Introduction
Testicular cancer is the most common solid malignancy in males aged 15–29, with 1200 cases and 35 deaths projected in Canada in 2021.1 More than 90% of testicular cancers are primary germ cell tumors (GCTs), which are histologically divided into seminomas and non-seminomas (NSGCT).2 The incidence of both types is rising, with seminoma rising at a faster rate.3 Testicular GCT remains a highly curable disease but requires a multidisciplinary and coordinated approach to deliver optimal and timely treatment. Studies show that GCT care performed in, or coordinated with, experienced centers that manage high volumes of GCT patients leads to improved survival and minimization of toxicity or overtreatment.4–6 Potential benefits include experienced pathological review, specialist radiographic assessment, guideline-based recommendations, and timely delivery of multidisciplinary care.
At the Canadian GCT meeting held in Toronto in 2018, Canadian GCT providers prioritized updating the Canadian consensus document, which was last updated in 2010.7
Methods
A multidisciplinary group of Canadian testicular cancer experts (nine medical oncologists, five uro-oncologists, two radiation oncologists, one genitourinary pathologist, and one genitourinary radiologist) participated in updating this guideline. Participants were assigned stage-specific topics to update and the project leads (RH, CC, LW) participated in updating all topics. Five individual stage-specific meetings with each subgroup of experts were conducted in April 2021 to discuss and develop key recommendations. Once recommendations were compiled, all participants voted via SurveyMonkey to determine their level of agreement. Modifying methods used by the European Association of Urology and European Society for Medical Oncology Guidelines Committees, each recommendation was followed by a five-point Likert scale: completely disagree, somewhat disagree, undecided, somewhat agree, completely agree, abstain (out of scope of my practice and/or I don’t feel comfortable answering).8 A priori, agreement was defined as ≥75% of experts voting somewhat agree or completely agree. Results from this initial vote are shown in Supplementary Figure 1 (available at cuaj.ca).
In June 2021, a full group virtual meeting was held to discuss one recommendation that did not reach agreement, along with 22 other recommendations that, despite ≥75% agreement, some experts had indicated disagreement or undecided opinions. Proposed changes were made accordingly, including the creation of one new recommendation. In July 2021, a second and final vote was conducted including all of the recommendations where any changes had been proposed. Agreement was achieved in all recommendations. Throughout the guideline, after each recommendation, the percent of experts who voted “completely agree” or “somewhat agree” with each statement is shown in parentheses.
1. Diagnosis and staging
Clinical presentation of GCT
Prompt recognition, diagnostic workup, and treatment initiation is of paramount importance for men with GCTs. Most patients present with a palpable testicular mass that may or may not be painful. Occasionally, the initial presentation reflects the presence of symptomatic metastatic disease, such as pain from a retroperitoneal or neck mass, respiratory symptoms, thrombus, or constitutional symptoms, such as weight loss.9 In approximately 5% of patients, the primary tumor is extragonadal (i.e., retroperitoneum or mediastinum).10
Rarely, in advanced GCTs, the testicular primary tumor will spontaneously regress without treatment and at orchiectomy is described pathologically as a “burned out” primary. 11 Occasionally, prior to orchiectomy, the radiologist may label changes in the ipsilateral testis as appearing as a burned out primary or Azzopardi lesion.12 In such cases where chemotherapy is about to commence, an orchiectomy can be deferred or even avoided altogether depending on clinical suspicion.
Consensus recommendations
-
There are mandatory diagnostic and staging examinations (Table 1).
These include scrotal examination, determination of the serum tumor markers alpha-fetoprotein (AFP), ß-human chorionic gonadotropin (HCG), and lactate dehydrogenase (LDH), ultrasound to image the testicles, and computed tomography (CT) scan of the thorax, abdomen, and pelvis (agreement: 100%)
-
Bone scan and brain imaging (preferably magnetic resonance imaging [MRI]) are indicated in patients with symptoms suggestive of bone or central nervous system involvement, as well as patients with International Germ Cell Cancer Collaborative Group (IGCCCG) poor prognosis (agreement: 100%).
Brain imaging should also be considered in those with extensive lung metastases, pure choriocarcinoma, and/or HCG >5000 IU/L at diagnosis13 (agreement: 100%).
Other imaging procedures, such as body MRI and positron emission tomography (PET), should not be routinely used for staging (agreement: 94%).
With rare exception, radical orchiectomy is the initial diagnostic and treatment maneuver and should be performed through an inguinal incision, removing the testicle and spermatic cord to the level of the internal inguinal ring (agreement: 100%).
Insertion of a testicular prosthesis at the time of orchiectomy should be discussed with patients prior (agreement: 83%).
Orchiectomy may be deferred in patients with life-threatening metastatic disease when a confirmed diagnosis of NSGCT (e.g., an unequivocally elevated AFP and/or HCG >5000 IU/L) or seminoma (e.g., biopsy of metastatic site) is made so as not to delay the start of chemotherapy. In such cases, orchiectomy should be performed after chemotherapy14,15 (agreement: 100%).
Tumor markers should be drawn prior to orchiectomy and repeated postoperatively within 1–3 weeks, and repeated to ensure return to normal, given the known half-life kinetics of AFP (<7 days) and ß-HCG (<3 days) (agreement: 100%).
In very rare cases where there is possibility of a benign tumor, excisional biopsy with a frozen section should be performed prior to definitive orchiectomy in an experienced center to allow for the possibility of organ-sparing partial orchiectomy16 (agreement: 94%).
In patients with synchronous bilateral tumors, metachronous contralateral tumors, or solitary testicles with normal preoperative testosterone levels, partial orchiectomy by an experienced surgeon may be an alternative procedure to orchiectomy in very select patients (agreement: 92%).
If organ-preserving surgery is performed and germ cell neoplasia in situ (GCNIS) is found in the remaining testicular tissue, options include completion orchiectomy, adjuvant radiotherapy, or surveillance, and discussion should include risk of cancer recurrence, hypogonadism, and fertility (agreement: 100%).
A full discussion on semen cryo-preservation for all patients undergoing therapy (surgery, chemotherapy, and/or radiation) for GCT should take place (agreement: 100%).
The histopathological report should document the following points: procedure, specimen laterality, tumor focality, tumor size, tumor extension (rete testis, hilar soft tissue, epididymis, tunica vaginalis, spermatic cord, scrotum), histological type, margin status, presence or absence of lymphovascular invasion, number of lymph nodes involved and examined, pathology stage classification (pTNM, according to most recent AJCC/UICC, 8th edition17 [Supplementary Table 1; available at cuaj.ca]), and additional pathological findings (GCNIS, microlithiasis, Sertoli cell nodule, atrophy). Mixed GCTs should have the estimated proportion of each component reported as a percentage (%). Immunohistochemistry is considered a useful adjunct to the diagnosis of testicular tumors (agreement: 100%).
It is recommended testicular tumors be assessed by a pathologist experienced in testis cancer pathology18 (agreement: 100%).
Patients with metastatic disease should be classified according to the IGCCCG classification system19 (Table 2) (agreement: 100%).
Table 1.
Mandatory investigations
| Complete history and physical exam, including scrotal exam Laboratory
|
Bone scan and brain imaging in patients with symptoms or poor prognosis metastatic disease.
CT: computed tomography.
Table 2.
The International Germ Cell Cancer Collaborative Group classification
| Histology | Prognostic category | Clinical factors |
|---|---|---|
| NSGCT | Good | Testes/retroperitoneal primary AND no non-pulmonary visceral metastases AND good markers (S1)
|
| Intermediate | Testes/retroperitoneal primary no non-pulmonary visceral metastases AND intermediate markers (S2)
or
|
|
| Poor | Mediastinal primary AND/OR non-pulmonary visceral metastases AND/OR any poor marker (S3)
|
|
| Seminoma | Good | Any primary site AND no non-pulmonary visceral metastases AND normal AFP, any ß-HCG, any LDH |
| Intermediate | Any primary site AND non-pulmonary visceral metastases AND normal AFP, any ß-HCG, any LDH |
LDH elevation alone for IGCCCG classification is controversial.
AFP: alpha-fetoprotein; ß-HCG: beta human chorionic gonadotrophin; LDH: lactate dehydrogenase; NSGCT: non-seminoma germ cell tumor; ULN: upper limit of normal. Adapted from International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group.19
2. Clinical stage I (CSI) and clinical stage II (CSII) seminoma
CSI seminoma
CSI seminoma requires an orchiectomy specimen containing pure seminoma only, normalized markers post-orchiectomy, no history of an elevated AFP, and normal staging imaging. The risk of relapse in such cases is 15%, with most occurring in the retroperitoneum.20–22 Treatment options include surveillance, para-aortic ± pelvic radiotherapy or chemotherapy (carboplatin x 1–2), as shown in Table 3. All patients should have a shared discussion balancing relapse risk and side effects of adjuvant treatment. However, given that adjuvant treatment is associated with acute toxicities, risk of late side effects, potential overtreatment in 85%, and no improvement in survival, surveillance for all CSI seminoma patients is the preferred approach.
Table 3.
Treatment options for CSI seminoma
| Option | Relapse rate | Cancer-specific survival |
|---|---|---|
| Surveillance | 15% | 99% |
| Carboplatin x 1 | 5% | 99% |
| Radiation | 4% | 99% |
Surveillance
Several prospective, non-randomized studies of surveillance in CSI seminoma have been performed (Supplementary Table 2; available at cuaj.ca). Relapse rates at five years range from 11–20%.23,24 The predominant site of relapse is the para-aortic lymph nodes (e.g., 82% in the Danish Testicular Cancer Study Group study; 97% in the Princess Margaret series).25,26 Median time to relapse is 12–18 months, but late relapses (>4 years) have been reported. Disease-specific survival is >99%, comparable to adjuvant radiation or carboplatin.
Risk factors associated with relapse have not been consistent among studies and are of limited prognostic value. In a pooled analysis of 638 patients from four centers, tumor size >4 cm and rete testes involvement were the only factors associated with relapse.27 Other large, pooled studies have shown inconsistent results, suggesting relapse associations with only rete testes involvement, only tumor size, or both.20,21,28
Despite the limitations of these risk factors, the Spanish Germ Cell Cancer Cooperative Study Group and the Swedish and Norwegian Testicular Cancer Group (SWENOTECA) have offered a risk-adapted strategy with surveillance for low-risk patients (0 risk factors) and adjuvant therapy for high-risk patients (1–2 risk factors).21,29 These studies confirmed that low-risk patients had a small risk of relapse (4–6%) and the risk-adapted strategy lowered the relapse risk for the high-risk patients. As patients in the high-risk group have a >75% chance of being relapse-free on surveillance, adjuvant therapy may only lead to a modest absolute reduction in relapse risk.20,21 Nearly all relapses on surveillance may be salvaged with radiotherapy or chemotherapy and result in a cancer-specific survival of 99%. Accordingly, a recent systematic review by the European Testicular Cancer Guidelines Panel concluded the prognostic utility of size and rete testes invasion have significant limitations.23
One concern regarding non-risk-adapted surveillance is the potential for increased chemotherapy use to salvage patients who relapse. However, as most relapses occur in the retroperitoneum, radiotherapy is an effective sole salvage approach. For example, at Princess Margaret, 78% of CSI seminoma relapses were managed with radiation, with only 9% of those requiring chemotherapy for subsequent second relapses.26
An optimal followup surveillance strategy has yet to be determined. Several retrospective studies suggest serial tumor marker assessments do not contribute to relapse detection and have been dropped from some CSI seminoma surveillance protocols.30 Results suggest MRI imaging was non-inferior to CT scans, and a three-scan schedule was non-inferior to a seven-scan schedule. Further interpretation and discussion of these results will be required to recommend a more optimal surveillance schedule to minimize radiation exposure without compromising outcomes.31
Adjuvant radiotherapy
Adjuvant retroperitoneal radiotherapy is now limited to the occasional circumstance where surveillance is not feasible.32,33 Large series and randomized trials of adjuvant radiotherapy report a 4% (range 0.8–5%) relapse rate (Supplementary Table 3; available at cuaj.ca).34 Relapse >3 years after radiotherapy appears rare, occurring in only three of 1893 patients entered onto the MRC TE10, TE18, and TE19 adjuvant trials.35 Evidence from randomized trials demonstrates similar overall relapse rates for para-aortic nodal radiotherapy vs. extended volume to include ipsilateral pelvic nodes (3.4% vs. 4%).36 Continued imaging to detect pelvic recurrence is recommended if this is not included in the initial treatment volume.
Adjuvant chemotherapy
Adjuvant chemotherapy is limited to the occasional circumstance where surveillance is not feasible. If given, 1–2 cycles of carboplatin are options but whether one or two cycles is better remains unclear. The only randomized study (MRC TE19) compared adjuvant radiotherapy to one cycle of carboplatin and observed similar five-year relapse (4.0% vs. 5.3%).37 However, non-randomized data suggest a reduction in relapses with two cycles (1.5–3% vs. 5%).38,39 As well, population-based SWENOTECA data shows relatively small risk reductions with only one cycle of carboplatin compared to surveillance, especially with higher-risk patients (relapse rate 2.2% vs. 4.0% in lower-risk patients and 9.3% vs. 15.5% in higher-risk patients).21 There are many potential disadvantages with this adjuvant approach. The reduction in relapse risk is not that impressive (15% down to 5%) and most relapses occur in the retroperitoneum and therefore CT scans of the abdomen and pelvis are still required, similar to patients on surveillance. Furthermore, recurrence after carboplatin may have a worse biology, as reported in a recent series.40 Compared to large surveillance series, these 185 patients appeared to have a higher burden of disease at relapse, a greater reliance on bleomycin, etoposide, cisplatin (BEP) as salvage, and a greater incidence of second relapse and death. Finally, the long-term (i.e., beyond 15 years) toxicity of carboplatin is unknown since the series with the longest toxicity information had only a nine-year median followup.41
Consensus recommendations (Supplementary Figure 2; available at cuaj.ca)
Patients with rising tumor markers after orchiectomy despite normal imaging are considered stage IS and should be treated with chemotherapy according to the corresponding IGCCCG group (agreement: 100%).
Patients should be informed of all treatment options (surveillance, adjuvant chemotherapy, adjuvant radiotherapy), including the potential benefits and side effects of each treatment, and should be involved in the decisionmaking process (agreement: 100%).
In a patient willing and able to adhere to a surveillance program, surveillance should be the preferred option (agreement: 100%).
A risk-adapted approach with surveillance for low-risk patients and adjuvant treatment for those at higher risk of relapse is not recommended (agreement: 93%).
If adjuvant therapy is chosen, carboplatin chemotherapy and radiation therapy are options (agreement: 94%).
If adjuvant therapy is given, post-therapy imaging is still required, similar to surveillance schedules (agreement: 93%).
At present, there is no role for primary retroperitoneal lymph node dissection (RPLND) in stage I seminoma (agreement: 93%).
CSII seminoma
In patients with CSIIA seminoma without marker elevation, an initial period of surveillance with repeat imaging in 6–8 weeks is recommended, as up to 30% of cases end up being false-positive nodal enlargement.
In CSIIA seminoma, radiotherapy or chemotherapy are options. No randomized trial has compared these two options in this setting. Radiation therapy given to the paraaortic and ipsilateral pelvic nodes with doses ranging from 30–35 Gy show five-year relapse-free rates in excess of 90% in most modern series.42
In CSIIB disease, depending on the bulk of disease and location of lymph nodes, radiation therapy or chemotherapy are options.42 The relapse-free rate with radiation therapy is close to 90% and most relapses are cured with salvage chemotherapy.43 With primary chemotherapy, there are very few relapses. The overall disease-specific survival is close to 100% with either management approach.43
In a meta-analysis of four prospective and nine retrospective studies, chemotherapy and radiation achieved similar disease control in CSIIA disease, but chemotherapy fared modestly better in CSIIB disease (relapse rate 5% vs. 12%), with a lower incidence of late toxicity and secondary cancers.44
CSIIC disease should be managed with chemotherapy, as the relapse rate with radiation therapy approaches 50% in most series, and not all patients can be salvaged with chemotherapy.42
Chemotherapy for all CSII seminoma patients is the same as that for IGCCCG good-prognosis patients. The role of RPLND and of reduced volume radiation therapy in combination with single-course of carboplatin in CSII seminoma is the subject of clinical trials and not an established option outside of a trial setting.45,46
Consensus recommendations
In patients with CSIIA seminoma without marker elevation, an initial period of surveillance with repeat imaging in 6–8 weeks is recommended (agreement: 94%).
In CSIIA seminoma, radiation therapy or chemotherapy are standard treatment options and should be discussed (agreement: 100%).
In CSIIB seminoma, radiation therapy or chemotherapy are treatment options. Chemotherapy is the preferred option in most cases (agreement: 94%).
In CSIIC seminoma, chemotherapy is the standard treatment approach (agreement: 100%).
Chemotherapy in this setting is the same as for IGCCCG good-prognosis metastatic patients (agreement: 100%).
The role of RPLND in stage II seminoma is investigational (agreement: 94%).
3. Clinical stage I NSGCT
Testicular cancer is classified as NSGCT if, histologically, the tumor contains any component of embryonal carcinoma, yolk sac tumor, choriocarcinoma, or teratoma. Patients with histologically pure seminoma but elevated serum AFP or markedly elevated HCG (generally regarded as >5000 mIU/ml) are considered to have NSGCT. Patients with persistently elevated or rising markers 4–6 weeks after orchiectomy with normal imaging are considered stage IS, and should be treated with chemotherapy according to their corresponding IGCCCG group.
Treatment options for CSI NSGCT are summarized in Table 4 and include surveillance, chemotherapy (typically BEP x 1–2), or RPLND. Approximately 20–30% of stage I NSGCT patients relapse without adjuvant treatment.22,47 Although adjuvant therapy reduces relapse risk, cancerspecific survival is equivalent to surveillance at 99% and so discussions with patients should go beyond relapse risk to include potential long-term toxicities of adjuvant therapy and the equivalent cancer-specific survival. Surveillance for all CSI NSGCT patients is the preferred approach.
Table 4.
Treatment options for CSI NSGCT
| Option | Relapse rate | Cancer-specific survival |
|---|---|---|
| Surveillance | 26% | 99% |
| BEPx1 | 2–7% | 99% |
| RPLND | 10%* | 99% |
This includes giving adjuvant chemotherapy to select patients with positive nodes at RPLND.
NSGCT: non-seminomatous germ cell tumor; RPLND: retroperitoneal lymph node dissection.
In some countries, patients with high-risk characteristics are treated with adjuvant therapy. The main factor associated with increased risk of relapse is the presence of lympho-vascular invasion (LVI) in the orchiectomy specimen, as it upstages NSGCT from pT1 to pT2 and overall CSIA to CSIB.22,48 Some data show embryonal predominance is also associated with relapse. The cutoff for defining embryonal predominance varies in the published series from >50% to 100%. Whether LVI, embryonal predominance, or both are present, the associated risk of relapse is approximately 50%.47
Surveillance
Published series report on 6179 CSI patients on surveillance, with 1603 (25.9%) relapses and 67 (1.1%) deaths (Supplementary Table 4; available at cuaj.ca). These series confirm LVI and embryonal predominance as risk factors, increasing relapse up to 50% when present, but highlight the overall safety of surveillance, with 74% of patients in the published literature not requiring any further therapy after orchiectomy.
Those relapsing on surveillance have traditionally been treated with 3–4 cycles of chemotherapy.47 As such, an argument against surveillance for high-risk CSI NSGCT is the higher treatment burden for the 50% who experience a relapse compared to a strategy of upfront adjuvant therapy (e.g., BEPx1). A recent series of 162 relapsed patients demonstrated the safety of salvaging CSI NSGCT surveillance relapses with RPLND.49 The majority relapsed within the retroperitoneum and 38% were treated with RPLND, of whom 73% did not require any subsequent chemotherapy. In their modelling exercise, a theoretical cohort of 100 high-risk patients treated with surveillance and salvaged preferentially with RPLND had similar chemotherapy burden to a group treated with adjuvant BEPx1.
Adjuvant chemotherapy
A historical systematic review of non-randomized trials and a single randomized trial using adjuvant chemotherapy in CSI NSGCT demonstrated a recurrence rate of 3.8% using a variety of platinum-based regimens (Supplementary Table 5; available at cuaj.ca).50 The MRC prospectively evaluated BEPx2 in patients deemed high-risk for relapse based on multiple histopathological features. With long-term followup, 1.8% (2/114) of patients recurred.51 Other trials have focused on high-risk patients by virtue of LVI or embryonal histology predominance. After two cycles of BEP chemotherapy, relapse ranged from 2.2–2.9%.52,53
Using a single cycle of BEP has also been explored. The SWENOTECA group studied surveillance vs. BEPx1 in a prospective cohort. In patients with LVI, 41.7% of patients on surveillance relapsed compared to 3.2% receiving BEPx1. The five-year cause-specific survival was 100% for the chemotherapy arm, with no treatment-related mortality.54 Most recently, the U.K. single-arm “111 Study” confirmed the efficacy of BEPx1 in CSIB NSGCT (3.1% relapse rate and one death with 49-month median followup).55
While cause-specific mortality is excellent after BEP x 1–2, detailed information is limited on the very long-term followup (>20 years) relating to complications, such as cardiovascular health, thus, shared decision-making regarding adjuvant chemotherapy vs. other strategies is critical.56
Adjuvant RPLND
Studies directly comparing adjuvant RPLND to surveillance are lacking. Most studies of adjuvant RPLND gave two cycles of chemotherapy to patients harboring pathological nodal disease, rendering the benefits of RPLND alone unclear when compared to a strategy of BEPx1 as upfront adjuvant therapy for high-risk CSI NSGCT. In the only randomized trial comparing adjuvant RPLND to BEPx1 in 382 patients, RPLND had a significantly higher relapse rate (8% vs. 0.5%, p=0.003).57 Among the patients who received RPLND, 18.5% had pathological stage II disease at surgery and received BEPx2. In those receiving two cycles, no relapses were observed. In patients managed with RPLND alone, 13 (9.2%) recurrences were observed, seven in the retroperitoneum. There were no significant quality of life differences at six months between the arms.58
This trial has been criticized for a number of reasons, including that 57% of the CSI patients did not have LVI or embryonal predominance; the overuse of adjuvant chemotherapy among patients with low-volume node-positive disease at RPLND (pN2A); the number of RPLNDs performed at low-volume centers leading to a much higher than expected retroperitoneal relapse rate; and high rates (10%) of patients not receiving the treatment allocated. However, the trial has highlighted that if RPLND is completed, it should be done at high-volume centers.
The remaining series exploring adjuvant RPLND are retrospective in nature (Supplementary Table 6; available at cuaj. ca). These series total over 2000 patients and highlight that only about a third harbor disease in the retroperitoneum, about 10% relapse post-RPLND, and <1% die of disease. The relapse rate for those with pathological nodal disease found at RPLND who do not receive adjuvant chemotherapy is approximately 20%. RPLND use in this setting has been decreasing over time in the U.S., Canada, and Europe.59–62
Despite this trend, advantages to RPLND include the fact it provides the most accurate staging and 80–90% can avoid the potential long-term side effects of chemotherapy. The in-field relapse rate at high-volume centers is <1%, allowing omission of abdominal imaging after the early postoperative period.63 RPLND also offers surgical excision of chemo-resistant teratoma, which is present in the retroperitoneum in 15% of patients with pathological nodal disease.63 Similarly, it has been suggested that patients with malignant transformation of teratoma in the orchiectomy specimen should be preferentially offered RPLND because, if metastatic, these elements are chemo-resistant;64 however, no study has directly addressed this paradigm and it remains as expert opinion.65
Long-term complications of RPLND can include loss of antegrade ejaculation, ventral hernia, and bowel obstruction, although these are rare if performed in centers of excellence.66 Thus, in patients who choose adjuvant treatment, RPLND remains an acceptable option in the properly selected and informed individual.
Consensus recommendations (Supplementary Figure 3; available at cuaj.ca)
Patients with rising tumor markers after orchiectomy despite normal imaging are considered stage IS and should be treated with chemotherapy according to the corresponding IGCCCG group (agreement: 100%).
Patients should be informed of all treatment options (surveillance, adjuvant chemotherapy, RPLND), including the potential benefits and side effects of each treatment, and should be involved in the decision-making process (agreement: 100%).
In a patient willing and able to adhere to a surveillance program, for all stage I risk groups, surveillance should be the preferred option (agreement: 100%)
For patients who prefer immediate treatment or who are unsuitable for primary surveillance, adjuvant chemotherapy or RPLND are both options (agreement: 93%).
If adjuvant chemotherapy is chosen, one cycle of BEP is the preferred option (agreement: 100%).
If RPLND is chosen, surgery should be performed by surgeons who are experienced with the procedure. Full bilateral templates and nerve-sparing techniques should be employed (agreement: 100%).
4. Clinical stage IIA/IIB NSGCT
The cure rate for CS IIA and IIB NSGCT approaches 98% and thus priority should be given to minimizing long-term morbidity.
CS IIA patients without marker elevation represent a dilemma. The retroperitoneal nodal enlargement can be benign, teratoma, or active germ cell cancer. Neither PET nor MRI can reliably discriminate between these. Thus, three options exist: surveillance, primary RPLND, or chemotherapy.
Initial surveillance is recommended in most and repeat imaging should be performed after 6–8 weeks to determine lesion change. A shrinking lesion is likely not malignant and should be further observed. A stable or growing lesion can indicate teratoma or malignant tumor and a primary RPLND is both diagnostic and therapeutic. The pathological stage can be verified immediately, although 30% will have non-malignant histology.67–69 Patients with a rapidly growing lesion and/or increased markers should be treated with primary chemotherapy according to the IGCCCG.
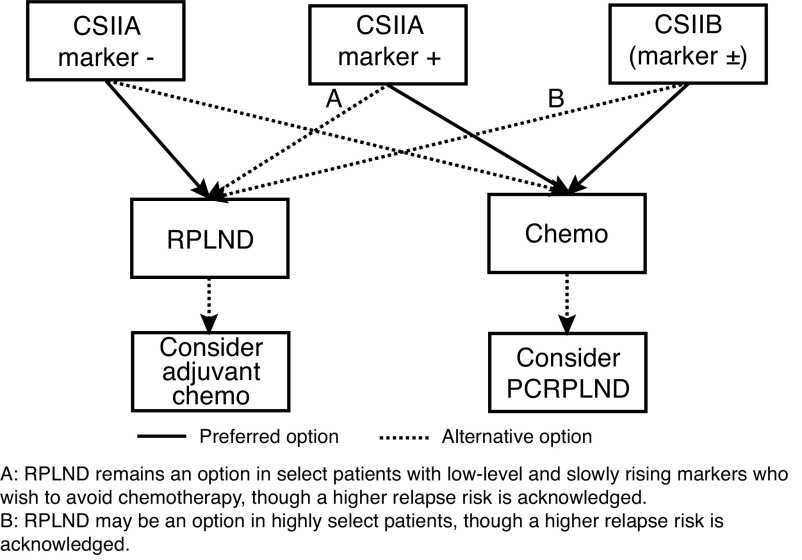
In patients with confirmed CS IIA or B disease, two treatment options exist: primary RPLND or primary chemotherapy. If patients undergo RPLND, they may be offered adjuvant chemotherapy if viable germ cell elements are identified in their pathology. If patients initiate primary chemotherapy, they may require a post-chemotherapy (PC)-RPLND if residual disease >1 cm is seen on post-chemotherapy imaging. The choice of initial therapy is based on multiple factors, but the main drivers are stage (IIA vs. IIB) and the presence/absence of elevated tumor markers. Treatment recommendations are shown in Figure 1.
Figure 1.
Clinical stage IIA/IIB non-seminomatous germ cell tumor treatment algorithm. PCRPLND: post-chemotherapy retroperitoneal lymph node dissection.
Primary RPLND alone has demonstrated relapse rates of 10% for patients with pathological stage IIA and 35–50% for pathological stage IIB disease.63,70,71 Primary RPLND followed by adjuvant chemotherapy with BEPx2 reduces the relapse risk to approximately 1%, but exposes patients to two treatment modalities and is overtreatment in 50–70% of patients. 68,72,73 When performed at centers with experience in RPLND, relapses after RPLND occur nearly exclusively outside the retroperitoneum.
Primary chemotherapy with BEPx3 or, if contraindications for bleomycin, four cycles of etoposide and cisplatin (EP), induce a complete response in 83–91% of patients with clinical stage IIA and in 61–87% of patients with clinical stage IIB.68,74 Most of these patients can be spared RPLND provided a satisfactory radiological response is obtained (i.e., residual mass ≤1 cm). Relapse rates after primary chemotherapy are low, with 4–9% for clinical stage IIA and 11–15% for clinical stage IIB disease, though these quoted rates include approximately a third of patients requiring PC-RPLND.68,74 In patients with elevated markers and/or CS IIB, primary chemotherapy is preferred given the higher rate of relapse with RPLND.67,75
In select cases of low-level and either stable or slowly rising markers, or smaller CS IIB disease, RPLND may be an option in patients wishing to avoid chemotherapy and willing to accept a higher relapse risk with RPLND alone.63 When RPLND is performed, this should be done using a full bilateral template with nerve-sparing technique, where feasible at centers with experience in performing this operation.67
Consensus recommendations
In patients with stage IIA disease without marker elevation, an initial period of surveillance with repeat imaging in 6–8 weeks is recommended (agreement: 94%).
In patients with confirmed stage IIA disease and negative markers, primary RPLND is the preferred approach. Primary chemotherapy is also an acceptable option (agreement: 100%).
-
In patients with stage IIA but tumor marker-positive disease, primary chemotherapy according to the IGCCCG recommendation is the preferred option (agreement: 100%).
RPLND remains an option in select patients with low-level and slowly rising markers who wish to avoid chemotherapy, though a higher relapse risk is acknowledged (agreement: 94%).
-
In patients with stage IIB disease irrespective of marker status, primary chemotherapy according to the IGCCCG criteria is the recommended treatment (agreement: 100%).
RPLND may be an option in highly select patients, though a higher relapse risk is acknowledged (agreement: 94%).
If RPLND is chosen, surgery should be performed by surgeons who are experienced with the procedure. Full bilateral templates and nerve-sparing techniques should be employed (agreement: 100%)
5. Treatment of advanced or metastatic disease
Patients with advanced GCTs should always be considered curable. Survival outcomes appear better in specialized centers and this may be related to experience, case selection, volume, and/or the organization of multidisciplinary care.76–78 Therefore, referral of all patients with advanced GCTs for consultation to an experienced center is strongly recommended. Patients with advanced disease must be stratified into a prognostic group using the IGCCCG criteria (Table 2)19,79,80 based on histology, site of primary, and degree of post-orchiectomy tumor marker elevation (AFP, β-HCG, and LDH). LDH elevation alone for IGCCCG classification is controversial and these cases should be discussed with oncologists in experienced centers. A recent IGCCCG update confirmed the prognostic ability of the IGCCCG groupings and reported improved five-year overall survival (OS) across all groups in seminoma (95% and 88% in good and intermediate risk, respectively) and NSGCT (96%, 89%, and 67% for good, intermediate, and poor risk, respectively).79,80
Standard chemotherapy for most patients is BEP given over five days every 21 days.81–83 The efficacy of etoposide 100 mg/m2/day and cisplatin 20 mg/m2/day for five days and bleomycin 30 IU weekly is of equivalent efficacy to the same drugs given on a three-day schedule but has less short-term gastrointestinal toxicity and long-term ototoxicity. 84 Carboplatin should not be substituted for cisplatin due to inferior outcomes.85–88 A summary of the randomized trials in advanced disease is shown in Supplementary Table 7 (available at cuaj.ca).
In patients with IGCCCG good-prognosis disease, BEPx3 is the preferred option.81,82,89 If there is a contraindication to bleomycin, EPx4 can be given, but has been associated with a non-statistically significant but higher death rate in one randomized controlled trial.83
Patients with intermediate or poor-prognosis disease should be considered oncological emergencies and treated urgently. Even brief treatment delays should be avoided, as these tumors can progress rapidly, particularly choriocarcinoma. BEPx4 is considered the standard therapy.90 BEP has been compared to etoposide, cisplatin, and ifosfamide (VIP) in this patient population with similar cancer outcomes, but VIP has more genitourinary toxicity and myelosuppression. VIP represents an alternative to BEP for patients with contraindications to bleomycin.91,92 VIP should be considered the preferred choice for patients with extensive pulmonary disease, a mediastinal primary, and/or brain metastases. Also, if intermediate- or poor-risk patients develop pulmonary toxicity during BEP, VIP (not EP) is recommended to complete four cycles of three drugs. For intermediate- or poor-prognosis patients, there is no evidence to date that first-line high-dose chemotherapy (HDCT) with autologous stem cell transplant (ASCT) is superior to BEPx4.93–95
Chemotherapy should be given without dose reductions at 21-day intervals regardless of the neutrophil count on day 1. Routine use of granulocyte colony-stimulating factor (G-CSF) for primary prophylaxis is not recommended in all patients. However, the risk of febrile neutropenia and its complications is higher in intermediate- and poor-risk patients. Primary prophylaxis should be considered in these patients or those at high risk based on age, comorbidities, and disease characteristics. 96 Patients who receive ifosfamide must have primary prophylaxis. Secondary prophylaxis is recommended for patients with prior infectious or neutropenic complications.97
Supportive care and monitoring during chemotherapy
Monitoring tumor markers prior to each chemotherapy cycle is mandatory. Routine radiological imaging is not mandatory during chemotherapy, however, may be useful in patients with no elevated tumor markers, a significant amount of teratoma in the primary, large retroperitoneal masses, or new/progressive symptoms.
Patients with GCTs treated with chemotherapy are at an increased risk for vascular thromboembolic events (VTEs) which can be fatal.98 Prophylactic anticoagulation with low molecular weight heparins or Factor X inhibitors reduces the risk of VTE, with a minor increase in bleeding risk. The use of prophylactic anticoagulation should be individualized. Identified risk factors include increased tumor bulk, retroperitoneal lymph nodes >3.5 cm, stage, chemotherapy exposure, Khorana score ≥3, and the presence of vascular access devices.99 In suitable patients, anticoagulation should start as soon as possible prior to the initiation of chemotherapy.
Bleomycin-related pneumonitis is a known but rare complication of BEP, however, data supporting best practices for monitoring on therapy are limited. Pneumonitis risk increases with bleomycin exposure and age, so baseline pulmonary function tests (PFTs) may be useful prior to chemotherapy, particularly in patients with pre-existing pulmonary disease, age >40, renal impairment, or those planned to receive BEPx4. For patients with limited pulmonary reserve, alternatives to BEP should be considered. History (including shortness of breath and cough) and physical exams are important, as PFTs during treatment are not useful for the early identification of pneumonitis. Patients who develop pneumonitis of clinical concern should discontinue bleomycin and complete alternative chemotherapy providing similar therapeutic dose intensity.
In patients with poor-prognosis NSGCT, a suboptimal decline in AFP and/or β-HCG after the first cycle of BEP has been validated as a poor prognostic factor.100,101 Such patients may be considered for treatment intensification based on one randomized trial, however, the regimen is complex, associated with increased neurotoxicity and no OS benefit has been proven.101
If there is an unequivocal tumor marker rise in AFP and/or β-HCG, radiological imaging is required, including imaging of the brain. Primary cisplatin resistance is rare, but in the presence of radiological progression, a switch to salvage chemotherapy may be necessary and the patient must be referred to an experienced center. Patients in this setting have a worse prognosis and will be managed as per “Treatment of relapsed and refractory disease.” If there is an expected tumor marker decline but metastases are growing radiologically, “growing teratoma syndrome” should be considered.102,103 In most cases, the full course of chemotherapy should be completed and resection of the growing and residual masses should be done promptly post-chemotherapy. Very rarely, rapid radiological progression despite decreasing tumor markers may require interruption of chemotherapy for surgical resection.
Post-chemotherapy, radiological restaging should be performed in all patients 4–8 weeks after day 21 of the last chemotherapy cycle. Tumor markers that plateau at a low level may be followed closely. It is not uncommon for patients with a markedly elevated β-HCG prior to treatment to have a delay in normalization of β-HCG.104 Unless tumor markers are rising, all residual masses should be treated as per the sections below.
Post-chemotherapy residual masses: NSGCT
Post-chemotherapy patients with normal tumor markers may have residual masses (≥1 cm) on repeat radiological imaging. Histology of residual masses after first-line chemotherapy is necrosis in 40–50%, teratoma in 35–40%, and viable cancer in 10–15%.105,106 The incidence of viable cancer is declining in more recent series and maybe as low as 6%.107
Some series have identified factors associated with the absence of viable tumor in the residual mass, including absence of teratoma in the primary tumor, normal markers pre-chemotherapy, a small pre-chemotherapy mass, significant shrinkage of the mass with chemotherapy, and size of residual mass ≤10 mm.106,108,109 Several multivariate models have been published in an attempt to identify patients in whom PC-RPLND can be safely avoided. However, to date, none are sufficiently discriminative to be used clinically and there is no universal definition on what constitutes “significant shrinkage” with chemotherapy.108,110–113
Similarly, PET scan use in the post-chemotherapy NSGCT setting has limited utility due to the inability to detect teratoma. 114 Decision-making regarding post-chemotherapy management should be done in a multidisciplinary setting with imaging reviewed by experienced radiologists. In patients with normal tumor markers and residual retroperitoneal masses ≥1 cm in dimension, PC-RPLND should occur. It should be noted that in select post-chemotherapy cases with low-level but stable marker elevations, PC-RPLND is still indicated, as some cystic masses may harbor residual AFP/HCG that slowly leaks into circulation.115 PC-RPLND in such patients can result in cure without the need for second-line chemotherapy.
For masses <1 cm, surveillance is the preferred option, with several large series demonstrating relapse rates of 6–9%.116–118 The largest series to date reported on 191 patients followed for a median 81 months with a relapse rate of 8.4%. Of the relapses, over half were in the retroperitoneum and salvaged successfully with delayed PC-RPLND. Four (2.1%) died from testis cancer.116
Bilateral nerve-sparing RPLND has been the recommended surgery in this post-chemotherapy setting.119 A nerve-sparing approach is feasible and although ejaculation recovery rates are lower due to the desmoplastic reaction around the post-ganglionic sympathetic fibers, a bilateral template dissection does not automatically equate to loss of ejaculation.120,121 More recent series have shown that in select patients with small volume (<5 cm) tumor limited to the primary landing zone before and after chemotherapy, a modified unilateral template resection is safe, with no infield relapse.122,123 The advantage of a modified template is to minimize complications.122 However, the disadvantage is the risk of leaving residual disease and that continued abdominal imaging will be required. For these reasons, bilateral RPLND with nerve-sparing, when feasible, is the preferred approach in the post-chemotherapy setting.
If the decision to perform a PC-RPLND is made, most centers advocate performing surgery within 12 weeks after completing chemotherapy. However, in the case of IGCCCG good-risk disease, negative markers, and shrinking masses, or in patients with chemotherapy complications, there is less urgency to proceed to PC-RPLND and a delay may be appropriate. Tumour markers must be checked in the period after chemotherapy up to and including the week of PC-RPLND to ensure normality. Complete resection of the residual masses impacts prognosis and every attempt at complete surgical resection must be made.124–126 Surgery should be performed by experienced uro-oncologists. Increasingly, data supports a relationship between lymph node yield, hospital surgical volume, and improved outcomes.127
Resection of residual masses outside the retroperitoneum must also be considered. In most cases, the retroperitoneum should be operated on first. However, concomitant resections involving retrocrural, mediastinal, hepatic, thoracic, and supraclavicular disease have been described with acceptable morbidity.128 Concordance in the pathology between the retroperitoneum and other metastatic sites ranges from 50–89%.129–133 Thus, while finding complete necrosis in resected retroperitoneal masses is reassuring, surgeons should maintain a low threshold to resect extra-retroperitoneal disease because of the imperfect concordance.134 Concordance between bilateral residual lung masses, however, appears sufficiently high (85–95%) to allow careful surveillance of contralateral lung masses if necrosis is found in masses resected from one lung.134,135
Post-chemotherapy residual masses: Seminoma
Post-chemotherapy residual masses in advanced seminoma are common and most do not require additional treatment. In patients with residual masses <3 cm, the use of fluorodeoxyglucose (FDG)-PET scanning is not recommended, and patients can be safely surveyed. In patients with residual masses ≥3 cm, an FDG-PET scan may be considered but its clinical relevance is debatable.136,137 Recent data show a 23% positive predictive value of FDG-PET imaging in this setting, which is much lower than initially reported.138 Thus, only a negative FDG-PET is helpful in predicting necrosis, and surveillance is the standard of care in these cases. The surveillance algorithm in this case can be identical to patients who achieved a complete response. Positive FDG-PET scans need to be interpreted cautiously and should not lead to immediate treatment in all cases. This is especially true in cases of mild PET scan positivity, as it is common for these masses to continue to reduce in size and decrease in FDG avidity over months, and thus, continued close observation is the preferred option. There is no clearly defined observation schedule. If ordered, the PET scan should be done at least eight weeks after day 21 of the last chemotherapy cycle.
In cases where the FDG-PET scan remains strongly positive over time or there is a high suspicion of viable disease, complete surgical resection or biopsy is the management of choice and should only be performed at an experienced centre.139–141
Radiation therapy may be given in select cases, however, identification of those who may benefit remains challenging, as this has been inadequately studied.142,143 The advantages of surgery include the ability to assess the response to chemotherapy, obtain histology, and potentially provide cure. The disadvantage of surgery is the high morbidity. This stems from the intense desmoplastic reaction when seminoma is treated with chemotherapy. Complete resection is difficult and associated with much higher complication rates.141 The extent of surgical resection in seminoma is usually a resection of the residual mass or multiple biopsies and does not usually include a full or modified RPLND.140
Consolidation chemotherapy after post-chemotherapy surgery
If the pathology from completely resected residual masses shows necrosis or mature teratoma, no further treatment is required. If viable cancer is found, the role of further chemotherapy is not clear, as there are no prospective data. A discussion with the treating surgeon should be had to understand the extent of dissection and concern for any residual unresected disease. Some retrospective data have shown consolidative chemotherapy improved disease-free survival but not OS.105,144,145 Three independent predictive factors for improved progression-free and OS are complete surgical resection, less than 10% viable malignant cells, and a good IGCCCG prognostic group.
If post-surgery chemotherapy is given, no specific chemotherapy regimen or number of cycles can be recommended based on evidence, however, most oncologists would choose two cycles of a different regimen, as the viable cells may represent at least partial chemoresistance. If the viable cells represent a transformation, such as sarcoma or adenocarcinoma, chemotherapy specific to that histological subtype should be chosen.
Brain metastases
Brain metastases are uncommon, occurring in only 2–3% of patients with metastatic GCT at presentation. Greater than 95% are NSGCT histology.146 Patients with synchronous brain metastases at initial diagnosis have better survival than patients who develop metachronous disease (three-year OS of 48% vs. 27%).146 These patients are preferentially treated with VIP chemotherapy, as ifosfamide crosses the blood brain barrier whereas bleomycin does not. Prognosis is dictated by IGCCCG prognostic factors and may be influenced by the number of brain metastases.146 Local treatment of post-chemotherapy residual masses in the brain with radiation therapy or surgery may be considered, but the routine use of multimodality therapy is of uncertain benefit, particularly in patients who have brain metastases at initial diagnosis.146
Consensus recommendations
All patients with advanced GCT should be treated for cure and referral to (or consultation with) experienced centers should be strongly considered (agreement: 100%).
For patients with IGCCC good-prognosis disease, three cycles of BEP is the preferred option (agreement: 100%).
For patients with IGCCC intermediate- and poor-risk disease, four cycles of BEP or VIP are both options (agreement: 100%).
Tumor markers should be regularly monitored during and after chemotherapy (agreement: 100%).
Post-chemotherapy, all patients should have biochemical and radiological re-staging to assess response and identify residual masses (agreement: 100%).
Prior to initiation of chemotherapy, prophylactic anticoagulation should be considered for patients at higher risk for VTE and a low risk for bleeding (agreement: 88%).
During chemotherapy, primary prophylaxis with G-CSF is required for patients receiving ifosfamide. It may be considered for patients with intermediate-/poor-risk disease or those at high risk based on age, comorbidities, and disease characteristics, or as secondary prophylaxis (agreement: 100%).
In NSGCT, post-chemotherapy residual masses ≥1 cm and normal tumor markers should be resected (agreement: 100%).
In NSGCT, post-chemotherapy residual masses <1 cm and normal tumor markers can be safely surveyed (agreement: 100%).
If surgery is performed for NSGCT retroperitoneal residual disease, in most cases, a full bilateral RPLND should be performed by experienced surgeons. It is acknowledged that more recent retrospective series have demonstrated low relapse rates in modified unilateral template surgery in select good-risk patients with ipsilateral primary landing-site disease <5 cm (agreement: 92%).
In patients where a decision to resect residual disease is made, a low threshold to also resect residual disease outside of the retroperitoneum should be considered regardless of anatomic location because of the imperfect pathological concordance between sites (agreement: 93%).
-
Post-chemotherapy residual masses in seminoma are common.
If they are ≤3 cm, PET scans are not recommended and the patient should be surveyed (agreement: 100%).
-
If they are >3 cm, a PET may be considered:
If the PET scan is negative, patients can be surveyed (agreement: 100%).
If the PET scan is positive, continued close observation is the preferred option (agreement: 100%).
If the PET scan remains strongly positive over time, surgical resection or biopsy of the residual mass is the preferred option (agreement: 100%).
If the post-chemotherapy residual mass is growing on radiological imaging, surgical resection of the mass should be performed if technically feasible (agreement: 94%).
The role of further chemotherapy in patients who have viable cancer cells in the pathological specimen from a post-chemotherapy residual mass resection is controversial. These cases require an individualized approach in an experienced center (agreement: 93%).
The management of patients with brain metastases is often multimodal and should be individualized and managed in an experienced center. VIP is the preferred option for first-line chemotherapy (agreement: 100%).
6. Treatment of relapsed and refractory disease
Patients may relapse while on surveillance, post-radiation therapy, post-RPLND, or post-chemotherapy. The optimal treatment for relapsed GCTs depends on the initial treatment modality, response to prior therapy, the extent and timing of the relapse, and tumor histology.
It is important to ensure that elevated tumor markers and/or radiological abnormalities during followup unequivocally represent relapsed GCT before embarking on therapy. For example, mild transient elevations of tumor markers during followup may occur (e.g., from hypogonadism) or a slowly declining β-HCG post-primary chemotherapy may not indicate persistent disease. Also, not all new radiographical changes may represent relapsed disease (e.g., sarcoidosis). Thus, the complete picture — including the clinical context, tumor markers, radiological imaging, and often biopsy — is required to confirm a diagnosis of relapse.
Relapse post-active surveillance or locoregional therapy only
For patients on active surveillance who relapse in the retroperitoneum only, treatment options include locoregional therapy or standard cisplatin-based chemotherapy. Locoregional therapy includes radiotherapy for relapsed seminoma and RPLND for relapsed NSGCT as per the sections on “Stage II seminoma and Stage IIA/B NSGCT management.”
For patients on active surveillance who have any relapse sites outside of the retroperitoneum, standard first-line chemotherapy, as per the section “Treatment of advanced or metastatic disease,” is recommended.
For relapsed patients previously treated with locoregional therapy (radiation for stage I or II seminoma and primary RPLND for stage I and II NSGCT), the recommended treatment should be chemotherapy, as per “Treatment of advanced or metastatic disease.”
Relapse post-adjuvant chemotherapy
In Canada, the use of adjuvant chemotherapy post-orchiectomy for stage I GCT is very uncommon, as active surveillance is the recommended approach.
For patients with stage I seminoma who relapse after adjuvant carboplatin in the retroperitoneum only, treatment options include radiotherapy or chemotherapy.147 If these patients relapse with any sites outside of the retroperitoneum, curative-intent chemotherapy according to their IGCCCG prognostic group is recommended.
For patients with stage I NSGCT who relapse after adjuvant chemotherapy, treatment may include RPLND, standard chemotherapy (BEP/VIP), or salvage chemotherapy. The treatment choice will depend on factors, including whether they received one or two cycles of adjuvant cisplatin-based chemotherapy, the timing and location of relapse, tumor marker elevation, and doubling time. RPLND alone is an option if the relapse is limited to the retroperitoneum and is marker-negative.54,55,148
Relapse post-cisplatin-based chemotherapy for advanced disease
Patients who relapse after cisplatin-based chemotherapy for advanced disease represent a heterogeneous group with varying outcomes based on prognostic factors that are similar but not identical to those identified for treatment of chemotherapy-naive patients. The International Prognostic Factors Study Group (IPFSG) identified seven variables associated with favorable outcome at relapse: seminoma histology, gonadal primary, response to primary therapy, progressionfree interval from primary therapy >6 months, AFP <1000 ng/mL, HCG <1000 u/L, and absence of bone/liver/brain metastases at relapse. Based on these variables, patients are categorized into very low-, low-, intermediate-, high-, and very high-risk prognostic groups, as shown in Table 5.149 Two-year progression-free survival (PFS) and three-year OS rates are 75% and 77%, respectively for patients in the very low-risk category, and decline to 5% and 6%, respectively, for the very high-risk cohort. These risk factors have since been validated in a number of other cohorts.150–152
Table 5.
Prognostic score for patients who relapse after cisplatin-based first-line chemotherapy
| Score points | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | 0 | 1 | 2 | 3 |
| Primary site | Testis | Extragonadal | Mediastinal non-seminoma | |
| Prior response | CR or PR marker - |
PR marker + or SD |
PD | |
| Progression-free interval, months | >3 | ≤3 | ||
| AFP at relapse | Normal | ≤1000 ug/ml | >1000 ug/ml | |
| β-HCG at relapse | ≤1000 IU/L | >1000 IU/L | ||
| Mets to liver, brain, bone | No | Yes | ||
| Score sum (values from 0–10) | ||||
| Regroup score sum into categories: (0)=0; (1 or 2)=1; (3 or 4)=2; (5 or more)=3 | ||||
| Add histology score points: pure seminoma=−1; non-seminoma or mixed tumors=0 | ||||
| Final prognostic score (−1=very low risk; 0=low risk; 1=intermediate risk; 2=high risk; 3=very high risk) | ||||
CR: complete remission; PD: progressive disease; PRm-: partial remission, negative markers; PRm+: partial remission, positive markers; SD: stable disease.149
Salvage treatment options include further conventional-dose chemotherapy (CDCT) or HDCT with ASCT. While risk-stratifying patients based on the IPFSG risk category may help define the preferred treatment option, it must be noted that there is no conclusive randomized data identifying which type of salvage treatment yields the best survival outcome for a given prognostic group. The only randomized trial reported no benefit from HDCT vs. CDCT but this trial has been criticized for being underpowered, not using contemporary chemotherapy or transplant regimens, and only 73% of patients in the HDCT arm received the high-dose chemotherapy.153 Non-randomized data report more favorable outcomes with HDCT. A large, retrospective review found that initial salvage with HDCT may be superior to CDCT across all IPFSG prognostic groups except in patients with low-risk disease, where outcomes were similar.150 However, several biases inherent in retrospective reports must be acknowledged. The ongoing TIGER trial randomizes patients to salvage CDCT with paclitaxel, ifosfamide, and cisplatin (TIP) for four cycles vs. paclitaxel and ifosfamide for two cycles, followed by three cycles of high-dose carboplatin and etoposide (TI-CE), which will hopefully formally address which strategy is superior (NCT02375204).
Acknowledging the lack of solid, prospective data, for patients in the IPFSG very low- or low-risk groups, both CDCT or HDCT are reasonable options. For patients in all other IPFSG risk categories, HDCT is the preferred treatment option.150 There may be some circumstances where CDCT is a reasonable option in intermediate-risk patients. It should be noted that HDCT in the first-line salvage setting appears more effective than in second-line or subsequent settings (two-year PFS 63% vs. 49%).154 Therefore, successful upfront salvage therapy is imperative in terms of the likelihood of achieving cure.
Most patients who relapse with primary mediastinal GCT have NSGCT and would be classified as IPFSG very highrisk, therefore, HDCT would be the preferred option. In one series, three of 12 (25%) patients treated with HDCT achieved complete remission155 and in another, three of 22 (14%) patients are disease-free.156 In retrospective reviews, the long-term survival has ranged from 11–22%.157,158
CDCT
CDCT salvage regimens should consist of a three-drug regimen, generally with cisplatin plus ifosfamide as the back-bone of therapy, plus either etoposide or paclitaxel.159–164
Definitive randomized comparisons of salvage CDCT regimens are lacking. Long-term disease control can be seen in 15–60% of patients but there is significant prognostic heterogeneity in these studies. Chemotherapy with TIP has some of the highest disease control rates published but these trials also included better prognostic patients (e.g., gonadal primary and cisplatin-responsive). Thus, there is less certainty if the TIP regimen is as active in patients with worse prognostic features at relapse; however, most of these patients should be offered HDCT. If CDCT is chosen, TIPX4 is the preferred option in most cases.
HDCT and ASCT
HDCT with ASCT is a complex process with different protocols and processes.150,154,165–168 It must be performed in experienced centers where there is adequate volume and expertise to offer the best supportive and pre/post-transplant care. HDCT requires early close coordination between the transplant hematology and medical oncology teams to optimize survival outcomes.
If HDCT is chosen, two consecutive courses of high-dose carboplatin and etoposide followed by ASCT is the recommended treatment.154 In some centers, one or two cycles of CDCT salvage chemotherapy (e.g., TIP) may be needed first to facilitate the organization of the transplant. In this setting, being refractory to CDCT does not indicate one will be refractory to HDCT and thus, demonstration of cisplatin chemo-sensitivity is not a prerequisite for planned HDCT.154 Some centers may have the capacity to collect stem cells from poor-risk patients during first-line chemotherapy to optimize the timing of salvage HDCT if required.
Subsequent relapses following salvage chemotherapy
In patients who relapse after salvage CDCT, HDCT has been shown to be curative in some patients with second or subsequent relapses and should be offered before declaring the patient incurable.166,167,169 For patients who relapse after HDCT with ASCT or who are ineligible for this therapy, chemotherapy with agents such paclitaxel, gemcitabine, oxaliplatin, or combinations may be an option. In most patients, treatment is palliative but when combined with subsequent surgical resection, 10–15% long-term survival has been reported.170–172 While infrequent, an occasional patient may also have long-term disease control or cure with aggressive local therapy.
Patients relapsing with brain metastases
Patients may relapse in the brain only or with multiple sites including the brain. Prognosis is poor overall and appears to be influenced by the presence of liver or bone metastases and tumor marker levels. Improved survival is associated with the use of multimodality therapy and HDCT, so the use of chemotherapy should always be considered if the intent of treatment is curative.146 Local therapy may include surgery or radiation but should not delay chemotherapy. With regards to radiation therapy, data supporting the use of stereotactic radiosurgery in GCT is limited, however, if possible, it is preferred over whole-brain radiation to avoid the long-term cognitive effects.173
Salvage surgery
In patients who normalize their markers with salvage chemotherapy (CDCT or HDCT ) but have residual disease radiographically, all residual masses should be considered for post-chemotherapy surgical resection.105,124 For patients with viable disease after salvage chemotherapy and surgery, the role of further chemotherapy is unclear.174 For some patients with solitary sites of relapse, surgical resection alone may be the optimal treatment.
Patients who fail to normalize their markers or have progressive disease post-salvage systemic treatment may be candidates for salvage or “desperation” surgery if it is felt that all radiological disease can be resected. This will often require a multidisciplinary surgical team and should be performed in experienced centers.
Late relapses
Late relapse is defined as disease recurrence more than two years after complete response to initial therapy. The risk of late relapse is approximately 1.4% for seminoma and 3.2% for NSGCT patients.175 One must rule out a new primary GCT with metastases vs. a late relapse by performing a physical exam and ultrasound of the contralateral testicle. Other possibilities include somatic transformation of teratoma or a new non-GCT malignancy, thus tissue confirmation needs to be obtained, especially in the setting of normal tumor markers.176 Management of patients with late relapse is complex and often multimodal.175,177–179
However, late relapses may have disease that is more chemo-resistant and thus, surgical resection is an integral component.176,178,180 Chemotherapy may still be required preor postoperatively, depending on the site of relapse, tumor marker elevation, what the prior treatment was, and ability for complete resection. These patients require lifelong followup.180
Consensus recommendations
All patients with relapsed GCT should be treated for cure and referral to (or consultation with) experienced centers should be strongly considered (agreement: 100%).
It is important to ensure that elevated tumor markers and/or radiological abnormalities during followup unequivocally represent relapsed GCT before embarking on therapy (agreement: 100%).
Stage I seminoma patients who relapse in the retroperitoneum only while on surveillance may be candidates for radiation therapy if they meet the criteria for radiation therapy as per stage II seminoma recommendations; otherwise, chemotherapy as per the IGCCCG prognostic group is recommended (agreement: 100%).
Stage I seminoma patients who relapse in the retroperitoneum only after adjuvant carboplatin may be candidates for radiation therapy if they meet the criteria for radiation therapy as per stage II seminoma recommendations; otherwise, chemotherapy as per the IGCCCG prognostic group is recommended (agreement: 94%).
Stage I NSGCT patients who relapse in the retroperitoneum only while on surveillance may be candidates for RPLND if they meet the criteria for RPLND as per stage II NSGCT recommendations; otherwise, chemotherapy as per the IGCCCG prognostic group is recommended (agreement: 100%).
In the rare situation where stage I NSGCT patients are treated with adjuvant chemotherapy, should they relapse, treatment may include RPLND (for retroperitoneal relapses only), standard first-line chemotherapy, or salvage chemotherapy (agreement: 100%).
All stage I patients who have relapsed outside of the retroperitoneum should be treated with curative-intent chemotherapy according to their IGCCCG prognostic group (with the exception of stage I NSGCT patients who received adjuvant BEP; see statement above) (agreement: 100%).
Stage I or II seminoma patients treated with radiation, or stage I or II NSGCT patients treated with RPLND, and subsequently relapse should be treated with chemotherapy according to the IGCCCG prognostic group (agreement: 100%).
-
Patients who relapse after initial cisplatin-based chemotherapy and require salvage chemotherapy should be stratified by the IPFSG criteria (agreement: 100%).
For patients in the very low or low IPFSG risk group, either CDCT or HDCT with ASCT are reasonable options (agreement: 91%).
For patients in the intermediate IPFSG risk group, HDCT with ASCT is the preferred treatment option. There may be some circumstances where CDCT is a reasonable option (agreement: 100%).
For patients in the high and very high IPFSG risk groups, HDCT with ASCT is the preferred treatment option (agreement: 100%).
If CDCT is chosen, TIPX4 is the preferred option (agreement: 90%).
If HDCT and ASCT is chosen, two consecutive courses of high-dose carboplatin and etoposide followed by tandem ASCT is the preferred option (agreement: 100%).
For patients relapsing after salvage CDCT, HDCT with ASCT should be offered (agreementHDCT with ASCT should be delivered in experienced centers with close coordination between the transplant hematology and medical oncology teams (agreement: 100%).
The optimal treatment of primary mediastinal GCT relapse is unknown but these patients should not be excluded from receiving HDCT and ASCT (agreement: 100%).
Surgical resection of residual disease after salvage chemotherapy (either CDCT or HDCT with ASCT) should be performed (agreement: 100%).
Late relapses will require multimodal therapy but surgical resection of disease is an integral part of the management (agreement: 100%).
Supplementary Information
Footnotes
See related commentary on page 174
Appendix available at cuaj.ca
Competing interests: Dr. Hamilton has been an advisory board member for Astellas, Bayer, Janssen, TerSera; and has participated in clinical trials supported by Astellas, Bayer, Janssen. Dr. Jiang has been an advisory board member for EMD Serono and Pfizer; and has received payment from Amgen, Bayer, EMD Serono, Ipsen, and Janssen Oncology. Dr. Chung has received honoraria from AbbVie, Boston Scientific, TerSera, and Verity; and has participated in clinical trials supported by CTG, ICON, Medivation, Mount Sinai Health Systems, NRG, and Princess Margaret Cancer Centre. Dr. Hotte has been an advisory board member for AAA/Novartis, Astellas, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, Pfizer, and Seagen; has received honoraria from Astellas, Bayer, BMS, and Janssen; and has participated in clinical trials supported by AAA/Novartis, Astellas, BMS, CCTG, Eisai, Merck, Pfizer, SeaGen, and SignalChem. Dr. Winquist has been an advisory board member for Amgen, Bayer, Eisai, Merck, and Roche; has received an unrestricted educational grant from Eiasi; and has participated in clinical trials supported by Ayala, Eisai, Merck, and Roche. Dr. Aprikian has been an advisory board member for Abbvie, Astellas, and Bayer; and has received honoraria from Abbvie, Astellas, Bayer, Sanofi, and TerSera. Dr. Soulières has been an advisory board member for Adlai-Nortye, BMS, Eisai, Ipsen, Merck, and Pfizer; and has received research grants to his institution from BMS, Eisai, Ipsen, Merck, and Pfizer. Dr. Tyldesley has received honoraria from Bayer, Janssen, and TerSera (prostate cancer); and has participated in clinical trials supported by Janssen (prostate cancer). Dr. So has been an advisory board member for Abbvie, Astellas, Bayer, Janssen, Merck, and TerSera. Dr. Rendon has been an advisory board member for and received honoraria from Abbvie, Astellas, Bayer, Ferring, Janssen, Sanofi, TerSera, and Tolmar; holds investments in Myovant; and has participated in clinical trials supported by Abbvie, Astellas, Bavarian Nordic, Bayer, Ferring, Janssen, Myovant, and Sanofi. Dr. Wood has been an advisory board member for AstraZeneca BMS, Ipsen, Merck, and Pfizer (with no financial compensation); and has participated in clinical trials supported by AstraZeneca, BMS, and Merck (compensation to institution). The remaining authors do not report any competing personal or financial interests related to this work.
Prior to publication, this guideline was reviewed by the CUA Guidelines Committee, an external expert, and the CUA Board of Directors.
References
- 1.Committee CCSA, Society CC, Canada S, et al. [Accessed November 16, 2021];Canadian Cancer Statistics. 2021 2021 Available at: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-statistics/2021-pdf-en-final.pdf?rev=2b9d2be7a2d34c1dab6a01c6b0a6a32d&hash=01DE85401DBF0217F8B64F2B7DF43986&_ga=2.89172571.1525507927.1637097416-1129242265.1637097416. [Google Scholar]
- 2.CC Society. Cancerous tumors of the testicle. [Accessed November 16, 2021]. Available at: https://cancer.ca/en/cancer-information/cancer-types/testicular/what-is-testicular-cancer/cancerous-tumours.
- 3.Gurney JK, Florio AA, Znaor A, et al. International trends in the incidence of testicular cancer: Lessons from 35 years and 41 countries. Eur Urol. 2019;76:615–23. doi: 10.1016/j.eururo.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harzstark AL, Altschuler A, Amsden LB, et al. Implementation of a multidisciplinary expert testicular cancer tumor board across a large integrated healthcare delivery system via early case ascertainment. Jco Clin Cancer Informatics. 2021;5:187–93. doi: 10.1200/CCI.20.00114. [DOI] [PubMed] [Google Scholar]
- 5.Albany C, Adra N, Snavely AC, et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol. 2018;29:341–6. doi: 10.1093/annonc/mdx731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woldu SL, Matulay JT, Clinton TN, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urologic Oncol Seminars Orig Investigations. 2018;36:14.e7–14.e15. doi: 10.1016/j.urolonc.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Wood L, Kollmannsberger C, Jewett M, et al. Canadian consensus guidelines for the management of testicular germ cell cancer. Can Urol Assoc J. 2010;4:e19–38. doi: 10.5489/cuaj.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witjes JA, Babjuk M, Bellmunt J, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-an international collaborative multistakeholder effort under the auspices of the EAU-ESMO guidelines committees. Eur Urol. 2020;77:223–50. doi: 10.1016/j.eururo.2019.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4:29. doi: 10.1038/s41572-018-0029-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmoll HJ. Extragonadal germ cell tumors. Ann Oncol. 2002;13:265–72. doi: 10.1093/annonc/mdf669. [DOI] [PubMed] [Google Scholar]
- 11.Dorantes-Heredia R, Motola-Kuba D, Murphy-Sanchez C, et al. Spontaneous regression as a ‘burned-out’ non-seminomatous testicular germ cell tumor: A case report and literature review. J Surg Case Reports. 2019. p. rjy358. [DOI] [PMC free article] [PubMed]
- 12.Azzopardi JG, Mostofi FK, Theiss EA. Lesions of testes observed in certain patients with widespread choriocarcinoma and related tumors. The significance and genesis of hematoxylin-staining bodies in the human testis. Am J Pathology. 1961;38:207–25. [PMC free article] [PubMed] [Google Scholar]
- 13.Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: Ddiagnosis, treatment, and followup. Ann Oncol. 2018;29:1658–86. doi: 10.1093/annonc/mdy217. [DOI] [PubMed] [Google Scholar]
- 14.Ondruš D, Horňák M, Breza J, et al. Delayed orchiectomy after chemotherapy in patients with advanced testicular cancer. Int Urol Nephrol. 2001;32:665. doi: 10.1023/A:1014466110566. [DOI] [PubMed] [Google Scholar]
- 15.Leibovitch I, Little JS, Foster RS, et al. Delayed orchiectomy after chemotherapy for metastatic non-seminomatous germ cell tumors. J Urology. 1996;155:952–4. doi: 10.1016/S0022-5347(01)66355-9. [DOI] [PubMed] [Google Scholar]
- 16.Nason GJ, Aditya I, Leao R, et al. Partial orchiectomy: The Princess Margaret Cancer Centre experience. Urol Oncol Seminars Orig Invest. 2020;38:605e19–24. doi: 10.1016/j.urolonc.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. Ca Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 18.Nason GJ, Sweet J, Landoni L, et al. Discrepancy in pathology reports upon second review of radical orchiectomy specimens for testicular germ cell tumors. Can Urol Assoc J. 2020;14:411–5. doi: 10.5489/cuaj.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 20.Chung P, Daugaard G, Tyldesley S, et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 2014;4:155–60. doi: 10.1002/cam4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tandstad T, Ståhl O, Dahl O, et al. Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy: A report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA) Ann Oncol. 2016;27:1299–1304. doi: 10.1093/annonc/mdw164. [DOI] [PubMed] [Google Scholar]
- 22.Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2014;33:51–7. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 23.Boormans JL, Castro JM, de Marconi L, et al. Testicular tumor size and rete testis invasion as prognostic factors for the risk of relapse of clinical stage I seminoma testis patients under surveillance: A systematic review by the testicular cancer guidelines panel. Eur Urol. 2018;73:394–405. doi: 10.1016/j.eururo.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Pierorazio PM, Cheaib JG, Patel HD, et al. Comparative effectiveness of surveillance, primary chemotherapy, radiotherapy, and retroperitoneal lymph node dissection for the management of early-stage testicular germ cell tumors: A systematic review. J Urol. 2021;205:370–82. doi: 10.1097/JU.0000000000001364. [DOI] [PubMed] [Google Scholar]
- 25.Hans von der M, Lena S, Grete Krag J, et al. Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer. 1993;29:1931–4. doi: 10.1016/0959-8049(93)90446-M. [DOI] [PubMed] [Google Scholar]
- 26.Leung E, Warde P, Jewett M, et al. Treatment burden in stage I seminoma: A comparison of surveillance and adjuvant radiation therapy. BJU Int. 2013;112:1088–95. doi: 10.1111/bju.12330. [DOI] [PubMed] [Google Scholar]
- 27.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: A pooled analysis. J Clin Oncol. 2002;20:4448–52. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Tomomi K, Toshiyuki K, Kazutoshi O, et al. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int J Urol. 2010;17:980–7. doi: 10.1111/j.1442-2042.2010.02645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio J, Germà JR, del Muro XG, et al. Risk-adapted management for patients with clinical stage I seminoma: The second Spanish germ cell cancer cooperative group study. J Clin Oncol. 2005;23:8717–23. doi: 10.1200/JCO.2005.01.9810. [DOI] [PubMed] [Google Scholar]
- 30.Vesprini D, Chung P, Tolan S, et al. Utility of serum tumor markers during surveillance for stage I seminoma. Cancer. 2012;118:5245–50. doi: 10.1002/cncr.27539. [DOI] [PubMed] [Google Scholar]
- 31.Joffe JK, Cafferty FH, Murphy L, et al. Imaging modality and frequency in surveillance of stage I seminoma testicular cancer: Results from a randomized, phase 3, non-inferiority trial (TRISST) J Clin Oncol. 2022. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 32.Mistretta FA, Mazzone E, Palumbo C, et al. Contemporary assessment of survival rates in stage I testicular seminoma: A population-based comparison between surveillance and active treatment after orchiectomy. Clin Genitourin Canc. 2019;17:e793–e801. doi: 10.1016/j.clgc.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Frankel JK, Caumont F, DeBerg HA, et al. Contemporary trends in management of stage 1 seminoma. Urol Oncol Sem Orig Invest. 2021;39:240.e1–8. doi: 10.1016/j.urolonc.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Chung P, Mayhew LA, Warde P, et al. Management of stage I seminomatous testicular cancer: A systematic review. Clin Oncol. 2010;22:6–16. doi: 10.1016/j.clon.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Mead GM, Fossa SD, Oliver RTD, et al. Randomized trials in 2466 patients with stage I seminoma: Patterns of relapse and followup. J Nat Cancer Inst. 2011;103:241–9. doi: 10.1093/jnci/djq525. [DOI] [PubMed] [Google Scholar]
- 36.Fosså SD, horwich A, Russell JM, et al. Optimal planning target volume for stage I testicular seminoma: A medical research council randomized trial. J Clin Oncol. 1999;17:1146. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- 37.Oliver RTD, Mead GM, Rustin GJS, et al. Randomized trial of carboplatin vs. radiotherapy for stage I seminoma: Mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214) J Clin Oncol. 2011;29:957–62. doi: 10.1200/JCO.2009.26.4655. [DOI] [PubMed] [Google Scholar]
- 38.Group GTCS, Dieckmann KP, Dralle-Filiz I, et al. Testicular seminoma clinical stage 1: Treatment outcome on a routine care level. J Cancer Res Clin. 2016;142:1599–1607. doi: 10.1007/s00432-016-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aparicio J, del Muro XG, Maroto P, et al. Patterns of relapse and treatment outcome after active surveillance or adjuvant carboplatin for stage I seminoma: A retrospective study of the Spanish germ cell cancer group. Clin Transl Oncol. 2021;23:58–64. doi: 10.1007/s12094-020-02393-9. [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Tandstad T, Wheater M, et al. Outcome of men with relapse after adjuvant carboplatin for clinical stage I seminoma. J Clin Oncol. 2017;35:194–200. doi: 10.1200/JCO.2016.69.0958. [DOI] [PubMed] [Google Scholar]
- 41.Powles T, Robinson D, Shamash J, et al. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol. 2008;19:443–7. doi: 10.1093/annonc/mdm540. [DOI] [PubMed] [Google Scholar]
- 42.Chung PWM, Gospodarowicz MK, Panzarella T, et al. Stage II testicular seminoma: Patterns of recurrence and outcome of treatment. Eur Urol. 2004;45:754–60. doi: 10.1016/j.eururo.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Kollmannsberger C, Tyldesley S, Moore C, et al. Evolution in management of testicular seminoma: Population-based outcomes with selective utilization of active therapies. Ann Oncol. 2011;22:808–14. doi: 10.1093/annonc/mdq466. [DOI] [PubMed] [Google Scholar]
- 44.Giannatempo P, Greco T, Mariani L, et al. Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: a systematic review and meta-analysis of patient outcomes. Ann Oncol. 2015;26:657–68. doi: 10.1093/annonc/mdu447. [DOI] [PubMed] [Google Scholar]
- 45.Horwich A, Dearnaley DP, Sohaib A, et al. Neoadjuvant carboplatin before radiotherapy in stage IIA and IIB seminoma. Ann Oncol. 2013;24:2104–7. doi: 10.1093/annonc/mdt148. [DOI] [PubMed] [Google Scholar]
- 46.Hu B, Daneshmand S. Retroperitoneal lymph node dissection as primary treatment for metastatic seminoma. Adv Urol. 2018;7978958 doi: 10.1155/2018/7978958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daugaard G, Gundgaard MG, Mortensen MS, et al. Surveillance for stage I non-seminoma testicular cancer: Outcomes and long-term followup in a population-based cohort. J Clin Oncol. 2014;32:3817–23. doi: 10.1200/JCO.2013.53.5831. [DOI] [PubMed] [Google Scholar]
- 48.Sturgeon JF, Moore MJ, Kakiashvili DM, et al. Non-risk-adapted surveillance in clinical stage I non-seminomatous germ cell tumors: The Princess Margaret Hospital’s experience. Eur Urol. 2011;59:556–62. doi: 10.1016/j.eururo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton RJ, Nayan M, Anson-Cartwright L, et al. Treatment of relapse of clinical stage I non-seminomatous germ cell tumors on surveillance. J Clin Oncol. 2019;37:1919–26. doi: 10.1200/JCO.18.01250. [DOI] [PubMed] [Google Scholar]
- 50.Hotte SJ, Mayhew LA, Jewett M, et al. Management of stage I non-seminomatous testicular cancer: A systematic review and meta-analysis. Clin Oncol. 2010;22:17–26. doi: 10.1016/j.clon.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Cullen MH, Stenning SP, Parkinson MC, et al. Short-course adjuvant chemotherapy in high-risk stage I non-seminomatous germ cell tumors of the testis: A Medical Research Council report. J Clin Oncol. 1996;14:1106–13. doi: 10.1200/JCO.1996.14.4.1106. [DOI] [PubMed] [Google Scholar]
- 52.Pont J, Albrecht W, Postner G, et al. Adjuvant chemotherapy for high-risk clinical stage I non-seminomatous testicular germ cell cancer: Long-term results of a prospective trial. J Clin Oncol. 1996;14:441–8. doi: 10.1200/JCO.1996.14.2.441. [DOI] [PubMed] [Google Scholar]
- 53.Chevreau C, Mazerolles C, Soulié M, et al. Long-term efficacy of two cycles of BEP regimen in high-risk stage I non-seminomatous testicular germ cell tumors with embryonal carcinoma and/or vascular invasion. Eur Urol. 2004;46:209–15. doi: 10.1016/j.eururo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Tandstad T, Ståhl O, Håkansson U, et al. One course of adjuvant BEP in clinical stage I non-seminoma mature and expanded results from the SWENOTECA group. Ann Oncol. 2014;25:2167–72. doi: 10.1093/annonc/mdu375. [DOI] [PubMed] [Google Scholar]
- 55.Cullen M, Huddart R, Joffe J, et al. The 111 study: A single-arm, phase 3 trial evaluating one cycle of bleomycin, etoposide, and cisplatin as adjuvant chemotherapy in high-risk, stage 1 non-seminomatous or combined germ cell tumors of the testis. Eur Urol. 2020;77:344–51. doi: 10.1016/j.eururo.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huddart RA, Norman A, Shahidi M, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003;21:1513–23. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 57.Albers P, Siener R, Krege S, et al. Randomized phase 3 trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I non-seminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–72. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 58.Flechtner HH, Fischer F, Albers P, et al. Quality-of-life analysis of the German, prospective, multicenter trial of single-cycle adjuvant BEP vs. retroperitoneal lymph node dissection in clinical stage I non-seminomatous germ cell tumors. Eur Urol. 2016;69:518–25. doi: 10.1016/j.eururo.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Hugen CM, Hu B, Jeldres C, et al. Utilization of retroperitoneal lymph node dissection for testicular cancer in the United States: Results from the National Cancer Database (1998–2011) Urol Oncol Sem Orig Invest. 2016;34:487.e7–11. doi: 10.1016/j.urolonc.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 60.Yap SA, Yuh LM, Evans CP, et al. Evolving patterns of care in the management of stage I non-seminomatous germ cell tumors: Data from the California Cancer Registry. World J Urol. 2017;35:277–83. doi: 10.1007/s00345-016-1870-y. [DOI] [PubMed] [Google Scholar]
- 61.Leveridge MJ, Siemens DR, Brennan K, et al. Temporal trends in management and outcomes of testicular cancer: A population-based study. Cancer. 2018;124:2724–32. doi: 10.1002/cncr.31390. [DOI] [PubMed] [Google Scholar]
- 62.Groeben C, Koch R, Nestler T, et al. Centralization tendencies of retroperitoneal lymph node dissection for testicular cancer in Germany? A total population-based analysis from 2006–2015. World J Urol. 2020;38:1765–72. doi: 10.1007/s00345-019-02972-8. [DOI] [PubMed] [Google Scholar]
- 63.Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for non-seminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol. 2005;23:2781–8. doi: 10.1200/JCO.2005.07.132. [DOI] [PubMed] [Google Scholar]
- 64.Donadio AC, Motzer RJ, Bajorin DF, et al. Chemotherapy for teratoma with malignant transformation. J Clin Oncol. 2003;21:4285–91. doi: 10.1200/JCO.2003.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson A, Eggener SE, Bass EB, et al. Diagnosis and treatment of early-stage testicular cancer: AUA guideline. J Urol. 2019;202:272–81. doi: 10.1097/JU.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 66.Heidenreich A, Albers P, Hartmann M, et al. Complications of primary nerve-sparing retroperitoneal lymph node dissection for clinical stage I non-seminomatous germ cell tumors of the testis: Experience of the German Testicular Cancer Study Group. J Urol. 2003;169:1710–4. doi: 10.1097/01.ju.0000060960.18092.54. [DOI] [PubMed] [Google Scholar]
- 67.Stephenson AJ, Bosl GJ, Bajorin D, et al. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. J Urol. 2005;174:557–60. doi: 10.1097/01.ju.0000165163.03805.37. [DOI] [PubMed] [Google Scholar]
- 68.Weissbach L, Bussar-Maatz R, Flechtner H, et al. RPLND or primary chemotherapy in clinical stage IIA/B non-seminomatous germ cell tumors? Eur Urol. 2000;37:582–94. doi: 10.1159/000020197. [DOI] [PubMed] [Google Scholar]
- 69.Sheinfeld J, Motzer RJ, Rabbani F, et al. Incidence and clinical outcome of patients with teratoma in the retroperitoneum following primary retroperitoneal lymph node dissection for clinical stages I and IIA non-seminomatous germ cell tumors. J Urol. 2003;170:1159–62. doi: 10.1097/01.ju.0000087822.71739.9. [DOI] [PubMed] [Google Scholar]
- 70.Rabbani F, Sheinfeld J, Farivar-Mohseni H, et al. Low-volume nodal metastases detected at retroperitoneal lymphadenectomy for testicular cancer: Pattern and prognostic factors for relapse. J Clin Oncol. 2001;19:2020–5. doi: 10.1200/JCO.2001.19.7.2020. [DOI] [PubMed] [Google Scholar]
- 71.Eggener SE, Carver BS, Sharp DS, et al. Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA non-seminomatous germ cell testicular cancer. J Urol. 2007;177:937–42. doi: 10.1016/j.juro.2006.10.045. -discussion 942-3. [DOI] [PubMed] [Google Scholar]
- 72.Williams SD, Stablein DM, Einhorn LH, et al. Immediate adjuvant chemotherapy vs. observation with treatment at relapse in pathological stage II testicular cancer. N Engl J Med. 1987;317:1433–8. doi: 10.1056/NEJM198712033172303. [DOI] [PubMed] [Google Scholar]
- 73.McHugh DJ, Funt SA, Silber D, et al. Adjuvant chemotherapy with etoposide plus cisplatin for patients with pathologic stage II non-seminomatous germ cell tumors. J Clin Oncol. 2020;38:1332–7. doi: 10.1200/JCO.19.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuczyk M, Machtens S, Stief C, et al. Management of the post-chemotherapy residual mass in patients with advanced stage non-seminomatous germ cell tumors (NSGCT) Int J Cancer. 1999;83:852–5. doi: 10.1002/(SICI)1097-0215(19991210)83:6<852::AID-IJC30>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 75.Stephenson AJ, Bosl GJ, Motzer RJ, et al. Non-randomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB non-seminomatous germ cell testicular cancer. J Clin Oncol. 2007;25:5597–602. doi: 10.1200/JCO.2007.12.0808. [DOI] [PubMed] [Google Scholar]
- 76.Laurence C, Richard JS, Sally PS, et al. Impact of the treating institution on survival of patients with “poor-prognosis” metastatic non-seminoma. J Nat Cancer Inst. 1999;91:839–46. doi: 10.1093/jnci/91.10.839. [DOI] [PubMed] [Google Scholar]
- 77.Aass N, Klepp O, Cavallin-Stahl E, et al. Prognostic factors in unselected patients with non-seminomatous metastatic testicular cancer: A multicenter experience. J Clin Oncol. 1991;9:818–26. doi: 10.1200/JCO.1991.9.5.818. [DOI] [PubMed] [Google Scholar]
- 78.Feuer EJ, Frey CM, Brawley OW, et al. After a treatment breakthrough: A comparison of trial and population-based data for advanced testicular cancer. J Clin Oncol. 1994;12:368–77. doi: 10.1200/JCO.1994.12.2.368. [DOI] [PubMed] [Google Scholar]
- 79.Gillessen S, Sauvé N, Collette L, et al. Predicting outcomes in men with metastatic non-seminomatous germ cell tumors (NSGCT): Results from the IGCCCG update consortium. J Clin Oncol. 2021;39:1563–74. doi: 10.1200/JCO.20.03296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beyer J, Collette L, Sauvé N, et al. Survival and new prognosticators in metastatic seminoma: Results from the IGCCCG Update Consortium. J Clin Oncol. 2021;39:1553–62. doi: 10.1200/jco.20.03292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Einhorn LH, Williams SD, Loehrer PJ, et al. Evaluation of optimal duration of chemotherapy in favorableprognosis disseminated germ cell tumors: A Southeastern Cancer Study Group protocol. J Clin Oncol. 1989;7:387–91. doi: 10.1200/JCO.1989.7.3.387. [DOI] [PubMed] [Google Scholar]
- 82.de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: A randomized study of the European organization for research and treatment of cancer genitourinary tract cancer cooperative group and the medical research council. J Clin Oncol. 2001;19:1629–40. doi: 10.1200/JCO.2001.19.6.1629. [DOI] [PubMed] [Google Scholar]
- 83.Culine S, Kerbrat P, Kramar A, et al. Refining the optimal chemotherapy regimen for good-risk metastatic non-seminomatous germ-cell tumors: A randomized trial of the Genito-Urinary Group of the French Federation of Cancer Centers (GETUG T93BP) Ann Oncol. 2007;18:917–24. doi: 10.1093/annonc/mdm062. [DOI] [PubMed] [Google Scholar]
- 84.Fosså SD, deWit R, Roberts T, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: A prospective study of the European organization for research and treatment of cancer genitourinary group/medical research council testicular cancer study group (30941/TE20) J Clin Oncol. 2003;21:1107–18. doi: 10.1200/JCO.2003.02.075. [DOI] [PubMed] [Google Scholar]
- 85.Horwich A, Sleijfer DT, Fosså SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic non-seminomatous germ cell cancer: A multiinstitutional medical research council/European organization for research and treatment of cancer trial. J Clin Oncol. 1997;15:1844–52. doi: 10.1200/JCO.1997.15.5.1844. [DOI] [PubMed] [Google Scholar]
- 86.Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: A multi-institutional study. J Clin Oncol. 1993;11:598–606. doi: 10.1200/JCO.1993.11.4.598. [DOI] [PubMed] [Google Scholar]
- 87.Horwich A, Oliver RT, Wilkinson PM, et al. A Medical Research Council randomized trial of single agent carboplatin vs. etoposide and cisplatin for advanced metastatic seminoma. Brit J Cancer. 2000;83:1623–29. doi: 10.1054/bjoc.2000.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide, and bleomycin (PEB) vs. carboplatin, etoposide and bleomycin (CEB) for patients with ‘good-risk’ metastatic non-seminomatious germ cell tumors. Ann Oncol. 1996;7:1015–21. doi: 10.1093/oxfordjournals.annonc.a010493. [DOI] [PubMed] [Google Scholar]
- 89.Toner G, Stockler M, Boyer M, et al. Comparison of two standard chemotherapy regimens for goodprognosis germ cell tumors: A randomized trial. Lancet. 2001;357:739–45. doi: 10.1016/S0140-6736(00)04165-9. [DOI] [PubMed] [Google Scholar]
- 90.Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–84. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- 91.deWit R, Stoter G, TSleijfer D, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediateprognosis metastatic testicular non-seminoma: A randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. Brit J Cancer. 1998;78:828–32. doi: 10.1038/bjc.1998.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: An Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B study. J Clin Oncol. 1998;16:1287–93. doi: 10.1200/JCO.1998.16.4.1287. [DOI] [PubMed] [Google Scholar]
- 93.Motzer RJ, Nichols CJ, Margolin KA, et al. Phase 3 randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007;25:247–56. doi: 10.1200/JCO.2005.05.4528. [DOI] [PubMed] [Google Scholar]
- 94.Droz JP, Kramar A, Biron P, et al. Failure of high-dose cyclophosphamide and etoposide combined with double-dose cisplatin and bone marrow support in patients with high-volume metastatic non-seminomatous germ-cell tumors: Mature results of a randomized trial. Eur Urol. 2007;51:739–48. doi: 10.1016/j.eururo.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 95.Daugaard G, Skoneczna I, Aass N, et al. A randomized phase 3 study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974) Ann Oncol. 2011;22:1054–61. doi: 10.1093/annonc/mdq575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 97.Fosså SD, Kaye SB, Mead GM, et al. Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J Clin Oncol. 1998;16:716–24. doi: 10.1200/JCO.1998.16.2.716. [DOI] [PubMed] [Google Scholar]
- 98.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tran B, Ruiz-Morales JM, Gonzalez-Billalabeitia E, et al. Large retroperitoneal lymphadenopathy and increased risk of venous thromboembolism in patients receiving first-line chemotherapy for metastatic germ cell tumors: A study by the global germ cell cancer group (G3) Cancer Med-us. 2020;9:116–24. doi: 10.1002/cam4.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fizazi K, Culine S, Kramar A, et al. Early predicted time to normalization of tumor markers predicts outcome in poor-prognosis non-seminomatous germ cell tumors. J Clin Oncol. 2004;22:3868–76. doi: 10.1200/JCO.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 101.Fizazi K, Pagliaro L, Laplanche A, et al. Personalized chemotherapy based on tumor marker decline in poor prognosis germ-cell tumors (GETUG 13): A phase 3, multicenter, randomized trial. Lancet Oncol. 2014;15:1442–50. doi: 10.1016/S1470-2045(14)70490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Logothetis CJ, Samuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer. 1982;50:1629–35. doi: 10.1002/1097-0142(19821015)50:8<1629::AID-CNCR2820500828>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 103.Paffenholz P, Pfister D, Matveev V, et al. Diagnosis and management of the growing teratoma syndrome: A single-center experience and review of the literature. Urol Oncol. 2018;36:529.e23–30. doi: 10.1016/j.urolonc.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Zon RT, Nichols C, Einhorn LH. Management strategies and outcomes of germ cell tumor patients with very high human chorionic gonadotropin levels. J Clin Oncol. 1998;16:1294–7. doi: 10.1200/JCO.1998.16.4.1294. [DOI] [PubMed] [Google Scholar]
- 105.Fox EP, Weathers TD, Williams SD, et al. Outcome analysis for patients with persistent non-teratomatous germ cell tumor in post-chemotherapy retroperitoneal lymph node dissections. J Clin Oncol. 1993;11:1294–9. doi: 10.1200/JCO.1993.11.7.1294. [DOI] [PubMed] [Google Scholar]
- 106.Toner GC, Panicek DM, Heelan RT, et al. Adjunctive surgery after chemotherapy for non-seminomatous germ cell tumors: Recommendations for patient selection. J Clin Oncol. 1990;8:1683–94. doi: 10.1200/JCO.1990.8.10.1683. [DOI] [PubMed] [Google Scholar]
- 107.Carver BS, Serio AM, Bajorin D, et al. Improved clinical outcome in recent years for men with metastatic non-seminomatous germ cell tumors. J Clin Oncol. 2007;25:5603–8. doi: 10.1200/JCO.2007.13.6283. [DOI] [PubMed] [Google Scholar]
- 108.Carver BS, Bianco FJ, Shayegan B, et al. Predicting teratoma in the retroperitoneum in men undergoing post-chemotherapy retroperitoneal lymph node dissection. J Urol. 2006;176:100–3. doi: 10.1016/S0022-5347(06)00508-8. ; discussion 103-4. [DOI] [PubMed] [Google Scholar]
- 109.Donohue JP, Rowland RG, Kopecky K, et al. Correlation of computerized tomographic changes and histological findings in 80 patients having radical retroperitoneal lymph node dissection after chemotherapy for testis cancer. J Urol. 1987;137:1176–9. doi: 10.1016/S0022-5347(17)44439-9. [DOI] [PubMed] [Google Scholar]
- 110.Debono DJ, Heilman DK, Einhorn LH, et al. Decision analysis for avoiding post-chemotherapy surgery in patients with disseminated non-seminomatous germ cell tumors. J Clin Oncol. 1997;15:1455–64. doi: 10.1200/JCO.1997.15.4.1455. [DOI] [PubMed] [Google Scholar]
- 111.Steyerberg EW, Keizer HJ, Fosså SD, et al. Prediction of residual retroperitoneal mass histology after chemotherapy for metastatic non-seminomatous germ cell tumor: Multivariate analysis of individual patient data from six study groups. J Clin Oncol. 1995;13:1177–87. doi: 10.1200/JCO.1995.13.5.1177. [DOI] [PubMed] [Google Scholar]
- 112.Vergouwe Y, Steyerberg EW, Foster RS, et al. Predicting retroperitoneal histology in post-chemotherapy testicular germ cell cancer: A model update and multicenter validation with more than 1000 patients. Eur Urol. 2007;51:424–32. doi: 10.1016/j.eururo.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 113.Leão R, Nayan M, Punjani N, et al. A new model to predict benign histology in residual retroperitoneal masses after chemotherapy in non-seminoma. Eur Urol Focus. 2018;4:995–1001. doi: 10.1016/j.euf.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 114.Oechsle K, Hartmann M, Brenner W, et al. [18F]Fluorodeoxyglucose positron emission tomography in non-seminomatous germ cell tumors after chemotherapy: The German multicenter positron emission tomography study group. J Clin Oncol. 2008;26:5930–5. doi: 10.1200/JCO.2008.17.1157. [DOI] [PubMed] [Google Scholar]
- 115.Beck SDW, Patel MI, Sheinfeld J. Tumor marker levels in post-chemotherapy cystic masses: Clinical implications for patients with germ cell tumors. J Urol. 2004;171:168–71. doi: 10.1097/01.ju.0000099714.16082.78. [DOI] [PubMed] [Google Scholar]
- 116.Nason GJ, Jewett MAS, Bostrom PJ, et al. Long-term surveillance of patients with complete response following chemotherapy for metastatic non-seminomatous germ cell tumor. Eur Urol Oncol. 2021;4:289–96. doi: 10.1016/j.euo.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 117.Kollmannsberger C, Daneshmand S, So A, et al. Management of disseminated non-seminomatous germ cell tumors with risk-based chemotherapy followed by response-guided post-chemotherapy surgery. J Clin Oncol. 2010;28:537–42. doi: 10.1200/JCO.2009.23.0755. [DOI] [PubMed] [Google Scholar]
- 118.Ehrlich Y, Brames MJ, Beck SDW, et al. Long-term followup of cisplatin combination chemotherapy in patients with disseminated non-seminomatous germ cell tumors: Is a post-chemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol. 2010;28:531–6. doi: 10.1200/JCO.2009.23.0714. [DOI] [PubMed] [Google Scholar]
- 119.Carver BS, Shayegan B, Eggener S, et al. Incidence of metastatic non-seminomatous germ cell tumor outside the boundaries of a modified post-chemotherapy retroperitoneal lymph node dissection. J Clin Oncol. 2007;25:4365–9. doi: 10.1200/JCO.2007.11.2078. [DOI] [PubMed] [Google Scholar]
- 120.Wahle GR, Foster RS, Bihrle R, et al. Nerve sparing retroperitoneal lymphadenectomy after primary chemotherapy for metastatic testicular carcinoma. J Urology. 1994;152:428–30. doi: 10.1016/S0022-5347(17)32755-6. [DOI] [PubMed] [Google Scholar]
- 121.Pettus JA, Carver BS, Masterson T, et al. Preservation of ejaculation in patients undergoing nerve-sparing post-chemotherapy retroperitoneal lymph node dissection for metastatic testicular cancer. Urology. 2009;73:328–31. doi: 10.1016/j.urology.2008.08.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho JS, Kaimakliotis HZ, Cary C, et al. Modified retroperitoneal lymph node dissection for post-chemotherapy residual tumor: A long-term update. BJU Int. 2017;120:104–8. doi: 10.1111/bju.13844. [DOI] [PubMed] [Google Scholar]
- 123.Heidenreich A, Pfister D, Witthuhn R, et al. Post-chemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: Radical or modified template resection. Eur Urol. 2009;55:217–26. doi: 10.1016/j.eururo.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 124.Hendry WF, Norman AR, Dearnaley DP, et al. Metastatic non-seminomatous germ cell tumors of the testis. Cancer. 2002;94:1668–76. doi: 10.1002/cncr.10440. [DOI] [PubMed] [Google Scholar]
- 125.Stenning SP, Parkinson MC, Fisher C, et al. Post-chemotherapy residual masses in germ cell tumor patients. Cancer. 1998;83:1409–19. https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291097-0142%2819981001%2983%3A7%3C1409%3A%3AAID-CNCR19%3E3.0.CO%3B2-8 . [PubMed] [Google Scholar]
- 126.Shayegan B, Carver BS, Stasi J, et al. Clinical outcome following post-chemotherapy retroperitoneal lymph node dissection in men with intermediate- and poor-risk non-seminomatous germ cell tumor. BJU Int. 2007;99:993–7. doi: 10.1111/j.1464-410X.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 127.Bhanvadia RR, Rodriguez J, 3rd, Bagrodia A, et al. Lymph node count impacts survival following post-chemotherapy retroperitoneal lymphadenectomy for non-seminomatous testicular cancer: A population-based analysis. BJU Int. 2019;124:792–800. doi: 10.1111/bju.14798. [DOI] [PubMed] [Google Scholar]
- 128.Nason GJ, Donahoe L, de Perrot M, et al. Simultaneous vs. sequential retroperitoneal, thoracic, and cervical resection of post-chemotherapy residual masses in patients with metastatic non-seminomatous germ cell tumors of the testis. Urology. 2020;138:69–76. doi: 10.1016/j.urology.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 129.Hartmann JT, Candelaria M, Kuczyk MA, et al. Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumors. Eur J Cancer. 1997;33:843–7. doi: 10.1016/S0959-8049(96)00517-5. [DOI] [PubMed] [Google Scholar]
- 130.Steyerberg EW, Donohue JP, Gerl A, et al. Residual masses after chemotherapy for metastatic testicular cancer: The clinical implications of the association between retroperitoneal and pulmonary histology. J Urology. 1997;158:474–8. doi: 10.1016/S0022-5347(01)64506-3. [DOI] [PubMed] [Google Scholar]
- 131.Tognoni PG, Foster RS, McGraw P, et al. Combined post-chemotherapy retroperitoneal lymph node dissection and resection of chest tumor under the same anesthetic is appropriate based on morbidity and tumor pathology. J Urol. 1998;159:1833–5. doi: 10.1097/00005392-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 132.Gels ME, Hoekstra HJ, Sleijfer DT, et al. Thoracotomy for post-chemotherapy resection of pulmonary residual tumor mass in patients with non-seminomatous testicular germ cell tumors: Aggressive surgical resection is justified. Chest. 1997;112:967–73. doi: 10.1378/chest.112.4.967. [DOI] [PubMed] [Google Scholar]
- 133.Brenner PC, Herr HW, Morse MJ, et al. Simultaneous retroperitoneal, thoracic, and cervical resection of post-chemotherapy residual masses in patients with metastatic non-seminomatous germ cell tumors of the testis. J Clin Oncol. 1996;14:1765–9. doi: 10.1200/JCO.1996.14.6.1765. [DOI] [PubMed] [Google Scholar]
- 134.Donahoe LL, Nason GJ, Bedard PL, et al. Pathologic concordance of resected metastatic non-seminomatous germ cell tumors in the chest. J Thorac Cardiovasc Surg. 2021;161:856–68e1. doi: 10.1016/j.jtcvs.2020.10.158. [DOI] [PubMed] [Google Scholar]
- 135.Besse B, Grunenwald D, Fléchon A, et al. Non-seminomatous germ cell tumors: Assessing the need for post-chemotherapy contralateral pulmonary resection in patients with ipsilateral complete necrosis. J Thorac Cardiovasc Surg. 2009;137:448–52. doi: 10.1016/j.jtcvs.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 136.Santis MD, Bokemeyer C, Becherer A, et al. Predictive impact of 2-18fluoro-2-deoxy-D-glucose positron emission tomography for residual post-chemotherapy masses in patients with bulky seminoma. J Clin Oncol. 2001;19:3740–4. doi: 10.1200/JCO.2001.19.17.3740. [DOI] [PubMed] [Google Scholar]
- 137.Santis MD, Becherer A, Bokemeyer C, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in post-chemotherapy seminoma: An update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004;22:1034–9. doi: 10.1200/JCO.2004.07.188. [DOI] [PubMed] [Google Scholar]
- 138.Cathomas R, Klingbiel D, Bernard B, et al. Questioning the value of fluorodeoxyglucose positron emission tomography for residual lesions after chemotherapy for metastatic seminoma: Results of an International Global Germ Cell Cancer group registry. J Clin Oncol. 2018;36:3381–7. doi: 10.1200/JCO.18.00210. [DOI] [PubMed] [Google Scholar]
- 139.Ravi R, Ong J, Oliver RTD, et al. The management of residual masses after chemotherapy in metastatic seminoma. BJU Int. 1999;83:649–53. doi: 10.1046/j.1464-410x.1999.00974.x. [DOI] [PubMed] [Google Scholar]
- 140.Puc HS, Heelan R, Mazumdar M, et al. Management of residual mass in advanced seminoma: Results and recommendations from the Memorial Sloan-Kettering Cancer Center. J Clin Oncol. 1996;14:454–60. doi: 10.1200/JCO.1996.14.2.454. [DOI] [PubMed] [Google Scholar]
- 141.Rice KR, Beck SDW, Bihrle R, et al. Survival analysis of pure seminoma at post-chemotherapy retroperitoneal lymph node dissection. J Urol. 2014;192:1397–1402. doi: 10.1016/j.juro.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 142.Duchesne GM, Stenning SP, Aass N, et al. Radiotherapy after chemotherapy for metastatic seminoma — a diminishing role. Eur J Cancer. 1997;33:829–35. doi: 10.1016/S0959-8049(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 143.Murthy V, Karmakar S, Carlton J, et al. Radiotherapy for post-chemotherapy residual mass in advanced seminoma: A fluorodeoxyglucose positron emission tomography-computed tomography-based risk-adapted approach. Clin Oncol. 2021;33:e315–21. doi: 10.1016/j.clon.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 144.Fizazi K, Tjulandin S, Salvioni R, et al. Viable malignant cells after primary chemotherapy for disseminated non-seminomatous germ cell tumors: prognostic factors and role of post-surgery chemotherapy — results from an international study group. J Clin Oncol. 2001;19:2647–57. doi: 10.1200/JCO.2001.19.10.2647. [DOI] [PubMed] [Google Scholar]
- 145.Fizazi K, Oldenburg J, Dunant A, et al. Assessing prognosis and optimizing treatment in patients with post-chemotherapy viable non-seminomatous germ-cell tumors (NSGCT): Results of the sCR2 international study. Ann Oncol. 2008;19:259–64. doi: 10.1093/annonc/mdm472. [DOI] [PubMed] [Google Scholar]
- 146.Feldman DR, Lorch A, Kramar A, et al. Brain metastases in patients with germ cell tumors: Prognostic factors and treatment options-an analysis from the global germ cell cancer group. J Clin Oncol. 2016;34:345–51. doi: 10.1200/JCO.2015.62.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chau C, Cathomas R, Wheater M, et al. Treatment outcome and patterns of relapse following adjuvant carboplatin for stage I testicular seminomatous germ-cell tumor: Results from a 17-year U.K. experience. Ann Oncol. 2015;26:1865–70. doi: 10.1093/annonc/mdv254. [DOI] [PubMed] [Google Scholar]
- 148.Fischer S, Tandstad T, cohn-Cedermark G, et al. Outcome of men with relapses after adjuvant bleomycin, etoposide, and cisplatin for clinical stage I non-seminoma. J Clin Oncol. 2020;38:1322–31. doi: 10.1200/jco.19.01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lorch A, Beyer J, Bascoul-Mollevi C, et al. International Prognostic Factors Study Group. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. 2010;28:4906–11. doi: 10.1200/JCO.2009.26.8128. [DOI] [PubMed] [Google Scholar]
- 150.Lorch A, Bascoul-Mollevi C, Kramar A, et al. Conventional-dose vs. high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: Evidence from a large international database. J Clin Oncol. 2011;29:2178–2184. doi: 10.1200/JCO.2010.32.6678. [DOI] [PubMed] [Google Scholar]
- 151.Agarwala AK, Perkins SM, Abonour R, et al. Salvage chemotherapy with high-dose carboplatin and etoposide with peripheral blood stem cell transplant in patients with relapsed pure seminoma. Am J Clin Oncol. 2011;34:286–8. doi: 10.1097/COC.0b013e3181d6b518. [DOI] [PubMed] [Google Scholar]
- 152.Berger LA, Bokemeyer C, Lorch A, et al. First salvage treatment in patients with advanced germ cell cancer after cisplatin-based chemotherapy: analysis of a registry of the German Testicular Cancer Study Group (GTCSG) J Cancer Res Clin. 2014;140:1211–20. doi: 10.1007/s00432-014-1661-z. [DOI] [PubMed] [Google Scholar]
- 153.Pico JL, Rosti G, Kramar A, et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumors. Ann Oncol. 2005;16:1152–9. doi: 10.1093/annonc/mdi228. [DOI] [PubMed] [Google Scholar]
- 154.Einhorn LH, Williams SD, Chamness A, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Medicine. 2007;357:340–8. doi: 10.1056/NEJMoa067749. [DOI] [PubMed] [Google Scholar]
- 155.Suleiman Y, Siddiqui BK, Brames MJ, et al. Salvage therapy with high-dose chemotherapy and peripheral blood stem cell transplant in patients with primary mediastinal non-seminomatous germ cell tumors. Biol Blood Marrow Tr. 2013;19:161–3. doi: 10.1016/j.bbmt.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 156.Giorgi UD, Demirer T, Wandt H, et al. Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: The EBMT experience. Ann Oncol. 2005;16:146–51. doi: 10.1093/annonc/mdi017. [DOI] [PubMed] [Google Scholar]
- 157.Hartmann JT, Einhorn L, Nichols CR, et al. Second-line chemotherapy in patients with relapsed extragonadal non-seminomatous germ cell tumors: Results of an international multicenter analysis. J Clin Oncol. 2001;19:1641–8. doi: 10.1200/JCO.2001.19.6.1641. [DOI] [PubMed] [Google Scholar]
- 158.Caso R, Jones GD, Bains MS, et al. Outcomes after multidisciplinary management of primary mediastinal germ cell tumors. Ann Surg. 2021;274:e1099–107. doi: 10.1097/sla.0000000000003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Motzer RJ, Sheinfeld J, Mazumdar M, et al. Paclitaxel, ifosfamide, and cisplatin second-line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol. 2000;18:2413–8. doi: 10.1200/JCO.2000.18.12.2413. [DOI] [PubMed] [Google Scholar]
- 160.Loehrer PJ, Gonin R, Nichols CR, et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol. 1998;16:2500–4. doi: 10.1200/JCO.1998.16.7.2500. [DOI] [PubMed] [Google Scholar]
- 161.Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549–55. doi: 10.1200/JCO.2005.19.638. [DOI] [PubMed] [Google Scholar]
- 162.Loehrer PJ. Salvage therapy in recurrent germ cell cancer: Ifosfamide and cisplatin plus either vinblastine or etoposide. Ann Intern Med. 1988;109:540–6. doi: 10.7326/0003-4819-109-7-540. [DOI] [PubMed] [Google Scholar]
- 163.Mardiak J, Salek T, Sycová-Milá Z, et al. Paclitaxel plus ifosfamide and cisplatin in second-line treatment of germ cell tumors: A phase 2 study. Neoplasma. 2005;52:497–501. http://www.aepress.sk/_downloads/dl.php?from=pubmed&journal=NEO&file=2005_06_497.pdf . [PubMed] [Google Scholar]
- 164.Mead GM, Cullen MH, Huddart R, et al. A phase 2 trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: A medical research council trial. Brit J Cancer. 2005;93:178–84. doi: 10.1038/sj.bjc.6602682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beyer J, Stenning S, Gerl A, et al. High-dose vs. conventional-dose chemotherapy as first-salvage treatment in patients with non-seminomatous germ cell tumors: A matched-pair analysis. Ann Oncol. 2002;13:599–605. doi: 10.1093/annonc/mdf112. [DOI] [PubMed] [Google Scholar]
- 166.Motzer RJ, Mazumdar M, Sheinfeld J, et al. Sequential dose-intensive paclitaxel, ifosfamide, carboplatin, and etoposide salvage therapy for germ cell tumor patients. J Clin Oncol. 2000;18:1173–80. doi: 10.1200/JCO.2000.18.6.1173. [DOI] [PubMed] [Google Scholar]
- 167.Vaena DA, Abonour R, Einhorn LH. Long-term survival after high-dose salvage chemotherapy for germ cell malignancies with adverse prognostic variables. J Clin Oncol. 2003;21:4100–4. doi: 10.1200/JCO.2003.06.067. [DOI] [PubMed] [Google Scholar]
- 168.Lorch A, Kollmannsberger C, Hartmann JT, et al. Single vs. sequential high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: A prospective randomized multicenter trial of the German Testicular Cancer Study Group. J Clin Oncol. 2007;25:2778–84. doi: 10.1200/JCO.2006.09.2148. [DOI] [PubMed] [Google Scholar]
- 169.Lorch A, Neubauer A, Hackenthal M, et al. High-dose chemotherapy (HDCT) as second-salvage treatment in patients with multiple relapsed or refractory germ-cell tumors. Ann Oncol. 2010;21:820–5. doi: 10.1093/annonc/mdp366. [DOI] [PubMed] [Google Scholar]
- 170.Kollmannsberger C, Beyer J, Liersch R, et al. Combination chemotherapy with gemcitabine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: A study of the German testicular cancer study group. J Clin Oncol. 2004;22:108–14. doi: 10.1200/JCO.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 171.Oechsle K, Kollmannsberger C, Honecker F, et al. Long-term survival after treatment with gemcitabine and oxaliplatin with and without paclitaxel plus secondary surgery in patients with cisplatin-refractory and/or multiply relapsed germ cell tumors. Eur Urol. 2011;60:850–5. doi: 10.1016/j.eururo.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 172.Einhorn LH, Brames MJ, Juliar B, et al. Phase 2 study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol. 2007;25:513–6. doi: 10.1200/JCO.2006.07.7271. [DOI] [PubMed] [Google Scholar]
- 173.Khalifa J, Fléchon A, Chevreau C. Brain metastases from germ cell tumor: Time to reconsider radiotherapy? Crit Rev Oncol Hemat. 2020;150:102946. doi: 10.1016/j.critrevonc.2020.102946. [DOI] [PubMed] [Google Scholar]
- 174.Hartmann JT, Schmoll HJ, Kuczyk MA, et al. Post-chemotherapy resections of residual masses from metastatic non-seminomatous testicular germ cell tumors. Ann Oncol. 1997;8:531–8. doi: 10.1023/A:1008200425854. [DOI] [PubMed] [Google Scholar]
- 175.Oldenburg J, Martin JM, Fosså SD. Late relapses of germ cell malignancies: Incidence, management, and prognosis. J Clin Oncol. 2006;24:5503–11. doi: 10.1200/JCO.2006.08.1836. [DOI] [PubMed] [Google Scholar]
- 176.Sharp DS, Carver BS, Eggener SE, et al. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol. 2008;26:5524–9. doi: 10.1200/JCO.2007.15.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baniel J, Foster RS, Gonin R, et al. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170–6. doi: 10.1200/JCO.1995.13.5.1170. [DOI] [PubMed] [Google Scholar]
- 178.Gerl A, Clemm C, Schmeller N, et al. Late relapse of germ cell tumors after cisplatin-based chemotherapy. Ann Oncol. 1997;8:41–7. doi: 10.1023/A:1008253323854. [DOI] [PubMed] [Google Scholar]
- 179.Shahidi M, Norman AR, Dearnaley DP, et al. Late recurrence in 1263 men with testicular germ cell tumors. Cancer. 2002;95:520–30. doi: 10.1002/cncr.10691. [DOI] [PubMed] [Google Scholar]
- 180.George DW, Foster RS, Hromas RA, et al. Update on late relapse of germ cell tumor: A clinical and molecular analysis. J Clin Oncol. 2003;21:113–22. doi: 10.1200/JCO.2003.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.