Abstract
Lactococcus lactis subsp. cremoris C60 is a probiotic strain that induces diverse functional modifications in immune cells. In this report, as a novel effect of C60 on myeloid lineage cells, we show that C60 enhances the immunological function of macrophages that consequently promotes CD4+ T cell activity in an antigen-dependent manner. Heat-killed (HK) C60 induced the production of pro-inflammatory cytokines in thioglycolate-elicited peritoneal macrophages (TPMs) much stronger than Toll-like receptor (TLR) ligand stimulation. The HK-C60 treatment also augmented the expression of antigen-presenting and co-stimulatory molecules, such as major histocompatibility complex (MHC) class II, CD80, and CD86, as well as antigen uptake in TPMs. These HK-C60-mediated functional upregulations in TPMs resulted in the promotion of CD4+ T cell activation in an antigen-dependent manner. Interestingly, the TPMs that originated from the mice fed the HK-C60 diet showed pre-activated characteristics, which was confirmed by the upregulation of cytokine production and antigen presentation-related molecule expression under lipopolysaccharide (LPS) stimulation. Furthermore, the antigen-dependent CD4+ T cell activation was also enhanced by the TPMs. This implied that antigen presentation activity was enhanced in the TPMs that originated from the HK-C60 diet mice. Thus, C60 effectively upregulates the immunological function of macrophages that directly connects to CD4+ T cell-based adaptive immunity.
Keywords: lactic acid bacteria, macrophages, CD4+ T cells, IFN-γ, IL-6, IL-12/IL-23p40, TNF-α, probiotics, antigen presentation
INTRODUCTION
Macrophages are innate immune cells that originate from myeloid lineage-derived precursors produced in bone marrow [1]. They show diverse function in various biological events depending on their immunological characters [2]. One of the primary functions of macrophages is as an innate defense against various pathogens [3, 4]. For instance, macrophages effectively eliminate bacteria in infected sites by phagocytosis, opsonization, and the production of anti-bacterial agents and cytokines that collaborate with neutrophils [5,6,7]. In these responses, pattern recognition receptors (PPRs), such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), and Fc receptors, participate in the recognition of/binding to bacteria prior to incorporation and degradation in the cytosol [8, 9]. Once bacteria are recognized and incorporated in a phagocytotic manner, macrophages are able to directly eliminate them in their phagosomes using various hydrolases to digest the pathogens [10]. In adaptive immunity, macrophages also have an important role in regulation of T cells activity [11]. Since macrophages are characterized as antigen-presenting cells (APCs), as well as dendritic cells (DCs), B cells, and neutrophils, they activate both CD4+ and CD8+ T cells by presenting antigens and producing cytokines in peripheral tissues and lymphoid organs [12]. The function of macrophages is thought to be much more focused on than that of DCs, especially for antigen-dependent reactivation of effector and memory T cells in the peripheral tissues [13].
Lactic acid bacteria (LAB) are major commensal bacteria, especially in the intestine, and are frequently utilized in fermented foods [14, 15]. Since some LAB strains have been identified as nonpathogenic bacteria (beneficial bacteria for our health), those strains are frequently utilized in supplements or food ingredients that aim to maintain proper internal homeostasis and to augment intestinal immunity [14,15,16]. We previously reported that Lactococcus lactis subsp. cremoris C60, a probiotic LAB strain, promoted the generation and activation of CD4+ T cells in the small intestine [17]. As an underlying mechanism, we revealed that a heat-killed C60 (HK-C60) diet promoted not only cytokine production but also antigen-presenting activity for CD4+ T cells in intestinal DCs.
We have identified an immunological function of C60: this strain induced a strong immuno-modification in DCs that consequently promoted the activation of effector T cells [17]. While, previous studies have reported that various LAB strains have the potential to enhance the functions of macrophages, and this prompted us to investigate the novel immunological functions of C60 in macrophages [18, 19]. In this report, we show that HK-C60 activated and modified immunological functions of macrophages. HK-C60-mediated macrophage activation consequently promoted CD4+ T cell activation in an antigen-specific manner. This is the first report showing a direct contribution of C60 to the immunological activities of macrophages.
MATERIALS AND METHODS
Mice
C57BL/6J and OT-II transgenic mice were purchased from Clea Japan (Tokyo, Japan). The mice were maintained under specific pathogen-free (SPF) conditions with a 12 hr day/night cycle and were allowed free access to food and water. Gender- and age-matched adult mice (8–12 weeks) were used for each experiment. To maintain similar microbiota in the gut, all mice used in the HK-LAB diet study were bred for at least 6 months in the same facility. All experiments protocols were reviewed and approved by the Animal Welfare Committee of AIST (protocol No. 109) and Jichi Medical University (protocol No. 20036-01, 20037-01).
Lactic acid bacteria
L. lactis subsp. cremoris C60 was provided by the National Agriculture and Food Research Organization (NARO). L. lactis subsp. cremoris SK-11 and HP were both purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). C60 was cultured by following the method described in a previous report [17]. The SK-11 and HP strains were cultured by following their product data sheets. Briefly, the bacteria were cultured in MRS broth (BD DifcoTM, BD Biosciences, Franklin Lakes, NJ, USA) at 37°C for 24 hr, and the bacterial colony-forming units per milliliter (CFU/mL) was calculated for each culture. To prepare HK-C60, the bacteria were heated at 95°C for 10 min, and then the bacterial cells were precipitated by centrifugation at 5,000 g for 10 min at 4°C. After washing with PBS, the cell pellet was resuspended in 0.9% NaCl (saline) or PBS and stored at −80°C until use. The sample was used as HK-C60.
LAB diet
To investigate the effect of a diet containing HK-C60, the mice were fed AIN-93G pellet (control; Oriental Yeast Co., Ltd., Tokyo, Japan) or HK-C60 kneaded pellet (containing 1.0×109 bacterial cells/g, AIN-93G based; Oriental Yeast Co., Ltd.) by following a protocol described in a previous report [17]. Briefly, the mice were fed the control diet for 1 week (habituation) followed by HK-C60 kneaded or control pellet for the next 2 weeks.
Preparation of murine macrophages
Thioglycolate-elicited peritoneal macrophages (TPMs) were prepared by following a protocol described in a previous report [20]. Briefly, the mice received an intraperitoneal (i.p.) injection of 2.5 mL of 3% thioglycolate (BD Biosciences). After 84–96 hr, the infiltrated leukocytes in the peritoneal cavity were collected with PBS, and the cells were seeded in a cell culture plate after washing with a cell culture medium comprised of RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 μM 2-mercaptoethanol (2-ME), 10 mM HEPES, 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells were then incubated at 37°C for 2 hr, and the adherent cells were harvested as TPMs. The purity of TPMs was analyzed by flow cytometry. Samples with CD11b+F4/80+ > 90% were used for the experiment.
Macrophage stimulation assay
TPMs (1.0×106/mL) were seeded on a 24-well plate and then treated with lipopolysaccharide (LPS, 100 ng/mL; Sigma Aldrich, St. Louis, MO, USA), peptidoglycan (PGN, 10 µg/mL; InvivoGen, San Diego, CA, USA), polyinosinic-polycytidylic acid (poly(I:C), 50 µg/mL; InvivoGen), HK-C60 (0.1, 0.5, 1.0, 2.5, or 5.0×107 CFU/mL), other HK-LABs (SK-11 or HP, 5.0×107 CFU/mL), or the vehicle control (PBS: phosphate buffered saline). The cultures were incubated at 37°C for 24 hr, and then the cultured medium was harvested and stored at −80°C until use. The cytokine concentration in the cultured medium was measured by ELISA (enzyme-linked immunosorbent assay). In some experiments, TPMs (1.0×106/mL) were seeded on a 12-well plate and treated with LPS (100 ng/mL), HK-C60 (5.0×107/mL), or the vehicle control (PBS) at 37°C for 6 hr, and then the cells were analyzed by flow cytometry.
Cytokine production assay in primary macrophages
Splenocytes, liver mononuclear cells (MNCs), and colon lamina propria (LP) leukocytes were isolated from both HK-C60 and control diet mice by following protocols described in previous reports [17, 21]. The isolated cells (3.0×106/mL) were treated with LPS (100 ng/mL) or the vehicle control (PBS) at 37°C for 6 hr in the presence of GolgiStopTM. TNF-α production was analyzed by flow cytometry.
In vitro antigen uptake
TPMs (1.0×106/mL) were seeded on a 12-well plate, and then the culture was treated with OVA (ovalbumin) -Alexa 488 (500 ng/mL, Thermo Fisher Scientific, Waltham, MA, USA) in the presence or absence of HK-C60 (5.0×107/mL). The culture was incubated at 37°C overnight, and then OVA uptake was analyzed by flow cytometry. The OVA-Alexa 488 signal was detected in the CD11b+F4/80+ gate.
Preparation of splenic CD4+ T cells
Splenocytes were prepared from spleens of OT-II transgenic mice. Spleens were crushed on a 70 μm cell strainer, and then the cells were washed with cell culture medium. After precipitating the cells by centrifugation at 300 g for 5 min, the cell pellet was treated with 1×RBC lysis buffer (Thermo Fisher Scientific) at room temperature for 10 min. After washing them twice with cell culture medium, the cells were collected by centrifugation at 300 g for 5 min. The CD4+ T cells in the cell suspension were enriched by using a mouse CD4+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and following the manufacturer’s instructions. The purity of CD4+ T cells was analyzed by flow cytometry. Samples with 90% > CD4+ T cells were used for the experiment.
In vitro antigen presentation assay
TPMs (2.0×106/mL) and CD4+ T cells (1.0×107/mL) were mixed and seeded on a 96-well plate in the presence of OVA323-339 peptide (100 ng/mL; Thermo Fisher Scientific). The culture was also treated with HK-C60 (5.0×108/mL) or the vehicle control. It was then incubated at 37°C for 24 hr, and the cellular activation and cytokine production in the CD4+ T cells were subsequently analyzed by flow cytometry.
Flow cytometry
Cell surface markers and intracellular cytokines were analyzed by flow cytometry (LSRFortessa SORP, FACSAria I; BD Biosciences) with fluorochrome-conjugated monoclonal antibodies. For intracellular cytokine staining of CD4+ T cells, samples were restimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL; Sigma Aldrich) and ionomycin (1 µg/mL; Sigma Aldrich) in the presence of GolgiStopTM (BD Biosciences) at 37°C for 6 hr before staining. The cells were first incubated in PBS/2% FBS with FcR blocker (anti-CD16/CD32 mAb; BioLegend, San Diego, CA, USA) at 4°C for 10 min. For surface marker staining, the cells were incubated with the antibody in PBS/2% FBS at 4°C for 30 min. For intracellular staining, the surface-stained samples were treated with Cytofix/Cytoperm (BD Biosciences) at 4°C for 20 min followed by staining for intracellular cytokines at 4°C for 30 min. All procedures for fixation and permeabilization were performed by following the Cytofix/Cytoperm manufacturer’s instruction manual. Dead cells were excluded by using forward scatter (FSC)/side scatter (SSC) and 7-aminoactinomycin D (7-AAD) staining in each analysis. All data were analyzed by BD FACSDiva (BD Biosciences) or FlowJo (BD Biosciences). Anti-mouse CD3ε (145-2C11), anti-mouse CD4 (GK1.5), anti-mouse CD8α (53-6.7), anti-mouse CD25 (3C7), anti-mouse CD69 (H1.2F3), anti-mouse IFN-γ (XMG1.2), anti-mouse TNF-α (MP6-XT22), anti-mouse IL-4 (11B11), anti-mouse IL-13 (W17010B), anti-mouse IL-17A (TC11-18H10.1), anti-mouse IL-10 (JES5-16E3), anti-mouse CD11b (M1/70), and anti-mouse F4/80 (BM8) were all purchased from BioLegend.
ELISA
Cytokines produced from stimulated TPMs were measured by DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) for each target. All procedures were performed by following the kit manufacturer’s instruction manual.
Statistics
Student’s t-test and one-way analysis of variance (ANOVA) were used to analyze the data for significant differences. Values of p<0.05, p<0.01, and p<0.001 were regarded as indicating significance.
RESULTS
C60 induces cytokine production and cellular activation in macrophages
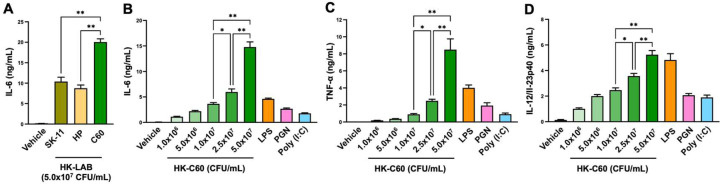
Since there were no reports about C60 in terms of the functional modification of macrophages, we first decided to investigate the effect of this LAB strain on the immunological activities of macrophages. When we compared the ability to induce cytokines among strains of L. lactis subsp. cremoris, C60 showed significantly high IL-6 production in TPMs compared with other strains (Fig. 1A). Next, TPMs were stimulated with LPS, PGN, Poly(I:C), or HK-C60, and then the cytokine production was measured by ELISA. The TPMs produced IL-6, TNF-α, and IL-12/IL-23p40 under TLR ligand stimulation, respectively. HK-C60 stimulation also induced these cytokines in a dose-dependently increased manner (Fig. 1B–D). We also analyzed the expression of surface molecules on TPMs, which correlated with antigen presentation, by flow cytometry. The expression levels of CD80, CD86, and I-A/I-E, which are generally upregulated in activated APCs [22, 23], were all significantly increased under HK-C60 stimulation compared with those of the vehicle control (Fig. 2A, B). The antigen uptake activity was also analyzed in the TPMs. OVA uptake was significantly promoted in the TPMs treated with HK-C60 compared with the vehicle control. The mean fluorescence intensity (MFI) of OVA-Alexa 488 incorporated into the TPMs was significantly higher in HK-C60-treated TPMs than TPMs treated with vehicle control (Fig. 2C, D).
Fig. 1.
HK-C60 induces pro-inflammatory cytokine production in macrophages.
A) Comparison of cytokine production between C60 and other strains of LAB. TPMs (1.0×106/mL) were treated with HK-LAB (5.0×107 CFU/mL) or the vehicle control (PBS) at 37°C for 24 hr, and the concentration of IL-6 in the cultured medium was measured by ELISA.
B–D) Comparison of cytokine production in HK-C60 and TLR ligand treatments. TPMs (1.0×106/mL) were treated with LPS (100 ng/mL), PGN (10 µg/mL), Poly(I:C) (50 µg/mL), HK-C60 (0.1, 0.5, 1.0, 2.5, or 5.0×107 CFU/mL), or the vehicle control (PBS). The cultures were incubated at 37°C for 24 hr, and the concentrations of IL-6 (B), TNF-α (C), and IL-12/IL-23p40 (D) in the cultured medium were measured by ELISA. The cumulative data are shown as the mean ± SEM of five samples in two independent experiments. *p<0.01 and **p<0.001 were regarded as indicating significance, respectively.
Fig. 2.
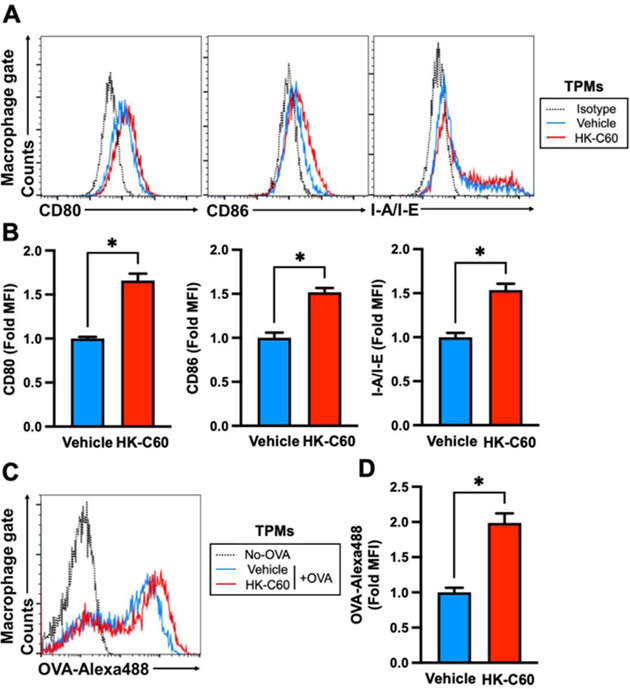
HK-C60 stimulation upregulates antigen presentation-related cellular activities in macrophages.
A, B) HK-C60-mediated cellular activation in macrophages. TPMs (1.0×106/mL) were treated with HK-C60 (5.0×107 CFU/mL) or the vehicle control (PBS) at 37°C for 6 hr. The expression levels of CD80, CD86, and I-A/I-E were analyzed by flow cytometry. A) Representative histogram of CD80, CD86, and I-A/I-E expression. (B) MFIs were calculated from the flow cytometry analysis. C, D) HK-C60-mediated upregulation of antigen uptake in macrophages. TPMs (1.0×106/mL) were treated with OVA-Alexa 488 (500 ng/mL), and then the culture was further treated with HK-C60 (5.0×107 CFU/mL) or the vehicle control and incubated at 37°C overnight. The OVA uptake was analyzed by flow cytometry. C) Representative histogram of OVA uptake in flow cytometry analysis. D) MFIs were calculated from the flow cytometry analysis. CD80, D86, I-A/I-E, and OVA-Alexa 488 signals were detected in the CD11b+F4/80+ gate in the flow cytometry analysis, respectively. The cumulative data are shown as the mean ± SEM of five samples in two independent experiments. *p<0.01 was regarded as indicating significance.
Taken together, HK-C60 treatment promoted immunological activities, such as cytokine production, antigen presentation-related molecule expression, and antigen uptake, in the macrophages.
HK-C60 enhances antigen presentation activity in macrophages that promotes functional differentiation and activation of CD4+ T cells
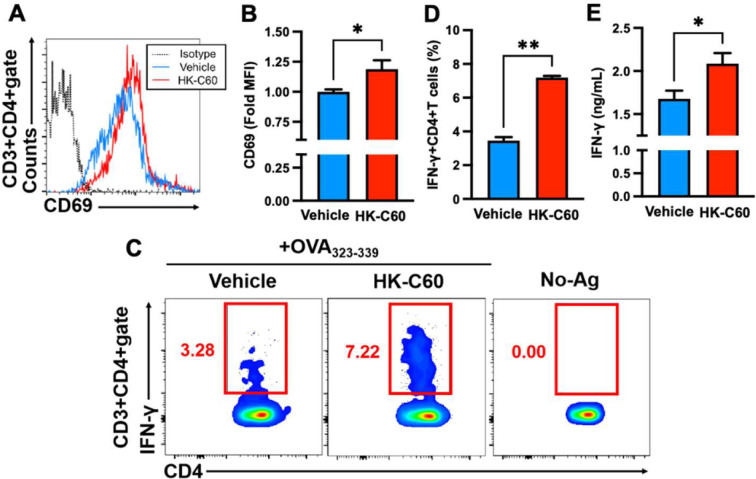
Since we found that HK-C60 enhanced immunological activities of macrophages, we next investigated the contribution of this HK-C60-mediated functional modification to subsequent immunological activities, such as the generation and activation of T cells. In vitro co-culture of TPMs and OT-II CD4+ T cells revealed that the expression of CD69 was significantly upregulated by the HK-C60 treatment compared with the vehicle control upon antigen-dependent stimulation (Fig. 3A, B). The differentiation of IFN-γ+CD4+ T (Th1) cells was promoted in the co-culture treated with HK-C60 (Fig. 3C). The percentage of IFN-γ-producing CD4+ T cells was significantly increased in the culture treated with HK-C60 compared with that treated with the vehicle control (Fig. 3D). IFN-γ production in the culture was measured by ELISA, and we confirmed that it was significantly increased by culture with HK-C60 compared with culture with the vehicle control (Fig. 3E). The productions of other cytokines were also analyzed in the co-cultured CD4+ T cells (Supplementary Fig. 1). Upregulation of TNF-α was induced in CD4+ T cells by the antigen-dependent activation in the presence of HK-C60 (Supplementary Fig. 1A). On the other hand, the productions of IL-4, IL-13, IL-17A, and IL-10 were all at similar levels for the cultures with or without HK-C60 (Supplementary Fig. 1B–E). We also found that HK-C60 treatment itself did not directly affect CD4+ T cell activity. The CD4+ T cells treated only with HK-C60 did not show aggressive differentiation into Th1 cells, and the percentage was similar to that of the CD4+ T cells treated with the vehicle control. In addition, the CD4+ T cells stimulated with anti-CD3/CD28 mAb showed identical percentages of Th1 cells between the cultures with and without HK-C60. However, the CD4+ T cells co-cultured with TPMs in the presence of OVA323-339 peptide showed that HK-C60 treatment significantly increased the percentage of IFN-γ-producing CD4+ T cells compared with the vehicle control treatment (Supplementary Fig. 2).
Fig. 3.
CD4+ T cell activities are enhanced through antigen presentation by macrophages in the presence of HK-C60.
A–E) HK-C60-mediated upregulation of effector CD4+ T cell generation and activation. Splenic CD4+ T cells were isolated from OT-II mice by magnetic sorting. The CD4+ T cells (2.0×106/mL) were cultured with TPMs (1.0×107/mL) in the presence of OVA323-339 peptide (100 ng/mL). No-Ag (antigen) indicates culture without OVA323-339. The cells were further treated with HK-C60 (5.0×108 CFU/mL) or the vehicle control and incubated at 37°C for 24 hr. After incubation, the percentage of IFN-γ production and CD69 expression in CD4+ T cells were analyzed by flow cytometry. The cultured medium was used for cytokine ELISA. A, B) Expression of CD69 in the CD4+ T cell population. A) Representative histogram of flow cytometry analysis for CD69 expression in CD4+ T cells. B) The MFI of CD69 in CD4+ T cells was calculated from the flow cytometry analysis. C, D) IFN-γ production in CD4+ T cells. C) Representative image of flow cytometry analysis for IFN-γ+CD4+ T cells. D) The percentage of IFN-γ+CD4+ T cells was calculated from the flow cytometry analysis. E) IFN-γ concentration in the cultured medium. IFN-γ production and CD69 expression were detected in the CD3+CD4+ gate in the flow cytometry analysis. The cumulative data are shown as the mean ± SEM of five samples in two independent experiments. *p<0.05 and **p<0.001 were regarded as indicating significance, respectively.
Thus, HK-C60 promoted antigen-specific effector CD4+ T cell generation and activation through the upregulation of macrophage activity.
HK-C60 diet augments the potential immunological activities of macrophages
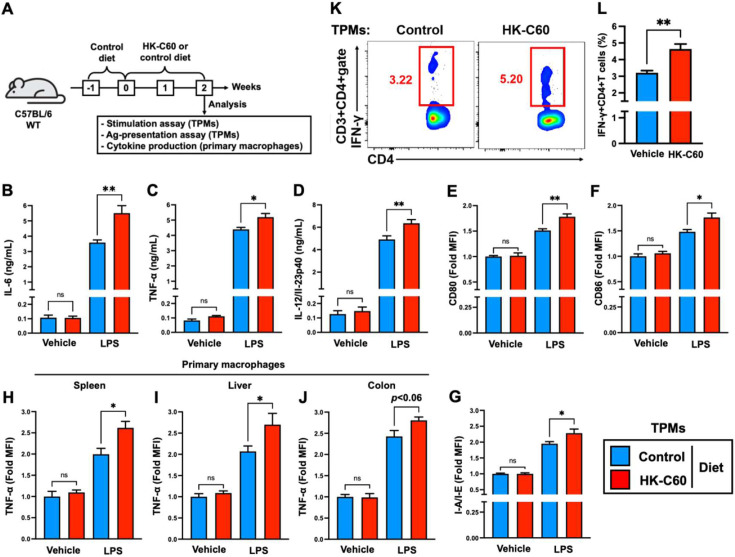
Finally, we investigated the possibility that the HK-C60 diet influences the potential immunological activities of macrophages under physiological conditions. TPMs were prepared from mice fed the HK-C60 or control diet. The activities of the TPMs were evaluated with LPS stimulation as well as antigen presentation to CD4+ T cells (Fig. 4A). Under LPS stimulation, cytokine productions were significantly increased in TPMs that originated from HK-C60 diet mice as compared with those that originated from control mice (Fig. 4 B–D). Furthermore, the expression levels of CD80, CD86, and I-A/I-E were all significantly upregulated in LPS-stimulated TPMs that originated from HK-C60 diet mice compared with those that originated from control mice (Fig. 4E–G). However, the HK-C60 diet did not influence the basal production of cytokines or expression of surface molecules of the TPMs, respectively. Similar results were obtained by using primary macrophages. The macrophages in the spleen, liver, and colon LP of HK-C60 diet mice showed higher levels of LPS stimulation-mediated TNF-α production compared with those of control mice (Fig. 4H–J).
Fig. 4.
The HK-C60 diet enhances differentiated macrophage activity, leading to CD4+ T cell activation in an antigen-dependent manner.
A) Experimental design for the HK-C60 diet and immune activation assays. The mice were first fed the control diet for 1 week (habituation) and then received the HK-C60 or control diet for the next 2 weeks. TPMs were prepared from the mice in each group and used for each assay. Cytokine production was analyzed in primary macrophages of the spleen, liver, and colon. B–D) Cytokine production in macrophages. TPMs (1.0×106/mL) were treated with LPS (100 ng/mL) or the vehicle control (PBS) at 37°C for 24 hr, and then the concentrations of IL-6 (B), TNF-α (C), and IL-12/IL-23p40 (C) in the cultured medium were measured by ELISA. E–G) Surface marker expression in macrophages. TPMs (1.0×106/mL) were treated with LPS (100 ng/mL) or the vehicle control (PBS) at 37°C for 6 hr, and then the expression levels of CD80 (E), CD86 (F), and I-A/I-E (G) were analyzed by flow cytometry. H–J) Cytokine production in primary macrophages. The splenocytes, liver mononuclear cells, and colon LP cells were isolated from each mouse and treated with LPS (100 ng/mL) or the vehicle control (PBS) at 37°C for 6 hr in the presence of GolgiStopTM. The TNF-α production in macrophages was analyzed by flow cytometry. K, L) Antigen presentation assay for CD4+ T cells. TPMs (1.0×107/mL) were co-cultured with splenic CD4+ T cells (2.0×106/mL, isolated from OT-II mice) in the presence of OVA323-339 peptide (100 ng/mL) at 37°C for 24 hr. K) Representative image of IFN-γ+CD4+ T cells in flow cytometry analysis. L) Percentage of IFN-γ+CD4+ T cells calculated in the flow cytometry analysis. In the flow cytometry analysis, CD80, CD86, and I-A/I-E expression (E–G) and TNF-α production (H–J) were detected in the CD11b+F4/80+gate, and IFN-γ production (K, L) was detected in the CD3+CD4+gate. The cumulative data are shown as the mean ± SEM of six samples in two independent experiments. *p<0.05 and **p<0.01 were regarded as indicating significance, respectively. ns, not significant.
The antigen presentation assay showed that TPMs prepared from HK-C60 diet mice significantly promoted the generation of IFN-γ+CD4+ T cells in an antigen-dependent manner compared with the TPMs prepared from control diet mice (Fig. 4 K, L).
Thus, the HK-C60 diet induced a pre-activated status in macrophages under physiological conditions, and this background enhances the immunological responses against subsequent exogenous stimuli in the macrophages.
DISCUSSION
It has been well proven that LAB modulate the activity of immune cells and that this immunological modification subsequently provides a beneficial effect on biological events in our bodies [15, 16]. Some studies have focused on myeloid lineage innate immune cells, represented as DCs, but the evidence for probiotic LAB-related immunological activities in macrophages is still insufficient [14,15,16,17, 24,25,26,27,28,29]. Therefore, we decided to investigate the immunomodulatory effects of C60 in macrophages.
We showed that HK-C60 enhanced the activity of TPMs, which ultimately upregulated the generation of effector CD4+ T cells (Figs. 1–3). We have previously reported that an HK-C60 diet promoted the immunological function of DCs and that this effect eventually contributed to the upregulation of CD4+ T cell function in the intestine of the mouse [17]. That report also provided sufficient evidence indicating that HK-C60 promotes the function of APCs, which directly upregulate CD4+ T cell-based adaptive immunity. In this study, we mostly focused on the antigen presentation activity of macrophages, which was augmented by HK-C60 exposure. HK-C60-mediated upregulation of cytokine production leads to the functional maturation of macrophages (Fig. 1B–D). Notably, IL-12/IL-23p40 is reported to promote the activity of APCs in an autocrine manner, as well as to promote the differentiation of CD4+ T cells toward Th1 cells [30, 31]. This functional enhancement might have induced the upregulation of antigen-presenting and co-stimulatory molecules on the macrophages (Fig. 2A, B). The HK-C60 treatment also enhanced antigen uptake in the macrophages (Fig. 2C, D). It is possible that these HK-C60-mediated functional upregulations of the macrophages comprehensively contributed to promotion of the generation and activation of effector CD4+ T cells in an antigen-dependent manner (Fig. 3). These results suggest that the HK-C60 diet positively influences the differentiation step of macrophages and/or the characteristics of precursors, such as monocytes and immature hematopoietic cells, that consequently activate the activity of differentiated macrophages in the peripheral [32]. These results imply that a crosstalk mechanism clearly exists between the intestinal and BM (bone marrow) environments in the regulation of myeloid cell differentiation. In this mechanism, probiotic LAB-mediated modifications in commensal bacteria may increase specific signals to the BM that promote a dynamic functional alteration in myeloid cells during their differentiation. Therefore, we hypothesized that the HK-C60 diet could be a trigger to induce a modification in commensal bacteria that ultimately contributes to the functional enhancement of macrophages [15, 33].
Through our current and previous studies, we revealed that HK-C60 enhances both innate and T cell-based adaptive immune responses. HK-C60-mediated activation of APCs seems to be an indispensable step that subsequently activates the function of T cells via enhanced antigen presentation activity in the APCs. As a next step, we need to investigate the detailed mechanism of the HK-C60-mediated functional upregulation in the APCs. An understanding of this will allow us to effectively and safely utilize HK-C60 as a supplement or food medicine for the maintenance of homeostasis and prevention of inflammation in the intestinal environment.
AUTHOR CONTRIBUTIONS
Conceptualization, S.S., A.O., and N.M.T.; methodology, S.S.; experiments, S.S., A.O., and N.K.; data analysis, S.S.; resources, A.O. and N.M.T.; discussion, S.S., A.O., N.K., T.M., and N.M.T.; writing manuscript, S.S. and N.M.T.; supervision, S.S. and N.M.T.; project administration, S.S. and N.M.T; funding acquisition, S.S. and N.M.T. All authors have read and agreed to the published version of the manuscript.
FUNDING
This research was funded by the Japan Society for the Promotion of Science (19H04042, N.M.T.), a grant for AIST-Shizuoka industrial innovation for the next generation (N.M.T.), and the Mishima Kaiun Memorial Foundation (S.S.).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Dr. Hiromi Kimoto for helpful discussion and the National Agriculture and Food Research Organization (NARO) for kindly providing Lactococcus lactis subsp. cremoris C60.
REFERENCES
- 1.Wynn TA, Chawla A, Pollard JW. 2013. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oishi Y, Manabe I. 2018. Macrophages in inflammation, repair and regeneration. Int Immunol 30: 511–528. [DOI] [PubMed] [Google Scholar]
- 3.Ellis MJ, Tsai CN, Johnson JW, French S, Elhenawy W, Porwollik S, Andrews-Polymenis H, McClelland M, Magolan J, Coombes BK, Brown ED. 2019. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat Commun 10: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikitina E, Larionova I, Choinzonov E, Kzhyshkowska J. 2018. Monocytes and macrophages as viral targets and reservoirs. Int J Mol Sci 19: 2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karavitis J, Kovacs EJ. 2011. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol 90: 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Hoppe AD, Swanson JA. 2010. Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis. Proc Natl Acad Sci USA 107: 19332–19337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acharya D, Li XRL, Heineman RE, Harrison RE. 2020. Complement receptor-mediated phagocytosis induces proinflammatory cytokine production in murine macrophages. Front Immunol 10: 3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 9.Bournazos S, Gupta A, Ravetch JV. 2020. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol 20: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell DG, Vanderven BC, Glennie S, Mwandumba H, Heyderman RS. 2009. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol 9: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao T, Bernstein KE, Fang J, Shen XZ. 2017. Angiotensin-converting enzyme affects the presentation of MHC class II antigens. Lab Invest 97: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntjewerff EM, Meesters LD, van den Bogaart G. 2020. Antigen cross-presentation by macrophages. Front Immunol 11: 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu YL, Harrison RE. 2021. Microbial phagocytic receptors and their potential involvement in cytokine induction in macrophages. Front Immunol 12: 662063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima T, Ikari N, Kouchi T, Kowatari Y, Kubota Y, Shimojo N, Tsuji NM. 2018. The molecular mechanism for activating IgA production by Pediococcus acidilactici K15 and the clinical impact in a randomized trial. Sci Rep 8: 5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, Kaneko D, Kumagai Y, You DJ, Carreras J, Uematsu S, Jang MH, Takeuchi O, Kaisho T, Akira S, Miyake K, Tsutsui H, Saito T, Nishimura I, Tsuji NM. 2013. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 38: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 16.Shahbazi R, Sharifzad F, Bagheri R, Alsadi N, Yasavoli-Sharahi H, Matar C. 2021. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients 13: 1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S, Kakizaki N, Okuno A, Maekawa T, Tsuji NM. 2020. Lactococcus lactis subsp. Cremoris C60 restores T cell population in small intestinal lamina propria in aged interleukin-18 deficient mice. Nutrients 12: 3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabersani E, Abeijon-Mukdsi MC, Ross R, Medina R, González S, Gauffin-Cano P. 2017. Specific strains of lactic acid bacteria differentially modulate the profile of adipokines in vitro. Front Immunol 8: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren C, Zhang Q, de Haan BJ, Zhang H, Faas MM, de Vos P. 2016. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci Rep 6: 34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao DY, Spivia WR, Veiras LC, Khan Z, Peng Z, Jones AE, Bernstein EA, Saito S, Okwan-Duodu D, Parker SJ, Giani JF, Divakaruni AS, Van Eyk JE, Bernstein KE. 2020. ACE overexpression in myeloid cells increases oxidative metabolism and cellular ATP. J Biol Chem 295: 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito S, Kawamura T, Higuchi M, Kobayashi T, Yoshita-Takahashi M, Yamazaki M, Abe M, Sakimura K, Kanda Y, Kawamura H, Jiang S, Naito M, Yoshizaki T, Takahashi M, Fujii M. 2015. RASAL3, a novel hematopoietic RasGAP protein, regulates the number and functions of NKT cells. Eur J Immunol 45: 1512–1523. [DOI] [PubMed] [Google Scholar]
- 22.Mehta AK, Kadel S, Townsend MG, Oliwa M, Guerriero JL. 2021. Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol 12: 643771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertho N, Drénou B, Laupeze B, Berre CL, Amiot L, Grosset JM, Fardel O, Charron D, Mooney N, Fauchet R. 2000. HLA-DR-mediated apoptosis susceptibility discriminates differentiation stages of dendritic/monocytic APC. J Immunol 164: 2379–2385. [DOI] [PubMed] [Google Scholar]
- 24.Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56 Suppl 4: S21–S26. [DOI] [PubMed] [Google Scholar]
- 25.Aattour N, Bouras M, Tome D, Marcos A, Lemonnier D. 2002. Oral ingestion of lactic-acid bacteria by rats increases lymphocyte proliferation and interferon-gamma production. Br J Nutr 87: 367–373. [DOI] [PubMed] [Google Scholar]
- 26.Perdigón G, Fuller R, Raya R. 2001. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol 2: 27–42. [PubMed] [Google Scholar]
- 27.Kanayama M, Kato Y, Tsuji T, Konoeda Y, Hashimoto A, Kanauchi O, Fujii T, Fujiwara D. 2018. Enhancement of immunomodulative effect of lactic acid bacteria on plasmacytoid dendritic cells with sucrose palmitate. Sci Rep 8: 3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. 2006. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci 89: 3306–3317. [DOI] [PubMed] [Google Scholar]
- 29.Laiño J, Villena J, Kanmani P, Kitazawa H. 2016. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grohmann U, Belladonna ML, Vacca C, Bianchi R, Fallarino F, Orabona C, Fioretti MC, Puccetti P. 2001. Positive regulatory role of IL-12 in macrophages and modulation by IFN-gamma. J Immunol 167: 221–227. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y, Li N, Wei F. 2015. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res 4: F1000 Faculty Rev, 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yahara Y, Ma X, Gracia L, Alman BA. 2021. Monocyte/Macrophage lineage cells from fetal erythromyeloid progenitors orchestrate bone remodeling and repair. Front Cell Dev Biol 9: 622035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Filippis F, Pasolli E, Ercolini D. 2020. The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol Rev 44: 454–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.