Abstract
Context: Detrusor underactivity (DUA) in women can result in urinary retention and the need for chronic bladder drainage management. Without a cure for urinary retention due to DUA, treatment options are focused on effective bladder drainage most often by intermittent or continuous catheter drainage. The inFlow intraurethral valve pump was FDA approved for use in women with this condition in 2014.
Methods: Using a literature search, this clinical review sought to explore the epidemiology and commonly used treatment options for women with DUA and critically examine all available studies of the inFlow urinary prosthesis.
Results: Due to a lack of effective treatments to improve detrusor function, DUA is generally considered incurable and there are limited treatment options which mostly focus on effective bladder drainage. The inFlow urinary prosthesis is a unique technology which utilizes a nonsurgically inserted urethral device for females to assist with bladder drainage due to DUA. The inFlow urinary prosthesis has been used in Europe and elsewhere for 20 years and is the subject of seven peer-reviewed clinical studies. For those that tolerate the device (about half), the inFlow urinary prosthesis has a low infection rate and side effect profile, is easy to use, and can normalize urination by returning autonomy to patients and thus improve their quality of life.
Conclusion: There is no cure for women with DUA. Bladder drainage can be managed by intermittent or continuous catheterization. Appropriately selected women interested in an alternative to catheterization may be offered an on-device trial of the inFlow urinary prosthesis.
Keywords: Detrusor underactivity, Impaired detrusor contractility, Urinary retention, inFlow urinary prosthesis, Female voiding dysfunction, Neurogenic bladder
Introduction
Detrusor underactivity (DUA), as defined by the International Continence Society (ICS), is a diagnosis based on urodynamic investigations, generally with relevant symptoms and signs, manifested by low detrusor pressure or short detrusor contraction in combination with a low urine flow rate resulting in prolonged bladder emptying and/or failure to achieve complete bladder emptying within a normal time span.1 Some women with detrusor underactivity (DUA) may have permanent chronic urinary retention. This debilitating condition is most often a consequence of neurogenic dysfunction, affecting the afferent pathways, efferent pathways, or brain circuits involved in the micturition reflex, or myogenic dysfunction, affecting the cellular function of detrusor myocytes or the surrounding extracellular matrix.2,3 DUA is generally incurable with clinical management focused on adequate bladder drainage to prevent sequelae of urinary retention. When women with DUA are unable to void at all or unable to empty well enough to prevent urinary tract infections (UTIs), overflow incontinence, bladder stones, or damage to upper urinary tracts, they must employ one of the few clinical options available for bladder drainage. The most common options are urinary catheters, either indwelling urethral or suprapubic catheter, or clean intermittent catheterization (CIC), however, this can lead to a significant change in quality of life, toileting behaviors, and may not be feasible for some to perform independently.
Urinary catheters may be the most commonly used medical device, however, they are associated with UTIs, low quality of life, and encrustation, especially with chronic use.4 In women with DUA, who must use urinary catheters daily for the rest of their lives, these problems can be amplified and become frequent. Additionally, CIC, the gold standard for bladder management in this population, provides particular challenges for certain groups of patients, particularly the elderly, visually impaired, mentally handicapped, and those with limited manual dexterity.
The inFlow™ Intraurethral Valve-Pump and Activator (collectively, the inFlow urinary prosthesis) has been designed to assist women with impaired detrusor contractility (IDC) seeking an alternative to CIC or indwelling urinary catheter. While “DUA” is the most up-to-date preferred term for this condition according to the ICS,5 “IDC” was used throughout testing of the inFlow urinary prosthesis and many of the studies discussed in this review, and thus it is used interchangeably throughout.6 The inFlow urinary prosthesis does require manual dexterity of the user to be able to place the remote device over the pelvis and activate the remote, but the required coordination for this maneuver is less than what it required for self-intermittent catheterization. The inFlow urinary prosthesis has already been in use in Europe for more than twenty years and was approved by the U.S. Food and Drug Administration (FDA) for use in the United States in 2014. The objective of this review article is to examine peer-reviewed literature available for the applicability, use, and safety of the inFlow urinary prosthesis.
Epidemiology
DUA is a serious medical problem that, without proper bladder drainage, can result in urinary retention, overflow incontinence, recurrent UTIs, bladder stones, hydronephrosis, and impaired renal function. DUA has been reported in up to 48% of men and 45% of women who received urodynamic assessment for lower urinary tract symptoms, with prevalence increasing with age.7 A population based, cross-sectional survey performed in Detroit found nearly a quarter of survey respondents reported difficulty emptying their bladder.8 A 2017 study examining the prevalence of urinary retention and bladder catheterization in female Medicare beneficiaries found the prevalence of retention to be 1532 per 100,000 and one in six of these subjects had undergone some form of bladder catheterization.9
While the prevalence of DUA due to neurologic etiology among women in the United States is not precisely known,10 it can be estimated based on the prevalence of neurologic conditions in the US, including multiple sclerosis, diabetes, spina bifida, Parkinson’s disease, multiple systems atrophy, and spinal cord injury. DUA is estimated to affect more than 468,000 women based on calculations of the prevalence of these conditions.3
Despite the high prevalence of DUA in women, it has not been studied extensively in this population. One main problem with investigating DUA is that DUA is a diagnosis based on urodynamic criteria. It is impractical to perform widespread invasive tests at a community level leading to significant limitations in our ability to understand the incidence, prevalence, underlying risk factors, and natural history of DUA.11 Furthermore, symptoms of DUA can oftentimes be indistinguishable from symptoms caused by other lower urinary tract dysfunctions, such as bladder outlet obstruction (BOO). For example, a low urinary flow rate or elevated post-void residual (PVR) volume may be due to either BOO or DUA. In women, urinary retention and high PVR are more likely due to DUA due to the very low incidence of BOO in women (2.7–8%).11,12
Traditional treatment options
For women with urinary retention due to DUA, there are currently no surgical or pharmaceutical remedies to restore detrusor contractility. These patients are most commonly directed towards bladder management with either indwelling urinary catheter per urethra, suprapubic tube, or CIC. Intermittent catheterization has become a standard treatment for persons with spinal cord injuries and other forms of chronic urinary retention.13,14 This procedure can be performed by patients or their caregivers using sterile or clean catheters to provide intermittent routine bladder emptying every three to six hours. While a thorough review of the limitations of CIC is beyond the scope of this paper, it is important to note that CIC is not without complications, including urethral strictures, false passages, hematuria, bacteriuria, and labial erosion.15 It also requires the patient to have adequate manual dexterity and visual and cognitive ability or have a reliable caretaker to perform catheterization 4–8 times per day.
The FDA approved the use of sacral neuromodulation (SNM) for the treatment nonobstructive urinary retention in 1999. In patients with nonobstructive urinary retention, SNM can be effective in increasing voiding volumes and reducing PVRs,16 however the mechanism of action remains unknown and response rates for this indication can vary greatly ranging from 33% to 90%.17 Therefore, it is important to proceed with SNM trial prior to full implantation. Additionally, not all patients with nonobstructive urinary retention are appropriate candidates for SNM depending on a variety of other comorbidities, their fitness for surgery, and other clinical needs, including the need for MRI imaging in the future.
Intraurethral valve-pump
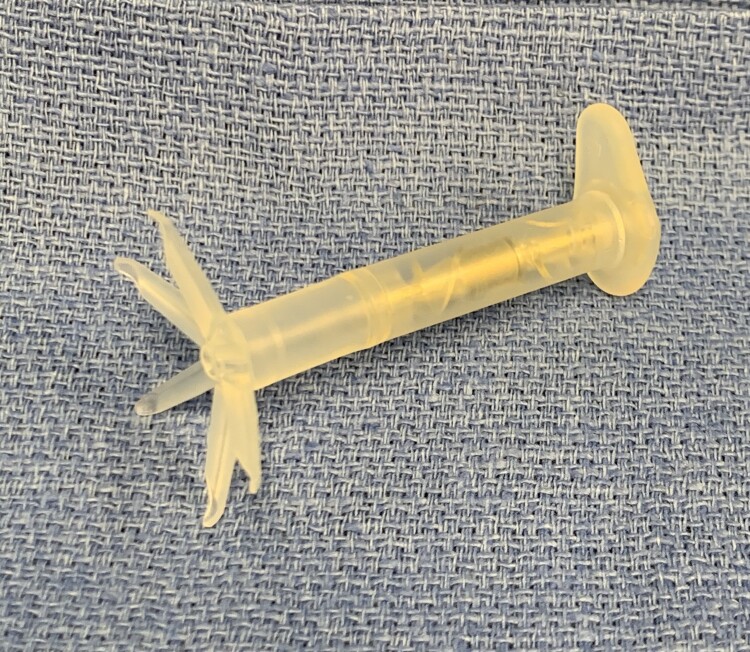
The inFlow intraurethral valve pump is a non-surgical urinary prosthesis that is comprised of a short self-retaining silicone catheter containing an internal valve and pump mechanism which uses a miniature magnetically-coupled pump activated by a hand-held remote control (see Fig. 1).6 It is inserted into the female urethra and remains in place for 29 days when it can then be removed and replaced with a new device. The inFlow urinary prosthesis works to empty the bladder when the patient holds the remote control over her pelvis and activates it by pushing the button. The remote then activates the miniature magnetically-coupled pump within the urinary prosthesis and urine is actively pumped from the bladder to mimic normal urination (see Fig. 2). When the button is released at the conclusion of voiding, a valve is engaged within the prosthesis that blocks further flow of urine.
Figure 1.
The inFlow urinary prosthesis is comprised of a short self-retaining silicone catheter containing an internal valve and pump mechanism which is magnetically activated by remote control. The intravesical portion has flexible fins that open like flower petals and hold the device in a fixed position at the bladder neck.
Figure 2.
To activate the inFlow urinary prosthesis, the patient holds the magnetically-coupled remote control remote near her pelvis in place while she sits on a toilet. This activates the device to pump urine out of the bladder.
The silicone catheter is 24Fr diameter and is available in lengths ranging from 3 to 7 cm. The pump speed operates at 10,000 RPM (revolutions per minute) with a flow rate of 14 mL per second. Prior to initial device insertion, the inFlow sizing device, a sterile single-use device, is used to determine the appropriate device length. As with any indwelling urinary catheter, the inFlow urinary prosthesis should be removed and replaced monthly (specifically every 29 days), which can be done by pulling on the external tab of the device to externalize it from the urethra. A new device can be replaced immediately.
Patients that have an active UTI should not have the prosthesis inserted, however, the inFlow urinary prosthesis can be used once the infection has been appropriately treated. Additionally, patients that are allergic to or otherwise cannot take any oral antibiotics should not undergo insertion of the inFlow urinary prosthesis. There are also several warnings when using the inFlow urinary prosthesis. Patient should ensure they have an extra inFlow urinary prosthesis device and an alternative method of bladder drainage available at all times in case the prosthesis is expelled, removed, or not working properly. Patients using the device should also be instructed to void every three to four hours when awake, even if they do not have a sensation of fullness and the desire to void. The device should be removed if a patient needs to undergo an MRI or radiation procedures. There are also several conditions in which the inFlow urinary prosthesis has not been evaluated for safety and effectiveness. These include pregnancy, small bladder capacity (<200 cc), history of impaired kidney function, recurrent pyelonephritis, moderate to severe hydronephrosis, vesicoureteral reflux grade II or higher, detrusor overactivity not controlled with medication, recent history of urolithiasis, neoplastic or inflammatory processes involving the lower urinary tract, uterus, cervix, or vagina, urinary tract fistula, bladder diverticula, compromised immune system, concurrent use of other medical devices with electronic or magnetic components (e.g. pacemakers), and significant pelvic organ prolapse (grade III/IV) requiring surgical treatment.
Available evidence
The FDA initially classified the inFlow urinary prosthesis as a Class III device and required that a pivotal trial be conducted in support of a PMA (premarket approval) application, the process used to prove that a new device is safe and effective for the end user. Following the FDA’s analysis of all evidence presented in the PMA application, the inFlow urinary prosthesis was approved for use via the De Novo pathway in October 2014 and, in the process, was down-classified from Class III to Class II. While the inFlow urinary prosthesis received a CE mark, the approval process within the European Economic Area, in 1997 as a Class IIb device, the delay in receiving FDA approval in the United States was due to the need for the de novo review process. Because it is a unique device, both the FDA and the Global Medical Device Nomenclature (GMSN) created new product classifications for the inFlow urinary prosthesis. The lack of predicates contributed to its length review process and there was no clear path to approval until the FDA implemented use of its de novo review process.
A literature review was performed using the PubMed database. Results were screened for original literature investigating the inFlow urinary prosthesis device. There are no currently available alternative devices that are similar to also investigate in this review. There have been a total of seven clinical studies published in major peer-reviewed journals concerning the inFlow urinary prosthesis. One study is the pivotal trial (n = 157) and six others are independent investigator-sponsored studies (n = 228). All of these studies will be discussed in detail.
Pivotal trial
The pivotal trial published in 2005 compared the “safety, effectiveness, and patient satisfaction of the inFlow Urinary Prosthesis versus CIC in females with a hypocontractile or acontractile bladder”.18 This was an 18-site, single-arm, crossover study limited to females with a urodynamically confirmed diagnosis of atonic bladder (the extreme end of the spectrum of DUA) who were already successfully using CIC for bladder drainage. The crossover design of the pivotal trial had the advantage of allowing subjects to be their own control by incorporating two study phases with objective measurement of the primary and secondary endpoints during both phases.18
In the original study protocol, eligible subjects went through an 8-week baseline phase using CIC (their usual method of bladder drainage), 16-week inFlow urinary prosthesis treatment phase, and then a final 4-week treatment withdrawal phase with resumption of CIC.18 The protocol was later revised in order to better identify patients who would complete the study by including a 1-week inFlow tolerability trial in which patients who successfully demonstrated tolerance to the device then continued through the rest of the protocol. The primary study endpoint was evaluating PVR to indicate how effectively the inFlow urinary prosthesis performs its primary function, draining the bladder. PVRs were considered comparable for a subject if their median inFlow (treatment) PVR was no greater than their median CIC (baseline) PVR. Alternatively, PVRs were also considered comparable if a patient’s CIC PVR was >50cc and their inFlow PVR was less than or equal to their CIC PVR. The secondary endpoint was quality of life (QOL) as measured by the Wagner I-QOL, a validated 100-point scale instrument.19 The rate of urinary tract infections and other adverse events were also examined and compared between groups to assess safety.
In the pivotal study, 157 women were enrolled out of 273 women that were recruited.18 There were a total of 74 women that withdrew, most commonly siting discomfort (n = 34, 32.4%) or leakage (n = 29, 27.6%) as their reason for withdrawal, leaving a total of 77 subjects who completed the protocol. This study found 98% of evaluable subjects (113/115) had comparable PVRs, with median PVR at each visit during inFlow treatment ranging from 10 to 20cc, thus leading the authors to conclude that CIC and inFlow urinary prosthesis were equivalent in their ability to fully empty the bladder. Furthermore, there was a statistically significantly within subject difference of lower PVR with the inFlow urinary prosthesis compared to the same subjects’ performing CIC (median PVR 13cc CIC baseline vs. 10cc inFlow treatment, p = 0.02).
This study showed that inFlow was significantly superior to CIC in its effect on quality of life.18 Out of evaluable subjects (n = 85) that had both baseline and treatment QOL data, the mean score at baseline on Wagner I-QOL was 42.2 and increased to 67.4 with treatment, a 25-point improvement which is both clinically and statistically significant (P < 0.0001) for the 100-point scale.20 For inFlow urinary prosthesis users, the median percent improvement in QOL from baseline was 54%. There were no unanticipated, serious, or long-lasting adverse events in this study.18
As with any indwelling device within the urinary system, adverse events such as encrustation, infection, and migration are a concern. Despite the indwelling nature of the inFlow urinary prosthesis compared to the very short indwelling time of CIC, the pivotal trial showed no significant differences in adverse event rates between the CIC baseline and inFlow treatment periods except for clinically minor events. During the treatment phase, 8% of patients reported hematuria, all of which were mild or moderate in severity and did not warrant treatment or device removal. Asymptomatic bacteriuria was found in 56% of patients and UTIs, all mild in severity, were noted in 30% of patients. There were no instances of failure of the inFlow device due to encrustation. Compared to baseline, discomfort and leakage were found to increase during the inFlow treatment period and, while all cases were mild in severity, this was noted to be the reason for discontinuation of device use a combined 60% of subjects.
Investigator sponsored studies
There are several investigator-sponsored clinical studies that have been published in major peer-reviewed journals (see Table 1). Lynch et al. conducted a one-year study of 20 women with IDC to determine the subjective and objective benefits of the inFlow urinary prosthesis.21 While the etiology of the subjects’ IDC varied, all of the subjects in this study had no evidence of effective detrusor contraction during urodynamic assessment and, prior to enrollment, all were using standard bladder drainage techniques (CIC in five, indwelling urethral catheter in eight, and suprapubic catheterization in seven subjects). Subjects enrolled in the study had the inFlow urinary prosthesis inserted in the standard fashion and subsequently received detailed instruction in the use of the device. In an effort to adjust for a required period of accommodation to a new prosthetic device, subjects were offered access to a 24-hour helpline for support in becoming familiar with the use of the inFlow system. The first inFlow urinary prosthesis was exchanged at 4 weeks at which time urinary flow rates were obtained in order to afford patients the time needed to learn the technique of operating the device correctly. The investigators obtained PVR and urine culture at each inFlow urinary prosthesis exchange. Additionally, subjects completed the Wagner-QOL questionnaire in reference to their original bladder drainage method at baseline and at 1, 3, 6, and 12 months follow-up after initial inFlow urinary prosthesis insertion.
Table 1. Summary of investigator-sponsored clinical studies.
| Lead Author (Year) | Study size (n) | Follow up | Drop out rate (%) | UTI incidence (UTI/subject months) | Adverse Events (# of occurrences) | Other Key Findings |
|---|---|---|---|---|---|---|
| Lynch (2003) | 20 | 12 months | 35% | 0.01% |
|
|
| Mazouni (2004) | 60 | 3.2 months (range 1–29) | 50% | 3.3% |
|
|
| Madjar (2000) | 21 | 24.6 months (range 12–44) | * | 0.01% |
|
|
| Madjar (1999) | 92 | 7.6 months (range 2–26 months) | 49% | 3.9% |
|
|
| Schurch (1999) | 18 | 9.6 months (range 5–15) | 44% | 0.04% |
|
|
| Nativ (1997) | 17 | 8.4 months (range 2–16) | 12% | 0.025% |
|
|
Note: PVR: post void residual volume, *not specified or information not available.
Lynch et al. found the mean (range) flow rate to be 10.7 (9–16) mL/s and PVR 3 (0–17) mL. Investigators also found a significant improvement in patient reported QOL when using the inFlow as mean (range) QOL scores, in which a lower score correlates with better QOL, were 59.6 (35–91) at baseline, 11.2 (1–29) at 1 month, and 5.0 (1–9) at 12 months.21 In this study, the side-effect profile and risk of infection were low. There were two subjects that each had a single UTI after initial insertion of the inFlow urinary prosthesis and were successfully treated with antibiotics. An additional three subjects had asymptomatic bacteriuria and one subject had recurrent UTIs from the time she began parenteral nutrition for treatment of an unrelated condition. Only one subject discontinued device use due to reasons related to the device. Overall, Lynch et al. showed that a high rate of device acceptance can be achieved by providing pre-insertion patient education and post-insertion nursing support in order to guide patients through the accommodation period of a new prosthetic device, manage patient expectations, and provide reassurance that discomfort is likely temporary and unlikely to do harm.
Madjar et al. reported a long-term study in two parts on the inFlow urinary prosthesis in women with urinary retention due to “difficulty voiding” which yielded similar results to the pivotal study in terms of PVR, UTIs, complication rates, and subject withdrawal.22,23 In the first part, 45 out of 92 subjects discontinued device use <7 days due to discomfort or leakage. The remaining 47 subjects were followed for a mean 7.6 months (range 2–26 months; total 357 subject-months).22 In the second part of their study, longer-term follow-up of 21 subjects was available with a mean follow-up time of 24.6 months (range 12–44 months; total 517 subject-months).23 Subjects in this study had a wide range of etiologies of their urinary retention including previous pelvic surgery and external radiation (n = 11), diabetes (n = 7), multiple sclerosis (n = 9), spinal cord injury (n = 6), and unknown etiology (n = 59). Prior to enrollment, bladder drainage was managed by indwelling catheter in 21 subjects, no treatment in 16 subjects, and CIC in 55 subjects.
Following initial inFlow urinary prosthesis insertion, subjects returned for monthly follow-up with uroflow, PVR, urinalysis, urine culture, and symptoms and satisfaction assessment and questionnaire. In 45 subjects (49%), the device was removed within 4 months (mean 7.1 days) with most subjects citing local discomfort, urinary leakage, or difficulty with operation as reasons for discontinuing use. Of the remaining 51% of subjects who continued to use the device, all remained dry and experienced complete bladder emptying. Mean use time in these subjects was 7.6 months. In the first part of the study, asymptomatic bacteriuria was common and found in 47% of subjects but there were only 14 symptomatic UTIs in 357 subject-months (3.9% incidence), all of which were successfully treated with oral antibiotics. Similarly, in the second part of the study, 15 of 21 subjects (71.4%) followed for more than 1 year developed asymptomatic bacteriuria but there were only four symptomatic UTIs recorded (4 UTIs in 517 subject-months; 0.01% incidence). There was no change in a woman’s ability to be sexually active after treatment with the inFlow urinary prosthesis if she had been sexually active prior. All inFlow device users reported being “satisfied” or “very satisfied” with the device. By multivariate analysis in this study the only independent predictor of treatment failure was if the subject had had no prior treatment for difficulty voiding. Madjar et al. concluded that patients who were previously treated with other modalities for their voiding dysfunction preferred to use the inFlow urinary prosthesis over alternative treatment modalities. Additionally, no long-term harm was noted in patients who discontinued the inFlow urinary prosthesis device for any reason.
Mazouni et al. also conducted a study to assess the efficacy and safety of the inFlow urinary prosthesis for the management of chronic urinary retention in women as an alternative to CIC.24 Using inclusion and exclusion criteria similar to that in the pivotal trial, they studied 60 women with chronic urinary retention, median age of 61.9 years old (range 40–89 years). The inFlow urinary prosthesis was found to be effective in emptying the bladder with mean maximal peak flow after 1 month of use 14 mL/s (range 7–18 mL/s) and PVR of 15 mL (range 0–40 mL). The incidence of UTI was 3.3%, compared to a reported rate of 12% after 5 years of CIC, and there were few significant complications.24,25
Concordant with other studies, Mazouni et al. also found a high early dropout rate, 50%, as 30 out of 60 subjects discontinued inFlow urinary prosthesis use within the first 15 days. Most subjects noted discomfort, technical defect (including deposits clogging the pump mechanism or device migration), pain, and urinary leakage as causes for withdrawal. In this study, there were no statistically significant differences in patient characteristics that predisposed a subject to either success or withdrawal from device use. Overall, Mazouni et al. concluded that the inFlow urinary prosthesis is an attractive, alternative to catheterization as it can be easily inserted and removed, is associated with a low number of adverse events, and allows for the recovery of autonomy by the patient.
Although more recent studies discussed above are more applicable as the inFlow urinary prosthesis has been remodeled and corrected since it’s initial design, there are two earlier studies that should also be mentioned. Schurch et al.26 conducted a study in 1997–1998 investigating the inFlow urinary prosthesis in which they found that the device was easy to implant and “user-friendly” however, mostly due to technical problems with the device and incontinence, only 6 of 18 subjects continued inFlow urinary prosthesis use at mean 9.6 (range 5–15) months follow-up. The UTI rate in this study was not reported. This early study had a small number of patients and suffered from poor transition of a new technology to clinical practice as well as early product quality issues which have since been corrected to improve device migration and increase device reliability.
Nativ et al. published their early work on the inFlow urinary prosthesis in 17 women with a diagnosis of atonic bladder in 1997.27 Fifteen of these patients had a range of use from two weeks to sixteen months during which they were dry and had complete bladder emptying. Two subjects had uninhibited detrusor contractions and did not tolerate the device prompting removal five days after insertion. Nativ et al. reported that 14 evaluable subjects had a total of 3 symptomatic UTIs in 118 subject-months (0.025% incidence) which were successfully treated with oral antibiotics.
Discussion
The inFlow urinary prosthesis is a unique technology which utilizes a nonsurgically inserted urethral device for females to assist with bladder drainage due to DUA. The inFlow urinary prosthesis has been used in Europe and elsewhere for 20 years and has been the subject of seven peer-reviewed clinical studies. Unfortunately, peer-reviewed literature on the inFlow urinary prosthesis has not been published in several years but with the anticipated approval by nationwide Medicare coverage within the United States in the near future, there may be greater applicability of the device and hopefully more contemporary studies published. The pivotal trial as well as several clinical studies discussed above note approximately 50% of subjects discontinue use of the inFlow urinary prosthesis early due to device-related reasons. In the pivotal trial,18 discomfort and leakage increased during inFlow urinary prosthesis treatment and although all cases were mild in severity, this caused numerous subjects to discontinue device use. Discomfort after insertion, device awareness, and leakage are commonly cited reasons for discontinuation of use of the inFlow urinary prosthesis. However, leakage and discomfort are both also frequently reported in patients using indwelling urinary catheters. At this point, there are no studies available that have identified a reliable predictor of device success and patient tolerance of the device based on demographics or pathology. Based on results from the pivotal trial, most patients that are going to discontinue device use will do so in the first 1–4 days and an on-device trial of 1 week can be effective in identifying subjects that will tolerate the device long-term. There have not been any long-term consequences reported in patients who discontinue use of the inFlow urinary prosthesis.
Lynch et al. importantly showed that device acceptance can be improved by implementing pre-insertion patient education and post-insertion patient support to provide reassurance to patients and manage expectations.21 It is important to acknowledge that, like most prosthetic devices, the inFlow urinary prosthesis requires a period of accommodation and adjustment to work well for many patients. When it is disclosed to patients that they may experience discomfort and leakage during accommodation to the device but also understand that any problems are likely to be temporary and unlikely to do harm, they are more likely to tolerate the device long-term. It is also important to provide nursing support post-insertion to closely monitor for any problems, address patient-concerns, and make adjustments as needed. Overall, while device acceptance is difficult to predict, it is easy to test for with an on-device trial and can be increased with patient education and nursing support.
Frequent and recurrent UTIs can be common among women with DUA regardless the method they employ for bladder management. The inFlow urinary prosthesis was found to have even lower rates of UTI than CIC, the current standard of care. Using the crossover design of the pivotal trial, one can see that the baseline rate of UTI among subjects utilizing CIC of 0.12 is actually higher than the rate of UTIs during the first half of inFlow treatment period of 0.11 per patient month. Rates of UTI decreased even further to 0.08 per patient month during the second half of inFlow treatment.18 This finding is consistent with other studies as well as Mazouni et al. reported only 3% UTI incidence and Madjar et al. reported 3.9% UTI incidence with device use.23,24 Catheter-associated urinary tract infections (CAUTIs) are common and costly. The Centers for Disease Control and Prevention (CDC) estimates that annually CAUTIs cause over 13,000 deaths and add $1.85 billion in direct medical costs.28 Additionally, the emergence of resistant bacteria in UTIs is increasing and thus the most effective strategy for UTI management is prevention of UTIs in the first place.29 The lower rate of UTIs in women that use the inFlow urinary prosthesis compared to an alternative catheterization method is likely due to the former’s ability to return key aspects of normal bladder function to the user, specifically periodic, forceful, and complete evacuation of urine, mimicking normal micturition.
The inFlow urinary prosthesis is a unique method for bladder drainage as it affords women an autonomous way to mimic normal voiding and thus can improve quality of life. This is a characteristic unmatched by any other treatment alternative for women with DUA. The pivotal trial showed that the inFlow urinary prosthesis improved QOL by almost 60% compared to CIC.18 Chronic catheterization by indwelling urethral or suprapubic catheter can be psychologically devastating to patients and socially alienating. CIC can also be burdensome and uncomfortable for some, or even not feasible for others due to visual, manual, or cognitive limitations. If a woman cannot or will not use CIC, then an indwelling catheter and urinary drainage bag will likely be her method of bladder management. For women with urinary retention due to DUA, the inFlow urinary prosthesis can significantly impact QOL by allowing them to return to “normal” micturition.
There are many benefits in QOL for women with urinary retention due to DUA that tolerate the inFlow urinary prosthesis demonstrated in both the pivotal trial and study by Lynch et al. The inFlow urinary prosthesis can allow most users to void without assistance, increasing self-reliance, and eliminating the need for an indwelling catheter or intermittent catheterization multiple times daily.
The inFlow urinary prosthesis has been FDA approved for use in the United States since 2014, however, it has not been accepted for widespread use throughout the country. While there are a combination of reasons for a lack of acceptance, including high drop-out rate during on-device trial period and appropriate patient selection, a major contributing factor is likely the lack of nationwide Medicare coverage. Since a large percentage of the inFlow urinary prosthesis target population are Medicare beneficiaries as a result of age and/or disability, the lack of coverage for the device by this insurance carrier made commercialization unviable. Additionally, private insurance payers rarely cover devices that lack Medicare coverage. At the time of writing, Medicare recently revised its non-coverage decision and coverage is expected in 2020.
Conclusions
The inFlow urinary prosthesis is a safe and effective alternative for women with urinary retention due to DUA and has about a 50% acceptance rate following a one-week trial of the device to ensure patient tolerance. A relatively high drop-out rate is noted during the trial period due to discomfort and leakage. However, due to design, the device can be removed by the patient or a nurse with no long-term adverse events noted. Patients that do not tolerate the device trial period may resume their previous method of bladder management. One of the most important findings is the decreased UTI rate in women that use the inFlow urinary prosthesis compared to their alternative method of bladder drainage. For women that tolerate the device, the inFlow urinary prosthesis has been found to be easy to use with improved quality of life. Peer-reviewed articles show that the inFlow urinary prosthesis can be beneficial for women with urinary retention due to DUA who desire an alternative to CIC or indwelling urinary catheter.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest Authors have no conflict of interests to declare.
References
- 1.D'Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn 2019;38(2):433–477. doi: 10.1002/nau.23897 [DOI] [PubMed] [Google Scholar]
- 2.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 2014;65(2):389–398. doi: 10.1016/j.eururo.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 3.Chapple CR, Osman NI.. The underactive detrusor. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, (ed.) Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016. p. 1808–1819 [Google Scholar]
- 4.Niel-Weise BS, van den Broek PJ, da Silva EM, Silva LA.. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;8:CD004201. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21(2):167–178. doi: 10.1002/nau.10052 [DOI] [PubMed] [Google Scholar]
- 6.Vesiflo . Physician instructions for use. Available from http://vesiflo.com/.
- 7.Jeong SJ, Kim HJ, Lee YJ, Lee JK, Lee BK, Choo YM, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol 2012;53(5):342–348. doi: 10.4111/kju.2012.53.5.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valente S, DuBeau C, Chancellor D, Okonski J, Vereecke A, Doo F, et al. Epidemiology and demographics of the underactive bladder: a cross-sectional survey. Int Urol Nephrol 2014;46(Suppl 1):S7–10. doi: 10.1007/s11255-014-0811-1 [DOI] [PubMed] [Google Scholar]
- 9.Cohn JA, Ni S, Kaufman MR, Graves AJ, Penson DF, Dmochowski RR, et al. Urinary retention and catheter use among U.S. female Medicare beneficiaries: prevalence and risk factors. Neurourol Urodyn 2017;36(8):2101–2108. doi: 10.1002/nau.23248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman NI, Esperto F, Chapple CR.. Detrusor underactivity and the underactive bladder: a systematic review of preclinical and clinical studies. Eur Urol 2018;74(5):633–643. doi: 10.1016/j.eururo.2018.07.037 [DOI] [PubMed] [Google Scholar]
- 11.Aldamanhori R, Osman NI, Chapple CR.. Underactive bladder: pathophysiology and clinical significance. Asian J Urol 2018;5(1):17–21. doi: 10.1016/j.ajur.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr LK, Webster GD.. Bladder outlet obstruction in women. Urol Clin North Am 1996;23(3):385–391. doi: 10.1016/S0094-0143(05)70319-0 [DOI] [PubMed] [Google Scholar]
- 13.Hill VB, Davies WE.. A swing to intermittent clean self-catheterisation as a preferred mode of management of the neuropathic bladder for the dextrous spinal cord patient. Paraplegia 1988;26(6):405–412. [DOI] [PubMed] [Google Scholar]
- 14.Hunt GM, Oakeshott P, Whitaker RH.. Fortnightly review: intermittent catheterisation: simple, safe, and effective but underused. Br Med J 1996;312(7023):103–107. doi: 10.1136/bmj.312.7023.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schimke L, Connolly KM.. First report: U.S. patient and clinician experiences with the inFlow™ urinary prosthesis for permanent urinary retention in women. Urol Nurs 2020;40(2):61–84. doi: 10.7257/1053-816X.2020.40.2.61 [DOI] [Google Scholar]
- 16.Gross C, Habli M, Lindsell C, South M.. Sacral neuromodulation for nonobstructive urinary retention: a meta-analysis. Female Pelvic Med Reconstr Surg 2010;16(4):249–253. doi: 10.1097/SPV.0b013e3181df9b3f [DOI] [PubMed] [Google Scholar]
- 17.Lee SM, Hashim H.. Recent advances in the understanding and management of underactive bladder. F1000Res 2018;7:437. doi: 10.12688/f1000research.13660.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TY-H, Ponsot Y, Carmel M, Bouffard N, Kennelly MJ, Tu LM.. Multi-centre study of intraurethral valve-pump catheter in women with a hypocontractile or acontractile bladder. Eur Urol 2005;48(4):628–633. doi: 10.1016/j.eururo.2005.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DE.. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996;47(1):67–71. discussion -2. doi: 10.1016/S0090-4295(99)80384-7 [DOI] [PubMed] [Google Scholar]
- 20.Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagner TH, Buesching DP.. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I-QOL). Urology 1999;53(1):71–76. doi: 10.1016/S0090-4295(98)00454-3 [DOI] [PubMed] [Google Scholar]
- 21.Lynch WJ, Testa GA, Bell DF.. The subjective and objective benefits of a remote-controlled intraurethral device for managing the female acontractile bladder. BJU Int 2003;92(9):960–963. doi: 10.1111/j.1464-410X.2003.04525.x [DOI] [PubMed] [Google Scholar]
- 22.Madjar S, Sabo E, Halachmi S, Wald M, Issaq E, Moskovitz B, et al. A remote controlled intraurethral insert for artificial voiding: a new concept for treating women with voiding dysfunction. J Urol 1999;161(3):895–898. doi: 10.1016/S0022-5347(01)61800-7 [DOI] [PubMed] [Google Scholar]
- 23.Madjar S, Halachmi S, Wald M, Issaq E, Moskovitz B, Beyar M, et al. Long-term follow-up of the in-flowtrade mark intraurethral insert for the treatment of women with voiding dysfunction. Eur Urol 2000;38(2):161–166. doi: 10.1159/000020274 [DOI] [PubMed] [Google Scholar]
- 24.Mazouni C, Karsenty G, Bladou F, Serment G.. Urethral device in women with chronic urinary retention: an alternative to self-catheterization? Eur J Obstet Gynecol Reprod Biol 2004;115(1):80–84. doi: 10.1016/j.ejogrb.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 25.Sutton G, Shah S, Hill V.. Clean intermittent self-catheterisation for quadriplegic patients–a five year follow-up. Paraplegia 1991;29(8):542–549. [DOI] [PubMed] [Google Scholar]
- 26.Schurch B, Suter S, Dubs M.. Intraurethral sphincter prosthesis to treat hyporeflexic bladders in women: does it work? BJU Int 1999;84(7):789–794. doi: 10.1046/j.1464-410x.1999.00281.x [DOI] [PubMed] [Google Scholar]
- 27.Nativ O, Moskowitz B, Issaq E, Condrea A, Kastin A, Halachmi S, et al. A new intraurethral sphincter prosthesis with a self contained urinary pump. ASAIO J 1997;43(3):197–203. [PubMed] [Google Scholar]
- 28.Klevens RM, Edwards JR, Richards CL, Jr., Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122(2):160–166. doi: 10.1177/003335490712200205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koves B, Magyar A, Tenke P.. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS Infect Dis 2017;5:Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]