Introduction
Despite advances in the surgical management of urinary stone disease that have significantly reduced patient morbidity, new stone formation and recurrence remain significant health issues. Data from the United States National Health and Nutrition Examination Survey (NHANES) published in 2020 reported a kidney stone prevalence of 12% in men and 10% among women.1 Recent data from the U.S. also has noted a cumulative increase in stone incidence from 0.6% to 0.9% between 2005 and 2015.2 An increase in stone formation among women has also been observed such that the male-to-female ratio appears to be decreasing.3,4 Evidence is also emerging there may be an increase in the incidence of certain stone compositions, such as uric acid, a stone type linked to both dietary and metabolic risk factors. 5 Furthermore, the association with obesity, metabolic syndrome, diabetes mellitus, and stone formation suggests nephrolithiasis should be regarded as a systemic disease.
Recurrence rates after an initial symptomatic stone event are reported to be 30–50% within 10 years.6,7 Therefore, patients are generally motivated to explore prevention strategies. 1 Most (96.8%) recurrent stone formers or those with more than one concurrent stone will have a urinary metabolic abnormality.8 Epidemiological data from the U.S. show that only 7% of patients with a high risk of recurrent stone disease undergo metabolic evaluation by any physician.9 Eighty-one percent of patients interviewed would prefer to take prophylactic medication than undergo another stone episode, and 92% of respondents preferred medication to undergoing surgery. 10 Although patients indicate they would be interested in using medication to prevent future stones, compliance studies suggest otherwise. Close to 50% of patients prescribed pharmacological prevention were non-compliant, especially those on potassium citrate.11 This data stresses the importance of a comprehensive metabolic assessment with implementaiton of individualized prevention strategies. With directed therapy and proper patient education, patient compliance and therapeutic success may be optimized.
The economic burden of recurrent stone disease is also significant. Estimates of direct costs for patient care and the indirect costs related to lost work time exceed $5 billion USD.12,13 Given the rising rates of obesity and diabetes and their association with stone formation, the cost of managing stone disease is expected to increase an additional 1.24 billion dollars yearly in the U.S. by 2030.14
This guideline is an update of the 2016 document and aims to identify patients at heightened risk of stone recurrence, to outline the required investigations to assess these patients, and to provide contemporary advice on dietary and medical interventions of proven benefit in the Canadian context. This current guideline addresses the evaluation and medical prophylaxis of upper urinary tract stones and not bladder stones.
Literature review
The updated content included in this document is based on a review of the English-language literature. A PubMed search was conducted encompassing the period from January 1, 2015, to July 1, 2021, to include the following terms: “nephrolithiasis,” “urolithiasis,” “kidney stone,” “renal stone,” or “urinary stone.” In total, 11 640 article titles were reviewed and 293 were identified as potentially relevant for inclusion in the literature assessment for this guideline update. Management recommendations were modified if needed based on the most current literature since the last guideline was published in 2016. Studies were evaluated and recommendations made based on Oxford levels of evidence and grades of recommendation as per the CUA Guidelines Committee’s directive.15
Indications for metabolic evaluation
Recommendation: Basic metabolic screening should include a urinalysis, with or without a urine culture, serum electrolytes (Na, K, HCO3), calcium, creatinine, and a stone analysis when available (Level of evidence [LE] 4, Grade C recommendation).
A first-time stone former, without any identifiable risk factors for recurrent stone formation, should undergo a limited metabolic screen to rule out potential systemic disorders such as hyperparathyroidism and renal dysfunction.
Recommendation: An in-depth metabolic evaluation is recommended in patients with risk factors for recurrent stone disease (LE 3, Grade C recommendation).
An in-depth metabolic investigation may be considered for any patient who is motivated and interested in completing metabolic testing. There are, however, patient populations with clearly known risk factors where an in-depth metabolic investigation is recommended:16–23
– Children (<18 years of age)
– Bilateral or multiple stones
– Recurrent stones (≥2 kidney stone episodes)
– Non-calcium stones (e.g., uric acid, cystine)
– Pure calcium phosphate stones
– Any complicated stone episode that resulted in a severe (if even temporary) acute kidney injury, sepsis, hospitalization, or complicated hospital admission
– Any stone requiring percutaneous nephrolithotomy treatment
– Stones in the setting of a solitary (anatomical or functional) kidney
– Patients with renal insufficiency
– History of systemic disease that increases the risk of kidney stones (e.g., gout, osteoporosis, inflammatory bowel disorders, hyperparathyroidism, renal tubular acidosis, sarcoidosis, diabetes mellitus/metabolic syndrome)
– Occupation where public safety is at risk (e.g., pilots, air traffic controller, police officer, military personnel, firemen)
– Family history of kidney stones
Struvite stones typically form in the setting of recurrent urinary infection, anatomical anomalies, and foreign bodies but occasionally are associated with metabolic abnormalities. Metabolic evaluation maybe considered in these patients, although the prevention focus should be on addressing the risks mentioned with periodic imaging surveillance if risk factors are not modifiable. The urease inhibitor acetohydroxamic acid (AHA) has been used with limited success and with significant side effects.24–26 This agent is not currently available in Canada.
In-depth evaluation
For those patients where an in-depth evaluation is indicated, the workup should include serum and 24-hour urine tests, as well as a thorough dietary history. These tests should include:
-
Serum:
– Creatinine, sodium, potassium, calcium, albumin, uric acid, and bicarbonate
– Parathyroid hormone (PTH) level if high or high-normal serum calcium, or if idiopathic hypercalciuria with normocalcemia
– Vitamin D level if PTH is elevated to rule out secondary hyperparathyroidism
-
24-hour urine collection:
– Volume, creatinine, calcium, sodium, potassium, oxalate, citrate, uric acid, magnesium
– Cystine if known or suspected cystine stone
-
Spot urine:
– Urinalysis with urine pH
Previously, it was suggested that PTH testing was only required if serum calcium was elevated.27 However, more recent data suggest that normocalcemic hyperparathyroidism is an important clinical variant that is associated with a high prevalence of nephrolithiasis.28 Screening for primary hyperparathyroidism in a tertiary stone clinic demonstrated a prevalence of 7.1%, with 71.7% of those cases having high-normal serum calcium.29 Therefore, we recommend serum PTH if the patient has a high or high-normal serum calcium or in patients with idiopathic hypercalciuria and normocalcemia.
Vitamin D deficiency is common in North American populations and new and evolving data has emerged regarding the impact of vitamin D on stone disease (see Vitamin D section below). Based on this evidence, we recommend that serum vitamin D be measured (if available in your health region) when a patient has an elevated PTH in order to rule out secondary hyperparathyroidism.
Number of 24-hour urine collections
Recommendation: Two 24-hour urine collections are preferred when possible but should be balanced by the practicality and importance of obtaining at least one collection (LE 3, Grade C recommendation).
There is some controversy regarding the number of 24-hour urine collections that are necessary in order to investigate patients.30,31 Data suggest that 47.6% of patients had their clinical management changed by an abnormality identified only when two samples were collected.32,33 Close to 25% of patients will have a 50% variation in at least one urinary parameter, and 25% of patients will have a 20% difference in three urine parameters.34 As such, it is currently recommended that two 24-hour urine collections be obtained. The benefit of two collections should be balanced, however, by the practicality and importance of obtaining at least one collection.
Importance of stone analysis
Recommendation: Stones collected by patients or removed at the time of surgical intervention should be submitted for analysis (LE 3, Grade C recommendation).
Identification of stone composition will aid in determining prevention and directing surgical options for future stones. Furthermore, identification of struvite, ammonium urate, uric acid, calcium phosphate, or cystine stones would alter the indication for in-depth metabolic evaluation. If a patient continues to form new stones, a repeat stone analysis should be performed, as this may change management. Stone composition changed in 21.2% of patients over time.35
Reports have demonstrated a strong correlation between pure stone composition and metabolic abnormalities.36 Specifically, hypercalciuria was associated with brushite and calcium oxalate dihydrate stones, whereas hyperoxaluria was found most commonly in calcium oxalate monohydrate stone formers.37 In addition, apatite stones were correlated with both hypercalciuria and hypocitraturia, and uric acid stones were found in patients with low urinary pH.36 Stone composition may be a useful tool to help guide empiric medical therapy for patients who are unable or unwilling to perform an in-depth medical evaluation.37
General dietary measures
Recommendation: When possible, specific dietary assessments and recommendations should be made with the involvement of a registered dietician (LE 3, Grade C recommendation).
General dietary and fluid intake advice has been shown to be effective in reducing stone recurrence rates and is warranted for even the first-time stone former without identifiable risk factors. The “stone clinic effect” has shown that counselling on appropriate fluid intake and dietary excesses can significantly reduce stone recurrence.38
The involvement of a registered dietician, if available, in the counselling of patients with recurrent renal stones should be incorporated into the management of these patients. Assessment with a registered dietician is strongly suggested where there is a history of compromised nutritional status, complex medical situations, or patients who need assistance implementing dietary recommendations.39,40 Evidence suggests that patients who received specific dietary recommendations based on a comprehensive evaluation had fewer stone recurrences over three years than those who only received general dietary advice.41
Fluid intake
Recommendation: All stone formers should be counselled to achieve a daily urine output of 2.5 liters (LE 2, Grade B recommendation).
Adequate fluid intake to produce a urine output of 2.5 L daily has been shown to reduce the risk of stone formation by 60–80% in several meta-analyses.42–46 Conversely, studies have shown that poor fluid intake increases lithogenic risk, particularly with 1.4 L of urine or less.45,47–49 Adequate fluid intake in the form of water is suggested as the first-line treatment to prevent stones, with each 200 mL increase in fluid intake reducing the risk of stones by 13%.50
Other fluids, including orange juice, caffeinated beverages (or caffeine alone), coffee, wine, and beer may have a protective effect against stone formation that must be weighed against other potential health effects.51–54 The consumption of low-calorie orange juice has been shown to have a protective effect by increasing urinary citrate levels.55 Additionally, sugar-sweetened non-colas and colas may increase the risk of stone formation.52 Milk does not increase the risk of stones unless consumed in excess. Lastly, no conclusive evidence exists regarding the specific properties of water (e.g., water hardness) and lithogenic risk.48
Recent studies assessing smart technology, including smart water bottles and associated digital applications, have shown the accuracy of these technologies to measure fluid intake; however, the impact on urine output may be similar to counselling.56–58 A systematic review showed that most interventions can improve fluid intake by approximately 500 mL, with the most effective strategies being instruction and self-monitoring using urine dipstick or 24-hour urine volume.59
Counselling regarding fluid intake should clearly state the risk reduction associated with increased fluid intake; explore barriers to fluid consumption, such as occupational barriers, taste factors related to water; and urinary voiding difficulties, such as overactive bladder and bladder outlet obstruction.60,61 Practical ways to increase fluid intake include drinking at set times during the day and hourly during working hours, maintaining a water bottle in all places where significant time is spent, and eating foods higher in water content, such as fruits and vegetables.61 Caution should be exercised when counselling patients with congestive heart failure or chronic renal insufficiency.
Calcium
Recommendation: The goal for dietary calcium intake should be 1000–1200 mg/day, and if calcium supplementation is required, it should be taken at mealtimes (LE 3, Grade B recommendation).
A public misconception persists that stone formers should restrict calcium in their diet. On the contrary, multiple large, prospective cohort studies have demonstrated higher dietary intakes of calcium are correlated with a lower risk of stone formation in both men and women.46,62–64 Previous studies examining the effect of calcium supplementation on stone risk have shown conflicting results, with one study demonstrating a slightly increased risk in stone formation63 and another study showing no change in risk.47 The difference in these findings may be attributed to the timing of the calcium supplement administration, since calcium not consumed at mealtimes in one study may have decreased its ability to chelate oxalate.64
The recommended daily intake of calcium is 1000–1200 mg separated into two doses and ideally with meals. Calcium would preferably be obtained through diet, as some studies suggest supplementation may also increase cardiovascular risk.65 Where supplementation is required, it should be taken with meals, as this results in the greatest oxalate sequestration and is not associated with an increased risk of hypercalciuria.65
Vitamin D and bone health
Recommendations:
In calcium stone formers with vitamin D deficiency, repletion is appropriate; however, monitoring of vitamin D levels and hypercalciuria on repeat testing is necessary (LE 2–3, Grade C recommendation).
Consider bone mineral density (BMD) testing in calcium stone formers with evidence of hypercalciuria and/or distal renal tubular acidosis (dRTA) (LE 2–3, Grade C recommendation).
Treatment of calcium stone formers with either a thiazide diuretic, alkali citrate, or ideally both has been shown to reduce stone recurrence risk and increase BMD and should be considered in patients with documented low BMD (LE 2–3, Grade C recommendation).
Vitamin D deficiency is common among stone formers in northern latitudes, with 80.2% of patients in a Canadian metabolic stone clinic having vitamin D insufficiency.66–68 Patients with low vitamin D levels have been shown to have an increased number of metabolic abnormalities, specifically, secondary hyperparathyroidism, which was found in 26% of a Canadian cohort.67 Studies examining serum vitamin D levels and urinary calcium excretion are conflicting. Some studies noted an association between higher vitamin D levels and hypercalciuria,66,69 whereas others have failed to find a relationship between vitamin D and hypercalciuria70 or stone reccurrence.71
The impact of vitamin D supplementation on hypercalciuria and stone risk is unclear. The largest study to date examining the effect of vitamin D in the general population evaluated over 200 000 men and women and found no association between vitamin D intake and urolithiasis.72 Similarly, a meta-analysis of 10 randomized controlled trials of vitamin D supplementation including 2010 participants showed no increased incidence of kidney stones.73 Several other large studies have shown an increased risk of stone formation with vitamin D supplementation; however, these studies were confounded by the co-administration of calcium supplementation.74–76
Previous studies in stone formers with vitamin D deficiency have found mixed effects of vitamin D repletion on urinary calcium excretion. Two prospective cohort studies of vitamin D supplementation in patients with vitamin D deficiency did not show an increase in mean calcium excretion.66,77 A recent randomized trial showed no effect of vitamin D administration on urinary calcium excretion or the supersaturation of calcium oxalate or calcium phosphate crystals.78
Although we do not recommend routinely testing vitamin D levels (see Metabolic evaluation above), chronic depletion of vitamin D may lead to secondary hyperparathyroidism, thereby increasing the risk of bone loss and recurrent stones. Consequently, vitamin D levels should be measured in the settings of an elevated PTH level in order to rule out secondary hyperparathyroidism. Repletion of vitamin D in stone formers is appropriate with followup monitoring for vitamin D response and the development of hypercalciuria.
The association between low BMD and calcium nephrolithiasis has been well-investigated. Several studies have demonstrated that calcium stone disease correlates with low BMD, with a higher risk corresponding with increasing levels of hypercalciuria.79–82 Furthermore, the prevalence of osteopenia or osteoporosis is higher in stone formers with concomitant vitamin D deficiency.80 Chronic metabolic acidosis in patients with complete dRTA increases bone resorption and places these patients at very high risk of low BMD; one series demonstrated rates of osteopenia and osteoporosis as high as 43% and 21%, respectively.83 Multiple studies have shown an increased risk of fractures in patients with urolithiasis (hazard ratio [HR] 1.13–2.32).84,85
Dietary recommendations for stone reduction, including a low-sodium and normal-calcium diet, have also been shown to improve BMD.86 Thiazide diuretics also reduce urinary calcium levels, decrease stone recurrence,86 improve BMD in stone formers85,87,88 and reduce the risk of hip fractures.89 In addition, alkali citrate therapy has a positive effect on both stone recurrence and bone health by reducing bone resorption and buffering acid production. Treatment with potassium citrate monotherapy effectively increases BMD in calcium stone formers and is effective in patients with dRTA.87,90,91 Several studies have examined combination therapy with both thiazides and potassium citrate and found significant improvements in both BMD and stone recurrence.87,92 While more prospective studies are required, combination therapy may be more effective than either monotherapy. Bisphosphate therapy has also been shown to be safe and effective in increasing BMD in patients with urinary stones.86
Animal protein
Recommendation: Patients with recurrent calcium or uric acid stones should moderate their animal protein intake and avoid purine-rich foods (LE 2–3, Grade C recommendation).
In some populations, high animal protein is associated with a slight increase in the risk of nephrolithiasis.63 A large, prospective cohort study of men showed that dietary protein consumption was directly associated with the risk of stone formation (relative risk [RR] 1.33).62 Similarly, idiopathic calcium stone formers have been noted to have an increased consumption of protein (animal and vegetable) and purine-rich foods compared to non-stone formers, resulting in elevated urinary oxalate and calcium, and lower levels of urinary citrate.93
However, other studies have failed to find a strong correlation between animal protein intake and the risk of nephrolithiasis.47,48,94 Regardless, diets high in animal protein are associated with increased uric acid excretion and decreased urinary citrate and pH levels, predisposing these individuals to uric acid nephrolithiasis.95 A vegetarian diet has been demonstrated to reduce the risk of uric acid crystallization by 93% compared to a typical Western diet.96
Sodium
Recommendation: Patients with recurrent calcium nephrolithiasis should limit their sodium intake to 1500 mg daily and not exceed 2300 mg daily (LE 1–2, Grade B recommendation).
Dietary sodium excess is associated with hypercalciuria,97 and high urinary sodium levels increase calcium excretion and decrease urinary citrate.35,38 High sodium intake was associated with up to 61% increase in stone risk in a large, prospective cohort of women.47 Furthermore, reduction in dietary sodium can improve urinary parameters and decrease stone recurrence. In a randomized trial of hypercalciuric calcium stone formers, a low-sodium diet resulted in lower urinary sodium, calcium, and oxalate, and resulted in normalization of urine calcium excretion for one-third of patients.98 An additional randomized trial demonstrated that a low-sodium and animal protein diet resulted in fewer stone recurrences compared with a low-calcium diet.99
Fruits and vegetables
Recommendation: A diet high in fiber, fruits, and vegetables may offer a small protective effect against stone formation (LE 2–3, Grade C recommendation).
Low dietary intake of fiber, fruit, and vegetables increases the risk of kidney stones in women.100 In stone forming patients with hypocitraturia, introducing these foods resulted in increased excretion of citrate, potassium, and magnesium, and a reduction in the supersaturation of calcium oxalate and calcium phosphate crystals.101
Vitamin C
Recommendation: Vitamin C supplementation of more than 1000 mg daily is not recommended due to the associated risk of hyperoxaluria and nephrolithiasis (LE 2–3, Grade C recommendation).
It is theorized that the excess vitamin C is converted to oxalate and may increase stone risk. In population-based studies, intake of over 1000 mg of vitamin C daily caused a slight increase in the risk of nephrolithiasis.63 Vitamin C supplementation of 1–2 g was associated with increased urinary oxalate in stone forming patients.102,103
Metabolic syndrome
Recommendation: Stone disease highly correlates with obesity, diabetes, and metabolic syndrome; patients should be counselled that proper management of these conditions may reduce their future stone risk (LE 2–3, Grade D recommendation).
Patients with one or more features of metabolic syndrome have significantly increased risks of heart disease, myocardial infarction, angina, and coronary bypass.104 Stone disease is also linked to metabolic syndrome, where patients with metabolic syndrome have 2.13 times increased odds of developing stones.105 In addition, the risk of stone disease increases with the number of metabolic syndrome traits present.105
Patients with metabolic syndrome and stones should be counselled to adopt healthier lifestyles, including dietary practices that promote low sodium intake and consumption of fresh fruit and vegetables. The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be effective in reducing cardiovascular risks, as well kidney stone recurrence in three large epidemiological studies.106
Obesity and diabetes have been shown to be independent risk factors for stone formation, likely secondary to the development of acidic urine promoting uric acid crystal formation.107–110 The underlying insulin resistance in these patients leads to impaired glutamine metabolism, ammonia production, and ammonium excretion. This results in unbuffered hydrogen ions and a lowering of the urinary pH.111 Recent evidence suggests pioglitazone, a thiazolidinedione used in the treatment of type 2 diabetes, can reduce insulin resistance, leading to increases in ammonium excretion and more alkaline urinary pH values, which may be protective against stone disease.112
Oxalate
Recommendations:
Patients with hyperoxaluria should minimize their intake of high-oxalate foods. Vitamin B6 supplementation can be considered to lower urinary oxalate levels when dietary modification has been unsuccessful (LE 2–3, Grade C recommendation).
In patients with enteric hyperoxaluria, elemental calcium or calcium citrate should be given with meals to bind with dietary oxalate to reduce its intestinal absorption (LE 2–3, Grade C recommendation).
Hyperoxaluria can result from dietary, enteric, or idiopathic causes and is associated with an increased risk of calcium oxalate stone formation.113 Treatment of dietary and idiopathic hyperoxaluria with a low-oxalate diet has shown mixed results. Most studies have demonstrated that a low-oxalate diet reduces urinary oxalate levels; however, this did not consistently result in a decrease in calcium oxalate crystal supersaturation.114,115 Multiple studies, including several prospective cohort studies and a randomized controlled trial, have shown that vitamin B6 or pyridoxine supplementation (25 mg daily) are effective in reducing urinary oxalate levels.116
Enteric hyperoxaluria is due primarily to the malabsorption of intestinal fats in the ileum. This condition is most frequently found in patients with inflammatory bowel disease with small bowel involvement, short gut syndrome, and after bariatric surgery (not including restrictive bariatric procedures, such as vertical banded gastroplasty and sleeve gastrectomy).117 The intestinal fatty acids that are not absorbed cause saponification of calcium and magnesium in the intestinal lumen, leading to a decreased amount of calcium available to bind dietary oxalate.117 This results in increased availability and absorption of oxalate and hyperoxaluria. As these conditions are often associated with diarrhea, typical laboratory findings include low urine volume and pH, hypocitraturia, hypercalciuria (unique to malabsorptive disease), and hyperoxaluria.117 Treatment includes reduction of dietary oxalate, increased fluid intake, and calcium consumption.118 The calcium can be elemental calcium or calcium citrate, as this will help correct the hypocitraturia and low urinary pH as well. Importantly, the calcium should be taken with meals for it to bind to the dietary oxalate.118
Pediatric stone disease
Recommendation: All children with stone disease should undergo an in-depth medical evaluation and may benefit from a multidisciplinary approach with urology and nephrology (LE 3, Grade D recommendation).
While pediatric stone disease is uncommon, with 59.5 cases per 100 000 person-years in the U.S.,119 the incidence is increasing in many developed countries.120 The recurrence rate is high, with 50% of patients forming a recurrent stone within three years of the index stone.121 Dietary factors, such as sodium and purine intake, low urine volume, and climate are thought to play a role in increased stone risk. Obesity may not have the same effect on stone risk in children as it does in adults.122–124 Prematurity, medications (e.g., loop diuretics), and genetic factors also increase the risk of pediatric stone disease.
An in-depth metabolic evaluation has historically been recommended in all children. In toilet-trained children, an in-depth evaluation with a 24-hour urine collection is recommended by most experts. In non-toilet-trained children, or when a 24-hour urine collection is not possible in a toilet-trained child, a spot or random urine collection may suffice. This would include a urinalysis, spot urine calcium, oxalate, citrate, cystine, protein, and uric acid.125 The utility of a full metabolic stone evaluation has been called into question for first-time stone formers, as it changes management in only 11% of patients.126
In a population-based U.S. study, metabolic abnormalities in children were as follows: hypercalciuria (62%), hyperoxaluria (18%), and hypocitraturia (12%), with hyperuricosuria relatively rare compared to adults (9%).120 Low urine volume may be affected by poor intake, access to clean drinking water, diarrhea, and other gastrointestinal illnesses. Recommended fluid intake should target a urine output of >750 ml/day in infants, >1 L/day in children under age five, >1.5 L/day in children ages 5–10, and >2 L in children over age 10.127
Hypercalciuria is the most common metabolic abnormality and is often idiopathic. Management includes increasing fluid intake, and reducing sodium intake and thiazide diuretics if refractory. Hypocitraturia can be idiopathic or associated with other metabolic conditions (e.g., dRTA, high meat protein diet).128 Hyperoxaluria can be primary (increased endogenous production), dietary, or enteric.128 Hyperuricosuria is relatively uncommon, as are uric acid stones. Most children with urinary stones will benefit from a multidisciplinary approach with urology and nephrology involvement.129
Specific prophylaxis based on stone composition
In the following section, “index patients” have been created based on the predominant stone composition. Potential metabolic abnormalities will be described, and specific diet and medical interventions will be highlighted for each clinical scenario.
Index patient 1: Calcium oxalate or mixed calcium oxalate/calcium phosphate stones
Recommendations:
Thiazide diuretics decrease urinary calcium and stone recurrence in calcium stone forming patients (LE 1–3, Grade A–B recommendation).
Alkali citrates are effective in increasing urinary citrate and reducing stone recurrence in calcium stone formers (LE 1–3, Grade A–B recommendation).
-
In calcium stone formers, allopurinol is effective in reducing stone recurrence in patients with hyperuricemia but does not provide any benefit in patients with normal serum uric acid levels (LE 1–2, Grade B recommendation).
– Empiric treatment with either thiazide diuretics and/or alkali citrates reduces stone recurrence in calcium stone formers with active stone disease who have normal metabolic evaluations (LE 1–3, Grade B recommendation).
Patients with calcium oxalate or combined calcium oxalate/calcium phosphate stones may have normal 24-hour urine testing, hypercalciuria, hyperoxaluria, hypocitraturia, hyperuricosuria, low urine volume, or a combination of any of these features.130 Based on 24-hour urine results, various interventions can be considered (Figure 1).
Figure 1.
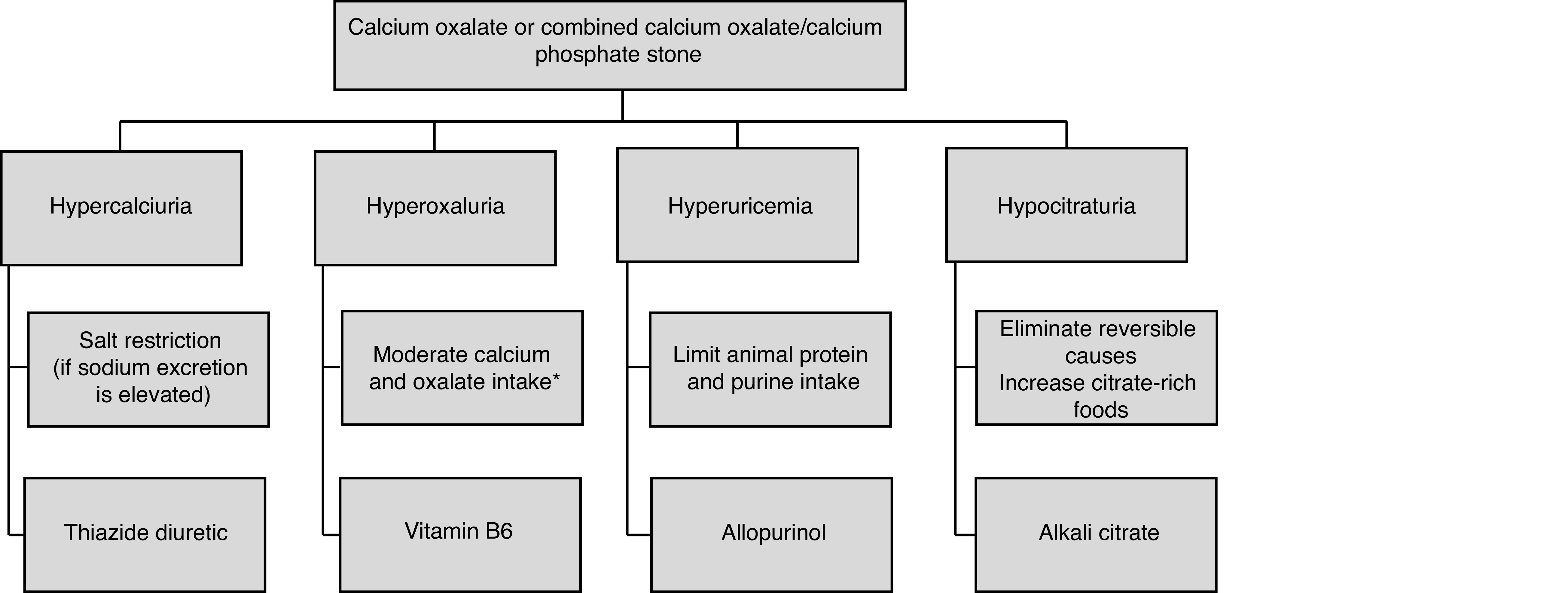
Specific dietary and medical treatments for patients with calcium oxalate or mixed calcium oxalate/calcium phosphate stones. *Calcium intake 1200 mg daily (with meals), moderation of foods high in oxalate, pair oxalate and calcium-containing foods.
Elevated urinary sodium can result in hypercalciuria and should be corrected through dietary reduction of sodium intake prior to initiating thiazide therapy.31,34,96 In patients with recurrent calcium stones with and without metabolic abnormalities, thiazide use decreases urinary calcium and stone recurrence.131–138
Dosages used in clinical trials are hydrochlorothiazide (25 mg orally twice daily or 50 mg orally once daily), chlorthalidone (25 mg orally once daily can be increased to 50 mg), and indapamide (1.25 mg orally once daily can be increased to 2.5 mg). The dose-dependent side effects of thiazide diuretics include hypokalemia, hyperglycemia, hyperlipidemia, hyperuricemia, hypomagnesemia, and hypocitraturia. Combining thiazide diuretics with potassium citrate or potassium chloride prevents hypokalemia and hypochloremic metabolic alkalosis, which can lead to hypocitraturia.139,140
Alkali citrate (potassium citrate, potassium magnesium citrate, sodium citrate) have been evaluated in several studies for the prevention of calcium nephrolithiasis. Alkali citrate increases urinary citrate levels and decreases the risk of recurrent nephrolithiasis.141–149 Potassium citrate is the most studied agent, with dosages in clinical trials ranging from 30–60 mEq in two to three divided doses daily. Gastrointestinal upset is the primary side effect. Overall, potassium citrate is preferred over sodium citrate, as the sodium load may increase urinary calcium excretion.150
Studies regarding hyperuricosuria in calcium oxalate stone disease show mixed results. In a large, cross-sectional study, elevated urinary uric acid levels were not associated with calcium oxalate stone formation.151 However, multiple studies have demonstrated treating hyperuricosuria in calcium oxalate stone formers reduces stone recurrence.152–154 Hyperuricemia may be treated with a xanthine oxidase inhibitor, most frequently allopurinol at a dose of 200–300 mg daily. Major side effects include rash, gastrointestinal upset, abnormal liver enzyme levels, and prolonged elimination in renal disease.
Empiric preventative treatment with a thiazide diuretic and or alkali citrate can be considered in calcium stone formers with a normal metabolic workup who continue to have active stone disease. Randomized controlled trials in calcium stone formers have demonstrated that thiazide diuretics were effective in reducing stone recurrence compared to placebo even in patients with normocalciuria.132,138 Similarly, a prospective, double-blind study demonstrated decreased stone events in calcium stone patients without hypocitraturia treated with potassium-magnesium citrate.146
Index patient 2: Pure calcium phosphate stones
Recommendation: Patients with incomplete or complete dRTA should be treated with alkali citrate therapy (LE 2–3, Grade C recommendation).
Patients who form pure calcium phosphate stones may have an underlying condition, such as dRTA or primary hyperparathyroidism. Suggestive biochemical features and treatment of these disorders are listed in Table 1.
Table 1.
Characteristic findings, investigations, and treatment of primary/secondary hyperparathyroidism and distal renal tubular acidosis
| Disorder | Suggestive features | Investigations | Treatment |
|---|---|---|---|
| Primary hyperparathyroidism | ↑ or ↑N serum calcium | Serum calcium | Treat vitamin D deficiency |
| ↑ or ↑N serum PTH | |||
| Hypercalciuria | PTH | Referral to endocrinology | |
| Calcium oxalate or calcium phosphate stone | |||
| ↓ Bone mineral density | Vitamin D | ||
| Secondary hyperparathyroidism | ↑ serum PTH | Serum calcium | Treat vitamin D deficiency |
| ↓ Vitamin D | |||
| ↓ or N serum calcium | PTH | Consider referral to endocrinology | |
| Hypercalciuria | |||
| ↓ Bone mineral density | Vitamin D | ||
| Complete distal renal tubular acidosis | Urine pH >5.8 | Serum electrolytes | Alkali citrate |
| ↓ serum bicarbonate | |||
| ↓ serum potassium | Urine pH | Thiazide | |
| Pure apatite stone | |||
| Hypocitraturia | 24h urine collection | ||
| Hypercalciuria | |||
| ↓ Bone mineral density | |||
| Incomplete distal renal tubular acidosis | Urine pH >5.3 | Serum electrolytes | Alkali citrate |
| ↓ N serum bicarbonate | |||
| ↓ N serum potassium | Urine pH | Thiazide | |
| Pure apatite stone | |||
| Hypocitraturia | 24h urine collection | ||
| Hypercalciuria |
PTH: parathyroid hormone, ↑ = high, ↑N = at the high end of normal range; ↓ = low, ↓N = at the low end of normal range.
Patients with primary hyperparathyroidism have a significantly increased risk of renal stone disease.155,156 Even in patients with normal serum calcium levels, an elevated PTH can increase stone risk and result in decreased BMD.157,158 Surgery for primary hyperparathyroidism results in decreased serum calcium, stone formation, and an improvement in BMD.159
Patients with dRTA may present with recurrent calcium phosphate stones, nephrocalcinosis, systemic acidosis, osteoporosis, failure to thrive, or sensorineural hearing loss.160 Patients with dRTA generally have severe hypocitraturia and are best treated with an alkali citrate.161,162 In patients with dRTA, treatment with potassium citrate resulted in correction of systemic acidosis, improvement in urinary parameters, reduced stone formation, and increased BMD.163 Potassium citrate has demonstrated superior effects on urinary indices as compared to sodium citrate in patients with dRTA.150
Incomplete dRTA is a condition characterized by defective urinary acidification similar to complete dRTA, without systemic metabolic acidosis.164 Patients with incomplete dRTA still have disturbed calcium, citrate, and bone metabolism resulting in recurrent stone formation and increased bone turnover. 165 Incomplete dRTA is likely a significantly under-recognized clinical condition due to the lack of a clear consensus on diagnostic criteria; however, the prevalence has been found to be as high as 15% in stone formers.166,167 Incomplete dRTA should be suspected in patients with persistently alkaline urine (pH>5.3), low urinary citrate, and low-normal serum potassium and bicarbonate levels.164,167 Similar to complete dRTA, the mainstay of treatment is alkali citrate therapy, which improves urinary citrate and reduces stone formation.162
Index patient 3: Uric acid stones
Recommendation: In patients with uric acid stones, urinary alkalization to a pH of 6.5 is first-line therapy. Allopurinol may be used as adjunctive therapy in patients with hyperuricemia or hyperuricosuria (LE 1–3, Grade B recommendation).
Uric acid stone formers are at significant risk of recurrence. Uric acid stones may form as the result of several underlying disorders, including obesity, diabetes mellitus, gout, excessive bicarbonate loss due to high output bowel disease, myeloproliferative disorders, and tumor lysis syndrome.5,168 Uric acid stone formation is most associated with low urinary pH and low urine volume rather than hyperuricosuria.151 The focus of prevention, therefore, should be to correct urine pH above 5.5 and increase urine volume (Figure 2).168 Xanthine oxidase inhibitors, such as allopurinol or febuxostat, can be used as adjunctive therapy in patients with demonstrated hyperuricemia, hyperuricosuria, or a history of gout after correction of urinary pH.169
Figure 2.
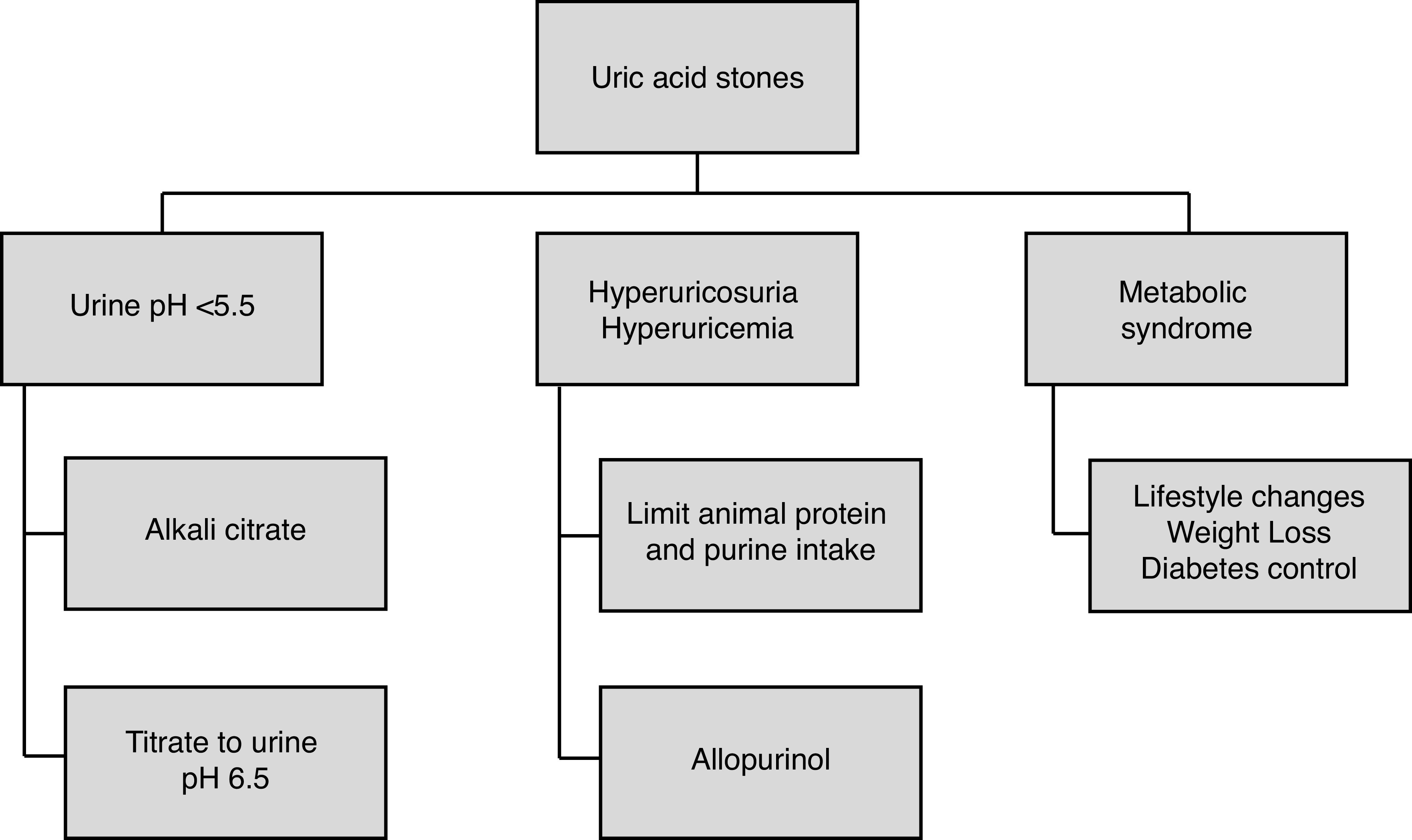
Specific dietary and medical treatments for patients with uric acid stones.
Success with in-situ uric acid stone dissolution has been demonstrated with alkali citrate and maybe an underused treatment strategy.170,171 In a recent prospective study, potassium citrate therapy was successful in dissolving 83% of stones (mean 1.4 cm3) after six months of treatment.172
Index patient 4: Cystine stones
Recommendation: Cystine stone formers should be counselled to target 3 L of urine output daily, restrict their sodium intake, and moderate their protein intake to reduce stone formation. Urinary alkalization of the urine targeting a urine pH of 7–7.5 is the initial therapy. Thiol-binding agents should be considered second-line therapy (LE 3–4, Grade C recommendation).
Cystinuria, caused by a deficiency in reabsorbing dibasic amino acids, is a common genetic disorder affecting 1/7000 individuals. Cystine stone formers often present in childhood or as teenagers and maybe plagued by recurrent stone formation and the need for repetitive surgical intervention, especially if prophylaxis is not optimized.
Patients with cystinuria should be encouraged to maintain a urine output of at least 3 L daily (often demanding oral intake of 3.5–4 L of fluid). This is a critical component of prevention in these patients, as even with adjunctive medical therapy the success of stone prevention will be poor in patients who do not comply with increased fluid intake.173,174 Sodium restriction is advised for all patients with cystinuria.174 In small studies, sodium restriction significantly decreased cystine excretion,175–177 reflecting the coupling of cystine to parallel sodium transport in the kidney. Elevated dietary protein is associated with increased urinary cystine and a reduction in overall protein intake can decrease urinary cystine levels.178
The solubility of cystine increases significantly between urine pH of 7.0–7.5. Urinary alkalinization is, therefore, the initial step in medical therapy, with the goal of achieving a urine pH of greater than 7.0.179 A urinary pH of greater than 7.5 should be avoided, as this may promote calcium phosphate stone formation. Acetazolamide may be used as an adjunct to urinary alkalinization when potassium citrate alone is ineffective.180 If alkalizing agents fail to adequately control cystine stone formation, thiol-binding agents, such as penicillamine 1–2 g or tiopronin 800–1200 mg in daily divided doses may be used.173,181–184 Side effects from penicillamine can be significant and include fever, arthralgias, rash, dysgeusia, leukopenia, and proteinuria. Where available, tiopronin, should be prioritized due to its better side effect profile (asthenia, gastrointestinal distress, rash, joint aches, and mental status changes) compared to penicillamine.182 A small study evaluated the role of captopril, however, significant reduction in stone formation was not achieved and captopril is no longer recommended.183 Dietary and medical prevention options are shown in Figure 3.
Figure 3.
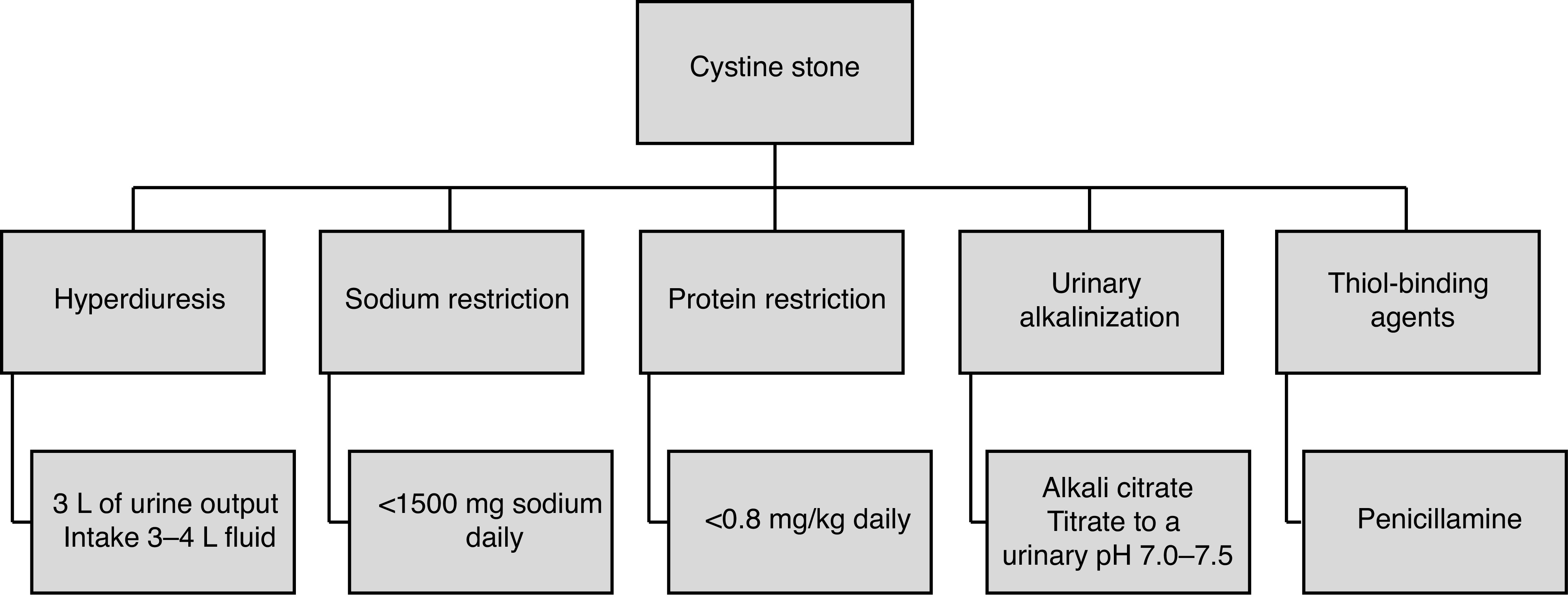
Specific dietary and medical treatments for patients with cystine stones.
Long-term compliance in patients with cystinuria can be difficult to achieve185 and consideration should be given to management of these patients at specialized multidisciplinary clinics with close followup.
Future directions: Genetic screening in recurrent nephrolithiasis
While not widely available, the genetic screening of patients with recurrent stone disease may have an increasing future role. It is likely that in addition to dietary and lifestyle choices, genetic factors are associated with recurrent nephrolithiasis; 35–65% of kidney stone patients have a family history of nephrolithiasis.186,187 Heritability of kidney stones and urinary calcium excretion have been found to be greater than 45% in twin studies.188,189 Underlying genetic causes are likely underdiagnosed, even in specialized renal stone clinics. In a study of nephrolithiasis and nephrocalcinosis patients recruited from renal stone clinics, 14.9% were found to have monogenic causative mutations, and 40% were novel.190
There have been more than 30 identified kidney stone genes representing different mechanisms leading to stone disease.191 These mutations represent renal tubular disorders, abnormal calcium and vitamin D metabolism, and other inborn errors of metabolism. Cystinuria is the most common of these genetic causes.190
Diagnosing genetic disorders may lead to targeted prevention and provide an opportunity for genetic counselling. Lumasiran, a novel RNAi therapy for primary hyperoxaluria type I, has been developed, shown promising results in phase 3 trials, and was recently approved by Health Canada.192 A multidisciplinary team approach including urologists, nephrologists, endocrinologists, and clinical geneticists can help coordinate biochemical and genetic testing and facilitate selection of optimal treatment strategies.193
Summary
For patients at risk of recurrent renal stones, a detailed medical evaluation and an individualized approach to dietary and pharmacological prevention are important aspects of their care. The frequency of followup and the need for repeat metabolic testing is not clearly defined in the literature and must, therefore, also be individualized. In patients where specific medical prophylaxis has been prescribed, re-evaluations with repeat metabolic testing within six months, and yearly thereafter, to monitor treatment efficacy and side effects are recommended.17 Periodic imaging is also recommended for those harboring small asymptomatic stones.
Urologists, in addition to providing state-of-the-art surgical care to our patients, should be capable of providing up-to-date metabolic assessment and optimal prevention strategies as part of a comprehensive approach to stone management.
Footnotes
Competing interests: Dr. Bhojani is a consultant for Boston Scientific, Olympus, and Procept BioRobotics; and participated in the WATER 2 trial supported by Procept BioRobotics. Dr. Chew has been an advisory board member for and has received honoraria from Auris Surgical, ADVA-Tec, Ambu, Becton Dickinson, Boston Scientific, Cook Medical, Olympus, Storz Medical, Sonomotion, and The Ureteral Stent Company; holds investments in Auris Surgical, Sonomotion, and The Ureteral Stent Company; and has participated in clinical trials supported by ADVA-Tec, Boston Scientific, Cook Medical, Olympus, Storz Medical, and Sonomotion. Dr. Razvi receives royalties from Cook Urological for a surgical device; is participating in clinical trials with Boston Scientific and Verity Pharma; and holds stock options with Histosonics. The remaining authors do not report any competing personal or financial interests related to this work.
Prior to publication, this guideline was reviewed by the CUA Guidelines Committee and the CUA Board of Directors.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tundo G, Vollstedt A, Meeks W, et al. Beyond prevalence: Cumulative incidence of kidney stones in the United States. J Urol. 2021;205:1704–9. doi: 10.1097/JU.0000000000001629. [DOI] [PubMed] [Google Scholar]
- 3.Penniston KL, McLaren ID, Greenlee RT, et al. Urolithiasis in a rural Wisconsin population from 1992 to 2008: Narrowing of the male-to-female ratio. J Urol. 2011;185:1731–6. doi: 10.1016/j.juro.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scales CD, Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979–82. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 5.Chou YH, Su CM, Li CC, et al. Difference in urinary stone components between obese and non-obese patients. Urol Res. 2011;39:283–7. doi: 10.1007/s00240-010-0344-8. [DOI] [PubMed] [Google Scholar]
- 6.Uribarri J, Oh MS, Carroll HJ. The first kidney stones. Ann Intern Med. 1989;111:1006–9. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Lieske JC, Li X, et al. The ROKS Nomogram for predicting a second symptomatic stone episode. J Amer Soc Nephrol. 2014;25:2685–7. doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaro CRPR, Goldberg J, Damasio PC, et al. An update on metabolic assessment in patients with urinary lithiasis. World J Urol. 2015;33:125–9. doi: 10.1007/s00345-014-1271-z. [DOI] [PubMed] [Google Scholar]
- 9.Milose JC, Kaufman SR, Hollenbeck BK, et al. Prevalence of 24-hour urine collection in high-risk stone formers. J Urol. 2014;191:376–80. doi: 10.1016/j.juro.2013.08.080. [DOI] [PubMed] [Google Scholar]
- 10.Bensalah K, Tuncel A, Raman JD, et al. How physician and patient perceptions differ regarding medical management of stone disease. J Urol. 2009;182:998–1004. doi: 10.1016/j.juro.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Dauw C, Yi Y, Bierlein M, et al. Medication non-adherence and effectiveness of preventative pharmacological therapy for kidney stones. J Urol. 2016;195:648–52. doi: 10.1016/j.juro.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 12.Saigal CS, Joyce G, Timilsina AR, et al. Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? Kidney Int. 2005;68:1808–14. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearle MS, Calhoun EA, Curhan GC, et al. Urologic diseases in America project: Urolithiasis. J Urol. 2005;173:848–57. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli JA, Maalouf NM, Pearle MS, et al. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. 2014;66:724–9. doi: 10.1016/j.eururo.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Evidence-Based Medicine (CEBM) [Accessed Dec 7, 2021];2018 Available at: https://www.cebm.net. [Google Scholar]
- 16.Skolarikos A, Straub M, Knoll T, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015;67:750–63. doi: 10.1016/j.eururo.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Pearle MS, Goldfarb DS, Assimos DG, et al. American Urological Association. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316–24. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Goldfarb DS, Arowojolu O. Metabolic evaluation of first-time and recurrent stone formers. Urol Clin North Am. 2013;40:13–20. doi: 10.1016/j.ucl.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maalouf N. Approach to the adult kidney stone former. Clin Rev Bone Miner Metab. 2012;10:38–49. doi: 10.1007/s12018-011-9111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadlec AO, Turk TM. Update on the evaluation of repeated stone formers. Curr Urol Rep. 2013;14:549–56. doi: 10.1007/s11934-013-0347-4. [DOI] [PubMed] [Google Scholar]
- 21.Eisner BH, Sheth S, Dretler SP, et al. Abnormalities of 24-hour urine composition in first-time and recurrent stone-formers. Urology. 2012;80:776–9. doi: 10.1016/j.urology.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Koyuncu HH, Yencilek F, Eryildirim B, et al. Family history in stone disease: How important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010;38:105–9. doi: 10.1007/s00240-009-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiselius HG, Daudon M, Thomas K, et al. Metabolic work-up of patients with urolithiasis: Indications and diagnostic algorithm. Eur Urol Focus. 2017;3:62–71. doi: 10.1016/j.euf.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Griffith DP, Khonsari F, Skurnick JH, et al. A randomized trial of acetohydroxamic acid for the treatment and prevention of infection-induced urinary stones in spinal cord injury patients. J Urol. 1988;140:318–24. doi: 10.1016/S0022-5347(17)41592-8. [DOI] [PubMed] [Google Scholar]
- 25.Griffith DP, Gleeson MJ, Lee H, et al. Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. Eur Urol. 1991;20:243–7. doi: 10.1159/000471707. [DOI] [PubMed] [Google Scholar]
- 26.Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med. 1984;311:760–4. doi: 10.1056/NEJM198409203111203. [DOI] [PubMed] [Google Scholar]
- 27.Eyre KS, Lewis F, Cui H, et al. Utility of blood tests in screening for metabolic disorders in kidney stone disease. BJU Int. 2021;127:538–43. doi: 10.1111/bju.15250. [DOI] [PubMed] [Google Scholar]
- 28.Pierreus J, Bravenboer B. Normocalcemic primary hyperparathyroidism: A comparison with a hypercalcemic form in a tertiary referral population. Horm Metab Res. 2018;50:797–802. doi: 10.1055/a-0752-4533. [DOI] [PubMed] [Google Scholar]
- 29.Boyd CJ, Wood KD, Singh N, et al. Screening for primary hyperparathyroidism in a tertiary stone clinic, a useful endeavor. Int Urol Nephrol. 2020;52:1651–5. doi: 10.1007/s11255-020-02476-0. [DOI] [PubMed] [Google Scholar]
- 30.Pak CY, Peterson R, Poindexter JR. Adequacy of a single stone risk analysis in the medical evaluation of urolithiasis. J Urol. 2001;165:378–81. doi: 10.1097/00005392-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Parks JH, Goldfisher E, Asplin JR, et al. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol. 2002;167:1607–12. doi: 10.1016/S0022-5347(05)65163-4. [DOI] [PubMed] [Google Scholar]
- 32.Healy KA, Hubosky SG, Bagley DH. 24-hour urine collection in the metabolic evaluation of stone formers: Is one study adequate? J Endourol. 2013;27:374–8. doi: 10.1089/end.2012.0216. [DOI] [PubMed] [Google Scholar]
- 33.Nayan M, Elkoushy MA, Andonian S. Variations between two 24-hour urine collections in patients presenting to a tertiary stone clinic. Can Urol Assoc J. 2012;6:30–3. doi: 10.5489/cuaj.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alruwaily AF, Dauw CA, Bierlein MJ, et al. How much information is lost when you only collect one 24-hour urine sample during the initial metabolic evaluation? J Urol. 2016;196:1143–8. doi: 10.1016/j.juro.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 35.Lee TT, Elkoushy MA, Andonian S. Are stone analysis results different with repeated sampling? Can Urol Assoc J. 2014;8:E317–22. doi: 10.5489/cuaj.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkman JE, Large T, Nottingham, et al. Clinical and metabolic correlates of pure stone subtypes. J Endourol. 2021;35:1555–62. doi: 10.1089/end.2020.1035. [DOI] [PubMed] [Google Scholar]
- 37.Goldfarb DS. Empiric therapy for kidney stones. Urolithiasis. 2019;47:107–13. doi: 10.1007/s00240-018-1090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosking DH, Erickson SB, Van den Berg CJ, et al. The stone clinic effect in patients with idiopathic calcium urolithiasis. J Urol. 1983;130:1115–8. doi: 10.1016/S0022-5347(17)51711-5. [DOI] [PubMed] [Google Scholar]
- 39.Penniston KL. The nutrition consult for recurrent stone formers. Curr Urol Rep. 2015;16:47. doi: 10.1007/s11934-015-0518-6. [DOI] [PubMed] [Google Scholar]
- 40.Damasio PC, Amaro CR, Padovani CR, et al. Influence of clinical therapy and nutritional counseling on the recurrence of urolithiasis. Acta Cir Bras. 2014;29:400–4. doi: 10.1590/S0102-86502014000600009. [DOI] [PubMed] [Google Scholar]
- 41.Kocvara R, Plasqura P, Petrik A, et al. A prospective study of non-medical prophylaxis after a first kidney stone. BJU Int. 1999;84:393–8. doi: 10.1046/j.1464-410x.1999.00216.x. [DOI] [PubMed] [Google Scholar]
- 42.Fink HA, Akornor JW, Garimella PS, et al. Diet, fluid, or supplements for secondary prevention of nephrolithiasis: A systematic review and meta-analysis of randomized trials. Eur Urol. 2009;56:72–80. doi: 10.1016/j.eururo.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheungpasitporn W, Rossetti S, Friend K, et al. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: A systematic review and meta-analysis. J Nephrol. 2016;29:211–9. doi: 10.1007/s40620-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borghi L, Meschi T, Amato F, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol. 1996;155:839–43. doi: 10.1016/S0022-5347(01)66321-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Xu X, Wu J, et al. Systematic review and meta-analysis of the effect of alcohol intake on the risk of urolithiasis including dose-response relationship. Urol Int. 2015;94:194–204. doi: 10.1159/000365358. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen MD, Kahn AJ, Reiner AP, et al. Impact of nutritional factors on incident kidney stone formation: a report from the WHI OS. J Urol. 2012;187:1645–9. doi: 10.1016/j.juro.2011.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curhan GC, Willett WC, Knight EL, et al. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004;164:885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 48.Ticinesi A, Nouvenne A, Borghi L, et al. Water and other fluids in nephrolithiasis: State of the art and future challenges. Crit Rev Food Sci Nutr. 2017;57:963–74. doi: 10.1080/10408398.2014.964355. [DOI] [PubMed] [Google Scholar]
- 49.Borghi L, Meschi T, Maggiore U, et al. Dietary therapy in idiopathic nephrolithiasis. Nutr Rev. 2006;64:301–12. doi: 10.1111/j.1753-4887.2006.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 50.Littlejohns TJ, Neal NL, Bradbury KE, et al. Fluid intake and dietary factors and the risk of incident kidney stones in UK Biobank: A population-based prospective cohort study. Eur Urol Focus. 2020;6:752–61. doi: 10.1016/j.euf.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Ferraro PM, Taylor EN, Gambaro G, et al. Caffeine intake and the risk of kidney stones. Am J Clin Nutr. 2014;100:1596–603. doi: 10.3945/ajcn.114.089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferraro PM, Taylor EN, Gambaro G, et al. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol. 2013;8:1389–95. doi: 10.2215/CJN.11661112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Zhang Y, Mao Z, et al. A meta-analysis of coffee intake and risk of urolithiasis. Urol Int. 2014;93:220–8. doi: 10.1159/000356559. [DOI] [PubMed] [Google Scholar]
- 54.Penniston KL, Steele TH, Nakada SY. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology. 2007;70:856–60. doi: 10.1016/j.urology.2007.06.1115. [DOI] [PubMed] [Google Scholar]
- 55.Large T, Williams J, Asplin JR, et al. Using low-calorie orange juice as a dietary alternative to alkali therapy. J Endourol. 2020;34:1082–7. doi: 10.1089/end.2020.0031. [DOI] [PubMed] [Google Scholar]
- 56.Conroy DE, West AB, Brunke-Reese D, et al. Just-in-time adaptive intervention to promote fluid consumption in patients with kidney stones. Health Psychol. 2020;39:1062–9. doi: 10.1037/hea0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borofsky MS, Dauw CA, York N, et al. Accuracy of daily fluid intake measurements using a “smart” water bottle. Urolithiasis. 2018;46:343–8. doi: 10.1007/s00240-017-1006-x. [DOI] [PubMed] [Google Scholar]
- 58.Wright HC, Alshara L, DiGennaro H, et al. The impact of smart technology on adherenc; rates and fluid management in the prevention of kidney stones. Urolithiasis. 2021. Epub ahead of print. [DOI] [PubMed]
- 59.Chua TX, Prasad NS, Rangan GK, et al. A systematic review to determine the most effective interventions to increase water intake. Nephrology. 2016;21:860–9. doi: 10.1111/nep.12675. [DOI] [PubMed] [Google Scholar]
- 60.McCauley LR, Dyer AJ, Stern K, et al. Factors influencing fluid intake behavior among kidney stone formers. J Urol. 2012;187:1282–6. doi: 10.1016/j.juro.2011.11.111. [DOI] [PubMed] [Google Scholar]
- 61.Meschi T, Nouvenne A, Borghi L. Urolithiasis. Springer; London: 2012. The importance of water and other fluids in the prevention of stone recurrence; pp. 745–749. [DOI] [Google Scholar]
- 62.Curhan GC, Willett WC, Rimm EB, et al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833–8. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 63.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of followup. J Am Soc Nephrol. 2004;15:3225–32. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- 64.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 65.Radford LT, Bolland MJ, Mason B, et al. The Auckland calcium study: 5-year post-trial followup. Osteoporos Int. 2014;25:297–304. doi: 10.1007/s00198-013-2526-z. [DOI] [PubMed] [Google Scholar]
- 66.Johri N, Haeger P, Ferraro P, et al. Vitamin D deficiency is prevalent among idiopathic stone formers but does correction pose any risk? Urolithiasis. 2017;45:535–43. doi: 10.1007/s00240-016-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elkoushy MA, Sabbagh R, Unikowsky B, et al. Prevalence and metabolic abnormalities of vitamin D-inadequate patients presenting with urolithiasis to a tertiary stone clinic. Urology. 2012;79:781–5. doi: 10.1016/j.urology.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Pipili C, Oreopoulos DG. Vitamin D status in patients with recurrent kidney stones. Nephron Clin Pract. 2012;122:134–8. doi: 10.1159/000351377. [DOI] [PubMed] [Google Scholar]
- 69.Rathod A, Bonny O, Guessous I, et al. Association of urinary calcium excretion with serum calcium and vitamin D levels. Clin J Am Soc Nephrol. 2015;10:452–62. doi: 10.2215/CJN.12511213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eisner BH, Thavaseelan S, Sheth S, et al. Relationship between serum vitamin D and 24-hour urine calcium in patients with nephrolithiasis. Urology. 2012;80:1007–10. doi: 10.1016/j.urology.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen S, Baggerly L, French C, et al. 25-Hydroxyvitamin D in the range of 20–100 ng/mL and incidence of kidney stones. Am J Public Health. 2014;104:1783–7. doi: 10.2105/AJPH.2013.301368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferraro PM, Taylor EN, Gambaro G, et al. Vitamin D intake and the risk of incident kidney stones. J Urol. 2017;197:405–10. doi: 10.1016/j.juro.2016.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:1443–63. doi: 10.1001/jama.2020.26498. [DOI] [PubMed] [Google Scholar]
- 74.Avenell A, Mak Jc, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014. p. Cd000227. [DOI] [PMC free article] [PubMed]
- 75.Prentice R, Pettinger M, Jackson R, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–80. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 77.Leaf DE, Korets R, Taylor EN, et al. Effect of vitamin D repletion on urinary calcium excretion among kidney stone formers. Clin J Am Soc Nephrol. 2012;7:829–34. doi: 10.2215/CJN.11331111. [DOI] [PubMed] [Google Scholar]
- 78.Ferroni MC, Rycyna KJ, Averch TD, et al. Vitamin D repletion in kidney stone formers — a randomized controlled trial. J Urol. 2016;197:1079–83. doi: 10.1016/j.juro.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 79.Tsuji H, Umekawa T, Kurita T, et al. Analysis of bone mineral density in urolithiasis patients. Int J Urol. 2005;12:335–9. doi: 10.1111/j.1442-2042.2005.01049.x. [DOI] [PubMed] [Google Scholar]
- 80.Tugcu V, Ozbek E, Aras B, et al. Bone mineral density measurement in patients with recurrent normocalciuric calcium stone disease. Urol Res. 2007;35:29–34. doi: 10.1007/s00240-006-0074-0. [DOI] [PubMed] [Google Scholar]
- 81.Letavernier E, Traxer O, Daudon M, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol. 2011;6:1149–54. doi: 10.2215/CJN.10191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasch A, Frey FJ, Eisenberger U, et al. PTH and 1,25-vitamin D response to a low-calcium diet is associated with bone mineral density in renal stone formers. Nephrol Dial Transplant. 2008;23:2563–70. doi: 10.1093/ndt/gfn091. [DOI] [PubMed] [Google Scholar]
- 83.Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, et al. Bone mineral density and histology in distal renal tubular acidosis. Kidney Int. 2001;59:1086–93. doi: 10.1046/j.1523-1755.2001.0590031086.x. [DOI] [PubMed] [Google Scholar]
- 84.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol. 2014;9:2133–40. doi: 10.2215/CJN.04340514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor EN, Feskanich D, Paik JM, et al. Nephrolithiasis and risk of incident bone fractures. J Urol. 2016;195:1482–6. doi: 10.1016/j.juro.2015.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prochaska M. Bisphosphates and management of kidney stones and bone disease. Curr Opin Nephrol Hypertens. 2021;30:184–9. doi: 10.1097/MNH.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 87.Alshara L, Batagello CA, Armanyous S, et al. The impact of thiazides and potassium citrate on bone mineral density evaluated by CT scan in stone formers. J Endourol. 2018;32:559–64. doi: 10.1089/end.2017.0940. [DOI] [PubMed] [Google Scholar]
- 88.Arrabal-Martin M, Gonzalez-Torres S, Cano-Garcia M, et al. Urine calcium and bone mineral density in calcium stone-forming patients treated with alendronate and hydrochlorothiazide. Urol Int. 2016;97:292–8. doi: 10.1159/000443484. [DOI] [PubMed] [Google Scholar]
- 89.Aung K, Htay T. Thiazide diuretics and the risk of hip fracture. Cochrane Database Syst Rev. 2011:CD005185. doi: 10.1002/14651858.CD005185.pub2. [DOI] [PubMed] [Google Scholar]
- 90.Sromicki JJ, Hess B. Abnormal distal renal tubular acidification in patients with low bone mass: prevalence and impact of alkali treatment. Urolithiasis. 2017;45:263–9. doi: 10.1007/s00240-016-0906-5. [DOI] [PubMed] [Google Scholar]
- 91.Vescini F, Buffa A, La Manna G, et al. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest. 2005;28:218–22. doi: 10.1007/BF03345376. [DOI] [PubMed] [Google Scholar]
- 92.Pak CY, Heller HJ, Pearle MS, et al. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol. 2003;169:465–9. doi: 10.1016/S0022-5347(05)63934-1. [DOI] [PubMed] [Google Scholar]
- 93.Trinchieri A, Mandressi A, Luongo P, et al. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. Br J Urol. 1991;67:230–6. doi: 10.1111/j.1464-410X.1991.tb15124.x. [DOI] [PubMed] [Google Scholar]
- 94.Hiatt RA, Ettinger B, Caan B, et al. Randomized controlled trial of a low animal protein, high-fiber diet in the prevention of recurrent calcium oxalate kidney stones. Am J Epidemiol. 1996;144:25–33. doi: 10.1093/oxfordjournals.aje.a008851. [DOI] [PubMed] [Google Scholar]
- 95.Breslau NA, Brinkley L, Hill KD, et al. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–6. doi: 10.1210/jcem-66-1-140. [DOI] [PubMed] [Google Scholar]
- 96.Siener R, Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur J Nutr. 2003;42:332–7. doi: 10.1007/s00394-003-0428-0. [DOI] [PubMed] [Google Scholar]
- 97.Damasio PC, Amaro CR, Cunha NB, et al. The role of salt abuse on risk for hypercalciuria. Nutr J. 2011;10:3. doi: 10.1186/1475-2891-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nouvenne A, Meschi T, Prati B, et al. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: A 3-mo randomized controlled trial. AJCN. 2010;91:565–70. doi: 10.3945/ajcn.2009.28614. [DOI] [PubMed] [Google Scholar]
- 99.Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 100.Sorensen MD, Hsi RS, Chi T, et al. Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women: A Women’s Health Initiative report. J Urol. 2014;192:1694–9. doi: 10.1016/j.juro.2014.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402–10. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 102.Baxmann AC, De OG Mendonca C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003(63):1066. doi: 10.1046/j.1523-1755.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- 103.Traxer O, Huet B, Poindexter J, et al. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol. 2003;170:397–401. doi: 10.1097/01.ju.0000076001.21606.53. [DOI] [PubMed] [Google Scholar]
- 104.Ferraro PM, Taylor EN, Eisner BH, et al. History of kidney stones and the risk of coronary heart disease. JAMA. 2013;310:408–15. doi: 10.1001/jama.2013.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–7. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 106.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20:2253–9. doi: 10.1681/ASN.2009030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Negri AL, Spivacow R, Del Valle E, et al. Clinical and biochemical profile of patients with “pure” uric acid nephrolithiasis compared with “pure” calcium oxalate stone formers. Urol Res. 2007;35:247–51. doi: 10.1007/s00240-007-0109-1. [DOI] [PubMed] [Google Scholar]
- 108.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: A novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–8. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 109.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–15. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Semins MJ, Shore AD, Makary MA, et al. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–5. doi: 10.1016/j.juro.2009.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maalouf NM, Cameron MA, Moe OW, et al. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–9. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 112.Maalouf N, Poindexter J, Adams-Huet B, et al. Increased production and reduced urinary buffering of acid in uric acid stone formers ins ameliorated by pioglitazone. Kidney Int. 2019;95:1262–76. doi: 10.1016/j.kint.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urol Res. 2004;32:311–6. doi: 10.1007/s00240-004-0437-3. [DOI] [PubMed] [Google Scholar]
- 114.Noori N, Honarkar E, Goldfarb D, et al. Urinary lithogenic risk profile in recurrent stone formers with hyperoxaluria: A randomized controlled trial comparing DASH (Dietary Approaches to Stop Hypertension)-style and low-oxalate diets. Am J Kidney Dis. 2014;63:456–63. doi: 10.1053/j.ajkd.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 115.Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010;78:1178–85. doi: 10.1038/ki.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mitwalli A, Ayiomamitis A, Grass L, et al. Control of hyperoxaluria with large doses of pyridoxine in patients with kidney stones. Int Urol Nephrol. 1988;20:353–9. doi: 10.1007/BF02549567. [DOI] [PubMed] [Google Scholar]
- 117.Witting C, Langman CB, Assimos D, et al. Pathophysiology and treatment of enteric hyperoxaluria. Clin J Am Soc Nephrol. 2021;16:487–95. doi: 10.2215/CJN.08000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sakhaee K, Griffith C, Pak CY. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis. 2012;8:67–72. doi: 10.1016/j.soard.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 119.Ward JB, Feinstein L, Pierce C, et al. Pediatric urinary stone disease in the United States: The urologic diseases in America project. Urology. 2019;129:180–7. doi: 10.1016/j.urology.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dwyer ME, Krambeck AE, Bergstralh EJ, et al. Temporal trends in incidence of kidney stones among children: A 25-year population based study. J Urol. 2012;188:247–52. doi: 10.1016/j.juro.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tasian GE, Kabarriti AE, Kalmus A, et al. Kidney stone recurrence among children and adolescents. J Urol. 2017;197:246–52. doi: 10.1016/j.juro.2016.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuroczycka-Saniutycz E, Porowski T, Protas PT, et al. Does obesity or hyperuricemia influence lithogenic risk profile in children with urolithiasis? Pediatr Nephrol. 2015;30:797–803. doi: 10.1007/s00467-014-2999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bandari J, Dangle PP, Lyon TD, et al. 24-hour urinary parameters in overweight and obese children with urolithiasis. J Urol. 2016;196:526–30. doi: 10.1016/j.juro.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 124.Issler N, Dufek S, Kleta R, et al. Epidemiology of pediatric renal stone disease: 22-year, single-centre experience in the U.K. BMC Nephrol. 2017;18:136. doi: 10.1186/s12882-017-0505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gillespie RS, Stapletone FB. Nephrolithiasis in children. Pediatr Rev. 2004;25:131–9. doi: 10.1542/pir.25-4-131. [DOI] [PubMed] [Google Scholar]
- 126.Carnes K, Howe A, Feustel PJ, et al. 24-hour urine collection for first time pediatric stone formers: Is it worth it? J Pediatr Urol. 2021;17:387. doi: 10.1016/j.jpurol.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 127.Hernandez JD, Ellison JS, Lendvay TS. Current trends, evaluation, and management of pediatric nephrolithiasis. JAMA Peditar. 2015;169:961–4. doi: 10.1001/jamapediatrics.2015.1419. [DOI] [PubMed] [Google Scholar]
- 128.Marra G, Taroni F, Berrettini A, et al. Pediatric nephrolithiasis: A systematic approach from diagnosis to treatment. J Nephrol. 2019;32:199–210. doi: 10.1007/s40620-018-0487-1. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez Cuellar CI, Wang PZT, Freundlich M, et al. Educational review: Role of the pediatric nephrologists in the workup and management of kidney stones. Pediatr Nephrol. 2020;35:383–97. doi: 10.1007/s00467-018-4179-9. [DOI] [PubMed] [Google Scholar]
- 130.Parvin M, Shakhssalim N, Basiri A, et al. The most important metabolic risk factors in recurrent urinary stone formers. Urol J. 2011;8:99–106. [PubMed] [Google Scholar]
- 131.Ohkawa M, Tokunaga S, Nakashima T, et al. Thiazide treatment for calcium urolithiasis in patients with idiopathic hypercalciuria. Br J Urol. 1992;69:571–6. doi: 10.1111/j.1464-410X.1992.tb15624.x. [DOI] [PubMed] [Google Scholar]
- 132.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977;87:404–10. doi: 10.7326/0003-4819-87-4-404. [DOI] [PubMed] [Google Scholar]
- 133.Brocks P, Dahl C, Wolf H, et al. Do thiazides prevent recurrent idiopathic renal calcium stones? Lancet. 1981;2:124–5. doi: 10.1016/S0140-6736(81)90302-0. [DOI] [PubMed] [Google Scholar]
- 134.Mortensen JT, Schultz A, Ostergaard AH. Thiazides in the prophylactic treatment of recurrent idiopathic kidney stones. Int Urol Nephrol. 1986;18:265–9. doi: 10.1007/BF02082712. [DOI] [PubMed] [Google Scholar]
- 135.Laerum S, Larsen S. Thiazide prophylaxis of urolithiasis: A double-blind study in general practice. Acta Med Scand. 1984;215:383–9. doi: 10.1111/j.0954-6820.1984.tb05023.x. [DOI] [PubMed] [Google Scholar]
- 136.Fernandez-Rodriguez A, Arrabal-Martin M, Garcia-Ruiz MJ, et al. The role of thiazides in the prophylaxis of recurrent calcium lithiasis. Actas Urol Esp. 2006;30:305–9. doi: 10.4321/S0210-48062006000300009. [DOI] [PubMed] [Google Scholar]
- 137.Borghi L, Meschi T, Guerra A, et al. Randomized prospective study of a non-thiazide diuretic, indapamide, in preventing calcium stone recurrences. J Cardiovasc Pharmacol. 1993;22:S78–86. doi: 10.1097/00005344-199306226-00014. [DOI] [PubMed] [Google Scholar]
- 138.Ettinger B, Citron JT, Livermore B, et al. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J Urol. 1988;139:679–84. doi: 10.1016/S0022-5347(17)42599-7. [DOI] [PubMed] [Google Scholar]
- 139.Nicar MJ, Peterson R, Pak CY. Use of potassium citrate as potassium supplement during thiazide therapy of calcium nephrolithiasis. J Urol. 1984;131:430–3. doi: 10.1016/S0022-5347(17)50438-3. [DOI] [PubMed] [Google Scholar]
- 140.Odvina CV, Preminger GM, Lindberg JS, et al. Long-term combined treatment with thiazide and potassium citrate in nephrolithiasis does not lead to hypokalemia or hypochloremic metabolic alkalosis. Kidney Int. 2003;63:240–7. doi: 10.1046/j.1523-1755.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- 141.Allie-Hamdulay S, Rodgers AL. Prophylactic and therapeutic properties of a sodium citrate preparation in the management of calcium oxalate urolithiasis: randomized, placebo-controlled trial. Urol Res. 2005;33:116–24. doi: 10.1007/s00240-005-0466-6. [DOI] [PubMed] [Google Scholar]
- 142.Reddy SV, Shaik AB, Bokkisam S. Effect of potassium magnesium citrate and vitamin B-6 prophylaxis for recurrent and multiple calcium oxalate and phosphate urolithiasis. Korean J Urol. 2014;55:411–6. doi: 10.4111/kju.2014.55.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barcelo P, Wuhl O, Servitge I, et al. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–4. doi: 10.1016/S0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 144.Hofbauer J, Hobarth K, Szabo N, et al. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis: a prospective randomized study. Br J Urol. 1994;73:362–5. doi: 10.1111/j.1464-410X.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 145.Mattle D, Hess B. Preventive treatment of nephrolithiasis with alkali citrate-a critical review. Urol Res. 2005;33:73–9. doi: 10.1007/s00240-005-0464-8. [DOI] [PubMed] [Google Scholar]
- 146.Ettinger B, Pak CY, Citron JT, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–73. doi: 10.1016/S0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 147.Robinson MR, Leitao VA, Haleblian GE, et al. Impact of long-term potassium citrate therapy on urinary profiles and recurrent stone formation. J Urol. 2009;181:1145–50. doi: 10.1016/j.juro.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 148.Lojanapiwat B, Tanthanuch M, Pripathanont C, et al. Alkaline citrate reduces stone recurrence and regrowth after shockwave lithotripsy and percutaneous nephrolithotomy. Int Braz J Urol. 2011;37:611–6. doi: 10.1590/S1677-55382011000500007. [DOI] [PubMed] [Google Scholar]
- 149.Soygür T, Akbay A, Küpeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: A randomized controlled trial. J Endourol. 2002;16:149–52. doi: 10.1089/089277902753716098. [DOI] [PubMed] [Google Scholar]
- 150.Preminger GM, Sakhaee K, Pak CY. Alkali action on the urinary crystallization of calcium salts: contrasting responses to sodium citrate and potassium citrate. J Urol. 1988;139:240–2. doi: 10.1016/S0022-5347(17)42374-3. [DOI] [PubMed] [Google Scholar]
- 151.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489–96. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 152.Ettinger B, Tang A, Citron JT, et al. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315:1386–9. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 153.Smith MJ. Placebo versus allopurinol for renal calculi. J Urol. 1977;117:690–2. doi: 10.1016/S0022-5347(17)58588-2. [DOI] [PubMed] [Google Scholar]
- 154.Pearle MS, Roehrborn CG, Pak CY. Meta-analysis of randomized trials for medical prevention of calcium oxalate nephrolithiasis. J Endourol. 1999;13:679–85. doi: 10.1089/end.1999.13.679. [DOI] [PubMed] [Google Scholar]
- 155.Suh JM, Cronan JJ, Monchik JM. Primary hyperparathyroidism: Is there an increased prevalence of renal stone disease? Am J Roentgenol. 2008;191:908–11. doi: 10.2214/AJR.07.3160. [DOI] [PubMed] [Google Scholar]
- 156.Mollerup CL, Vestergaard P, Frøkjær VG, et al. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective followup study. BMJ. 2002;325:807. doi: 10.1136/bmj.325.7368.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Amaral LM, Queiroz DC, Marques TF, et al. Normocalcemic vs. hypercalcemic primary hyperparathyroidism: More stone than bone? J Osteoporos. 2012;2012:128352. doi: 10.1155/2012/128352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tuna MM, Çalışkan M, Ünal M, et al. Normocalcemic hyperparathyroidism is associated with complications similar to those of hypercalcemic hyperparathyroidism. J Bone Miner Metab. 2015;34:331–5. doi: 10.1007/s00774-015-0673-3. [DOI] [PubMed] [Google Scholar]
- 159.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 160.Ferraro PM, D’Addessi A, Gambaro G. When to suspect a genetic disorder in a patient with renal stones, and why. Nephrol Dial Transplant. 2013;28:811–20. doi: 10.1093/ndt/gfs545. [DOI] [PubMed] [Google Scholar]
- 161.Sakhaee K, Alpern R, Poindexter J, et al. Citraturic response to oral citric acid load. J Urol. 1992;147:975–6. doi: 10.1016/S0022-5347(17)37437-2. [DOI] [PubMed] [Google Scholar]
- 162.Domrongkitchaiporn S, Khositseth S, Stitchantrakul W, et al. Dosage of potassium citrate in the correction of urinary abnormalities in pediatric distal renal tubular acidosis patients. Am J Kidney Dis. 2002;39:383–91. doi: 10.1053/ajkd.2002.30560. [DOI] [PubMed] [Google Scholar]
- 163.Preminger GM, Sakhaee K, Skurla C, et al. Prevention of recurrent calcium stone formation with potassium citrate therapy in patients with distal renal tubular acidosis. J Urol. 1985;134:20–3. doi: 10.1016/S0022-5347(17)46963-1. [DOI] [PubMed] [Google Scholar]
- 164.Dhayat NA, Gradwell MW, Pathare G, et al. Furosemide/fludrocortisone test and clinical parameters to diagnose incomplete distal rental tubular acidosis in kidney stone formers. Clin J Am Soc Nephrol. 2017;12:1507–17. doi: 10.2215/CJN.01320217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Osther PJ, Bollerslev J, Hansen AB, et al. Pathophysiology of incomplete renal tubular acidosis in recurrent renal stone formers: Evidence of disturbed calcium, bone, and citrate metabolism. Urol Res. 1993;21:169–73. doi: 10.1007/BF00590032. [DOI] [PubMed] [Google Scholar]
- 166.Osther PJ, Hansen AB, Rohl HF. Distal renal tubular acidosis in recurrent renal stone formers. Ban Med Bull. 1989;36:492–3. [PubMed] [Google Scholar]
- 167.Donnelly S, Kamel KS, Vasuvattakul S, et al. Might distal renal tubular acidosis be a proximal tubular cell disorder? Am J Kindey Dis. 1992;19:272. doi: 10.1016/S0272-6386(13)80009-1. [DOI] [PubMed] [Google Scholar]
- 168.Cameron MA, Sakhaee K. Uric acid nephrolithiasis. Urol Clin North Am. 2007;34:335–46. doi: 10.1016/j.ucl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 169.Goldfarb DS, MacDonald PA, Gunawardhana L, et al. Randomized controlled trial of febuxostat versus allopurinol or placebo in individuals with higher urinary uric acid excretion and calcium stones. Clin J Am Soc Nephrol. 2013;8:1960–7. doi: 10.2215/CJN.01760213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Trinchieri A, Esposito N, Castelnuovo C. Dissolution of radiolucent renal stones by oral alkalinization with potassium citrate/potassium bicarbonate. Arch Ital Urol Androl. 2009;81:188–91. [PubMed] [Google Scholar]
- 171.Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney Int. 1986;30:422–8. doi: 10.1038/ki.1986.201. [DOI] [PubMed] [Google Scholar]
- 172.Elsawy A, Elshal A, El-Naha A, et al. Can we predict the outcome of oral dissolution therapy for radiolucent renal calculi? A prospective study. J Urol. 2019;201:350–7. doi: 10.1016/j.juro.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 173.Barbey F, Joly D, Rieu P, et al. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol. 2000;163:1419–23. doi: 10.1016/S0022-5347(05)67633-1. [DOI] [PubMed] [Google Scholar]
- 174.Mattoo A, Goldfarb DS. Cystinuria. Semin Nephrol. 2008;28:181–91. doi: 10.1016/j.semnephrol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 175.Rodriguez LM, Santos F, Malaga S, et al. Effect of low sodium diet on urinary elimination of cystine in cystinuric children. Nephron. 1995;71:416–8. doi: 10.1159/000188761. [DOI] [PubMed] [Google Scholar]
- 176.Lindell A, Denneberg T, Edholm E, et al. The effect of sodium intake on cystinuria with and without tiopronin treatment. Nephron. 1995;71:407–15. doi: 10.1159/000188760. [DOI] [PubMed] [Google Scholar]
- 177.Norman RW, Manette WA. Dietary restriction of sodium as a means of reducing urinary cystine. J Urol. 1990;143:1193–5. doi: 10.1016/S0022-5347(17)40222-9. [DOI] [PubMed] [Google Scholar]
- 178.Rodman JS, Blackburn P, Williams JJ, et al. The effect of dietary protein on cystine excretion in patients with cystinuria. Clin Nephrol. 1984;22:273–8. [PubMed] [Google Scholar]
- 179.Claes DJ, Jackson E. Cystinuria: Mechanisms and management. Pediatr Nephrol. 2012;27:2031–8. doi: 10.1007/s00467-011-2092-6. [DOI] [PubMed] [Google Scholar]
- 180.Sterret SP, Penniston KL, Wolf JS, et al. Acetazolamide is an effective adjunct for urinary alkalinization in patients with uric acid and cystine stone formation recalcitrant to potassium citrate. Urology. 2008;72:278–81. doi: 10.1016/j.urology.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 181.Dahlberg PJ, van den Berg Kurtz SB, et al. Clinical features and management of cystinuria. Mayo Clin Proc. 1977;52:533–42. [PubMed] [Google Scholar]
- 182.Pak CY, Fuller C, Sakhaee K, et al. Management of cystine nephrolithiasis with alpha-mercaptopropionylglycine. J Urol. 1986;136:1003–8. doi: 10.1016/S0022-5347(17)45188-3. [DOI] [PubMed] [Google Scholar]
- 183.Chow GK, Streem SB. Medical treatment of cystinuria: Results of contemporary clinical practice. J Urol. 1996;156:1576–8. doi: 10.1016/S0022-5347(01)65451-X. [DOI] [PubMed] [Google Scholar]
- 184.Cohen TD, Streem SB, Hall P. Clinical effect of captopril on the formation and growth of cystine calculi. J Urol. 1995;154:164–6. doi: 10.1016/S0022-5347(01)67256-2. [DOI] [PubMed] [Google Scholar]
- 185.Pietrow PK, Auge BK, Weizer AZ, et al. Durability of the medical management of cystinuria. J Urol. 2003;169:68–70. doi: 10.1016/S0022-5347(05)64037-2. [DOI] [PubMed] [Google Scholar]
- 186.Resnick M, Pridgen DB, Goodman HO. Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med. 1968;278:1313–8. doi: 10.1056/NEJM196806132782403. [DOI] [PubMed] [Google Scholar]
- 187.Curhan GC, Willett WC, Rimm EB, et al. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–73. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 188.Goldfarb DS, Avery AR, Beara-Lasic L, et al. A twin study of genetic influences on nephrolithiasis in women and men. Kidney Int Rep. 2018;4:535–40. doi: 10.1016/j.ekir.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goldfarb DS, Fischer ME, Keich Y, et al. A twin study of genetic and dietary influences on nephrolithiasis: A report from th eVientam Era Twin Registry. Kidney Int. 2005;67:1053–61. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 190.Halbritter J, Baum M, Hynes AM, et al. Fourteen monogenic genes account from 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol. 2015;26:543–51. doi: 10.1681/ASN.2014040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Daga A, Majmundar AJ, Braun DA, et al. Whole exome sequencing frequently detects a mongenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018;93:204–13. doi: 10.1016/j.kint.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Garrelfs SF, Frishberg Y, Hulton SA, et al. Lumasiran, an RNAi therapetuic for primary hyperoxaluria type I. N Engl J Med. 2021;384:1216–26. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 193.Howles S, Thakker R. Genetics of kidney stone disease. Nat Rev Urol. 2020;17:407–21. doi: 10.1038/s41585-020-0332-x. [DOI] [PubMed] [Google Scholar]


