induced membrane depolarization of SMCs. 6 Whether AT1aR is also involved in pressure‐induced membrane depolarization or acts downstream of depolarization is not clear. In this regard, Gonzales and colleagues recently showed that pressure‐induced AT1R activation could lead to the opening of TRPC6 channels and influx of Ca2+ in SMCs, which in turn activates TRPM4 channels and results in SMC membrane depolarization. 7 Collectively, the literature supports an essential role of AT1aR, Piezo1 channels, TRPC6 channels, and TRPM4 channels in the development of myogenic vasoconstriction. Signaling interactions among these proteins will be an exciting area for future investigations.
Several ion channels in SMCs could potentially interact with AT1aRs to modulate myogenic vasoconstriction. Harraz et al demonstrated that Ca2+ influx through T‐type Ca2+ channels is essential for the development of myogenic vasoconstriction in mesenteric arteries. 8 Another TRP channel, TRP vanilloid 4 (TRPV4), is unlikely to be a direct mechanosensor, 9 but is activated by increased intraluminal pressure in SMCs. 10 Intriguingly, Swain and colleagues reported that Piezo1 channels can stimulate TRPV4 channel activity in pancreatic acinar cells. 11 Studies by Crnich and colleagues also support a role for the transient receptor potential mucolipin channels in the development of myogenic vasoconstriction. 12 Thus, multiple ion channels and receptors are involved in the development of intraluminal pressure‐induced vasoconstriction. Considering the findings of Cui and colleagues that the development of myogenic vasoconstriction is impaired in SMC‐specific AT1aR knockout mice, 4 it is conceivable that AT1aR interacts with 1 or more of these ion channels and other signaling elements involved in myogenic vasoconstriction.
Cui and colleagues propose that the effects of AT1aR are transduced by Gq/11 proteins. 4 However, the specific signaling events downstream of Gq/11 activation are unclear. For example, AT1aR stimulation will result in inositol 1,4,5‐trisphosphate (IP3) release and IP3 receptor (IP3R) activation in SMCs. 13 IP3R activation will increase Ca2+ release from the sarcoplasmic reticulum, ultimately contracting the SMCs. 13 Increased intracellular Ca2+ can also activate Ca2+‐regulated channels, including TRPM4 and TRPV4 channels, on the SMC membrane. Additionally, Gq/11 signaling will activate phospholipase C, thereby breaking down phosphatidylinositol 4,5‐bisphosphate and increasing the levels of diacylglycerol. 14 Diacylglycerol is the endogenous activator of TRPC6 channels and can promote myogenic vasoconstriction through TRPC6 channel activation. 15 Moreover, phosphatidylinositol 4,5‐bisphosphate has been identified as an endogenous inhibitor of TRPV4 channel activity. 16 Diacylglycerol also activates protein kinase C, which can phosphorylate and regulate the activity of several ion channels involved in the development of myogenic vasoconstriction. Protein kinase C has been shown to phosphorylate and activate L‐type Ca2+ channels and TRPV4 channels. 17 Thus, AT1aR signaling can potentially regulate the intricate network of ion channels involved in the development of myogenic vasoconstriction.
Co‐localization of AT1Rs with ion channels and other signaling elements will be a major consideration as we further investigate the AT1R‐dependent signaling pathways in SMCs. SMC membrane contains functionally important signaling nano/microdomains facilitated by spatial co‐localization of interacting proteins. The proximity of the signaling elements determines their coupling and, ultimately, the effect on SMC contraction. For example, the coupling of ryanodine receptors with Ca2+‐activated, large‐conductance K+ (BK) channels negatively regulates myogenic constriction, 18 whereas the coupling of voltage‐gated Ca2+ channels with IP3Rs promotes SMC contraction. 19 Similarly, spatial proximity of TRPV4 channels or T‐type Ca2+ channels with ryanodine receptors can limit SMC contraction. 8 , 18 Therefore, studies investigating the ion channels and other proteins in spatial proximity with AT1aR may provide new insights on the signaling linkages of AT1aR in SMCs. Chennupati and colleagues recently reported the involvement of G proteins 12/13 subunits (G12/G13)‐dependent pathways in modulating vasoconstriction and maintaining vascular resistance via Rho‐dependent pathway under physiological and pathological conditions (Figure). 20 Therefore, a role for the interaction between Gq/11‐ and G12/13‐dependent signaling in myogenic vasoconstriction cannot be ruled out.
Significant progress has been made in understanding the specific role of AT1aR in the development of myogenic vasoconstriction, although the role of AT1aR‐dependent signaling in resting blood pressure regulation remains unclear. Individual elements of AT1aR‐dependent signaling could also be involved in excessive vasoconstriction commonly observed in cardiovascular disorders, a possibility that remains unexplored. Considering the importance of AT1aR signaling in myogenic vasoconstriction, targeting individual elements of this signaling pathway may result in therapeutic benefit in hypertension. Future investigations in this direction are likely to further our understanding of AT1aR‐dependent mechanisms in myogenic vasoconstriction, their physiological significance, and involvement in disease pathogenesis.
Sources of Funding
This work was supported by grants from the National Institutes of Health (HL142808, HL146914, and HL147555) to Sonkusare.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 3.
See Article by Cui et al.
References
- 1. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou Y, Chen Y, Dirksen WP, Morris M, Periasamy M. AT1b receptor predominantly mediates contractions in major mouse blood vessels. Circ Res. 2003;93:1089–1094. doi: 10.1161/01.RES.0000101912.01071.FF [DOI] [PubMed] [Google Scholar]
- 3. Schleifenbaum J, Kassmann M, Szijarto IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, et al. Stretch‐activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res. 2014;115:263–272. doi: 10.1161/CIRCRESAHA.115.302882 [DOI] [PubMed] [Google Scholar]
- 4. Cui Y, Kassmann M, Nickel S, Zhang C, Alenina N, Anistan YM, Schleifenbaum J, Bader M, Welsh DG, Huang Y, et al. Myogenic vasoconstriction requires canonical Gq/11 signaling of the angiotensin II type 1 receptor. J Am Heart Assoc. 2022;10:e022070. doi: 10.1161/JAHA.121.022070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill‐Eubanks DC, Nelson MT, Earley S. A PLCγ1‐dependent, force‐sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal. 2014;7:ra49. doi: 10.1126/scisignal.2004732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR‐BKCa axis. Arterioscler Thromb Vasc Biol. 2015;35:1843–1851. doi: 10.1161/ATVBAHA.115.305736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen YL, Sonkusare SK. Endothelial TRPV4 channels and vasodilator reactivity. Curr Top Membr. 2020;85:89–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YL, Daneva Z, Kuppusamy M, Ottolini M, Klimentova E, Sonkusare SK. Regulation of blood pressure by smooth muscle TRPV4 channels under normal and hypertensive conditions. Circulation. 2021;144:A12772. doi: 10.1161/circ.144.suppl_1.12772 [DOI] [Google Scholar]
- 11. Swain SM, Romac JM, Shahid RA, Pandol SJ, Liedtke W, Vigna SR, Liddle RA. TRPV4 channel opening mediates pressure‐induced pancreatitis initiated by Piezo1 activation. J Clin Invest. 2020;130:2527–2541. doi: 10.1172/JCI134111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, Earley S. Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C682–C694. doi: 10.1152/ajpcell.00101.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexander RW, Brock TA, Gimbrone MA Jr, Rittenhouse SE. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension. 1985;7:447–451. doi: 10.1161/01.HYP.7.3.447 [DOI] [PubMed] [Google Scholar]
- 14. Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407 [DOI] [PubMed] [Google Scholar]
- 15. Aires V, Hichami A, Boulay G, Khan NA. Activation of TRPC6 calcium channels by diacylglycerol (DAG)‐containing arachidonic acid: a comparative study with DAG‐containing docosahexaenoic acid. Biochimie. 2007;89:926–937. doi: 10.1016/j.biochi.2006.10.016 [DOI] [PubMed] [Google Scholar]
- 16. Harraz OF, Longden TA, Hill‐Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife. 2018;7:e38689. doi: 10.7554/eLife.38689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE, Santana LF. Local control of TRPV4 channels by AKAP150‐targeted PKC in arterial smooth muscle. J Gen Physiol. 2014;143:559–575. doi: 10.1085/jgp.201311050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6 [DOI] [PubMed] [Google Scholar]
- 19. Ghosh D, Syed AU, Prada MP, Nystoriak MA, Santana LF, Nieves‐Cintron M, Navedo MF. Calcium channels in vascular smooth muscle. Adv Pharmacol. 2017;78:49–87. doi: 10.1016/bs.apha.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chennupati R, Wirth A, Favre J, Li R, Bonnavion R, Jin YJ, Wietelmann A, Schweda F, Wettschureck N, Henrion D, et al. Myogenic vasoconstriction requires G12/G13 and LARG to maintain local and systemic vascular resistance. eLife. 2019;8:e49374. doi: 10.7554/eLife.49374 [DOI] [PMC free article] [PubMed] [Google Scholar]


