Abstract
We developed a chemically defined medium called milieu proche du lait (MPL), in which 22 Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) strains exhibited growth rates ranging from 0.55 to 1 h−1. MPL can also be used for cultivation of other lactobacilli and Streptococcus thermophilus. The growth characteristics of L. bulgaricus in MPL containing different carbon sources were determined, including an initial characterization of the phosphotransferase system transporters involved. For the 22 tested strains, growth on lactose was faster than on glucose, mannose, and fructose. Lactose concentrations below 0.4% were limiting for growth. We isolated 2-deoxyglucose-resistant mutants from strains CNRZ397 and ATCC 11842. CNRZ397-derived mutants were all deficient for glucose, fructose, and mannose utilization, indicating that these three sugars are probably transported via a unique mannose-specific-enzyme-II-like transporter. In contrast, mutants of ATCC 11842 exhibited diverse phenotypes, suggesting that multiple transporters may exist in that strain. We also developed a protein labeling method and verified that exopolysaccharide production and phage infection can occur in MPL. The MPL medium should thus be useful in conducting physiological studies of L. bulgaricus and other lactic acid bacteria under well controlled nutritional conditions.
Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) and Streptococcus thermophilus are used in the dairy industry to transform milk into yogurt. However, despite the industrial interest in L. bulgaricus, little is known about its physiology and genetics. A chemically defined medium (CDM) is a prerequisite for physiological studies that allows experimentation under reproducible conditions via the control of all nutrient sources. So far, of the three CDMs published for L. bulgaricus, two involve an agarose-solidified medium (reference 22, as modified by reference 4 and T. Sasaki and Y. Sasaki, Abstr. Fifth Symp. Lactic Acid Bacteria, abstr. E16, 1996). The third CDM was developed for an L. bulgaricus strain (NCFB2772) which exhibited an anaerobic growth rate of around 0.2 h−1; this rate is at least three times lower than growth rates in rich and complex media such as milk or MRS (12, 13). In view of these drawbacks, we considered it necessary to develop a new CDM that would allow efficient growth of L. bulgaricus strains. This criterion was fulfilled by milieu proche du lait (MPL), since all L. bulgaricus strains tested (22 strains) grew in this medium with an average rate of 0.7 h−1 (corresponding to a doubling time of about 1 h).
The composition of MPL allowed us to examine sugar utilization in L. bulgaricus. Previous genetic characterization of the lactose transport system showed that the LacS permease in L. bulgaricus belongs to the galactose-pentose-hexuronides (GPH) family (23, 30). However, little is known about the transport of glucose, mannose, and fructose, the other three sugars catabolized by L. bulgaricus. There are no reports concerning mannose and fructose, two sugars that are transported by the phosphotransferase system (PTS) in other lactic acid bacteria (25, 29, 34). Biochemical studies have indicated that glucose and its analogue, 2-deoxyglucose (2DG), are internalized via the PTS in some L. bulgaricus strains (14). The PTS catalyzes concomitant transport and phosphorylation of sugars via a specific membrane-localized enzyme II complex (EII) which is phosphorylated by the general PTS enzymes HPr and enzyme I (for a review, see reference 31). Characterization of PTS transporters is of interest since (i) PTS sugars are often preferred carbon sources, and (ii) PTS enzymes like HPr exert regulatory functions affecting the catabolism of other sugars via a control of transport and/or glycolytic activities (15, 31). EIIMan (mannose-specific EII) is able to transport mannose, fructose, glucose, and 2DG in Escherichia coli and gram-positive bacteria (20, 25, 31, 37) and is found in many lactic acid bacteria, such as Lactobacillus sakei, Lactobacillus casei, Lactobacillus pentosus, Lactobacillus plantarum, Lactobacillus curvatus, and Lactococcus lactis (7, 35, 39, 40). It is therefore possible that glucose, mannose, and fructose are transported by an EIIMan homologue in L. bulgaricus.
In order to initiate the genetic characterization of PTS transporters, mutants resistant to 2DG were isolated. Transport of 2DG via EIIMan leads to accumulation of phosphorylated 2DG, which is toxic for the cell. Previous studies indicated that spontaneous 2DG-resistant mutants usually affect EIIMan-specific enzymes (10, 20, 36). We analyzed the phenotypes of mutants for their growth properties on various sugars in MPL.
Finally, to facilitate the use of MPL for various physiological studies, we developed a protocol to label neosynthesized proteins and verified that phage infection and exopolysaccharide (EPS) production occur in this medium.
MATERIALS AND METHODS
Strains and culture conditions.
L. bulgaricus strains used in this study were as follows: CNRZ208, which corresponds to the type strain ATCC 11842, CNRZ397, CNRZ1057, and 21 strains numbered from VI1001 to VI1021 which are natural isolates of various origins (T. Smokvina, unpublished data). Other lactic acid bacteria strains used were L. delbrueckii subsp. delbrueckii CNRZ225 and subsp. lactis CNRZ207, Lactobacillus acidophilus CNRZ204 and CNRZ462, Lactobacillus helveticus CNRZ223, Lactobacillus fermentum CNRZ209 and CNRZ236, Lactobacillus plantarum CNRZ211, Lactobacillus buchneri CNRZ214, Lactobacillus zeae ATCC 393, formerly classified as Lactobacillus casei (8), Streptococcus thermophilus CNRZ1358 and IL73, and Leuconostoc mesenteroides CNRZ1019. All CNRZ strains were provided by the INRA National Culture Collection (URLGA, Jouy-en-Josas, France). L. delbrueckii, L. acidophilus, L. fermentum, L. buchneri, and S. thermophilus cultures were incubated at 42°C, and L. plantarum, L. zeae, and L. mesenteroides were incubated at 30°C.
Liquid cultures were inoculated with dilutions (at least 100-fold) of (i) a fresh colony grown on MRS complex medium (Difco) plates and resuspended in 1 ml of medium or (ii) cells from a fresh culture that were first washed in 0.9% NaCl. Growth was estimated by measuring optical density at 600 nm (OD600) and/or by serial dilution and plating. Growth kinetics measurements were performed using a microplate reader (Microbiology Reader Bioscreen C; Labsystems). The culture volume was around 350 μl, and samples were overlaid with mineral oil to avoid aeration during shaking. For experiments in larger volumes (in which OD and pH were monitored), cultures were distributed into several tubes; each tube was used for one measurement to avoid aeration due to mixing.
Optimization of MPL component concentrations.
Strain CNRZ397 was cultured in MPL containing various concentrations of one of the following components: thioglycolate, riboflavin, cobalamine, calcium chloride, or a mixture of four vitamins (folic acid, calcium pantothenate, niacin, and pyridoxal). Growth kinetics were followed using a microplate reader. The final concentration used in MPL was higher (often 10-fold) than the concentration leading to decreased growth.
Phage infection.
Phage 19 was propagated on MRS or MPL stationary-phase cultures of indicator strain L. bulgaricus CNRZ1057, using standard procedures (1). The phage titer of each lysate was estimated by spotting serial dilutions on a lawn of CNRZ1057 in MRS top agar supplemented with 10 mM CaCl2.
Isolation and characterization of 2DG-resistant mutants.
Bacteria were grown in MPL plus 2% lactose (MPL-lactose) to an OD600 of 1.5, washed with MPL without sugar, and plated onto MPL agar containing 0.1, 0.2, or 0.4% lactose and 2, 5, or 10 mM 2DG. After 24 to 35 h of incubation at 42°C, colonies were purified on MPL agar containing lactose and 2DG. Phenotypic tests were performed as follows: MPL-lactose cultures of the mutants were serially diluted in 0.9% NaCl and spotted on MPL agar either containing a 1% concentration of lactose, glucose, fructose, or mannose or without sugar (control). Growth of colonies was monitored after 24 to 96 h of incubation at 42°C.
Protein labeling.
To favor [35S]methionine ([35S]Met) incorporation, we used MPL containing a limiting amount of methionine (40 μM), which allows growth to a final OD600 of 1. At an OD of about 0.7, 10 μCi of [35S]methionine (ICN Pharmaceuticals, Inc., Irvine, Calif.) per ml was added to the culture. After 2 to 30 min, the labeling was interrupted by the addition of 0.8 mM cold methionine and 1 volume of ice-cold MPL. Cells were collected by centrifugation for 3 min at 10,000 rpm and 4°C. The equivalent of 0.2 ml of culture was resuspended in 32 μl of TES (50 mM Tris [pH 8], 5 mM EDTA, 20% sucrose), lysozyme (0.2 mg/ml), and mutanolysin (100 U/ml; Sigma) and incubated for 2 min at 37°C. An 8-μl aliquot of 5× Laemmli buffer (19) was then added before a 5-min incubation at 95°C for lysis. Protein samples were analyzed by classical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (19) and autoradiography.
RESULTS
MPL medium. (i) Design of MPLR.
Growth of L. bulgaricus CNRZ397 was tested in CDM, described in the literature (4, 20). As growth was poor (at best reaching an OD600 of 0.8 after 24 h), even after addition of vitamins and Casamino Acids, we chose to develop another CDM, referred to as MPLR (Table 1). This medium was designed using components present in previously described CDMs as well as in milk. Concentrations of lactose, phosphate, calcium, potassium, sodium, citrate, chloride, and oleic acid (the major fatty acid in cow milk) were based on milk composition (2, 22, 26). We used several components previously reported as stimulatory for L. bulgaricus growth, i.e., formate, pyruvate, acetate, ascorbic acid, and nucleic acid bases (18, 22). Tween 80 was used as a putative lipid source, although it can also act as a detergent. The reducing agent thioglycolate was added, since our tests were not carried out under strict anaerobic conditions. Amino acids, bases, vitamins, and micronutrients were used according to concentrations previously described for various CDMs (Table 1). The resulting MPLR comprised 60 components (Table 1) and allowed the growth of CNRZ397 with an average doubling time of 1 h and a final OD600 of 2.
TABLE 1.
Composition of the CDM
| Constituentb | Final concn (mM)a
|
|
|---|---|---|
| MPLRc | MPL (amt/liter) | |
| Lactose | 58.4 | 58.4 (20 g) |
| Sodium thioglycolate* | 4.38 | 4.38 (0.5 g) |
| Tween 80* (% vol/vol) | 0.1 | 0.1 (1 g) |
| K2HPO4 · 3H2O* | 7 | |
| Na2HPO4 | 2.11 (0.3 g) | |
| NaH2PO4 · H2O | 5.8 (0.8 g) | |
| Potassium acetate* | 15 | 75 (7.35 g) |
| Ammonium citrate* | 10 | |
| Orotic acid* | 3.2 | |
| Pyruvic acid* | 0.9 | |
| Formic acid* | 0.2 | |
| Oleic acid* | 0.033 | |
| NaCl | 10 | |
| CaCl2* | 5 | 2.5 (0.27 g) |
| MgSO4 · 7H2O* | 0.8d | 0.8 (0.2 g) |
| MnSO4 · H2O | 0.12e | 0.12 (0.02 g) |
| FeSO4 · 7H2O (μM) | 70f | 0.23 (0.066 mg) |
| Micronutrients* (μM) | ||
| (NH4)6Mo7O24 · 4H2O | 0.003g | 0.003 (0.0037 mg) |
| CoCl2 · 6H2O | 0.03g | 0.03 (0.007 mg) |
| H3Bo3 | 0.4g | 0.4 (0.025 mg) |
| CuSO4 | 0.01g | 0.01 (0.0016 mg) |
| ZnSO4 · 7H2O | 0.01g | 0.01 (0.0029 mg) |
| Bases* | ||
| Adenine | 0.37e | 0.37 (0.05 g) |
| Guanine | 0.33e | 0.33 (0.05 g) |
| Xanthine | 0.32 | 0.32 (0.05 g) |
| Uracil | 0.45e | 0.45 (0.05 g) |
| 2′-Deoxyguanosine* | 0.19e | |
| Hypoxanthine* | 0.37 | |
| Amino acids | ||
| l-Alanine | 2.24d | 1.12i (0.1 g) |
| l-Arginine | 0.29d | 1.84i (0.32 g) |
| l-Asparagine | 1.9d | 2.27 (0.3 g) |
| l-Aspartate | 7.5 | 3.75i (0.5 g) |
| l-Cysteine · HCl | 2.28d | 1.6 (0.28 g) |
| Glycine | 2.66d | 2.13i (0.16 g) |
| l-Glutamate | 5.9d | 2.25i (0.38 g) |
| l-Glutamine | 2d | 2 (0.3 g) |
| l-Histidine | 3.22d | 1.3i (0.2 g) |
| l-Isoleucine | 3.8d | 2.75i (0.36 g) |
| l-Leucine | 1.52d | 4.57i (0.6 g) |
| l-Lysine · HCl | 0.27d | 2.4i (0.44 g) |
| l-Methionine | 3.35d | 0.8i (0.12 g) |
| l-Phenylalanine | 1.21d | 2.06i (0.34 g) |
| l-Proline | 4.7 | 8i (0.92 g) |
| l-Serine | 1.9d | 3.42i (0.36 g) |
| l-Threonine | 0.42d | 2.52i (0.3 g) |
| l-Tryptophan | 1d | 0.5i (0.1 g) |
| l-Tyrosine | 1.1d | 0.66i (0.12 g) |
| l-Valine | 4.27d | 4i (0.48 g) |
| l-Cystine | 0.42d | |
| Vitamins (μM) | ||
| B12 (cobalamin)* | 7.4h | 7.4 (0.01 mg) |
| Riboflavin* | 2.66e | 0.53 (0.2 mg) |
| Calcium pantothenate* | 2e | 0.2 (0.1 mg) |
| Niacin* | 14d | 1.4 (0.17 mg) |
| Folic acid* | 0.72d | 0.072 (0.032 mg) |
| Pyridoxal* | 2.45e | 0.245 (0.05 mg) |
| Pyridoxamine* | 0.83 | |
| p-Aminobenzoate* | 2.92d | |
| Biotin* | 0.04e | |
| Thiamine* | 3e | |
| l-Ascorbic acid* (mM) | 2.8 | |
| Spermine · 6H2O* | 2.5 | |
Concentrations are millimolar unless otherwise indicated.
*, component tested by single omission (all micronutrients and bases were omitted at once).
MPLR corresponds to the rich medium composition before optimization of concentrations. Concentrations used in previously described CDMs are indicated with a footnote.
According to Lauret et al. (20).
According to Ledesma et al. (21).
According to Sasaki and Sasaki, Abstr. Fifth Symp. Lactic Acid Bacteria.
According to Jensen and Hammer (16).
According to Atlan et al. (4).
According to D. Roy, 1995 online communication in Lactacid Forum (http://lactacid@searn.sunet.se).
(ii) Simplification and optimization of the medium.
Single-omission tests were carried out to reduce the number of medium constituents without affecting CNRZ397 growth; the final medium was called MPL (Table 1, column 3). Fourteen MPLR components were not required, and 14 elements (i.e., thioglycolate, Tween 80, acetate, phosphate, bases, 6 of 12 vitamins, magnesium, calcium, and micronutrients) were essential for efficient growth (Table 1, compare columns 2 and 3). Nucleoside bases and the micronutrient mix were not individually tested at this stage.
For eight of the “essential” compounds, concentrations affecting CNRZ397 growth were determined (Materials and Methods), and final optimal concentrations in MPL were adjusted accordingly. Moreover, acetate, iron, amino acid, and phosphate concentrations were modified as described below. (i) Acetate was increased to 75 mM, which allowed a higher growth yield. This is probably due to an increased buffering capacity, since addition of another buffer (morpholinoethanesulfonic acid [MES]) also increased the yield in the presence of 15 mM acetate. (ii) Elimination of manganese or iron did not affect growth as long as calcium and micronutrients were present. Therefore, we kept these elements but the amount of iron, which is potentially toxic, was reduced about 300 fold. (iii) Amino acid concentrations were modified according to Casamino Acid composition (Table 1). (iv) Potassium phosphate was replaced by sodium phosphate (7.91 mM) and adjusted to obtain an initial medium pH of 6.4.
All these modifications led to MPL, which contains 45 constituents including the 20 amino acids (Table 1) and allows the growth of CNRZ397 with a doubling time of about 70 min, with a final OD600 of 2 and pH of 4.2.
Lactic acid bacteria growth in MPL.
Since MPL allowed rapid growth of CNRZ397, we tested 21 other strains of L. bulgaricus that belong to several randomly amplified polymorphic DNA groups (E. M. Lim, personal communication). Growth kinetics measurements carried out in microplates showed that the doubling times of these strains ranged from 40 to 75 min (mean, 60 min). Final OD600 values for batch cultures ranged between 1.1 and 3 (mean, 2.4). Although the growth rates were only slightly lower in MPL than in MRS medium, the final OD in MPL was two to three times lower than the OD in MRS.
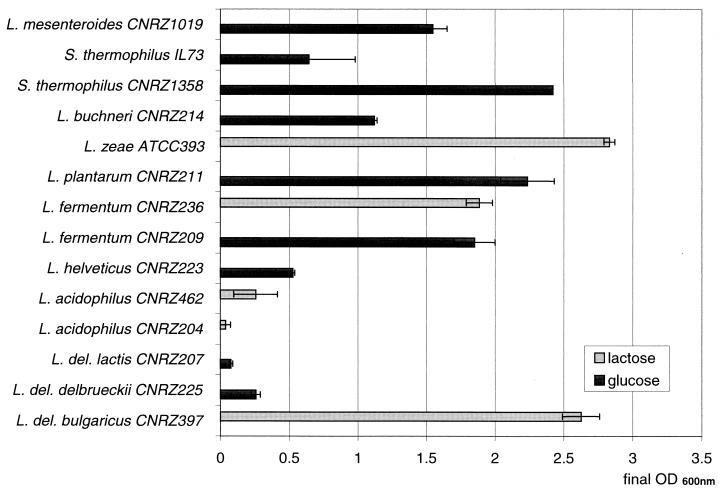
Growth of several other lactic acid bacteria was also tested in modified MPL (with the vitamins of MPLR) containing glucose or lactose (Fig. 1). The tested strains of L. fermentum, L. mesenteroides, L. plantarum, L. buchneri, L. zeae, and S. thermophilus CNRZ1358 grew well until the OD600 reached ≃1 to 2.5. In contrast, S. thermophilus IL73 as well as L. delbrueckii subsp. lactis CNRZ207, L. delbrueckii subsp. delbrueckii CNRZ225, L. helveticus CNRZ223, and L. acidophilus CNRZ204 and CNRZ462 did not grow in MPL or grew only poorly. These data indicate that MPL is a suitable CDM not only for L. bulgaricus strains but also for a wide range of other lactobacilli, including L. fermentum, for which no CDM was previously available. Moreover, MPL may allow coculture of L. bulgaricus with at least some S. thermophilus strains and may thus be useful for concomitant physiological studies of these two species, which are used together in yogurt production.
FIG. 1.
Growth characteristics of various lactic acid bacteria in MPL. Fresh colonies obtained on MRS plates were inoculated in MPL containing a 2% concentration of either lactose or glucose and the 12 vitamins indicated in Table 1, column 2. OD600 was measured after at least 48 h of incubation at either 42 or 30°C. The standard deviation is indicated when two independent experiments were performed.
Growth of L. bulgaricus on different carbohydrates.
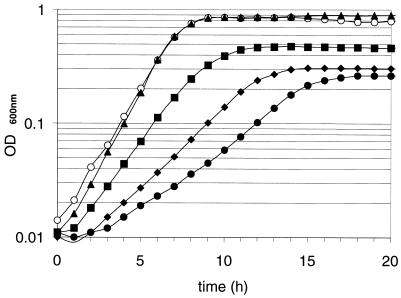
L. bulgaricus ferments lactose, glucose, fructose, and mannose (5, 12) and does not grow on galactose. The 22 L. bulgaricus strains had a doubling time of about 1 h in MPL-lactose, compared to 3 to 4 h in MPL containing 1% glucose (MPL-glucose). In both cases, cultures reached equivalent OD600 levels after 24 to 48 h of incubation at 42°C. Growth in MPL-mannose or -fructose was, for most of the tested strains, similar to that in MPL-glucose. This observation is surprising in view of the usual preference of bacteria for glucose, which is often transported via the PTS (15, 31). Galactose has been reported to accumulate in the medium after fermentation of the glucose moiety of lactose. To determine whether galactose accumulation might be responsible for the better growth on lactose, the effect of galactose on growth in MPL was tested. Supplementation of MPL-glucose (1%) with galactose (0.5 to 2%) did not modify growth, whereas addition of more than 1% galactose in MPL-lactose (1%) reduced the growth rate and the final biomass of the cultures (Fig. 2). Thus, while galactose may affect the efficiency of growth on lactose, it does not account for the growth differences observed between lactose and glucose. The efficiency of growth also depended on lactose concentration: we found that below 0.4% lactose, the growth rate was proportional to the sugar concentration. Doubling time increased from 68 to 160 min when lactose concentration varied from 0.4 to 0.1% (data not shown).
FIG. 2.
Effect of various concentrations of galactose on CNRZ397 growth. Media were inoculated by diluting a preculture (1/100) into MPL-lactose (1%) (open circles) supplemented with galactose at 1% (triangles), 2% (squares), 3% (diamonds), or 4% (filled circles). Growth was followed using a microplate reader as described in Materials and Methods.
We also examined growth when both glucose and lactose were present (the concentration of each sugar was between 0.1 and 2%). The addition of glucose did not affect the growth rate on lactose. Moreover, no diauxic growth curve could be observed, suggesting that both sugars are more probably simultaneously consumed (data not shown). These results suggest that lactose catabolism is not under catabolic repression by glucose in L. bulgaricus. This observation fits with the fact that growth on glucose is less efficient than on lactose.
Selection and characterization of 2DG-resistant mutants.
Strains ATCC 11842 and CNRZ397 were plated on MPL-lactose containing 2DG. After 2 days of incubation, colonies appeared with a frequency of 10−5. Sugar fermentation patterns of these mutants were studied on MPL plates containing different sugars as the sole carbon source. ATCC 11842-derived mutants exhibited four phenotypes: (i) two mutants were not affected on any sugars; (ii) one mutant was unable to grow on glucose and fructose and its growth was delayed on lactose; (iii) four mutants were greatly affected on glucose and slightly affected on mannose; and (iv) three mutants grew poorly, only on glucose. Therefore it appears that growth on glucose, mannose, or fructose can be dissociated in the ATCC 11842 strain.
We isolated and analyzed 74 2DG-tolerant mutants from CNRZ397. A majority (75.6%) grew normally on lactose and displayed a Glc− Man− Fru− phenotype; some (5.4%) displayed reduced growth on glucose, mannose, and fructose; and the remainder (19%) grew normally on each of the three sugars. These results show that, in contrast to the mutants derived from the ATCC 11842 strain, each CNRZ397-derived mutant had a similar growth on the three sugars tested, i.e., none, slow, or normal growth. We conclude that CNRZ397 is likely to have a single transporter for the three sugars, which is affected in the majority (around 80%) of the 2DGr mutants, whereas several sugar transporters probably coexist in ATCC 11842.
Protein labeling, phage infection, and EPS production in MPL. (i) De novo protein synthesis.
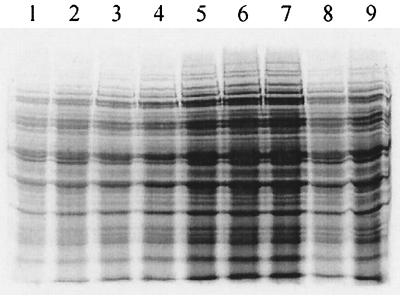
Radioactive labeling of proteins may be useful for a number of physiological studies, in particular in conjunction with two-dimensional gel electrophoresis. Here we showed that proteins may be labeled efficiently in MPL (Fig. 3). Incorporation of about 5 × 104 to 1 × 105 cpm for 0.2 OD600 units, obtained in 2 min, gave an easily detectable signal on X-ray film. Incorporation increased with a longer labeling time. As expected, use of higher amounts of [35S]Met (25 and 50 μCi/ml) resulted in a greater incorporation. Such labeling allows detection of induced or repressed proteins under different environmental conditions (24).
FIG. 3.
Protein labeling in MPL. CNRZ397 was grown in MPL containing 40 μM methionine. At an OD600 of 0.7, [35S]Met was added to the cultures, and samples were withdrawn at different time intervals. Samples equivalent to 0.2 OD600 units were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Lanes 1 to 7 correspond to samples incubated with [35S]Met at 10 μCi/ml for 1, 2, 5, 10, 15, 20, or 25 min, respectively. Lanes 8 and 9 correspond to samples labeled for 2 min with 25 or 50 μCi of [35S]Met/ml, respectively.
(ii) Phage infection.
To test phage development in MPL, strain CNRZ1057 was infected by phage 19 (33) using a multiplicity of infection of around 1/50 (Materials and Methods). Cells grown in MRS were used as a control. The resulting phage titers reached 5 × 106 and 3 × 108 PFU/ml in MPL and MRS, respectively. This result suggests that MPL can be used for phage infection studies but that phage development may be hampered.
(iii) EPS production.
EPS production is an important industrial trait of L. bulgaricus for yogurt quality (6), although the amounts of EPS produced by these lactobacilli are often small (12). The supernatants of MPL batch cultures of CNRZ416 and CNRZ1187 contained 30 to 50 mg of EPS/liter (S. Petry, personal communication). This is similar to the amounts detected in media optimized for EPS production and in which L. bulgaricus growth was much slower than in MPL (12, 28).
DISCUSSION
MPL is the first CDM described that allows a high growth rate for L. bulgaricus. Grobben et al. (12) reported growth rates of 0.18 to 0.23 h−1 (i.e., doubling times of 3 h 50 min to 3 h) in media designed for EPS production, and growth rates in MPL are close to 0.7 h−1 (doubling time of 60 min). Our work reveals that certain parameters are important for efficient growth of L. bulgaricus. (i) Lactose rather than glucose, as previously considered, markedly increases the growth rate of L. bulgaricus. (ii) A high proline concentration appears to be essential. We observed that increasing the proline concentration from 0.04 to 0.54 g/liter markedly improves growth (data not shown). It was previously established that proline plays a role in osmotic protection in some lactobacilli and in Lactococcus lactis (11, 17, 27). In our case, the requirement for a high proline concentration could reflect an osmotic protection role or a nutritional requirement due to inefficient uptake. (iii) Sodium thioglycolate (β-mercaptoacetate), a reducing agent, is important for efficient growth. It is known that during growth toxic oxygen derivatives are produced, but the enzymes required to eliminate them appear not to be expressed in L. bulgaricus (3). Reducing agents may provide protection against toxic products, particularly if growth conditions are not strictly anaerobic.
Our work confirmed some observations of Grobben et al. (12) concerning the nutritional requirements of L. bulgaricus. Single-omission growth tests confirmed that niacin, calcium pantothenate, riboflavin, and vitamin B12 were essential for growth and that biotin, thiamine, and p-aminobenzoic acid were dispensable in MPL. However, we also observed major differences in requirements, compared to those reported by Grobben et al. We found that folic acid, pyridoxal, and CaCl2 were important for efficient growth. Conversely, citrate and ammonium were eliminated from the MPL medium without affecting growth efficiency. These discrepancies could be due to other differences in medium composition or to strain-specific requirements.
L. bulgaricus does not use galactose but catabolizes the glucose moiety of lactose (14, 32). Enzymes responsible for glucose fermentation are thus present in L. bulgaricus. However, a rather important difference in doubling times was observed on MPL-lactose (1 h) and MPL-glucose (∼3 h). A possible explanation is that the lactose preference may be due to characteristics of the transporters involved in glucose or lactose uptake.
The lactose permease (LacS) of L. bulgaricus is homologous to LacS of S. thermophilus (30). This permease is a galactoside/H+ symporter which can transport either galactose or lactose. Under physiological conditions, LacS activity catalyzes the exchange of both sugars, leading to the entry of lactose and the efflux of galactose according to the gradient of sugars (9, 38). Our results, showing that the presence of galactose results in growth inhibition (if more than 1% galactose with 1% lactose is present) or slow growth at low lactose concentrations (below 0.4%), indicate that a similar exchange mechanism may exist in L. bulgaricus CNRZ397.
Previous studies (14) indicated that glucose is imported via a PTS in some strains of L. bulgaricus. Analyses of 2DGr mutants in this study suggest that the CNRZ397 strain imports glucose, mannose, and fructose via a single transporter. In many bacteria, the PTS-specific enzyme EIIMan is able to transport glucose, mannose, and fructose. Also, EIIMan is considered to be involved in 2DG sensitivity. Therefore we propose that EIIMan is present in L. bulgaricus CNRZ397 and that it transports glucose, mannose, and fructose. By contrast, the ATCC 11842 strain probably uses different systems to take up these sugars. ATCC 11842 gave a high background of growth on lactose-2DG plates, and this low 2DG sensitivity may reflect the absence of EIIMan in this strain. Therefore, transport systems of sugars other than lactose are likely to vary among L. bulgaricus strains.
The observations reported above strongly suggest that glucose import is inefficient in L. bulgaricus. This is in contrast with the general idea that glucose, transported via a PTS, is the most energetic or hierarchically preferred sugar for bacteria. We propose that this is an example of bacterial adaptation to a given growth medium, since L. bulgaricus is usually found and extensively cultured in milk in which lactose is present at a high concentration (around 5%).
In conclusion, our studies indicate that MPL may prove useful for biochemical studies and phage and EPS production as well as protein labeling in L. bulgaricus. Use of this medium should thus facilitate physiological and genetic studies of this important microorganism.
ACKNOWLEDGMENTS
D. Lebars and S. Petry are gratefully acknowledged for amino acid and EPS measurements, respectively. We thank P. Loubière and F. Rodolfe for helpful suggestions concerning MPL optimization, M. Zagorec for her expertise in sugar transport, and A. Gruss and M. van de Guchte for valuable suggestions concerning the manuscript. We also thank L. Benbadis, C. Fremaux, and A. Sépulchre for their interest in this study.
This work was supported, in part, by Danone (CIRDC) and Rhodia Food (Texel).
REFERENCES
- 1.Accolas J-P, Chopin M-C. Bacteriophages of lactic starters detection and enumeration. In: Bourgeois C M, Leveau J Y, editors. Techniques d'analyse et de contrôle dans les industries agroalimentaires. Le contrôle microbiologique. Vol. 3. Paris, France: Technique et Documentation APRIA; 1980. pp. 159–173. [Google Scholar]
- 2.Alais C. Science du lait. Principes des techniques laitières. 4th ed. Paris, France: Sepaic; 1984. [Google Scholar]
- 3.Archibald F S, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlan D, Laloi P, Portalier R. Isolation and characterization of aminopeptidase-deficient Lactobacillus bulgaricus mutants. Appl Environ Microbiol. 1989;55:1717–1723. doi: 10.1128/aem.55.7.1717-1723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan R E, Gibbons N E, editors. Bergey's manual of determinative bacteriology. 8th ed. Baltimore, Md: The Williams & Wilkins Co.; 1974. [Google Scholar]
- 6.Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Rev. 1990;7:113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaillou S, Pouwels P H, Postma P W. Transport of d-xylose in Lactobacillus pentosus, Lactobacillus casei, and Lactobacillus plantarum: evidence for a mechanism of facilitated diffusion via the phosphoenolpyruvate:mannose phosphotransferase system. J Bacteriol. 1999;181:4768–4773. doi: 10.1128/jb.181.16.4768-4773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dicks L M, Du Plessis E M, Dellaglio F, Lauer E. Reclassification of Lactobacillus casei subsp. casei ATCC 393 and Lactobacillus rhamnosus ATCC 15820 as Lactobacillus zeae nom. rev., designation of ATCC 334 as the neotype of L. casei subsp. casei, and rejection of the name Lactobacillus paracasei. Int J Syst Bacteriol. 1996;46:337–340. doi: 10.1099/00207713-46-1-337. [DOI] [PubMed] [Google Scholar]
- 9.Foucaud C, Poolman B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 1992;267:22087–22094. [PubMed] [Google Scholar]
- 10.Gauthier L, Thomas S, Gagnon G, Frenette M, Trahan L, Vadeboncoeur C. Positive selection for resistance to 2-deoxyglucose gives rise, in Streptococcus salivarius, to seven classes of pleiotropic mutants, including ptsH and ptsI missense mutants. Mol Microbiol. 1994;13:1101–1109. doi: 10.1111/j.1365-2958.1994.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 11.Glaasker E, Konings W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobben G J, Chin-Joe I, Kitzen V A, Boels I C, Boer F, Sikkema J, Smith M R, De Bont J A M. Enhancement of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a simplified defined medium. Appl Environ Microbiol. 1998;64:1333–1337. doi: 10.1128/aem.64.4.1333-1337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grobben G J, Sikkema J, Smith M R, De Bont J A M. Production of extracellular polysaccharide by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in a chemically defined medium. J Appl Bacteriol. 1995;79:103–107. [Google Scholar]
- 14.Hickey M W, Hillier A J, Jago G R. Transport and metabolism of lactose, glucose, and galactose in homofermentive lactobacilli. Appl Microbiol. 1986;51:825–831. doi: 10.1128/aem.51.4.825-831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 16.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jewell J B, Kashket E R. Osmotically regulated transport of proline by Lactobacillus acidophilus IFO 3532. Appl Environ Microbiol. 1991;57:2829–2833. doi: 10.1128/aem.57.10.2829-2833.1991. . (Erratum, 57:3683.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juillard V, Spinnzler M E, Desmazeaud M, Boquien C Y. Phénomènes de coopération et d'inhibition entre les bactéries lactiques utilisées en industrie laitière. Lait. 1987;67:149–172. [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lauret R, Morel-Deville F, Berthier F, Champomier-Verges M, Postma P, Ehrlich S D, Zagorec M. Carbohydrate utilization in Lactobacillus sake. Appl Environ Microbiol. 1996;62:1922–1927. doi: 10.1128/aem.62.6.1922-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledesma O V, De Ruiz Holgado A P, Oliver G, De Giori G S, Raibaud P, Galpin J V. A synthetic medium for comparative nutritional studies of lactobacilli. J Appl Bacteriol. 1977;42:123–133. doi: 10.1111/j.1365-2672.1977.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Le Graet Y, Brulé G. Les équilibres minéraux du lait: influence du pH et de la force ionique. Lait. 1993;73:51–60. [Google Scholar]
- 23.Leong-Morgenthaler P, Zwahlen M C, Hottinger H. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J Bacteriol. 1991;173:1951–1957. doi: 10.1128/jb.173.6.1951-1957.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim E-M, Ehrlich S D, Maguin E. Identification of stress-inducible proteins in Lactobacillus delbrueckii subsp. bulgaricus. Electrophoresis. 2000;21:2557–2561. doi: 10.1002/1522-2683(20000701)21:12<2557::AID-ELPS2557>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Meadow N D, Fox D K, Roseman S. The bacterial phosphoenolpyruvate:glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- 26.Mietton B, Desmazeaud M, de Roissart H, Webeer F. Transformation du lait en fromage. In: de Roissart H, Luquet F M, editors. Bactéries Lactiques. Vol. 2. Uriage, France: Lorica; 1994. pp. 55–132. [Google Scholar]
- 27.Molenaar D, Hagting A, Alkema H, Driessen A J, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petry S, Furlan S, Crepeau M-J, Cerning J, Desmazeaud M. Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl Environ Microbiol. 2000;66:3427–3431. doi: 10.1128/aem.66.8.3427-3431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poolman B. Energy transduction in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:125–147. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 30.Poolman B, Knol J, van der Does C, Henderson P J, Liang W J, Leblanc G, Pourcher T, Mus-Veteau I. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol Microbiol. 1996;19:911–922. doi: 10.1046/j.1365-2958.1996.397949.x. [DOI] [PubMed] [Google Scholar]
- 31.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Premi L, Sandine W E, Elliker P R. Lactose-hydrolyzing enzymes of Lactobacillus species. Appl Microbiol. 1972;24:51–57. doi: 10.1128/am.24.1.51-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sechaud L. Caractérisation de 35 bactériophages de Lactobacillus helveticus. Ph.D. thesis. Paris, France: University of Paris; 1990. [Google Scholar]
- 34.Thompson J. Sugar transport. In: Reizer J, Peterkofsky A, editors. Sugar transport and metabolism in gram-positive bacteria. London, United Kingdom: Ellis Horwood Ltd.; 1987. p. 13. [Google Scholar]
- 35.Thompson J, Chassy B M. Intracellular phosphorylation of glucose analogs via the phosphoenolpyruvate:mannose-phosphotransferase system in Streptococcus lactis. J Bacteriol. 1985;162:224–234. doi: 10.1128/jb.162.1.224-234.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson J, Saier M H., Jr Regulation of methyl-β-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981;146:885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 38.Veenhoff L M, Poolman B. Substrate recognition at the cytoplasmic and extracellular binding site of the lactose transport protein of Streptococcus thermophilus. J Biol Chem. 1999;274:33244–33250. doi: 10.1074/jbc.274.47.33244. [DOI] [PubMed] [Google Scholar]
- 39.Veyrat A, Gosalbes M J, Perez-Martinez G. Lactobacillus curvatus has a glucose transport system homologous to the mannose family of phosphoenolpyruvate-dependent phosphotransferase systems. Microbiology. 1996;142:3469–3477. doi: 10.1099/13500872-142-12-3469. [DOI] [PubMed] [Google Scholar]
- 40.Veyrat A, Monedero V, Perez-Martinez G. Glucose transport by the phosphoenolpyruvate:mannose phosphotransferase system in Lactobacillus casei ATCC 393 and its role in carbon catabolite repression. Microbiology. 1994;140:1141–1149. doi: 10.1099/13500872-140-5-1141. [DOI] [PubMed] [Google Scholar]