Graphical Abstract

1. Procedure
Caution: Dimethyl sulfide, a by-product of this reaction, is a highly flammable liquid and an irritant with an extremely unpleasant odor. The experimentalist should wear gloves, and the reaction and its workup should always be performed in a well-ventilated hood.
A 250-mL round-bottomed flask with a 24/40 joint equipped a Teflon-coated oval-shaped magnetic stir bar (3.2 cm x 1.5 cm) and topped with a rubber septum is flushed with nitrogen (Note 1). Dichloromethane (60 mL) is added via syringe (Note 2). Ethyl 2-isocyanoacetate (7.0 mL, 60.8 mmol) is added via syringe to afford a brown solution (Note 3). Dimethyl sulfoxide (5.4 mL, 76 mmol) is added via syringe (Note 4). The resulting solution is cooled to −78 °C in a dry ice/acetone bath, then trifluoroacetic anhydride (0.86 mL, 6.2 mmol) is added dropwise via syringe over 5 min (Note 5). The resulting solution is stirred for 5 min at −78 °C, then the dry ice/acetone bath is removed, and the dark brown solution is allowed to warm to rt over 1 h and stirred for an additional 5 min (Note 6). The magnetic stir bar is removed using a Teflon-coated magnet retriever and rinsed with dichloromethane (0.5 mL). The reaction mixture is concentrated in the hood by rotary evaporation at 20 mmHg. The resulting brown concentrate is dissolved in ice-cold dichloromethane (20 mL) and washed with three 10-mL portions of ice-cold deionized water. The organic layer is dried over anhydrous magnesium sulfate powder (1g) and filtered through a 30 mL sintered glass funnel using 3 x 5 mL portions of fresh solvent for rinsing (Note 7). The filtrate is concentrated by rotary evaporation (25 °C, 20 mmHg) (Note 8) and then under high vacuum (0.3 mmHg) for 30 min to afford 7.58–7.66 g (96–97%) of 2 as a dark brown oil (Notes 9 and 10).
Safety and Waste Disposal Information
All hazardous materials should be handled and disposed of in accordance with “Prudent Practices in the Laboratory”; National Academies Press; Washington, DC, 2011.
3. Discussion
Isocyanates, which embody the N═C═O functional group, undergo several synthetically useful reactions, including nucleophilic addition of alcohols and amines to produce urethanes and ureas, respectively. Isocyanates are also intermediates in several well-known organic reactions, including the Hofmann rearrangement of primary amides, the Curtius rearrangement of acyl azides, the Schmidt reaction of carboxylic acids with hydrazoic acid, and the Lossen rearrangement of hydroxamic acids. Diisocyanates are widely used in chemical industry to produce polyurethanes and other polymers.
The present method of synthesizing isocyanates from isonitriles is simple and easy to perform.3 Both reagents (DMSO as oxidant; trifluoroacetic anhydride as catalyst) are inexpensive and readily available. The reaction proceeds swiftly and efficiently at low temperature and produces volatile byproducts (dimethyl sulfide, residual anhydride) that are easily removed upon evaporative workup. Furthermore, washing with cold water completely eliminates any small quantities of residual DMSO from the desired product.
Ethyl 2-isocyanatoacetate has previously been prepared by the reaction of ethanol with 2-isocyanatoacetyl chloride.5 It has also been synthesized by the reaction of ethyl hydrogen malonate using diphenylphosphoryl azide via a Curtius rearrangement.6 Ethyl 2-isocyanatoacetate can also be prepared from various glycine derivatives, including the reaction of N-tosylglycine ethyl ester7 or N-trimethylsilylglycine ethyl ester4 with phosgene, or the reaction of glycine ethyl ester hydrochloride with bis(trichloromethyl)carbonate.8
Funding:
Funding was provided by the NIH (GM 008500 Training Grant Support for HVL). Support of the Cornell NMR Facility has been provided by NSF (CHE 7904825; PGM 8018643) and NIH (RR02002).
Biographies

Bruce Ganem was born in Boston, MA. He received his B.A. degree in chemistry from Harvard College and his Ph.D in organic chemistry from Columbia University. After completing postdoctoral work at Stanford, he joined the Cornell faculty in 1974, where he is the Franz and Elisabeth Roessler Professor of Chemistry and the J. Thomas Clark Professor of Entrepreneurship. A synthetic organic chemist by training, Ganem has broad scientific research interests ranging from organic and analytical chemistry to biochemistry. His current research focuses on the development of new synthetic reactions with applications to drug discovery and development.

Hoang V. Le was born and raised in Vietnam. He graduated from the University of California, Berkeley in 2008 with a B.S. degree in Chemistry. While at Berkeley, he worked in the lab of Professor Kenneth Raymond synthesizing dicationic bridges and investigating their stability in supramolecular systems. He is currently a fourth-year graduate student in the lab of Professor Bruce Ganem at Cornell University. His research aims to expand the scope of multicomponent reaction (MCR) chemistry by improving known MCRs and developing new MCRs.

Kenichi Kobayashi received his B.S. and M.S. degrees in chemistry from Rikkyo University where he conducted research with Professor Chiaki Kuroda. After having worked at KYORIN Pharmaceutical Co., Ltd. for six years, he joined the lab of Professor Hideo Kigoshi at University of Tsukuba and received his Ph.D. in 2011. He then moved to the University of Chicago, where he is currently a postdoctoral researcher in the lab of Professor Viresh H. Rawal.
Appendix. Chemical Abstracts Nomenclature; (Registry Number)
Ethyl 2-isocyanoacetate; (2999-46-4)
Ethyl 2-isocyanatoacetate; (2949-22-6)
2. Notes
The round-bottomed flask and the magnetic stir bar were dried in an oven, cooled to room temperature under high vacuum, and subsequently maintained under a positive pressure of nitrogen.
Dichloromethane (Optima® grade) was purchased from Fisher Scientific and dried over activated alumina solvent purification system (Innovative Technology Inc. Pure Solv™).
Ethyl 2-isocyanoacetate (95%) was purchased from Aldrich Chemical Company Inc. and the dark brown oil was used without further purification.
Dimethyl sulfoxide (DMSO, anhydrous, ≥99.9%) was purchased from Sigma-Aldrich and used without further purification.
Trifluoroacetic anhydride (≥99%) was purchased from Sigma-Aldrich and used without further purification.
After stirring at room temperature for 5 min, a few drops of the reaction solution were removed via Pasteur pipet and submitted to IR analysis to confirm that the reaction was complete (complete disappearance of isonitrile stretch at 2164 cm−1; appearance of isocyanate stretch at 2255 cm−1.
Magnesium sulfate anhydrous powder was purchased from Fisher Scientific and used without further purification.
Removing the bulk of the solvent by rotary evaporation was judged complete when the flask no longer felt cold to the touch after the water bath was lowered from the evaporator.
The product was a dark brown oil which appeared pure by 1H and 13C NMR spectroscopy. When stored at room temperature, the neat product decomposed with a half-life of ca. 12 h into unidentifiable solid. However, decomposition could be suppressed by wrapping the sample in aluminum foil and storing it neat at −10 °C. After 4 days, only traces of solid decomposition product were observed.
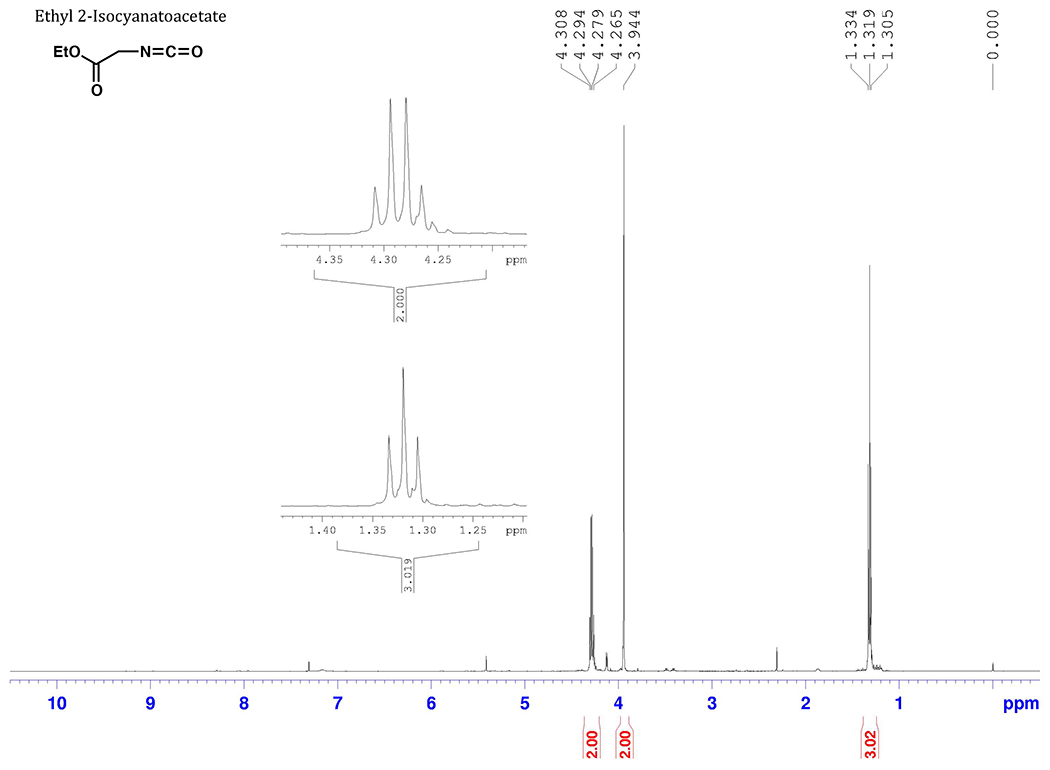
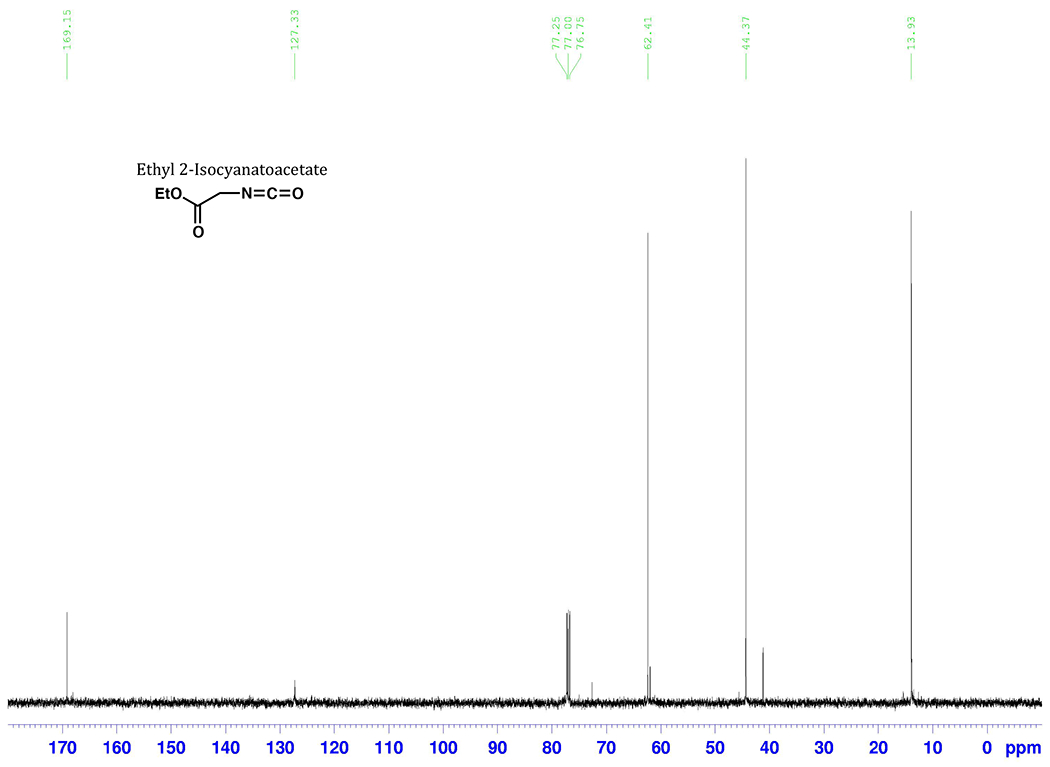
The product isocyanate displayed the following spectroscopic characteristics, which matched published2–4 spectra: 1H NMR (500 MHz, CDC13) δ: 1.32 (t, 3 H, J= 7.2 Hz), 3.94 (s, 2 H), 4.29 (q, 2 H, J= 7.2 Hz); 13C NMR (125 MHz, CDC13) δ: 13.9, 44.4, 62.4, 127.3 (weak), 169.2; IR (neat, cm−1): 3363 (m), 2987 (m), 2943 (m), 2255 (s), 1749 (s).
References
- 2.Barany MJ; Hammer RP; Merrifield RB; Barany G J. Am. Chem. Soc 2005, 127, 508. [DOI] [PubMed] [Google Scholar]
- 3.Le HV; Ganem B Org. Lett 2011, 13, 2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebedev AV; Lebedeva AB; Sheludyakov VD; Ovcharuk SN; Kovaleva EA; Ustinova OL Russ. J. Gen. Chem 2006, 76, 1069. [Google Scholar]
- 5.Iwakura Y; Uno K; Kang S J. Org. Chem 1965, 30, 1158. [Google Scholar]
- 6.Aoyama Y; Uenaka M; Konoike T; Hayasaki-Kajiwara Y; Naya N; Nakajima M Bioorg. Med. Chem. Lett 2001, 11, 1691. [DOI] [PubMed] [Google Scholar]
- 7.Schuemacher AC; Hoffmann RW Synthesis 2001, 243. [Google Scholar]
- 8.Huang J-M; Chen H; Chen R-Y. Synth. Commun 2002, 32, 1357. [Google Scholar]


