Abstract
Objective
To evaluate the effect of a tele-nutrition counseling program on diet quality, weight, waist circumference, and quality of life in people with spinal cord injury (SCI).
Design
Prospective observational study.
Participants
Fifteen participants with SCI were enrolled from an acute inpatient rehabilitation unit and outpatient SCI clinic; ten participants completed the intervention.
Interventions
Six tele-nutrition counseling sessions over 3 months, utilizing videoconferencing and a photographic food diary.
Outcome measures
Weight, waist circumference, Life Satisfaction Index A (LSIA), Knowledge and Nutrition Evaluation with Supplement on Eating Behavior, and Program Satisfaction Survey (PSS).
Results
Ten participants completed both baseline and 3-month follow-up evaluations and were used in this analysis. There were no statistically significant changes from baseline to 3-month follow up in weight, waist circumference, Knowledge and Nutrition Evaluation, and LSIA (P > .48). Using the Supplement on Eating Behavior total score to measure overall changes in healthy food choices, 9 out of 10 participants rated their healthy food choices as improving (P = .008). A post-hoc exploratory itemized analysis on the Supplement on Eating Behavior revealed significant improvements from baseline to 3-month follow-up in participant’s self-reported choice of balanced meals (P = .008), reading food labels (P = .031), logging meals (P = .007), and monitoring portions of eating favorite foods (P = .031). Participants endorsed a 97-100% satisfaction rating in relation to perceived health benefits, equipment, and program satisfaction.
Conclusion
This study provides preliminary data suggesting that tele-nutrition is an efficacious intervention that may improve diet quality for individuals with SCI.
Keywords: Nutrition, Spinal cord injury, Tele-nutrition, Weight management
Introduction
In the United States, approximately 291,000 persons are living with a spinal cord injury (SCI).1 A retrospective evidence shows persons with acute traumatic SCI, at the time of injury, have a higher prevalence of cardiovascular diseases (CVD) and greater odds of chronic obesity (4.05 odds ratio), heart disease (2.7 odds ratio), hypertension (2 odds ratio), and diabetes (1.7 odds ratio) compared to individuals with lower extremity fractures.2 These increased odds for poor health outcomes, paired with physical limitations and psychosocial stressors, may adversely affect recovery trajectories, mortality, and quality of life (QoL).2 CVD, which may be mediated through medical nutrition therapy (MNT), has become a leading cause of death (35–46%) beyond the first year post-SCI3; the mortality rate due to CVD is 228% higher than individuals without SCI.4 More broadly there is evidence that persons with SCI who considered themselves overweight have increased prevalence of secondary complications, such as pain, overuse injuries, fatigue, and depressive symptoms, as well as lower QoL compared to individuals who did not consider themselves overweight.5 Particularly for individuals with SCI, providing greater nutrition awareness, education, and treatment is warranted.
In the general population, it is well known that diet quality is inversely related to CVD risks6 and mortality.4 Although there is a paucity of research on nutrition in individuals with SCI, several studies have found that persons with SCI have poor diet quality (i.e. reliance on fast/convenient foods) and poor intake of healthy foods.3,7–11 Groah et al. has provided evidence showing that males with tetraplegia, the most common sub-group of individuals with SCI, tend to have higher than recommended fat, sodium, and alcohol intake.3 It has been observed that from the start of acute rehabilitation to five years after discharge the classification of overweight or obesity (BMI > 22 kg/m2) increased from 56% to 75%.12 Furthermore, in persons with chronic SCI, rates of 23% overweight and 44% obese have been observed.13 Physiologically, persons with SCI who are wheelchair-dependent have reduced energy expenditure, reduced resting metabolic rate, and reduced thermic effect of both food and physical activity.4,14 SCI-specialized MNT may be required to provide appropriate treatment for individuals with SCI across the continuum of care.
Primary prevention of chronic disease via nutrition education by a registered dietitian has been found to be effective in reducing morbidity and mortality, improving QoL, as well as being impactful in the prevention of CVD and other comorbidities.15 Yet, barriers to weight management in individuals with SCI have been identified and include: limited evidence-based guidelines for weight-management, limited wheelchair accessible space to receive group counseling and to engage in physical activity, shortage of staffing with expertise in nutrition and SCI, secondary medical conditions requiring more attention, and a “reactive” as opposed to a proactive weight management approach.16 A qualitative study at the Veterans’ Affairs (VA) found that patients also “desire empathetic and personalized discussions” with their providers about how weight may impact their health and other conditions.16 Behavioral intervention models like the Health Belief Model (HBM), which includes the patient’s current beliefs and proactive goals to change eating behavior, and utilizing evidence-based guidelines, such as the Academy of Nutrition and Dietetics’ SCI-toolbox, may be useful approaches to address patient buy-in and SCI-specific nutrition recommendations.17,18
Tele-health may provide a promising avenue for individuals with SCI to receive nutritional counseling19 as well as address barriers for SCI-specific treatment such as, physical, social, and geographical barriers.20 In a VA setting, 26 participants with SCI enrolled in a multidisciplinary CVD risk reduction program in 2012. The program consisted of “frequent telephone contact by a case manager and in-person visits by a dietitian, physical therapist and exercise physiologist.” Although significant drop-out was observed (10/26 completed the 2-year program), significant improvements were seen in weight, plasma insulin levels, and total cholesterol/HDL ratios21 suggesting that for individuals who engage in the program, there may be significant health outcomes. A previous study in women with obesity without physical disability showed tele-nutrition using e-mail and computer-based chat had a failure or drop-out rate half that of those in the traditional in-person consultation group and showed a small but statistically significant difference in weight loss.22 By reducing barriers to treatment through tele-nutrition, a study such as this may result in increased retention and subsequently improved outcomes.
This pilot study provided a tele-nutrition intervention tailored for individuals with SCI via iPad FaceTime. Although the primary aim of the study was to provide tele-nutrition, the study also collected data on weight and waist circumference, QoL, diet quality, and program satisfaction to explore program outcomes. We hypothesized that individuals with SCI would show no increases in waist circumference and weight yet show improvements in QoL (Life Satisfaction Index-A) pre- to post-intervention. A diet quality assessment, Knowledge and Nutrition Evaluation with Supplement on Eating Behavior, was developed at the study site and was hypothesized to show improvements pre- to post-intervention. Additionally, we hypothesized that individuals would be satisfied in relation to perceived health improvement, with the provided equipment, and with the program itself.
Methods
Participants
Fifteen participants (Table 1) were enrolled from an inpatient rehabilitation program prior to discharge (n = 10) as well as through the outpatient SCI clinic (n = 5) at a community hospital. Ten participants completed the baseline and 3-month follow up assessment and were included in the analysis. This study was approved by the Institutional Review Board at the community hospital in 2018. Enrollment and data collection were conducted from 2018-2019. The inclusion criteria were: (1) age older than 18 years, (2) traumatic or non-traumatic SCI at any neurological level; and (3) living at home or discharging to home for outpatient and inpatient, respectively. The exclusion criteria were: (1) medically unstable; (2) lives in a nursing home or hospitalized in a medical facility; (3) need to follow strict dietary guidelines (i.e. diabetic, heart failure, renal, ketogenic); and (4) inability to speak English. The dietitian who provided tele-nutrition counseling speaks only English and consultations cannot be provided with a translator, therefore, the ability to speak English was part of the recruitment criteria.
Table 1.
Provides a summary of participant demographics.
| Characteristics | Whole Group (n = 15) | Follow-Up Group (n=10) | |||
|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | ||
| Age at Enrollment | 41.7 (29.9–53.3) | 15 | 42.6 (30.2–55.1) | 10 | |
| Days from Injury | 207.0 (157.5–236.0) | 9 | 207.0 (157.5–236.0) | 9 | |
| Length of Stay | 37 (31–43) | 13 | 37 (30–43) | 9 | |
| Sex | |||||
| Male | 13 | 8 | |||
| Female | 2 | 2 | |||
| Ethnicity | |||||
| Caucasian | 6 | 4 | |||
| Hispanic | 4 | 3 | |||
| Asian | 4 | 3 | |||
| Other | 1 | 0 | |||
| Education | |||||
| Less than high school | 1 | 1 | |||
| High School/GED | 7 | 5 | |||
| Trade | 1 | 0 | |||
| Some College | 2 | 1 | |||
| Bachelors | 3 | 3 | |||
| Other | 1 | 0 | |||
| Etiology | |||||
| MVA | 4 | 3 | |||
| Sports | 1 | 1 | |||
| Fall | 3 | 3 | |||
| Other | 7 | 3 | |||
| Injury | |||||
| Cervical | 9 | 6 | |||
| Thoracic | 5 | 3 | |||
| Lumbar | 1 | 1 | |||
| AIS | |||||
| A (Complete) | 8 | 6 | |||
| B, C, D, E (Incomplete) | 6 | 3 | |||
| Unknown | 1 | 1 | |||
Abbreviations: IQR, interquartile range; n, count; GED, General Education Diploma; MVA, motor vehicle accident; AIS, American Spinal Injury Association Impairment Scale.
Tele-nutrition: equipment and the Ate application
Tele-nutrition consultations were conducted through an iPad’s FaceTime videoconferencing application. At enrollment, participants received an iPad, a 4-month Verizon data plan, a three-month photo diary membership used to document food and drink intake (Ate application), an iPad cover stand, a measuring tape, and adaptive accessories (i.e. mouth stick or wheelchair mount), if needed. In addition to equipment, each participant and their caregiver (if available), received basic training on how to use the iPad, FaceTime, and Ate applications.
The Ate application is a mindful and simple way for participants to document their intake for the review of the dietitian over the course of the project. Once a picture is taken, participants can choose whether the intake is on-path or off-path based on participants’ personal goals (described below). Participants have the option to include information about the intake in a notes section. Additionally, one-tap choices within the application further tailor participant’s mindful approach by selecting why the meal was chosen, where the meal was purchased/prepared, how the meal was made, and how the meal made them feel. Participants were asked to capture photos of all intake (meals, drinks, and snacks) consumed for the duration of the study, yet compliance was not assessed.
Tele-nutrition: counseling description
The interactive tele-nutrition counseling was conducted by a dietitian following the Nutrition Guidelines for Individuals with SCI set forth by the Academy of Nutrition and Dietetics’ Evidence-Based Analysis Toolkit.17 This SCI-specific toolkit aims to assist dietitians with providing evidence-based MNT for those with SCI and provides a summary of outcomes, assessment factors, expected outcomes, and ideal values for MNT. Scientific evidence is provided with a recommended strength of evidence: Strong, Fair, Weak, Consensus, and Insufficient Evidence. HBM served as the framework behind each counseling sessions to address “buy-in” when making changes to nutritional habits. Participants’ belief that incorporating healthier eating habits as well as the desire to prevent and/or reverse negative health consequences (e.g. perceived seriousness, susceptibility, threat, benefits, barriers, cues to action) resulting from a poor diet following SCI (e.g. weight gain) were a main portion of each tele-nutrition session.
At enrollment participants identified an overarching goal for the project (e.g. weight loss, weight maintenance, or desire to improve overall healthful intake). Between enrollment and their first tele-nutrition session (approximately 2–3 weeks), participants began using the Ate app to take pictures of all intake. Before each session, the dietitian would review the participants’ Ate app diary to review diet quality (e.g. amount of whole grains, vegetables, protein, food color variety) and provide specific feedback. The first sessions began with a discussion of each participant’s enrollment goal, current appetite, current medications, and any bowel or skin issues. Bowel and skin issues informed the dietitian to suggest changes in protein, fiber, and/or water intake. The dietitian assessed food availability, caregiver responsibility (if applicable), and psychosocial or economic issues. Based on this conversation, the dietitian would assess the participant and caregivers’ knowledge level, motivation, and readiness to modify diet behaviors. Furthermore, using the HBM framework, the dietitian assessed the participants’ level of confidence in their own ability to be successful at making positive dietary changes. The dietitian discussed and addressed modifying variables (e.g. perceived threat, benefits, barriers, self-efficacy), considering the participants’ needs and lifestyle, to collaboratively create a nutrition care plan with the participant that reduces potential barriers to behavior change and increase self-efficacy for making beneficial dietary lifestyle changes. Then the dietitian reviewed intake photographs from the ATE app with the participant as well as initial survey results from the Knowledge and Nutrition Evaluation with Supplement on Eating Behavior, noting any areas of improvement to help formulate the participants’ first SMART (Specific, Measurable, Assignable, Relevant, Time-specific) goal (e.g. include reduction of fast food to once per week, inclusion of 4 cups of leafy green vegetables into 1–2 meals each day, or inclusion of ½–1 cup of oatmeal with breakfast at least 3 times per week). The participant and dietitian would agree on 1–2 specific goals for the next two weeks.
Subsequent sessions with participants addressed appetite, bowel, and/or skin issues, and included a review of the ATE intake photographs to discuss improvements and progress with the participants’ SMART goal. Participants were then counseled on improvements to make for the next session (e.g. reducing sodium through comparing food labels, incorporating more leafy greens or whole grains to specific meals, or to reduce sugar-sweetened beverages). When appropriate the Academy of Nutrition and Dietetics’ Evidence-Based Analysis Toolkit was referenced. Positive encouragement was provided by the dietitian throughout and solutions for barriers to achieving goals was discussed using the HBM framework. If a participant achieved their SMART goal(s) and felt comfortable creating a new goal, a new goal was added. Otherwise, the participant would continue to work on existing goal(s) for the next session.
Tele-nutrition sessions via iPad’s FaceTime application were conducted twice a month for a total of 6 sessions per participant lasting approximately 30–60 min. If participants were not able to connect to the dietitian through FaceTime, a telephone counseling session was completed. Since all the participants had caregivers, these caregivers were involved in sessions and nutrition education.
Tele-nutrition: outcome measures
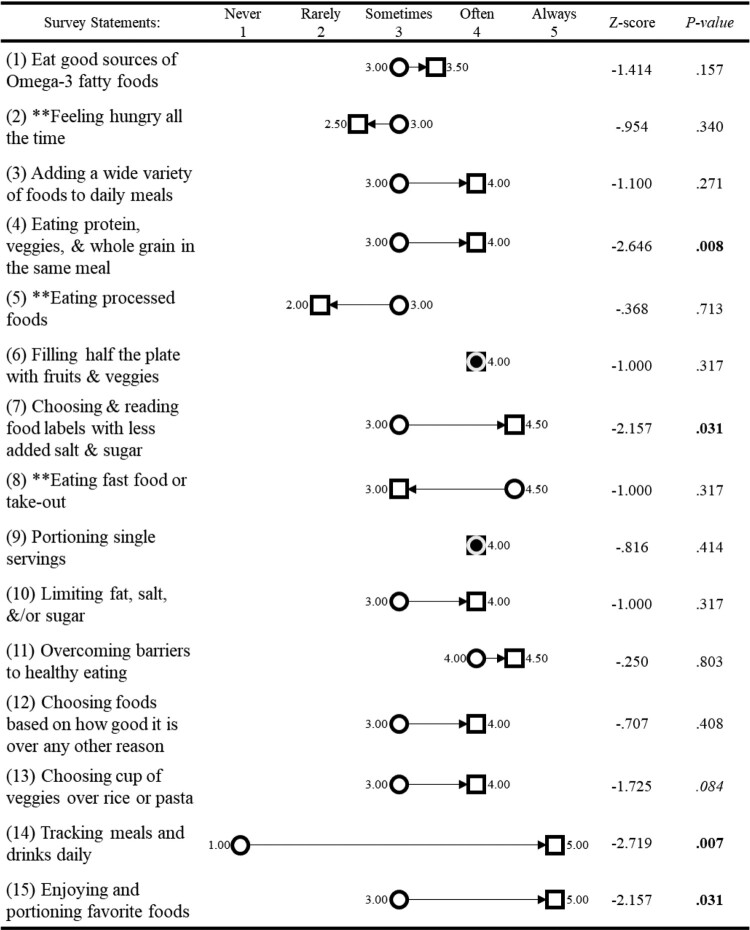
A research assistant collected demographic characteristics from an intake survey, Life Satisfaction Index A (LSIA), and Knowledge and Nutrition Evaluation with Supplement on Eating Behaviors (Supplemental Materials) were obtained and completed at enrollment and at the 3-month follow-up. Initial weight was collected via bed-scale at the end of admission or roll-on scale in the outpatient rehabilitation clinic (with wheelchair weight deducted) and follow-up weight was collected via roll-on scale in the clinic. Waist circumference was taken at enrollment and at the 3-month follow-up and was taken with participant in the sitting position. The Knowledge and Nutrition Evaluation with Supplement on Eating Behavior was created by two dietitians at the study site and is comprised of two parts. The first section, Knowledge and Nutrition Evaluation, includes 15 statements regarding healthy eating patterns that need to be correctly identified as “true”, “false”, or “don’t know”. The second section, Supplement on Eating Behavior, includes 15 questions with a 5-point scale (ranging from “never” to “always”) that assess the frequency of healthy eating behaviors (Figure 1). The Supplement on Eating Behavior questions are based around the intake patterns for the 2015–2020 U.S. Dietary Guidelines.23 At the 3-month follow-up, each participant completed a Program Satisfaction Survey (PSS) in addition to the follow-up surveys.
Figure 1.
Median scores for healthy food choices from the Supplement on Eating Behavior. Healthy food choices improved in 13 out of 15 statements, in 2 items (Statement 6 and 9) scores remained the same. Asterisked statements represent items that should decrease with healthy behaviors. Circles represent median scores at baseline and squares represent median scores at 3-month follow-up. A circle inscribed in a square indicates no change in median scores between time points. Italicized P-values indicates P < .1, and bolded P-values emphasize P < .05.
For participants unable to return to the clinic at follow up, questionnaires were administered over the phone and participants were asked to measure waist circumference by themselves or with assistance from a caregiver, if needed. The dietitian instructed participants and caregivers via FaceTime on how to take waist circumference measurements, while weight was obtained in a different clinic and reported. Two individuals used caregiver assistance for their measurements, while the remaining 8 individuals were measured at the study site outpatient rehabilitation clinic.
Data analysis
Non-parametric statistics (Wilcoxon Sign Ranked Tests) were used to investigate significant (P < .05) changes pre- to post-intervention. Life Satisfaction Index A was used to measure QoL changes, waist circumference (inches), and weight changes (lbs) were assessed as physical measures, and the Knowledge and Nutrition Evaluation with Supplement on Eating Behavior was used to measure changes in nutrition knowledge and eating behavior changes. The participant PSS was summarized to evaluate the overall satisfaction of the program.
Results
Fifteen persons with traumatic or non-traumatic SCI were enrolled and 10 completed the study (Table 1). Five participants dropped out of the study due to unresponsiveness (n = 2), unable to meet study obligations (n = 2), and lost iPad (n = 1). Study aims were assessed on the subset that completed the program (n = 10). Two individuals did not return their PSS and therefore only eight individuals were included in the evaluation of the overall satisfaction with the program. For each outcome variable only the 10 participants that completed both assessments are reported.
Waist circumference and weight
Ten participants completed both the baseline and follow-up assessments, there was no significant change in weight (Wilcoxon Signed Rank Test: Z = −.178, P = .859) or waist circumference (Wilcoxon Signed Rank Test: Z = −.059, P = .953; Table 2).
Table 2.
summarizes the outcome variables for the all individuals enrolled (baseline whole group) as well as outcome measures and test statistics for the group that completed the baseline and 3-month follow-up assessments.
| Survey/Variable | Baseline Whole Group (n = 15) Median (IQR) |
Baseline Follow-Up Group (n = 10) Median (IQR) |
3 Month Follow-Up Follow-Up Group (n = 10) Median (IQR) |
Z-score | P |
|---|---|---|---|---|---|
| Weight (lbs) | 209 (137–236) | 202 (135–217) | 191 (145–220) | −0.178 | 0.859 |
| Waist (in.) | 44.0 (38.0–47.5) | 40.3 (33.5–46.4) | 39.5 (36.6–49.3) | −0.059 | 0.953 |
| LSIA | 28.0 (22.0–34.0) | 31.0 (22.0–34.3) | 29.0 (20.5–36.5) | −0.119 | 0.906 |
| Knowledge and Nutrition Evaluation | 13.0 (12.0–13.0) | 13.0 (11.5–13.3) | 12.5 (11.5–14.0) | −0.707 | 0.48 |
| Supplement on Eating Behavior | 48.0 (42.0–52.0) | 50.0 (46.5–52.3) | 57.0 (53.0–59.0) | −2.668 | 0.008 |
Abbreviations: IQR, interquartile range; lbs, pounds; in, inches; LSIA, Life Satisfaction Index A.
Life satisfaction index A
Ten participants completed both the baseline and follow-up assessments, there was no significant change in LSIA total score (Wilcoxon Signed Rank Test: Z = −.119, P = .906; Table 2).
Knowledge and nutrition evaluation
For the ten participants that completed both assessments, there was no significant change in Knowledge and Nutrition Evaluation total score (Wilcoxon Signed Rank Test: Z = −.707, P = .480; Table 2). Median baseline scores (13.0 IQR:11.5–13.3) reflect high score at baseline.
Supplement on eating behavior
Using the Supplement on Eating Behavior total score to measure overall changes in healthy food choices, 9 out of 10 participants rated their healthy food choices as improving (Wilcoxon Signed Rank Test: Z = −2.67, P = .008; Figure 1); one individual did not change their rating. Doing a post-hoc exploratory itemized analysis, individuals significantly improved in their choice of balanced meals (Wilcoxon Signed Rank Test: Z = −2.65, P = .008), reading food labels (Wilcoxon Signed Rank Test: Z = −2.16, P = .031), logging meals (Wilcoxon Signed Rank Test: Z = −2.72, P = .007), and monitoring portions of eating favorite foods (Wilcoxon Signed Rank Test: Z = −2.16, P = .031). Although not significant, including a cup of vegetables over rice or pasta (Wilcoxon Signed Rank Test: Z = −1.73, P=.084), did marginally improve.
Program satisfaction survey
Overall, the participants that completed the Program Satisfaction Survey (PSS; n = 8) endorsed that they were satisfied with the program across three different domains (i.e. Perceived Health Benefits, Equipment Satisfaction, and Program Satisfaction; Table 3). Participants ranked the category of perceived health benefits as 100% agree, satisfied with the equipment as 97% agree, and satisfied with the program as 97% agree.
Table 3.
represents the percent of individuals who endorsed different levels on each question of the Program Satisfaction Survey.
| Satisfaction Statements | Agree | Neither Agree or Disagree | Disagree | N/A | |
|---|---|---|---|---|---|
| Perceived | A) Motivated to monitor health | 100.00% | 0.00% | 0.00% | 0.00% |
| Health | B) Health improved due to TM | 100.00% | 0.00% | 0.00% | 0.00% |
| Equipment | C) Sufficient training to use the iPad | 100.00% | 0.00% | 0.00% | 0.00% |
| Satisfaction | D) Ease of use of iPad | 100.00% | 0.00% | 0.00% | 0.00% |
| E) Quality of video and audio | 100.00% | 0.00% | 0.00% | 0.00% | |
| F) The iPad took too much time to use* | 0.00% | 12.50% | 87.50% | 0.00% | |
| G) Privacy was a concern* | 0.00% | 0.00% | 100.00% | 0.00% | |
| H) Satisfied with the use of the iPad | 100.00% | 0.00% | 0.00% | 0.00% | |
| I) Received sufficient adaptive equipment | 50.00% | 12.50% | 0.00% | 37.50% | |
| Program Satisfaction | J) TM was just as good as seeing an RD in person | 100.00% | 0.00% | 0.00% | 0.00% |
| K) Recommend TM | 100.00% | 0.00% | 0.00% | 0.00% | |
| L) Continue TM | 87.50% | 12.50% | 0.00% | 0.00% | |
| M) Staff Responded sufficiently | 100.00% | 0.00% | 0.00% | 0.00% | |
*Represent questions that were presented in a negative tone.
Discussion
The incidence of poor diet quality and cardiovascular disease in persons with SCI is known to be high,3,4,11 yet there are few weight management interventions tailored for persons with SCI. Interventions to reduce weight, which are needed to show effectiveness in reducing modifiable cardiovascular risk,14 are lacking. The intervention described in this study provides a disseminatable individualized model on how tele-nutrition counseling may be administered for a variety of patient groups, including individuals with SCI. Although there was a high drop-out rate in the study (10/15 completed), the subset that completed the study expressed high satisfaction with perceived health, equipment, and program satisfaction. Additionally, dietary changes were observed, which may be a precursor for weight, waist circumferences, and other health changes; weight, waist circumference, and QoL changes did not show significant changes. This study provided preliminary data suggesting that tele-nutrition may be an efficacious intervention to improve diet quality for individuals with SCI.
Real-time feedback, encouragement, and counseling that can be provided by tele-nutrition appear to be important factors for a successful nutrition intervention.24 The Health Belief Model provides a framework to address barriers to treatment adoption while honoring a patient’s individual beliefs. Discussing individual barriers and beliefs was a significant component in the creation of the participants’ inter-session SMART goals, which provided a digestible roadmap to meet their self-identified overarching enrollment goal (e.g. losing weight). Additionally, utilizing two-feedback approaches, photo-journaling application and tele-nutrition counseling, provided an immediate self-assessment on whether the food was on/off track as well as expert feedback from a dietitian with SCI-specific experience. The photo journaling app also provided the dietician and participant with a diary versus relying on an individual’s memory. The use of the Academy of Nutrition and Dietetics’ Evidence-Based Analysis Toolkit provided evidence-based MNT suggestions paired with the photo journal’s intake log augmented resources for SCI-specific and individualized care. These components provide a model that may be modifiable for other patient populations.
Tele-nutrition, along with other forms of telemedicine, delivers multiple advantages for post-rehabilitation care to persons with SCI. Potential benefits include reduced travel time, equitable access to health services, reduced caregiver burden, independence and privacy, reduced stigma, and improved access to SCI specialists.25 Participants that completed the satisfaction survey (n = 8) endorsed a 97–100% satisfaction rating in perceived health benefits, satisfaction with equipment, and program satisfaction (Table 3). Similar results were observed in prior telemedicine studies using iPad FaceTime for individuals with SCI, highlighting that telemedicine can be used for a variety of interventions and have a high patient satisfaction.26,27 From the perspective of an individual with SCI, the potential for reduced stress and inconvenience of travel away from home to get to a specialist appointment makes telemedicine an extremely favorable alternative to the current model of post-acute rehabilitative care delivery.
In the acute and sub-acute rehabilitation setting, priority is given to functional improvement and emotional adjustment, and it may be difficult for patients and their families to retain and apply concepts taught in a nutrition class while hospitalized and undergoing an active rehabilitation program. A tele-nutrition intervention post-discharge, like the one in this study, may provide individuals with SCI counseling in a setting that is convenient and during a time period when they may be most receptive to retain the information. Albeit a small sample and not correct for multiple comparisons, our exploratory analysis revealed improvements in eating behavior across a number of domains (Figure 1). Future studies with a larger sample, a control group, and post-intervention follow-up would be needed to assess the effectiveness of tele-nutrition on eating behaviors.
For this study, each participant had different goals, challenges, and approaches, which made each individual’s interventions tailored to meet participant-specific goals. Ethnic background, caregiver buy-in and influence, prior nutrition knowledge (or lack thereof) and beliefs, motivation, and desire to learn were variables that impacted our findings. Similarly, due to physiological status at and after the time of injury (e.g. abdominal muscle tone loss, neurogenic bowel, and reduction in metabolic needs), it was not surprising that improvements in waist circumference and weight were not observed. Future studies may benefit from selecting other outcome measures, such as a fasting lipid panel and blood metabolic chemistry, which may provide more sensitive measures. Similarly, QoL may be too broad a concept and selecting specific domains related to an individual with SCI’s QoL, such as questionnaires on bladder or bowel function, may be more appropriate. Additionally, as with many follow-up studies, this study was impacted by a one-third drop-out in participation. More research is recommended with larger sample sizes to understand the generalizability of providing this service.
Conclusion
The program successfully provided tele-nutrition counseling services for individuals with SCI. This program employed an innovative method (iPad FaceTime) to provide tele-nutrition counseling conveniently and privately in the participants’ home, and this tele-nutrition method may be easily disseminated to and implemented in other SCI centers. Although the main outcome measures (waist circumference, weight, and life satisfaction) did not show treatment effects, significant improvements in eating behavior were observed. Additionally, participants were satisfied with the intervention in relation to perceived health benefits, equipment, and the tele-nutrition program itself. Furthermore, this program may provide valuable information on the sample size needed to assess tele-nutrition programs in the future, while providing initial evidence to help inform future guidelines on tele-nutrition for individuals with SCI and acceptance of tele-nutrition in the SCI community.
Supplementary Material
Acknowledgments
This work was partially funded through a 2018 Christopher and Dana Reeve Foundation Quality of Life Grant. Thank you to the VMC foundation for your grant management support and Sarah Lavoie for her assistance in creating the Knowledge and Nutrition Evaluation with Supplement on Eating Behavior.
Disclaimer statements
Contributors None.
Funding This work was supported by Christopher & Dana Reeve Foundation (US).
Conflicts of interest There are no competing financial interests to disclose in relation to the work described.
References
- 1.National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance [Internet]. University of Alabama at Birmingham; 2018 [cited 2018 May 31]. Available from: https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202018.pdf
- 2.Selassie A, Snipe L, Focht K, Welldaregay W.. Baseline prevalence of heart diseases, hypertension, diabetes, and obesity in persons with acute traumatic spinal cord injury: potential threats in the recovery trajectory. Top Spinal Cord Inj Rehabil. 2013 Jul;19(3):172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med. 2009 Jan;32(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorgey A, Gater D.. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007 Apr;12(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetz SP, Latimer AE, Arbour-Nicitopoulos KP, Martin Ginis KA, SHAPE-SCI Research Group . Secondary complications and subjective well-being in individuals with chronic spinal cord injury: associations with self-reported adiposity. Spinal Cord 2011 Feb;49(2):266–72. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JA, McClelland JW, Goff DC, Racine E, Dulin MF, Bauman WA, et al. Nutrition education for cardiovascular disease prevention in individuals with spinal cord injuries: study protocol for a randomized controlled trial. Trials [Internet]. 2017 Dec [cited 2018 May 30];18(1). Available from: https://trialsjournal.biomedcentral.com/articles/ 10.1186/s13063-017-2263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Henson S, Jackson AB, Richards JS.. Obesity intervention in persons with spinal cord injury. Spinal Cord 2006 Feb;44(2):82–91. [DOI] [PubMed] [Google Scholar]
- 8.Crane DA, Little JW, Burns SP.. Weight gain following spinal cord injury: a pilot study. J Spinal Cord Med. 2011 Mar;34(2):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ.. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Spinal Cord 1992;30(12):880. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli SM, LaVela SL.. Documentation of weight management practices for individuals with spinal cord injuries and disorders. Spinal Cord 2016 Dec;54(12):1176–82. [DOI] [PubMed] [Google Scholar]
- 11.Pellicane A, Millis S, Zimmerman S, Roth E.. Calorie and protein intake in acute rehabilitation Inpatients with traumatic spinal cord injury versus other Diagnoses. Top Spinal Cord Inj Rehabil. 2013 Jul;19(3):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot S, Post M, Postma K, Sluis T, van der Woude L.. Prospective analysis of body mass index during and up to 5 years after discharge from inpatient spinal cord injury rehabilitation. J Rehabil Med. 2010;42(10):922–8. [DOI] [PubMed] [Google Scholar]
- 13.Hatchett PE, Mulroy SJ, Eberly VJ, Haubert LL, Requejo PS.. Body mass index changes over 3 years and effect of obesity on community mobility for persons with chronic spinal cord injury. J Spinal Cord Med. 2016 Jul 3;39(4):421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalil RE, Gorgey AS, Janisko M, Dolbow DR, Moore JR, Gater DR.. The role of nutrition in health status after spinal cord injury. Aging Dis 2013;4(1):9. [PMC free article] [PubMed] [Google Scholar]
- 15.Slawson DL, Fitzgerald N, Morgan KT.. Position of the academy of nutrition and dietetics: the role of nutrition in health promotion and chronic disease prevention. J Acad Nutr Diet. 2013 Jul;113(7):972–9. [DOI] [PubMed] [Google Scholar]
- 16.Locatelli S, Gerber B, Goldstein B, Weaver F, LaVela S.. Health care provider practices, barriers, and facilitators for weight management for individuals with spinal cord injuries and disorders. Top Spinal Cord Inj Rehabil. 2014 Oct;20(4):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Academy of Nutrition and Dietetics Evidence Analysis Library . Spinal Cord Injury Toolkit [Internet]. Academy of Nutrition and Dietetics Evidence Analysis Library. Spinal Cord Injury Toolkit. 2014 [cited 2018 May 23]. Available from: http://www.adaevidencelibrary.com/evidence.cfm?evidence_summary_id=250466.
- 18.Jones CJ, Smith H, Llewellyn C.. Evaluating the effectiveness of health belief model interventions in improving adherence: a systematic review. Health Psychol Rev 2014 Jul 3;8(3):253–69. [DOI] [PubMed] [Google Scholar]
- 19.Rimmer JH, Wang E, Pellegrini CA, Lullo C, Gerber BS.. Telehealth weight management intervention for adults with physical disabilities: a randomized controlled trial. Am J Phys Med Rehabil. 2013 Dec;92(12):1084–94. [DOI] [PubMed] [Google Scholar]
- 20.Galea MD. Telemedicine in rehabilitation. Phys Med Rehabil Clin N Am. 2019 May;30(2):473–83. [DOI] [PubMed] [Google Scholar]
- 21.Myers J, Gopalan R, Shahoumian T, Kiratli J.. Effects of customized risk reduction program on cardiovascular risk in males with spinal cord injury. J Rehabil Res Dev. 2012;49(9):1355. [DOI] [PubMed] [Google Scholar]
- 22.Kuzmar IE, Cortés-Castell E, Rizo M.. Effectiveness of telenutrition in a women’s weight loss program. PeerJ. 2015 Feb 3;3:e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2015-2020 Dietary Guidelines health.gov [Internet]. [cited 2020 Mar 19]. Available from: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/
- 24.Allen JK, Stephens J, Patel A.. Technology-assisted weight management interventions: systematic review of clinical trials. Telemed E-Health 2014 Dec;20(12):1103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo C, Guihan M, Frick C, Gill CM, Ho CH.. What’s happening now! Telehealth management of spinal cord injury/disorders. J Spinal Cord Med. 2011;34(3):322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sechrist S, Lavoie S, Khong C-M, Dirlikov B, Shem K.. Telemedicine using an iPad in the spinal cord injury population: a utility and patient satisfaction study. Spinal Cord Ser Cases 2018 Aug 8;4(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shem K, Sechrist SJ, Loomis E, Isaac L.. SCipad: effective Implementation of telemedicine using iPads with individuals with spinal cord injuries, a case series. Front Med [Internet] 2017 May 29;4:58, Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5447014/. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.