Abstract
Background:
As well as developing graft vasculopathy, coronary artery bypass grafts (CABG) have been proposed to accelerate native coronary atherosclerosis. We aimed to describe the potential of 18F-sodium fluoride positron emission tomography (18F-NaF PET) to identify graft vasculopathy and to investigate the influence of CABG surgery on native coronary artery disease activity and progression.
Methods:
Patients with established coronary artery disease underwent baseline 18F-NaF PET, coronary artery calcium scoring, coronary CT angiography, and one-year repeat coronary artery calcium scoring. We quantified the whole-vessel 18F-NaF PET coronary microcalcification activity (CMA) and change in calcium scores in those with and without CABG surgery.
Results:
Among 293 participants (65±9 years; 84% male), 48 (16%) had CABG surgery 2.7 [1.4–10.4] years previously. Although all arterial and the majority (120/128, 94%) of vein grafts showed no 18F-NaF uptake, 8 saphenous vein grafts in 7 subjects had detectable CMA.
Bypassed native coronary arteries had 3-times higher CMA values (2.1 (0.4–7.5) versus 0.6 (0–2.7), p<0.001) and greater progression of one-year calcium scores (118 [48–194] versus 69 [21–142] AU, p=0.01) compared to those who that had not undergone CABG: an effect largely confined to native coronary plaques proximal to the graft anastomosis. In sensitivity analysis, bypassed native coronary arteries had higher CMA (2.0 [0.4–7.5] vs 0.8 [0.3–3.2], p<0.001) and faster disease progression (24 [16–43] versus 8 [0–24] %, p=0.002) than matched patients (n=48) with comparable burdens of coronary artery disease and cardiovascular comorbidities in the absence of bypass grafting.
Conclusions:
Native coronary arteries that have been bypassed, demonstrate increased disease activity and more rapid disease progression than non-bypassed arteries, an observation that appears independent of the baseline atherosclerotic plaque burden. Microcalcification activity is not a dominant feature of graft vasculopathy.
Keywords: PET/CT, coronary artery disease, coronary artery bypass graft, CABG, 18F-NaF
Condensed Abstract
Coronary artery bypass grafts (CABG) have been proposed to accelerate native coronary atherosclerosis. In patients with established coronary artery disease, we quantified the whole-vessel 18F-sodium fluoride positron emission tomography (18F-NaF PET) coronary microcalcification activity (CMA) and change in calcium scores to compare disease activity and progression in those with and without CABG surgery. Despite a similar coronary plaque burden, bypassed native coronary arteries had 3-times higher CMA values and greater progression of one-year calcium scores compared to those who that had not undergone CABG. Bypassed native coronary arteries demonstrate increased disease activity and faster disease progression than non-bypassed arteries.
Introduction
Coronary artery bypass graft (CABG) surgery is the gold standard method of coronary revascularization in patients with advanced multivessel coronary artery disease. It improves symptoms, quality of life, clinical outcomes and overall survival. Saphenous vein grafts are the major conduits used for grafting but are prone to degeneration with a 10-year patency rate of around 30–60% (1–6). It has been hypothesized that bypass grafts might also impact the pathophysiology of native coronary artery disease, accelerating proximal disease progression due to reduced shear stress. This hypothesis has been investigated in several observational studies, but these observations were limited by a lack of assessment of disease activity and confounded by differences in plaque burden (7,8).
18F-Sodium fluoride (18F-NaF) positron emission tomography (PET) provides an assessment of calcification activity (active microcalcification) across a wide range of cardiovascular conditions including atherosclerosis (9–15). In the context of coronary artery disease, this tracer correlates with disease activity, predicting subsequent disease progression and future myocardial infarction in patients with advanced coronary atherosclerosis (13–15). In addition, CT calcium scoring (measure of macrocalcification) provides an assessment of established coronary plaque burden that is widely used and highly reproducible, with serial scans providing an assessment of coronary artery disease progression. However, the role of 18F-NaF PET and CT in patients with prior CABG surgery is relatively unexplored and whether advanced non-invasive cardiac imaging can help inform our understanding of bypass graft vasculopathy is unknown.
We aimed to describe the potential of 18F-NaF PET to identify graft vasculopathy and to investigate the influence of CABG surgery on native coronary artery disease activity and progression with coronary artery 18F-NaF PET and CT calcium scoring respectively.
Methods
Clinical Study Population
The imaging study was based on a cohort of patients with known coronary artery disease who underwent baseline 18F-NaF PET imaging within prospective observational studies at the University of Edinburgh and Cedars Sinai Medical Center (NCT01749254, NCT02110303, NCT02607748; 14). All participants underwent a comprehensive baseline clinical assessment including evaluation of their cardiovascular risk factor profile and calculation of the Secondary Manifestations of ARTerial disease (SMART) risk score (16). The ex-vivo analysis of saphenous vein bypass grafts was performed on tissue obtained from patients undergoing heart transplant (explanted hearts) at St. Paul’s Hospital. The studies were conducted with the approval of the local research ethics committee, in accordance with the Declaration of Helsinki, and with the written informed consent of each participant.
18F-Sodium Fluoride and CT imaging
Acquisition and reconstruction
18F-Sodium fluoride PET was performed on hybrid PET/CT scanners (128-slice Biograph mCT, Siemens Medical Systems, Erlangen, Germany or Discovery 710 GE Healthcare, Milwaukee, WI, USA) using harmonized imaging protocols 60 min following injection of 250 MBq of 18F-NaF (14). During the imaging session, we acquired a non-contrast CT attenuation correction scan followed by a 30-min PET emission scan in list mode, a low dose non-contrast ECG-gated CT for coronary calcium scoring and a contrast-enhanced, ECG-gated coronary CT angiogram which was obtained in mid-diastole on the same PET/CT system without repositioning the patient. The electrocardiogram (ECG)-gated list mode dataset was reconstructed using a standard ordered expectation maximization algorithm with time-of-flight, and point-spread-function correction. Using 4 cardiac gates, the data were reconstructed on a 256×256 matrix (with 75 or 47 slices using 2 iterations, 21 subsets and 5-mm Gaussian smoothing for Siemens mCT data and 4 iterations, 24 subsets and 5-mm gaussian smoothing for GE Discovery data) (17).
Motion correction and blood clearance correction
To compensate for coronary motion associated with heart contraction, we performed cardiac motion correction of the PET/CT images as described previously (18). Additionally, to offset for variation in the delay between tracer injection and scanning, we used a recently validated correction factor to harmonize the background activity to a reference 60-min injection-to-acquisition interval (19,20).
Coronary microcalcification activity
We employed FusionQuant (Cedars-Sinai Medical Center, Los Angeles, CA, USA) for PET image analysis (21). In the first step, PET and CT angiography reconstructions were reoriented, fused and systematically co-registered in 3 orthogonal planes (22). We used a recently described measure of coronary 18F-NaF uptake, coronary microcalcification activity (CMA) that quantifies PET activity across the entire coronary vasculature, is highly reproducible and is an independent predictor of myocardial infarction (14,23,24). We automatically extracted whole-vessel tubular and tortuous 3D volumes of interest from CT angiography datasets (Figure 1). These encompass all the main native epicardial coronary vessels, bypass grafts and their immediate surroundings (4-mm radius) facilitating per-vessel and per-patient uptake quantification. Within such volumes of interest, we measured CMA, which represents the overall disease activity in the vessel and is based upon both the volume and intensity of 18F-NaF PET activity within it. CMA was defined as the integrated activity in standardized uptake value (SUV) exceeding the corrected background blood-pool mean SUV + 2 standard deviations. We measured the background activity in the right atrium, drawing cylindrical volumes of interest (10-mm radius and 5-mm thickness) at the level of the right coronary artery ostium. The per-patient CMA was defined as the sum of the per-vessel CMA values. Previous studies have demonstrated excellent scan-rescan reproducibility for such measurements (24).
Figure 1. Measurement of 18F-sodium fluoride uptake and calculation of the coronary microcalcification activity in patients with prior coronary artery bypass graft surgery.
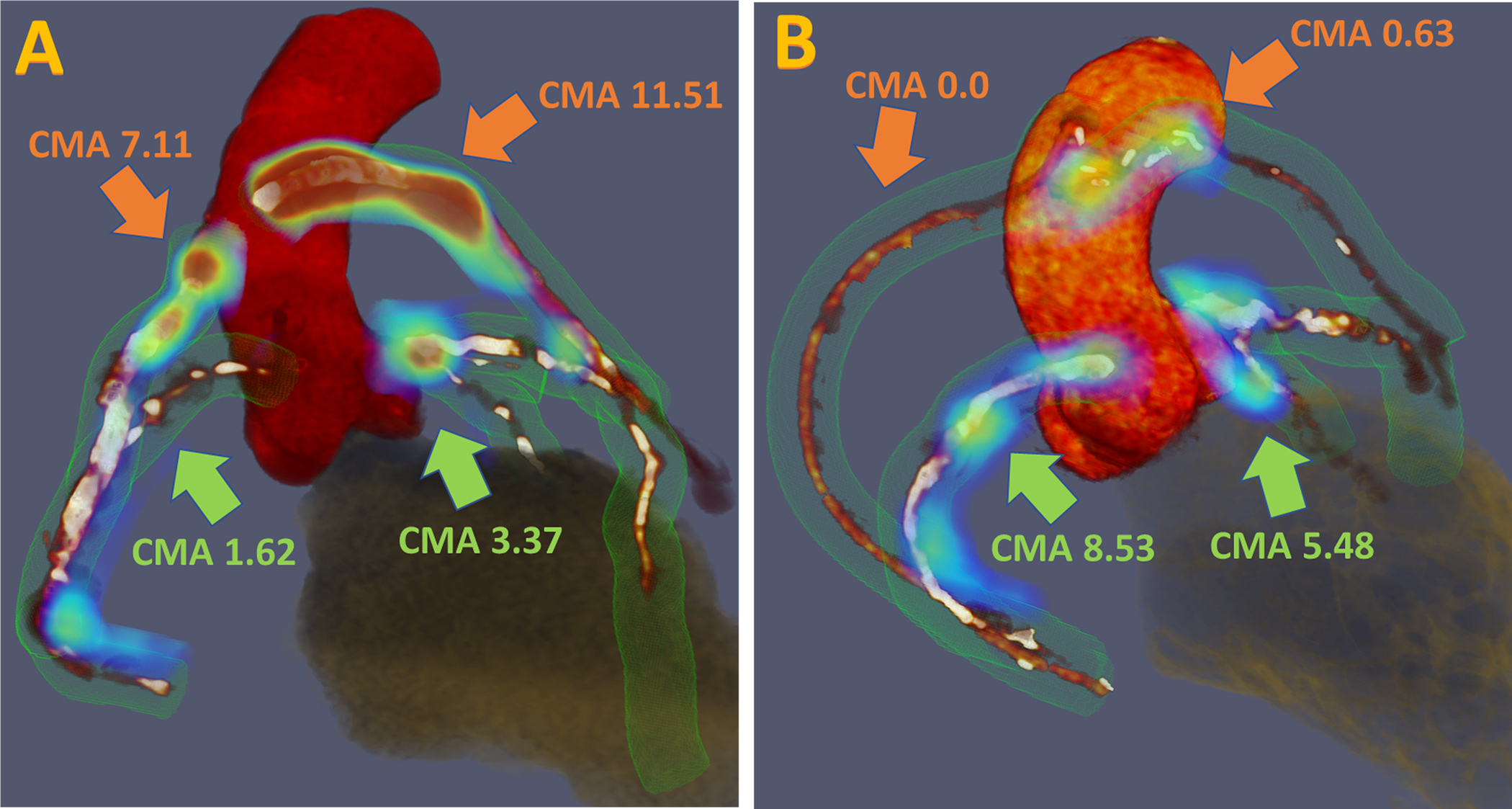
Three-dimensional rendering of coronary computed tomography (CT) angiography with superimposed tubular whole vessel volumes of interest (light green) employed for evaluation of 18F-sodium fluoride (18F-NaF) uptake (blue and red). The coronary microcalcification activity (CMA) is a summary measure of 18F-NaF activity across the entire coronary vasculature. (A) 63-year-old male with prominent uptake in stented saphenous vein bypass grafts (orange arrows) and native coronary arteries (green arrows) who experienced a non-fatal non-ST segment elevation myocardial infarction during follow-up. (B) 70-year old male with evident uptake in native coronary arteries (green arrows) and subtle 18F-NaF activity within coronary bypasses (orange arrows).
Computed Tomography
The coronary artery calcium score was measured in Agatston units (AU) as our primary marker of plaque burden using clinical software (NetraMD, ScImage, Los Altos, CA, USA) (25). The presence, extent and severity of coronary artery disease were also evaluated on contrast-enhanced CT angiography by defining the segment involvement score and the number of vessels with >50% luminal stenosis (26). Multivessel coronary artery disease was defined as at least 2 major epicardial vessels with any combination of either >50% stenosis, or previous revascularization. In a subset of patients who underwent follow-up non-contrast CT, we calculated the change in coronary calcium scores. Graft vasculopathy was defined as the presence of plaque or abnormal vessel wall thickening on CT angiography (4).
Plaque analysis
Quantitative plaque analysis of all coronary segments with a lumen diameter greater than 2 mm was performed using semi-automated software (AutoPlaque version 2.0, Cedars-Sinai Medical Center, Los Angeles, CA, USA) (27,28). Coronary CT angiography images were examined in multiplanar format, proximal and distal limits of lesions were manually marked by an experienced reader. Subsequent plaque quantification was fully automated using adaptive scan-specific thresholds. Total (TPV), calcified, non-calcified (NCP) as well as low attenuation plaque volumes were calculated. The plaque burden was calculated according to the following equation (plaque volume x 100%/vessel volume).
Ex Vivo Assessment of Saphenous Vein Coronary Bypass Grafts
Explanted saphenous vein bypass grafts were obtained from patients during heart transplant (explanted hearts). None of these patients underwent 18F-NaF prior to heart transplant. Specimens were fixed in 10% w/v buffered formalin phosphate pending histological analysis. Each sample was embedded in paraffin, sectioned and stained for architecture with hematoxylin and eosin as well as Movat’s pentachrome (29). High-resolution histological images were generated using an Aperio Slide Scanner and ImageScope software (Leica Biosystems, Germany). For autoradiography experiments, slide-mounted paraffin-embedded sections were rehydrated bathed in PBS and placed in 100 KBq/mL of 18F-NaF for 1 hour, before being washed in PBS again. The slides were then placed in the phosphor-images Amesham Typhoon (GE) at 4000 X 10 μm scale, the Fuji film high resolution film applied and left overnight for exposure. Autoradiography analysis was performed in the open-source software ImageJ (v2.0.0).
Statistical analysis
We assessed the distribution of data with the Shapiro-Wilk test. Continuous normally distributed variables were expressed as mean (SD) and compared using the Student’s t-test. Non-normally distributed data were presented as median [interquartile interval] and compared using the Mann-Whitney test. Fisher’s exact test or chi-squared test was used for analysis of categorical variables. In the analysis conducted on the per-bypass level we made no adjustments for multiple observations (grafts) within a single patient. Patients with prior CABG surgery were compared to patients with advanced coronary artery disease without a history of surgical revascularization and were matched by the coronary artery disease burden (coronary calcium scores, total plaque volumes on coronary CT angiography) and clinical co-morbidities. Coronary calcium score progression was presented in absolute values (Agatston units) in the unmatched analysis (as change from 1 to 2 AU translates into a 100% difference which is not comparable to progression from 500 to 1000 AU) and percent difference versus baseline in the matched analysis. In the analysis comparing bypassed versus non-bypassed vessels in patients with surgical revascularization we compared the change (expressed in percentage) in the coronary calcium score between the two groups with the Mann-Whitney test. Statistical analysis was performed with SPSS version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). A two-sided p<0.05 was considered statistically significant.
Results
Among 293 participants (65±9 years; 84% male), 48 (16%) patients had undergone CABG surgery a median of 2.7 [1.4–10.4] years prior to scanning (Table 1). As expected, patients with prior CABG surgery (n=48) were older, had more co-morbidities, higher SMART scores and more extensive native coronary artery disease than patients without prior CABG surgery (n=245). In particular, baseline native coronary calcium scores were higher (1135 [631–2120] versus 225 [59–542] AU, p<0.001), as were non-calcified (1606 [1095–2185] versus 1014 [582–1483], p<0.001) and total plaque volumes (1797 [1198–2615] versus 1103 [670–1561)], p<0.001).
Table 1.
Characteristics of patients with and without prior coronary artery bypass graft surgery.
| Patients with prior coronary artery bypass graft surgery n=48 |
Patients without prior coronary artery bypass graft surgery n=245 |
p value | |
|---|---|---|---|
|
| |||
| Age (years) | 68±8 | 64±9 | 0.13 |
|
| |||
| Men | 44 (92%) | 203 (83%) | 0.20 |
|
| |||
| Body-mass index (kg/m2) | 28.6± 4 | 29.4± 5 | 0.27 |
|
| |||
| Systolic blood pressure (mmHg) | 144±21 | 141±21 | 0.37 |
|
| |||
| Diastolic blood pressure (mmHg) | 80±11 | 79±11 | 0.72 |
|
| |||
| Cardiovascular history | |||
|
| |||
| Acute coronary syndrome | 29 (60%) | 133 (54.3%) | 0.21 |
|
| |||
| Percutaneous coronary intervention | 17 (35%) | 166 (67.8%) | <0.001 |
|
| |||
| Coronary artery bypass graft surgery | 48 (100%) | 0 | <0.001 |
|
| |||
| Patients with 4 bypass grafts | 12 (25%) | 0 | |
|
| |||
| Patients with 3 bypass grafts | 34 (71%) | 0 | |
|
| |||
| Patients with 2 bypass grafts | 2 (4%) | 0 | |
|
| |||
| Cerebrovascular accident/ Transient ischemic attack | 2 (4.2%) | 7 (2.9%) | 0.63 |
|
| |||
| Comorbidities/risk factors | |||
|
| |||
| Hypertension | 36 (75%) | 139 (57%) | 0.02 |
|
| |||
| Hyperlipidemia | 47 (98%) | 211 (86%) | 0.02 |
|
| |||
| Diabetes mellitus | 11 (23%) | 50 (20%) | 0.70 |
|
| |||
| Current smoking Ex-smoker |
5 (10%) 24 (50%) |
61 (25%) 121 (49%) |
0.03 0.94 |
|
| |||
| Atrial fibrillation | 2 (4%) | 8 (3%) | 0.75 |
|
| |||
| Peripheral vascular disease | 8 (17%) | 8 (3%) | <0.001 |
|
| |||
| Medications * | |||
|
| |||
| Aspirin | 46 (96%) | 223 (91%) | 0.27 |
|
| |||
| P2Y12 receptor antagonist | 3 (6%) | 44 (18%) | 0.04 |
|
| |||
| Statin | 46 (96%) | 217 (89%) | 0.13 |
|
| |||
| Beta Blocker | 30 (63%) | 167 (68%) | 0.44 |
|
| |||
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 34 (71%) | 166 (68%) | 0.68 |
|
| |||
| Insulin | 1 (2%) | 6 (2%) | 0.74 |
|
| |||
| Oral diabetic medications | 7 (15%) | 12 (5%) | 0.83 |
|
| |||
| Calcium channel blocker | 17 (35%) | 48 (20%) | 0.02 |
|
| |||
| Diuretics | 15 (31%) | 57 (23%) | 0.24 |
|
| |||
| Clinical Biochemistry | |||
|
| |||
| Total Cholesterol (mmol/L) | 3.9 [3.4–4.7] | 4.1 [3.6–4.8] | 0.10 |
|
| |||
| Low density lipoprotein (mmol/L) | 1.9 [1.3–2.5] | 1.9 [1.2–2.4] | 0.45 |
|
| |||
| High density lipoprotein HDL (mmol/L) | 1.2 [0.9–1.6] | 1.2 [1.0–1.7] | 0.17 |
|
| |||
| Triglycerides TAG (mmol/L) | 1.5 [1.2–2.3] | 1.5 [1.1–2.3] | 0.44 |
|
| |||
| Creatinine (mg/dL) | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 0.18 |
|
| |||
| eGFR (mL/min/1.73m2) | 62 [53–69] | 63 [56–70] | 0.35 |
|
| |||
| Patients with eGFR <60 mL/min/1.73m2 | 6 (13%) | 25 (10%) | 0.64 |
|
| |||
| Coronary Artery Disease | |||
|
| |||
| Single vessel disease | 7 (15%) | 85 (35%) | |
| Two vessel disease | 11 (23%) | 99 (40%) | |
| Three vessel disease | 22 (46.0%) | 44 (18%) | |
| Left main stem disease | 8 (17%) | 4 (2%) | <0.001 |
|
| |||
| Coronary Stent | 16 (33%) | 201 (82%) | <0.001 |
|
| |||
| Segment involvement score | 8 [6–9] | 5 [3–7] | <0.001 |
|
| |||
| 0–1 | 0 (0%) | 17 (7%) | <0.001 |
| 2–3 | 1 (2%) | 55 (22%) | <0.001 |
| 4–5 | 5 (10%) | 68 (28%) | 0.01 |
| >5 | 42 (88%) | 105 (43%) | <0.001 |
|
| |||
| Coronary Calcium Score (AU) | 1135 [625–2165] | 225 [59–543] | <0.001 |
|
| |||
| 0–99 | 1 (2%) | 83 (34%) | <0.001 |
| 100–399 | 2 (4%) | 74 (30%) | <0.001 |
| 400–999 | 17 (35%) | 57 (23%) | 0.08 |
| >1000 | 28 (59%) | 31 (13%) | <0.001 |
|
| |||
| Quantitative coronary plaque analysis | |||
|
| |||
| Non-calcified plaque volume (mm3) | 1606 [1095–2185] | 1014 [582–1483] | <0.001 |
|
| |||
| Low attenuation plaque volume (mm3) | 136 [72–263] | 81 [36–148] | 0.01 |
|
| |||
| Total plaque volume (mm3) | 1797 [1198–2615] | 1103 [670–1561] | <0.001 |
|
| |||
| Risk scores | |||
|
| |||
| Duke score | 5 [4–6] | 4 [3–5] | 0.01 |
|
| |||
| SMART risk score | 23 [17–30] | 17 [13–24] | 0.01 |
|
| |||
| 18 F-Sodium fluoride uptake | |||
|
| |||
| TBRmax | 1.42 [1.24–1.59] | 1.21 [1.09–1.39] | 0.003 |
|
| |||
| TBRmax – native coronaries | 1.40 [1.21–1.58] | 1.21 [1.09–1.39] | 0.008 |
|
| |||
| TBRmax – CABG | 1.61 [1.34–2.57] | - | |
|
| |||
| CMA total | 2.1 [0.37–7.83] | 0.64 [0.0–2.73] | <0.001 |
|
| |||
| CMA – native coronary arteries | 2.1 [0.37–7.53] | 0.64 [0.0–2.73] | <0.001 |
|
| |||
| CMA - CABG | 6.9 [0.63–11.58] | - | |
|
| |||
| No of positive plaques - total | 3 [1–4] | 1 [1–3] | 0.001 |
|
| |||
| No of positive plaques – native coronaries | 3 [1–4] | 1 [1–3] | 0.001 |
CABG – coronary artery bypass graft, CMA – coronary microcalcification activity, SMART Secondary Manifestations of Arterial Disease, TBR – target to background ratio
n (%), mean±SD, median [interquartile range]
medications at the time of scan
In a total of 154 grafts, 37 (24%) grafts showed evidence of vasculopathy on computed tomography and 20 (13%) were occluded. All arterial and the majority (120/128, 94%) of venous bypass grafts showed no 18F-NaF uptake and only 8 saphenous vein grafts (in 7 subjects) had detectable uptake (Online supplement). These grafts were older (3.2 [2.4–10.6] versus 1.8 [1.3–6.5] years, p=0.005) and three demonstrated no visible evidence of graft vasculopathy on CT (Figure 2). On explanatory ex vivo analysis, we observed 18F-NaF uptake on autoradiography co-localized with areas of fibro-intimal thickening within notable elastic fiber reduplication (Figure 3 and Online supplement).
Figure 2. Case examples of increased 18F-sodium fluoride uptake in saphenous vein bypass grafts.
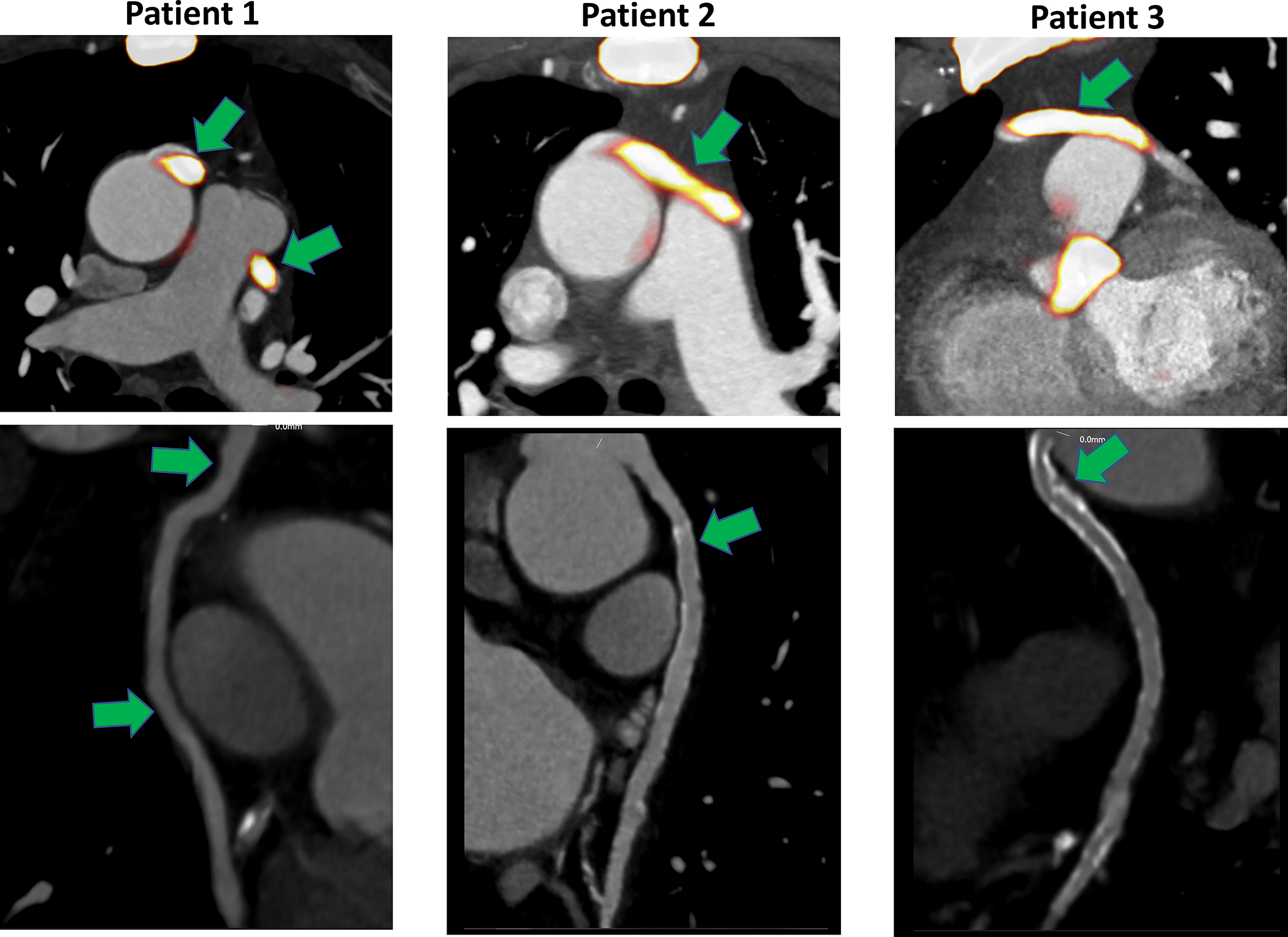
Green arrows indicate the areas of increased 18F-sodium fluoride (18F-NaF) uptake within saphenous bypass grafts (top row) and computed tomography angiography of the corresponding saphenous vein bypass grafts (bottom row). We observed increased 18F-NaF activity both in grafts with advanced atherosclerosis (Patient 2 and 3) and in the absence of such lesions (Patient 1).
Figure 3. 18F-Sodium fluoride positive saphenous vein graft.
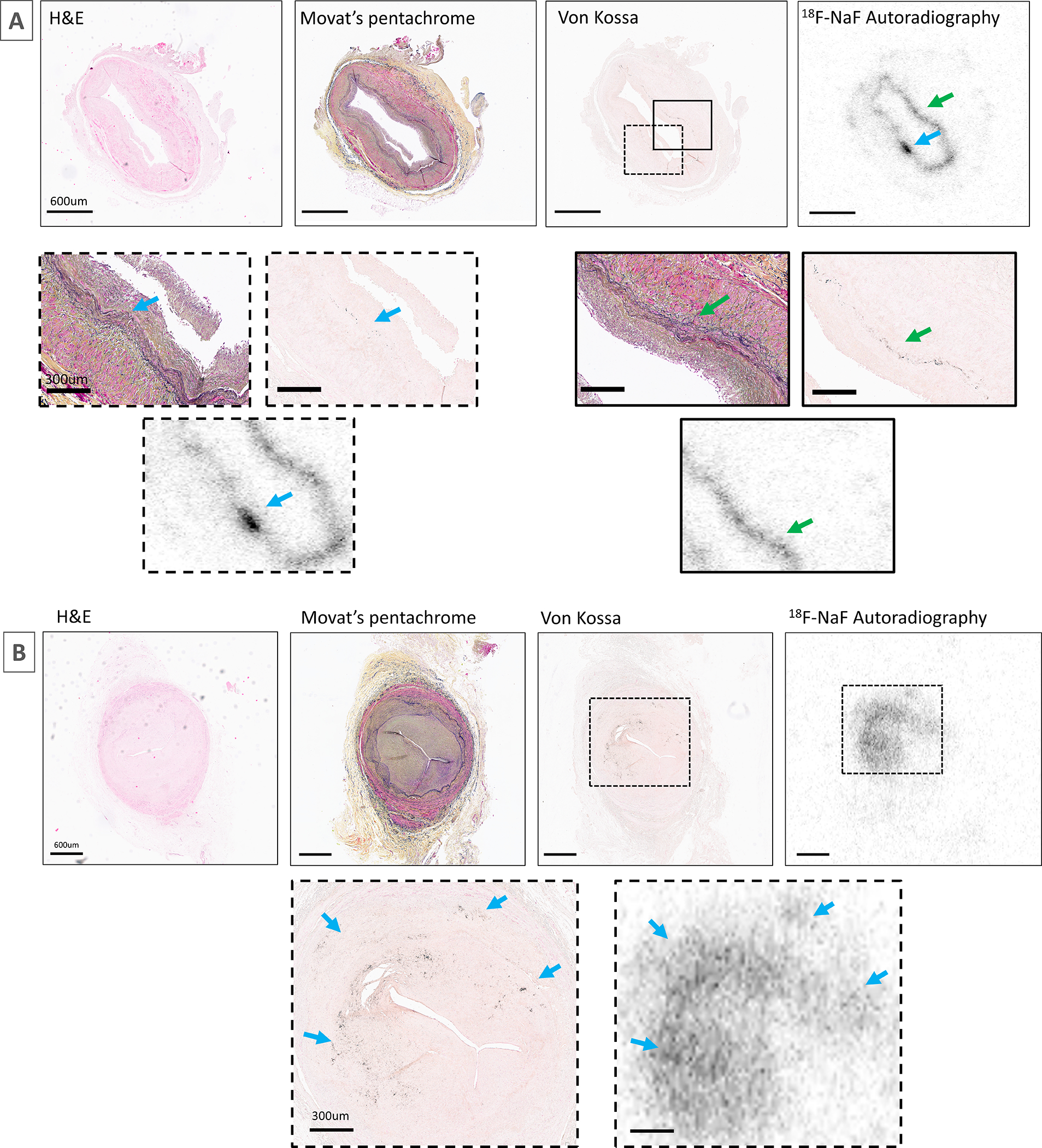
The samples were obtained at heart transplant from a 61-year-old male patient 4 years after coronary artery bypass grafting to left circumflex artery (A) and a 72-year-old male patient 12 years after coronary artery bypass grafting to right coronary artery (B). Both patients underwent surgical revascularization due to obstructive coronary artery disease. Magnified and matched to H+E as well as Movat’s Pentachrome staining demonstrating co-localization of 18F-sodium fluoride uptake with elastin as part of fibro-intimal thickening and microcalcification (black staining on von Kossa).
A higher proportion of patients with prior CABG surgery showed increased native coronary artery 18F-NaF uptake than patients without CABG surgery (83% versus 67%, p=0.02). Indeed, patients with prior CABG surgery had CMA values that were over 3 times higher (2.1 [0.4–7.5] versus 0.6 [0–2.7], p<0.001). This increased 18F-NaF uptake was most often observed within the proximal native coronary segments that had been bypassed with grafts (CMA 2.0 [0.4–7.5]). Only minimal uptake was seen distal to the graft anastomosis (CMA 0.2 [0.0–0.5]), indeed this was detectable in only 6 individuals (Figure 4). The difference in whole-coronary disease activity translated into faster progression of native coronary atherosclerosis compared to patients without CABG surgery (change in coronary calcium: 118 [48–194] versus 69 [21–142] AU, p=0.01; Online supplement).
Figure 4. Coronary 18F-sodium fluoride uptake in native coronary arteries in patients with or without prior coronary artery bypass graft.
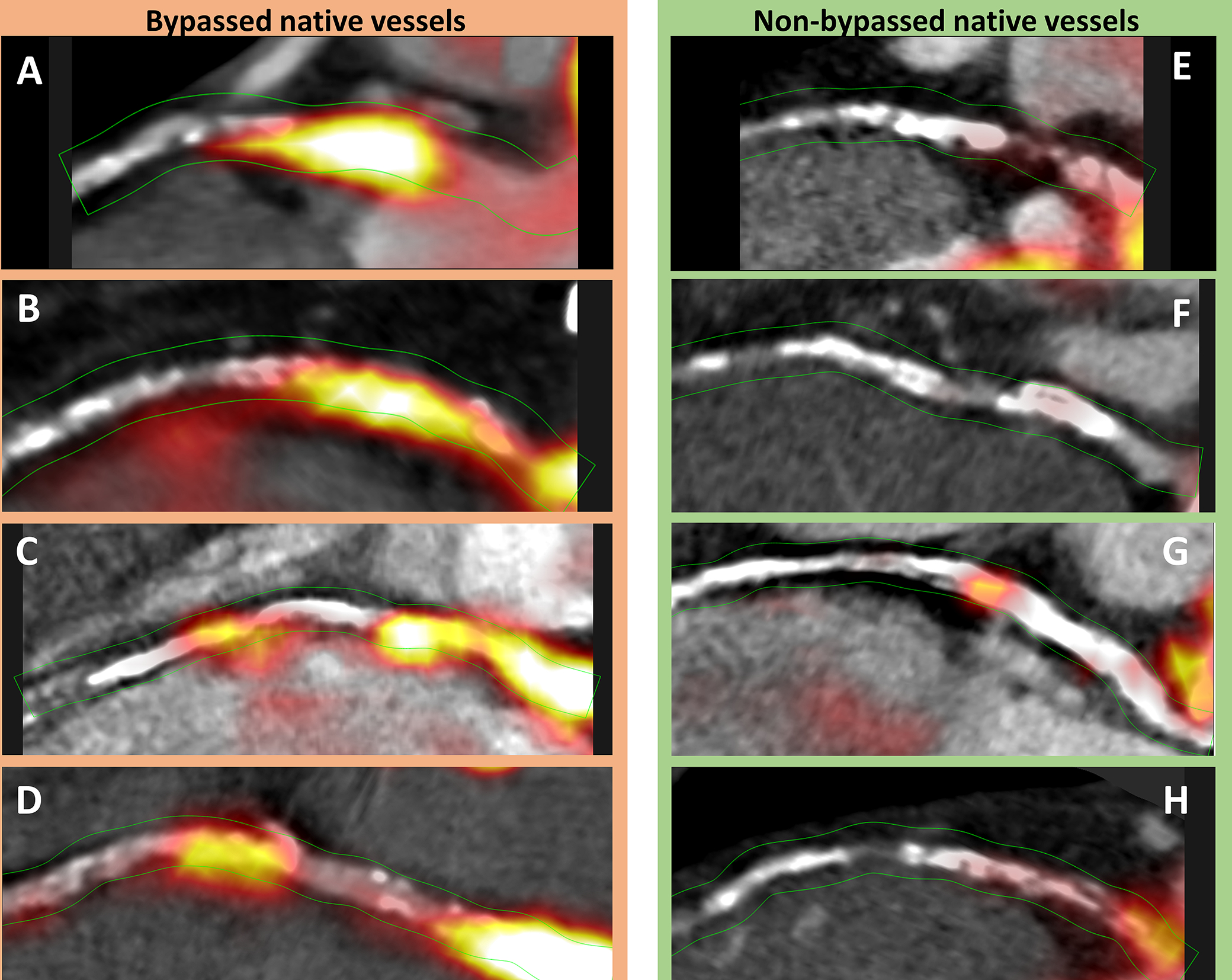
Case examples of 18F-sodium fluoride (18F-NaF) activity within native coronary segments proximal to a graft (A-D) and non-grafted coronary arteries with a similar plaque burden (E-H). While all the bypassed native vessels demonstrate intense 18F-NaF uptake (CMA > 2.0), the non-bypassed vessels showed no (CMA = 0), or only very subtle 18F-NaF activity (CMA = 0.32 and 0.47 in G and H respectively).
To account for baseline differences in disease severity, we identified a matched control group of 48 patients without a history of prior CABG but who had a similar baseline CT calcium scores, plaque burden on coronary CT angiography, segment involvement scores and cardiovascular co-morbidities (Table 2). Native coronary 18F-NaF activity remained twice as high in patients with a history of previous CABG surgery compared to matched patients without CABG surgery (CMA 2.0 [0.4–7.5] vs 0.8 [0.3–3.2], p<0.001; Table 2). Moreover, in the patients who underwent repeated non-contrast CT (n=33/48), calcium scores progressed three times more quickly within bypassed native coronary segments (proximal to the graft anastomosis n=116) compared to proximal coronary segments (n=116) of matched patients without a history of surgical revascularization (24 [16–43] vs 8 [0–24] %, p=0.002; Central Figure). Interestingly, such differences in disease progression were not observed when comparing coronary segments distal to the graft anastomosis with equivalent distal coronary segments in those without prior CABG surgery (7 [5–12] vs 11 [7–14] %, p=0.28). However, when comparing bypassed versus non-bypassed vessels in patients with surgical revascularization again disease progression was more pronounced in bypassed coronary arteries (24 [16–43]) vs 10 [1–26] %, p=0.01).
Table 2.
Characteristics of patient with and without prior coronary artery bypass graft surgery matched according to plaque burden and co-morbidities.
| Patients with prior coronary artery bypass graft surgery n=48 |
Patients without prior coronary bypass graft surgery n=48 |
p value | |
|---|---|---|---|
|
| |||
| Age (years) | 68±8 | 69±6 | 0.61 |
|
| |||
| Men | 44 (93%) | 44 (93%) | 1.00 |
|
| |||
| Body-mass index (kg/m2) | 28.6±4 | 29.2±6 | 0.62 |
|
| |||
| Systolic blood pressure (mmHg) | 144±21 | 145±19 | 0.81 |
|
| |||
| Diastolic blood pressure (mmHg) | 80±11 | 79±12 | 0.74 |
|
| |||
| Cardiovascular history | |||
|
| |||
| Acute coronary syndrome | 29 (60%) | 31 (65%) | 0.67 |
|
| |||
| Percutaneous coronary intervention | 17 (35%) | 36 (75%) | 0.001 |
|
| |||
| Coronary artery bypass graft surgery | 48 (100%) | 0 | <0.001 |
|
| |||
| Cerebrovascular accident/ Transient ischemic attack | 2 (4.2%) | 3 (6%) | 0.65 |
|
| |||
| Comorbidities/risk factors | |||
|
| |||
| Hypertension | 36 (75%) | 32 (67%) | 0.37 |
|
| |||
| Hyperlipidemia | 47 (98%) | 43 (89%) | 0.10 |
|
| |||
| Diabetes mellitus | 11 (23%) | 15 (31%) | 0.36 |
|
| |||
| Current smoking | 5 (10%) | 6 (12%) | 0.75 |
| Ex-smoker | 24 (50%) | 19 (40%) | 0.47 |
|
| |||
| Atrial fibrillation | 2 (4%) | 3 (6%) | 0.65 |
|
| |||
| Peripheral vascular disease | 8 (17%) | 5 (10%) | 0.37 |
|
| |||
| Medications * | |||
|
| |||
| Aspirin | 46 (96%) | 45 (94%) | 0.76 |
|
| |||
| P2Y12 receptor antagonist | 3 (6%) | 6 (12%) | 0.29 |
|
| |||
| Statin | 46 (96%) | 45 (94%) | 0.76 |
|
| |||
| Beta Blocker | 30 (63%) | 35 (73%) | 0.57 |
|
| |||
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 34 (71%) | 36 (75%) | 0.73 |
|
| |||
| Insulin | 1 (2%) | 1 (2%) | 1.00 |
|
| |||
| Oral diabetic medications | 7 (15%) | 9 (19%) | 0.78 |
|
| |||
| Calcium channel blocker | 17 (35%) | 12 (25%) | 0.27 |
|
| |||
| Diuretics | 15 (31%) | 10 (22%) | 0.24 |
|
| |||
| Clinical Biochemistry | |||
|
| |||
| Total Cholesterol (mmol/L) | 3.9 [3.4–4.7] | 4.0 [3.6–4.8] | 0.73 |
|
| |||
| Low density lipoprotein (mmol/L) | 1.9 [1.3–2.5] | 2.0 [1.5–2.6] | 0.62 |
|
| |||
| High density lipoprotein (mmol/L) | 1.2 [0.9–1.6] | 1.1 [1.0–1.5] | 0.40 |
|
| |||
| Triglycerides (mmol/L) | 1.5 [1.2–2.3] | 1.5 [1.1–2.3] | 0.48 |
|
| |||
| Creatinine (mgl/dL) | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 0.22 |
|
| |||
| eGFR (mL/min/1.73m2) | 62 [53–69] | 61 [52–67] | 0.58 |
|
| |||
| Patients with eGFR <60 mL/min/1.73m2 | 6 (13%) | 7 (15%) | 0.77 |
|
| |||
| Coronary Stent, n (%) | 16 [33%] | 38 [79%] | <0.001 |
|
| |||
| Segment involvement score | 8 [6–9] | 7 [5–9] | 0.14 |
|
| |||
| 0–1 | 0 (0%) | 0 (0%) | N/A |
| 2–3 | 1 (2%) | 2 (4%) | 0.21 |
| 4–5 | 5 (10%) | 7 (15%) | 0.18 |
| >5 | 42 (88%) | 39 (81%) | 0.64 |
|
| |||
| Coronary Calcium Score (AU) | 1135 [625–2165] | 1108 [894–1537] | 0.26 |
|
| |||
| Quantitative coronary plaque analysis | |||
|
| |||
| Non-calcified plaque volume (mm3) | 1606 [1095–2185] | 1566 [1006–1949] | 0.29 |
|
| |||
| Low attenuation plaque volume (mm3) | 136 [72–263] | 124 [78–211] | 0.34 |
|
| |||
| Total plaque volume (mm3) | 1797 [1198–2615] | 1820 [1144–2197] | 0.38 |
|
| |||
| Total plaque volume within bypassed coronary segments (mm3) | 1581 [1062–2297] | - | |
|
| |||
| Risk scores | |||
|
| |||
| Duke score | 5 [4–6] | 5 [3–6] | 0.42 |
|
| |||
| SMART risk score | 23 [17–30] | 24 [18–31] | 0.69 |
|
| |||
| 18 F-Sodium fluoride uptake | |||
|
| |||
| TBRmax | 1.42 [1.24–1.59] | 1.40 [1.23–1.55] | 0.50 |
|
| |||
| TBRmax – native coronaries | 1.40 [1.21–1.58] | 1.40 [1.23–1.55] | 0.83 |
|
| |||
| TBRmax – CABG | 1.61 [1.34–2.57] | - | |
|
| |||
| CMA total | 2.1 [0.37–7.83] | 0.8 [0.31–3.23] | <0.001 |
|
| |||
| CMA – native coronary arteries | 2.0 [0.37–7.53] | 0.8 [0.31–3.23] | <0.001 |
|
| |||
| CMA - CABG | 6.9 [0.63–11.58] | - | |
|
| |||
| No of positive plaques - total | 3 [1–4] | 3 [2–3] | 0.28 |
|
| |||
| No of positive plaques – native coronaries | 3 [1–4] | 3 [2–3] | 0.28 |
|
| |||
| Coronary artery disease progression | |||
|
| |||
| Change in calcium scores# (%) | 24 [16–43] | 8 [0–24] | 0.002 |
CABG – coronary artery bypass graft, CMA – coronary microcalcification activity, SMART Secondary Manifestations of Arterial Disease, TBR – target to background ratio
n (%), mean±SD, median [interquartile range]
medications at the time of scan
data available for 33 CABG individuals
Central Figure: Bypassed native coronary arteries demonstrate increased disease activity and faster disease progression.
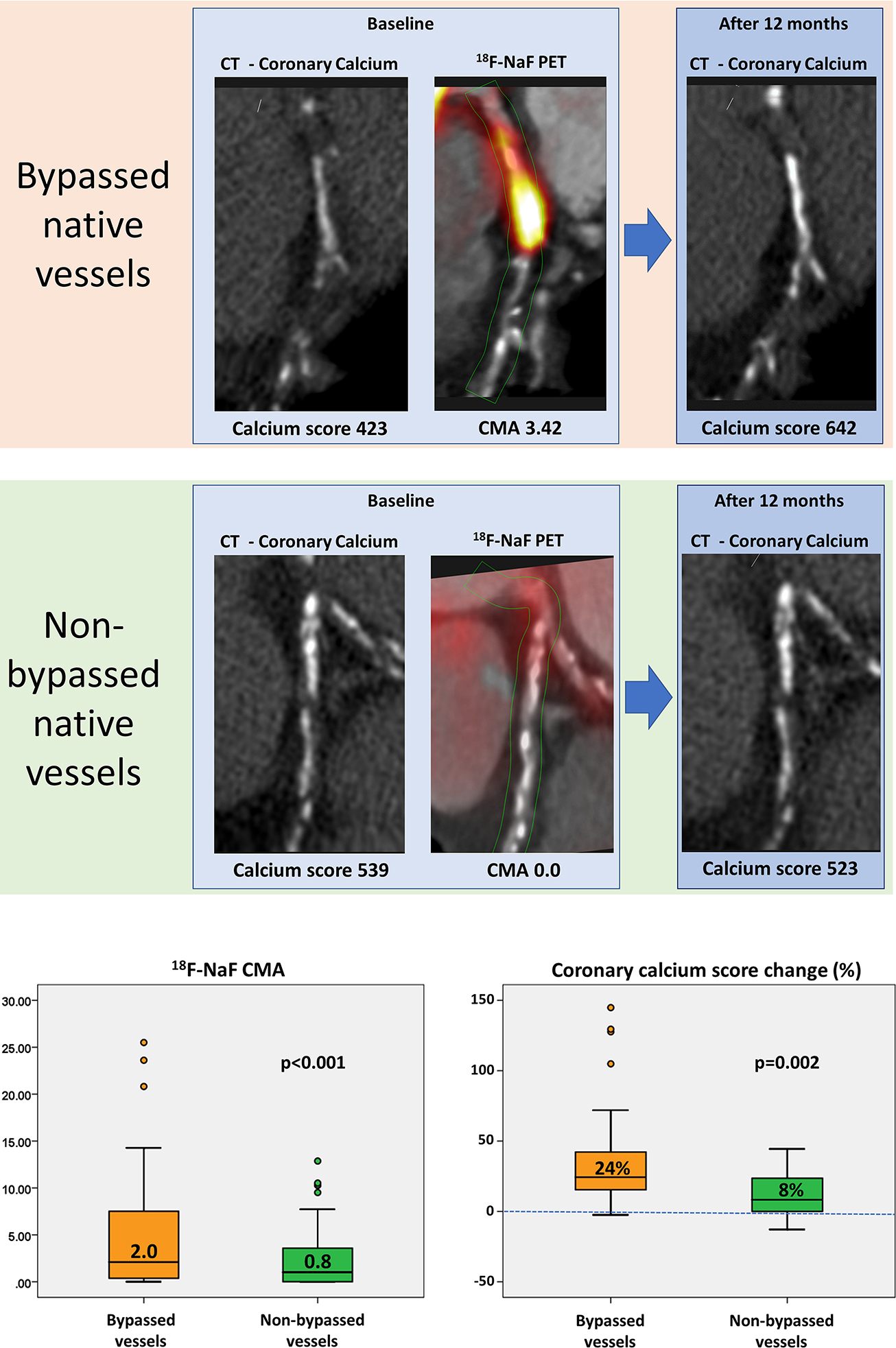
Co-registered coronary CT angiography with 18F-sodium fluoride (18F-NaF) positron emission tomography (PET) and non-contrast CT at baseline and after 12 months of follow-up in a patient with (top row) and without (bottom row) a history of surgical revascularization. Despite similar co-morbidities and plaque burdens bypassed native coronary vessels demonstrate both greater disease activity (coronary microcalcification activity [CMA] on 18F-NaF PET) and more rapid disease progression (change in the coronary calcium score on CT) than non-bypassed coronary arteries.
Discussion
This is the first study to investigate both coronary artery disease activity and progression in patients with and without prior CABG surgery using 18F-NaF PET and CT coronary artery calcium scoring. We have demonstrated that both disease activity and disease progression are higher in bypassed native vessels and that this is independent of the overall plaque burden. In contrast, 18F-NaF uptake was detectable in only 6% of saphenous vein grafts that could occur in the absence of detectable graft vasculopathy on coronary CT angiography. Our study supports the hypothesis that CABG surgery accelerates disease activity and progression in the native coronary vasculature and that 18F-NaF graft vasculopathy has distinct differences to native coronary artery disease.
CABG surgery is the gold-standard technique for coronary revascularization in subjects with multivessel coronary artery disease. However, it has been postulated that patients who undergo CABG develop accelerated progression of atherosclerosis in native coronary artery segments proximal to the graft anastomosis (30). Here, we have provided the first demonstration that coronary disease activity, as assessed by 18F-NaF PET and CMA, is higher in patients with prior CABG surgery. However, these results may simply reflect the fact that patients with advanced disease are more likely to undergo CABG surgery. To address this potential confounding issue, we undertook additional analyses where patients with prior CABG surgery were matched to patients with a similar extent of coronary plaque burden and prevalence, medication and prevalence of cardiovascular risk factors. Following this rigorous matching, we continue to see higher uptake and activity in the proximal segments of native coronary arteries which had undergone bypass grafting suggesting that this is not simply reflective of baseline differences in disease severity (Central Illustration).
We next explored whether there was any evidence of more rapid disease progression in the proximal native coronary segments that had been bypassed. Again, we observed that, following matching for co-morbidities, medication and disease severity, coronary artery calcium score progressed more rapidly in coronary arteries proximal to bypass anastomosis. Bypassed native coronary vessels therefore demonstrate both greater disease activity and more rapid disease progression than non-bypassed coronary arteries despite similar plaque burdens and co-morbidities (31).
While the mechanisms for atherosclerosis progression within bypassed native coronary arteries are not well understood, altered wall shear stress appears to be a contributing factor (32). Due to the presence of grafts, the pressure and wall shear stress can be markedly reduced proximal to the bypass anastomosis. In coronary plaques with a preserved lumen, low wall shear stress has been correlated to a reduction in smooth muscle cells, reduced collagen production, increased metalloproteinase activity, enlargement of plaque area, increased plaque eccentricity and reduction in lumen and vessel area (33, 34). Low wall shear stress also activates numerous inflammatory pathways, including inflammatory adhesion molecules, nuclear factor κB, and JNK-cAMP-dependent transcription factor ATF-2 (35). Within such an environment, ongoing inflammatory stimuli encourage plaque progression (36). Additionally, the reduction in coronary flow can reduce the flow-mediated and endothelium-dependent release of athero-protective nitric oxide into the subintimal and intimal space of the arterial wall favoring disease progression. This low shear stress hypothesis is supported by our observation here that increased disease activity and faster disease progression was observed in coronary plaque proximal but not distal to the graft anastomosis.
Our study explored 18F-NaF activity in arterial and venous bypass grafts. Our findings are consistent with previous studies demonstrating that, whilst arterial grafts demonstrate excellent longevity, vein grafts are subject to degeneration (37). Other than thrombosis which can occlude 10–25% of saphenous vein grafts, intimal hyperplasia is the dominant pathological process affecting vein grafts within the first year after surgery and is the foundation for subsequent atherosclerosis development (38). While coronary lesions develop over decades, the early stages of saphenous vein graft degeneration are detectable after just 1 year, with necrotic core formation present at 2–5 years and intraplaque hemorrhages or plaque ruptures present 5 years after surgery (39, 40). These pathological processes can trigger calcification, which can be non-invasively assessed with 18F-NaF PET (41–43). In our current study, only 7 patients (15%) and 8 venous grafts (6%) showed evident 18F-NaF bypass uptake. No uptake was observed in any arterial grafts. This low prevalence is likely a result of the relatively low median age of bypass grafts (2.7 years) and almost universal adherence to guideline-directed medical therapy within our study population. Importantly, similar to previous studies focused on native coronary arteries, we observed 18F-NaF bypass graft uptake in the absence of atherosclerotic plaque (44). Such PET-positive and CT-negative findings are likely to be the result of developing microcalcifications within atherosclerotic plaque which are beyond the resolution of CT angiography or fibro-intimal hyperplasia as part of the pathology and arterialization of the vein graft (41–43). Furthermore, whether such 18F-NaF uptake within saphenous vein graft predicts progression of disease or adverse events should be assessed within larger observational studies with long-term follow-up. In the future coronary artery disease progression in bypassed and non-bypassed vessels requires more attention. 18F-NaF coronary PET could serve as a tool for assessment of disease activity in studies investigating therapies targeting progression of the atherosclerotic plaque.
Our study has a number of limitations. Patients with prior CABG surgery only comprised 16% of the study population and the length of follow up was modest. Because of the limited sample size, we did not make adjustments for multiple observations (grafts) in a single patient. It would therefore be important to study larger cohorts of patients with older bypass grafts as well as examine the changes with more prolonged follow up to investigate the later stages of graft vasculopathy. Although we have attempted to match our patient populations for risk factors and disease burden in our sensitivity analyses, we acknowledge that there remains a possibility of residual confounding in our comparisons. Ideally, repeated 18F-NaF PET and calcium scoring before and years after CABG surgery with a matched contemporaneous control group without surgery would be needed to confirm our findings more definitively. The matched control group will however be challenging as percutaneous coronary intervention and coronary revascularization might also confound the results and nullify subsequent follow up comparisons.
In conclusion, native coronary arteries that have been bypassed, demonstrate increased disease activity and more rapid disease progression than non-bypassed arteries, an observation that appears independent of baseline atherosclerotic plaque burden. Microcalcification activity is not a dominant feature of graft vasculopathy.
Supplementary Material
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Surgically bypassed native coronary arteries demonstrate increased atherosclerotic activity and more rapid disease progression than non-bypassed arteries.
TRANSLATIONAL OUTLOOK:
Additional research with long-term follow-up is necessary to confirm our findings and evaluate their potential clinical relevance.
Funding and Acknowledgements
This research was supported in part by grants R01HL135557 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DEN (CH/09/002, RE/18/5/34216, RG/16/10/32375), ET (FS/CRTF/20/24086), AJF (FS/19/15/34155), AHB (RG/20/5/34796), MRD (FS/14/78/31020) and MCW (FS/ICRF/20/26002) are supported by the British Heart Foundation. PDA is supported by Heart Foundation of New Zealand Senior Fellowship (1844). EvB is supported by the Scottish Imaging Network (www.sinapse.ac.uk). ET was supported by a grant from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. DEN is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA) and MRD of the Sir Jules Thorn Award for Biomedical Research Award (2015). SLS is supported by fellowships from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research.
List of abbreviations:
- 18F-NaF
18F-sodium fluoride
- AU
Agatston units
- CABG
Coronary artery bypass grafts
- CMA
Coronary microcalcification activity
- NCP
Non-calcified plaque
- PET
Positron emission tomography
- SMART
Secondary Manifestations of ARTerial disease
- SUV
Standard uptake value
- TPV
Total plaque volume
Footnotes
Disclosures
The authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
References
- 1.Goldman S, Sethi G, Holman W, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: A randomized trial. JAMA. 2011. 305, 167–174. doi: 10.1001/jama.2010.1976. [DOI] [PubMed] [Google Scholar]
- 2.Desai ND, Cohen EA, Naylor CD, Fremes SE. Radial Artery Patency Study Investigators. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302–9. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 3.Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai Sedrakyan A, et al. , for the RADIAL Investigators. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. 2018; 378:2069–2077 DOI: 10.1056/NEJMoa171602 [DOI] [PubMed] [Google Scholar]
- 4.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149–2156. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 6.Bourassa MG, Fisher LD, Campeau L, Gillespie MJ, McConney M, Lespérance J. Long-term fate of bypass grafts: the Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation 1985. Dec;72(6 Pt 2):V71–8. [PubMed] [Google Scholar]
- 7.Robert EW, Guthaner DF, Wexler L, Alderman EL. Six-year clinical and angiographic follow-up of patients with previously documented complete revascularization. Circulation 1978;58:I194–I199. [PubMed] [Google Scholar]
- 8.Ivert T, Landou C. Changes in coronary artery disease five years after coronary bypass surgery. Scand J Thorac Cardiovasc Surg 1981;15:187–198. [DOI] [PubMed] [Google Scholar]
- 9.Dweck MR, Jenkins WSA, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circulation-Cardiovascular Imaging. 2014, 7(2), 371–378. [DOI] [PubMed] [Google Scholar]
- 10.Cartlidge TRG, Doris MK, Sellers SL, et al. Detection and prediction of bioprosthetic aortic valve degeneration. J Am Coll Cardiol 2019;73:1107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiecinski J, Tzolos E, Cartlidge TRG, et al. Native Aortic Valve Disease Progression and Bioprosthetic Valve Degeneration in Patients with Transcatheter Aortic Valve Implantation. Circulation. 2021. Aug 29 Online ahead of print; doi: 10.1161/CIRCULATIONAHA.121.056891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsythe RO, Dweck MR, McBride OMB, et al. F-18-Sodium fluoride uptake in abdominal aortic aneurysms: The SoFIA(3) Study. Journal of the American College of Cardiology, 71(5), 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13 [DOI] [PubMed] [Google Scholar]
- 14.Kwiecinski J, Tzolos E, Adamson PD et al. 18F-Sodium fluoride coronary uptake predicts outcome in patients with coronary artery disease. J Am Coll Cardiol 2020;75:3061–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiecinski J, Tzolos E, Meah M et al. Machine-learning with 18F-sodium fluoride PET and quantitative plaque analysis on CT angiography for the future risk of myocardial infarction. J Nucl Med. 2021. Apr 23:jnumed.121.262283. Online ahead of print. doi: 10.2967/jnumed.121.262283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorresteijn JA, Visseren FL, Wassink AM et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease:the SMART risk score. Heart 2013;99:866–72. [DOI] [PubMed] [Google Scholar]
- 17.Doris MK, Otaki Y, Krishnan SK, et al. Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol 2020;27(2):494–504. doi: 10.1007/s12350-018-1317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubeaux M, Joshi N, Dweck MR, et al. Motion correction of 18F-sodium fluoride PET for imaging coronary atherosclerotic plaques. J Nucl Med 2016;57:54–9 [DOI] [PubMed] [Google Scholar]
- 19.Kwiecinski J, Berman DS, Lee SE et al. Three-hour delayed imaging improves assessment of coronary 18F-sodium fluoride PET. J Nucl Med 2019; 60(4):530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassen ML, Kwiecinski J, Dey D, et al. Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary 18F-NaF PET. Eur J Nucl Med Mol Imaging. 2019;46:2610–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massera D, Doris MK, Cadet S et al. Analytical quantification of aortic valve 18F-sodium fluoride PET uptake. J Nucl Cardiol. 2020;27(3):962–972. doi: 10.1007/s12350-018-01542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiecinski J, Adamson PD, Lassen ML et al. Feasibility of coronary 18F-sodium fluoride PET assessment with the utilization of previously acquired CT angiography. 2018. Circ Cardiovasc Imaging 11(12): e008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwiecinski J, Cadet S, Dey D et al. Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020;47:1736–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzolos E, Kwiecinski J, Lassen ML. et al. Observer repeatability and interscan reproducibility of 18F-sodium fluoride coronary microcalcification activity. J. Nucl. Cardiol. (2020). 10.1007/s12350-020-02221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 26.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 27.Hell MM, Motwani M, Otaki Y et al. Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging 2017;18:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams M, Kwiecinski J, Doris M, et al. Low attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction. Circulation 2020;(18),1452–1462 DOI: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss AJ, Sim AM, Adamson PD, et al. Ex vivo 18 F-fluoride uptake and hydroxyapatite deposition in human coronary atherosclerosis. Sci Rep 2020;19;10(1):20172. doi: 10.1038/s41598-020-77391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang MH, Meadows WR, Palac RT, Piao ZE, Pifarre R, Loeb HS, Gunnar RM. Progression of native coronary artery disease at 10 years: insights from a randomized study of medical versus surgical therapy for angina, J Am Coll Cardiol, 1990, vol. 16 (pg. 1066–1070) 10.1016/0735-1097(90)90533-U [DOI] [PubMed] [Google Scholar]
- 31.Doris MK, Meah MN, Moss AJ et al. Coronary 18 F-fluoride uptake and progression of coronary artery calcification. Circ Cardiovasc Imaging 2020. Dec;13(12):e011438. doi: 10.1161/CIRCIMAGING.120.011438. Epub 2020 Dec 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Thompson EW, Lefieux A, et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J Am Coll Cardiol 2018;72:1926–1935. [DOI] [PubMed] [Google Scholar]
- 33.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 2011;124:779–788. [DOI] [PubMed] [Google Scholar]
- 34.Costopoulos C, Timmins LH, Huang Y, et al. Impact of combined plaque structural stress and wall shear stress on coronary plaque progression, regression, and changes in composition. Eur Heart J 2019;doi: 10.1093/eurheartj/ehz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakellarios AI, Bizopoulos P, Papafaklis MI, et al. Natural history of carotid atherosclerosis in relation to the hemodynamic environment. Angiology, 68 (2017), pp. 109–118 [DOI] [PubMed] [Google Scholar]
- 36.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation, 117 (2008), pp. 993–1002 [DOI] [PubMed] [Google Scholar]
- 37.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13:79. [DOI] [PubMed] [Google Scholar]
- 38.Motwani JG, Topol EJ. Aortocoronary Saphenous Vein Graft Disease: Pathogenesis, Predisposition, and Prevention. Circulation 1998;97:916–931. [DOI] [PubMed] [Google Scholar]
- 39.Walts AE, Fishbein MC, Matloff JM. Thrombosed, ruptured atheromatous plaques in saphenous vein coronary artery bypass grafts: Ten years’ experience. Am Heart J 1987;114:718–723. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Théroux P, Lespérance J, et al. Angiographic features of vein grafts versus ungrafted coronary arteries in patients with unstable angina and previous bypass surgery. J Am Coll Cardiol. 1996. Nov, 28 (6) 1493–1499. [DOI] [PubMed] [Google Scholar]
- 41.McKenney-Drake ML, Territo PR, Salavati A et al. 18F-NaF PET imaging of early coronary artery calcification. JACC Img 2016;9:627–628 [DOI] [PubMed] [Google Scholar]
- 42.Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creager MD, Hohl T, Hutcheson et al. (18)F-fluoride signal amplification identifies microcalcifications associated with atherosclerotic plaque instability in Positron emission tomography/computed tomography images. Circ Cardiovasc Imaging 2019;12:e007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwiecinski J, Dey D, Cadet S, et al. Predictors of 18F-sodium fluoride uptake in patients with stable coronary artery disease and adverse plaque features on computed tomography angiography. Eur Heart J Cardiovasc Imaging 2020. 21(1):58–66. doi: 10.1093/ehjci/jez152 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


