Abstract
Recent research has confirmed that moderate-intensity exercise affects the gut microbiome composition and improves cardiac function in an animal model after myocardial infarction (MI). However, few studies have investigated the effects of exercise on glucose and lipid metabolism in patients with coronary heart disease (CHD) receiving a statin treatment and successful percutaneous coronary intervention (PCI). Meanwhile, since statin therapy may lead to the risk of an increase in blood glucose level in CHD patients, we hypothesized that moderate-intensity exercise may be helpful for regulating glucose-lipid metabolism and stabilizing the blood glucose level in CHD patients. Therefore, to confirm our conjecture, we conducted a clinical retrospective study and animal experiment, respectively. The clinical study involved a total of 501 statin-treated patients with CHD after PCI. According to the study protocol, patients were divided into the following three groups: a non-exercise group, exercise at the recommended standard group, and exercise not at the recommended standard group. We found that qualified moderate-intensity exercise decreased blood glucose and lipid levels at follow-up at a mean of 2.2 years, and the incidence of new-onset diabetes showed a downward trend compared with the non-exercise and exercise not at the recommended standard groups. Furthermore, we used a high-fat rat model to explore an additional mechanism of the beneficial effects of exercise-based management on glucose-lipid metabolism apart from the known mechanism. We used 16S rRNA high-throughput sequencing technology to analyze the changes induced by exercise in the composition of intestinal flora in experimental rats. We found that rats that exercised with or without statin administration had lower plasma glucose and lipid levels and that these parameters were higher in the control and statin-treated rats that did not exercise. These results were consistent with the human study. The results from high-throughput sequencing of the intestinal flora of rats showed, to the best of our knowledge, that exercise leads to an increased relative abundance of Akkermansia muciniphila, which contributes to improved glucose and lipid metabolism. Based on our current results, we suggest that moderate-intensity exercise can improve glucose and lipid metabolism and prevent statin treatment-related side effects, such as hyperglycemia, in patients after PCI. Exercise could facilitate the applicability of statins for lower lipid levels. Exercise training also provides additional benefits, such as alteration of the gut microbiota, which contributes to improved glucose and lipid metabolism.
Keywords: exercise, statin, metabolism, percutaneous coronary intervention, coronary heart disease, intestinal microbiota
INTRODUCTION
Ischaemic heart disease (IHD) is the leading cause of death and disability-adjusted life-years (DALYs) at the national level in China in 2017 [1]. Abnormal lipid metabolism is recognized as the most important risk factor of atherosclerosis, which accelerates the development of IHD [2, 3]. Numerous evidences demonstrated that blood lipids management are crucial measures for delaying the development of coronary heart disease (CHD). Statins, well-known lipid-lowering drugs, commonly used as the primary- and secondary prevention of CHD coronary heart disease [4, 5]. However, treatment dose of statin is limited due to some drug related side effects, and statin treatment does not systematically reach lipid-lowering goals [6, 7]. High-dose statin administration may cause severe adverse effects including liver dysfunction, myopathy and even hyperglycemia. Increased blood glucose concentration can lead to occurrence of type 2 diabetes [8, 9]. These side effects counteract the benefits from statin treatment and decrease treatment adherence of patients.
Physical exercise exerts positive effects on diabetes, obesity, metabolic syndrome, and other conditions [10]. Aerobic exercise in particular reduces the risk of atherosclerotic cardiovascular disease. Committees of the American Heart Association (AHA) and American College of Cardiology (ACC) recommend 150 minutes of moderate-intensity or 75 minutes of high-intensity aerobic exercise per week [11]. Exercise rehabilitation programs significantly improve the clinical prognosis of CHD patients after percutaneous coronary intervention (PCI) [12,13,14]. In addition to a single statin treatment, the combination of aerobic exercise and statin therapy is thought to be more effective for lowering plasma lipid and glucose levels and improving prognosis and quality of life in patients with CHD [15]. However, it is still unclear whether physical exercise can facilitate the applicability of statins for lowering lipid levels and prevent new onset of type II diabetes in patients after PCI.
Therefore, we conducted a retrospective clinical study in post-PCI patients with CHD. These cases were treated with statins and assigned to three groups with different exercise plans. Furthermore, there is close relationship between exercise and microbiome composition, which influences cardiac function and prognoses of myocardial infarction (MI) [16]. Therefore, we performed an animal study and then used 16S rRNA high-throughput sequencing technology to explore the effects of exercise and statin administration on the composition of intestinal flora and further discuss an additional mechanism by which the combination of exercise and statin treatment exerts these beneficial effects on CHD patients after PCI.
MATERIALS AND METHODS
Patients who provided informed consent for the exercise programs were involved in the study, and the study protocol was approved by the ethics committee of Zhongshan Hospital of Dalian University (registration number 2019162). Our animal study was approved by the Animal Ethics Committee of Zhongshan Hospital of Dalian University (registration number 2019021002). Our study conforms to the 3R principles and the rules of the Animal Ethics Committee of Zhongshan Hospital Affiliated to Dalian University.
Patients and protocol of the clinical study
We selected 501 patients (201 women and 300 men; age 68.6 ± 9.9 years; mean follow-up 2.2 years, range 1–4 years) admitted to our department. These patients were treated with statins after a successful PCI for CHD between January 2016 and December 2018. We excluded patients who refused to give informed consent for the exercise program. The inclusion criteria were defined as follows: (1) Patients did not undergo systematic statin treatment before PCI. (2) Patients adhered to statin treatment after PCI with 20 mg atorvastatin per day or 10 mg rosuvastatin per day. (3) Patients underwent regular follow-up, including blood sugar, glycated hemoglobin, and blood lipid examinations. (4) Patients were not taking other drugs that might affect the efficacy and safety of the evaluation. (5) Patients did not have a history of severe arrhythmia, such as atrial fibrillation and sick sinus syndrome. (6) Patients had not received a cardiac pacemaker. (7) The language expression of the patients was clear. There were no hearing impairment or language communication disorders that might disturb telephone follow-up and medication supervision. (8) Patients did not have liver or kidney dysfunction, malignant tumors, or statin allergy.
The exercise procedures followed the guidelines of the AHA/ACC [17, 18]. The exercise program was carried out gradually in hospitalized CHD patients after PCI. The conditions of the patients were estimated to be stable after PCI. The following approaches were used: (1) 30 minutes of moderate-intensity aerobic exercise at least 5 times per week. (2) The target heart rate (HR) was 64% of the maximal HR. HR was measured for 10 seconds immediately after exercise, and the maximal HR was defined as 220 − age or 161 − 0.51 × age for patients using β-blockers. The HR and its counting formula could reflect the workload. (3) In addition to HR, we estimated the exercise intensity or amount by referring to the Borg scale. The recommended rating of perceived exertion (RPE) score was lower than 11 to 15, from the beginning of 50 kcal/time to the next course of 250 to 300 kcal/time [19, 20]. (4) Patients exercised voluntarily under the guidance of an experienced cardiologist or nurse, and they or their family members also supervised the process with photoplethysmography (PPG)-based smartwear and wearables [21]. (5) According to the requirements for routine follow-up after PCI, the patients needed to be admitted to our department every three months, and their HRs were measured at this time. Additionally, telephone-based follow-up was conducted for discharged patients.
The patients were divided into the following three groups: (1) a no exercise (NE) group, which included sedentary patients getting no exercise; (2) an exercise at the recommended standard (RS) group, which included patients who adhered to aerobic exercise and reached the recommended standard for the amount of exercise, and (3) an exercise not at the recommended standard (NS) group, which included patients who adhered to the aerobic exercise but did not reach the recommended standard in terms of exercise intensity or/and exercise duration (Fig. 1A).
Fig. 1.
Flow charts of the study design and treatments. (A) Clinical study. (B) Animal study. NE: no exercise group; NS: did not reach the exercise standard group; RS: reached the exercise standard group.
Serum samples were collected from the CHD patients at the time of follow-up, and corresponding serum biochemical indicators were analyzed in the clinical laboratory of our hospital. Apart from analyzing serum levels of glucose and lipids in the CHD patients, we also calculated the incidence of primary cardiovascular adverse events, such as cardiovascular death, recurrence of myocardial infarction, and recurrence of angina pectoris, and PCI at the time of follow-up in patients of the three groups.
Rats and protocol of the experimental study
Several studies have indicated that estrogen from female animals affects metabolism. Therefore, we used male rats in our study [22]. A total of 40 healthy specific pathogen-free (SPF) male Sprague Dawley (SD) rats (age range 6–8 weeks; weight 200 ± 10 g) were purchased from the Experimental Animal Center of Dalian Medical University. The animal certificate number was SYXK (Liao) 2018-0003. High-fat diets were purchased form Jiangsu Synergy Biomedical Engineering Co., Ltd. (Jiangsu, China). The rats were housed in isolated cages in a controlled environment, with a 12-hour light/12-hour dark cycle, relative humidity of 45–55%, and temperature of 22–26°C. The rats had free access to food and water. They were fed a high-fat diet containing cholesterol (2%), lard (10%), propylthiouracil (0.2%), and basic feed (87.8%) for one week.
Rats were randomly divided into four groups from the second week (n=10 each). The experimental groups were as follows: group A, control group; group B, statin group; group C, exercise and statin group; and group D, exercise group. In group A, rats were given a volume of physiological saline (0.9%) equivalent to that of the rosuvastatin solution given to the rats in group B. In group B, rats were orally treated with a single dose of rosuvastatin at 2 mg/kg per day at 9:00 AM from the 2nd to 6th weeks. In group C, rats were given an exercise training program in addition to the same rosuvastatin administration as group B. The exercise training program was discontinued at the end of the 6th week, and the rats continued receiving rosuvastatin administration for another 5 weeks. In group D, rats were given physiological saline in the same manner as group A and given the same exercise program as group C (Fig. 1B).
The exercise training program methods have previously been described in detail [23]. Briefly, we adopted a weight-free swimming exercise program for the rats in groups C and D. In the first week, the rats performed adaptive swimming exercise and swam for 10 minutes on the first day, and the duration was then increased by 10 minutes every day until reaching 60 minutes on the 6th and 7th days. From the second week, the rats exercised for 60 minutes per day until the end of the 6th week. The rats swam in a white bucket with the water temperature controlled to 33 ± 2°C and the water depth being 50 cm.
Serum samples were collected from rats at the end of 6th week (groups A–D) and 11th week (group C). The animals were fasted for 12 hours before sampling and anesthetized by ether inhalation, and blood was collected from the inner canthus. Following centrifugation at 2,000 g for 20 minutes, serum was removed and analyzed. The biochemical detection kits (Number: 200311101) were provided by Ningbo Medical System Biotechnology Co., Ltd. (Ningbo, Zhejiang, China). The procedure was performed according to the manufacturer’s instructions.
After the 6th week (groups A–D) and 11th week (group C), 3 to 5 pieces of fresh feces (amounting to about 1 g) were collected from the anus of the rats. A QIA amp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) was used to extract total DNA from the fecal samples.
Four stool DNA samples were randomly selected from each group, and 16S rRNA V3V4 region high-throughput sequencing was performed. A two-step PCR method was used to construct a DNA library. The purified DNA was used as a template, and the universal primers 338F (5′-ACTCCTACGGRAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the target fragment by PCR. An AxyPrep DNA Gel Extraction Kit (AP-GX-50, Axygen Scientific, Corning, Corning, NY, USA) was used to purify the PCR product, and the recovered product was used as a template to perform an 8-cycle PCR amplification. The adapters, sequencing primers, and tag sequences required for platform sequencing were added to both ends of the target fragment. The PCR products were further purified with an AxyPrep DNA gel recovery kit, and fluorescence quantification was performed with an FTC-3000TM Real-Time PCR instrument. Sequencing was performed on an Illumina-MiSeq system (Illumina, San Diego, CA, USA).
The Trimmomatic (version 0.35) software was used to remove low-quality sequences. The mothur (version 1.33.3) software was used to control and filter the sequences quality. The long and short sequences and some chimeras generated during the PCR process were removed to obtain the optimized sequences. OTU (operational taxonomic unit) clustering was performed with UPARSE (USEARCH version 8.1.1756), and sequences with a similarity greater than 97% were classified as the same taxon OTU. OTU representative sequences were compared with the silva 128 database for species annotation. Based on taxonomic information, community structure statistics were analyzed at the classification levels of the phylum, class, order, family, genus, and species. A Shannon analysis was performed for alpha diversity using mothur (version 1.33.2).
Statistical analysis
The raw data of plasma parameters from patients and model animals were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test, and normally distributed data were analyzed with Dunnett’s T3 test. For comparisons between pairs of groups, the paired t-test was used. All statistical analyses were performed using the IBM SPSS Statistics software package (version 20.0, IBM Corp., Armonk, NY, USA). Count data are expressed as a percentage (%). Composition and abundance for the gut microbiome were calculated by linear discriminant analysis Effect Size (LEfSe). The threshold for linear discriminant analysis (LDA) was defined as 2.0. A nonparametric test (Kruskal–Wallis rank sum test) was applied to analyze data that could not be analyzed using normal distribution. Statistical analyses were carried out in LEfSe software and R version 4.0.3 [24]. Measurement data are expressed as the mean ± standard deviation (mean ± SD). A p value lower than 0.05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics of the selected patients
In the clinical study, 94 patients (18.8%) were included in the NE group, 286 patients (57.1%) were included in the NS group, and 121 patients (24.2%) were included in the RS group. The baseline characteristics of the patients in the three groups are listed in Supplementary Table 1.
Exercise sufficiently regulates glucose and lipid metabolism in CHD patients after PCI
Table 1 shows that there were no significant differences in the baseline levels of biochemical parameters, including fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), in the three groups. Figure 1 and Table 1 show that there were no significant changes in the levels of FPG before and after treatment in the NE and NS groups. In contrast, a significant decrease in the level of FPG was observed in the RS group after treatment (from 7.38 ± 2.00 to 7.00 ± 1.70 mmol/L; p=0.02). The level of HbA1C significantly increased in the NE group (from 7.12 ± 1.65 to 7.38 ± 1.44 mmol/L; p=0.006), whereas there were no change in HbA1C in the NS and RS groups. The increased HbA1C concentration suggested that statin treatment led to a risk of hyperglycemia.
Table 1. Comparison of blood glucose, lipids, and creatine kinase before and after statin treatment in each group.
| Parameters (mmol/L) | NE (n=94) | NS (n=286) | RS (n=121) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| FPG | 7.28 ± 2.35 | 7.31 ± 2.30 | 7.23 ± 2.38 | 7.28 ± 2.14 | 7.38 ± 2.00 | 7.00 ± 1.70* |
| HbA1c (%) | 7.12 ± 1.65 | 7.38 ± 1.44# | 7.22 ± 1.65 | 7.29 ± 2.14 | 7.27 ± 1.53 | 7.25 ± 1.28 |
| TG | 1.85 ± 0.76 | 1.59 ± 0.59# | 1.83 ± 0.89 | 1.58 ± 0.55# | 1.85 ± 0.82 | 1.51 ± 0.74# |
| TC | 5.23 ± 0.92 | 4.34 ± 0.74# | 5.16 ± 0.93 | 4.01 ± 0.73# | 5.15 ± 0.90 | 3.55 ± 0.67* |
| HDL-C | 1.15 ± 0.31 | 1.19 ± 0.26 | 1.13 ± 0.28 | 1.16 ± 0.24 | 1.13 ± 0.27 | 1.17 ± 0.23 |
| LDL-C | 3.01 ± 0.86 | 2.07 ± 0.54# | 3.00 ± 0.93 | 1.82 ± 0.53# | 3.02 ± 0.96 | 1.61 ± 0.46# |
| CK (U/L) | 84.52 ± 25.63 | 86.63 ± 27.32 | 83.44 ± 32.52 | 84.18 ± 35.09 | 72.56 ± 30.21 | 73.26 ± 32.79 |
NE: no exercise group; NS: did not reach the exercise standard group; RS: reached the exercise standard group; FPG: fasting plasma glucose; HbA1C: hemoglobin A1C; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; CK: creatine kinase.
Furthermore, none of the involved patients presented statin-associated muscle symptoms (SAMS) during statin therapy and exercise. The creatine kinase (CK) results showed that the combination of exercise and statin treatment did not lead to a significant change in plasma CK levels compared with the other two groups. The results suggested that there were no additional statin-associated muscle side effects in patients with moderate exercise (Table 1).
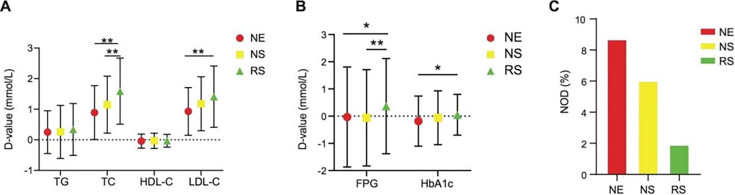
The levels of TG, TC, and LDL-C significantly decreased in the three groups after statin therapy or the combination of statin treatment and an exercise training program (Table 1). The decrease in TC in the RS group (1.59 ± 1.08 mmol/L) was 16.39% and 24.56% higher than those in the NS group (1.15 ± 0.93 mmol/L) and NE group (0.89 ± 0.88 mmol/L; p<0.01), respectively. The decrease in LDL-C in the RS group (1.41 ± 1.00 mmol/L) was 42.17% higher than that in the NE group (0.93 ± 0.78 mmol/L; p<0.01; Fig. 2A). These results suggest that exercise exerts additional beneficial effects that regulate lipid metabolism in addition to statins.
Fig. 2.
Exercise improves glucose and lipid metabolism in coronary heart disease (CHD) patients after percutaneous coronary intervention (PCI). The changes of corresponding metabolic parameters were compared between baseline and follow-up among the three groups. One-way ANOVA was performed followed by a t-test. The D-value denotes the deviation value (D-value = baseline value − value at the time of follow-up). (A) Lipid metabolism-related parameters including TC, TG, HDL-C, and LDL-C. (B) Glucose metabolism-related parameters including FPG and HbA1C. (C) Percentages of new-onset diabetes (NOD) among the three groups. NE: no exercise group; NS: did not reach the exercise standard group; RS: reached the exercise standard group; FPG: fasting plasma glucose; HbA1C: hemoglobin A1C; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol. *p<0.05; **p<0.01.
Exercise does not lead to a statistically significant decrease in the incidence of new-onset diabetes but leads to a downward trend in CHD patients after PCI. In our clinical study, the FPG levels of the NE and NS groups increased by 0.03 ± 1.84 mmol/L and 0.06 ± 1.77 mmol/L, respectively. The FPG level of the RS group decreased by 0.37 mmol/L and was 5.81% and 6.09%, higher than those of the other two groups (p<0.05; p<0.01; Fig. 2B), respectively. In the NE group, 8.7% of the patients treated with statins were diagnosed with new-onset diabetes. However, the incidences of new-onset diabetes were obviously lower in the NS group (6.03%) and RS group (1.92%). Although no statistical significance was found based on the current data, patients with qualified exercise showed a downward trend in new-onset diabetes (Fig. 2C). We considered the results to have been limited by sample size, and a large sample study is still needed in our subsequent research.
Exercise reduces the incidence of cardiovascular events
Primary cardiovascular events were measured among the three groups at the time of follow-up. The RS group (n=121) had a lower incidence of overall events (p<0.05), cardiovascular death (p<0.05), recurrence of myocardial infarction (p<0.05), and recurrence of angina pectoris and PCI (p<0.05) compared with the NE (n=94) and NS (n=286) groups (Table 2). The results suggested that sufficient exercise can reduce the incidence of cardiovascular events in CHD patients after PCI.
Table 2. Comparison of the incidences of cardiovascular events in the 3 groups.
| Cardiovascular events | NE (n=94) | NS (n=286) | RS (n=121) |
|---|---|---|---|
| Cardiovascular death | 4 (4.26) | 3 (1.05) | 0#* |
| Recurrence of myocardial infarction | 6 (6.38) | 5 (1.75) | 1 (0.83)#* |
| Recurrence of angina pectoris and PCI | 8 (8.51) | 20 (6.70) | 7 (5.79)# |
| The overall event | 18 (19.15) | 28 (9.79) | 8 (6.61)#* |
N (%) NE: no exercise group; NS: did not reach the exercise standard group; RS: reached the exercise standard group; PCI: percutaneous coronary intervention.
Statistical analyses were conducted using A One way ANOVA followed by a TURKEY or Dunnett’s 3 test. *p<0.05 vs. NE group; #p<0.05 vs. NS group.
Exercise counteracts rosuvastatin-induced hyperglycemia and decreases levels of serum lipids in a high fat–diet rat model
We used a high fat-diet rat model to explore the mechanism underlying the regulation of glucose and lipid metabolism by exercise. Correlating to our clinical study, rats fed a high-fat diet were assigned to four experimental groups. Statin administration without exercise (group B) led to a significant increase in the levels of FPG compared with the other three groups (Fig. 3A). This suggested that statin treatment can increase serum glucose levels in rats and that supplementation of statin treatment with exercise can reduce the increase in FPG concentration caused by statins. Furthermore, exercise without statin administration (group D) can also decrease the level of FPG in rats fed a high-fat diet. This suggested that exercise is beneficial for preventing the incidence of hyperglycemia.
Fig. 3.
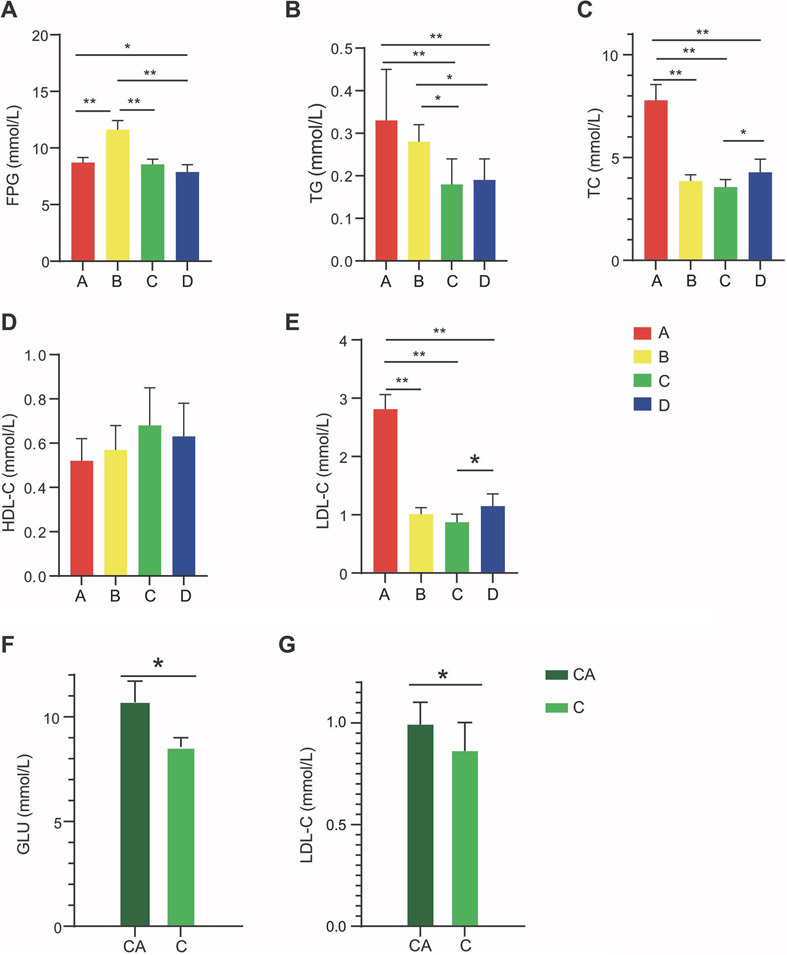
Exercise decreases the levels of serum glucose and lipids and prevents statin therapy-related hyperglycemia in rats fed a high-fat diet. (A to E) Levels of FPG, TC, TG, HDL-C, and LDL-C in rats of the four experimental groups (A, control group; B, statins group; C, exercise and statins group; D, exercise group; n=10 for each group). Statistical analyses were conducted using one-way ANOVA followed by a Tukey or Dunnett’s T3 test. The results are presented as the mean ± standard deviation (SD). (F, G) Levels of GLU and LDL-C in rats received the combination of exercise and statin administration (group C) for 5 weeks and then statin administration for 5 weeks without the exercise training program (CA). *p<0.05; **p<0.01. FPG: fasting plasma glucose; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; GLU: glucose.
In addition to the effect of exercise on the serum glucose level, significantly decreased the levels of serum TG, TC, and LDL-C were found in the rats treated with both exercise and statin administration (group C) or with exercise without statin administration (group D; Fig. 3B, C, E). The TG-lowing effects of the two treatments were superior to simple treatment with statin (group B; Fig. 3B). The TC- and LDL-C-lowing effects of exercise were weaker when statin administration was removed (Fig. 3C, E). Furthermore, there were no significant differences in HDL-C levels among the four experimental groups (Fig. 3D). These results suggested that the combination of statin treatment and exercise is more effective for reducing TG levels.
To further elucidate the relationship of statin treatment with hyperglycemia and exercise, the rats in group C were no longer exercised beginning in the 7th week and only received the statin treatment for the subsequent 5 weeks. Both the FPG and LDL-C levels significantly increased when the exercise training program was discontinued in the rats for 5 weeks (Fig. 3F, G). The results suggested that continuous exercise is necessary to maintain the beneficial effects of exercise on the levels of blood glucose and lipids.
Exercise regulates abundances of intestinal flora closely associated with improved glucose and lipid metabolism in rats fed a high-fat diet
A recent study demonstrated that the gut microbiome plays an important role in regulating cardiac function in rats after MI [16]. Exercise can regulate the composition of the gut microbiome [10]. However, the effects of combined exercise and statin therapy on the composition of the intestinal flora are largely unclear. The high fat-diet model is the most confirmed method for studying the pathological mechanisms of metabolic or cardiovascular diseases. Therefore, we examined the combined effects of exercise and statin therapy on the structure and composition of the intestinal flora in rats fed a high-fat diet, which could provide suggestions for studying CHD, as abnormal glucose and lipid metabolism are key risk factors associated with the development of CHD and both hyperglycemia and hyperlipidemia contribute to the formation of atherosclerosis. We used 16S rRNA high-throughput sequencing technology and metagenomic tools to detect the composition and abundance of the intestinal flora in the rats. We collected 20 rat feces samples, and each sample led to an average of 35,352 high-quality reads. Taxonomic identification led to 1,085 unique OTUs. The number of OTUs for each sample ranged from 370 to 987. OTUs were divided into 14 phyla, 20 classes, 29 orders, 42 families, 107 genera, and 117 species. The alpha diversity of the intestinal flora among the groups was calculated by Shannon index (Supplementary Fig. 1). The results revealed that the diversity of the microbial community in group C was the highest, but the difference compared with the other groups was not statistically significant (p=0.11).
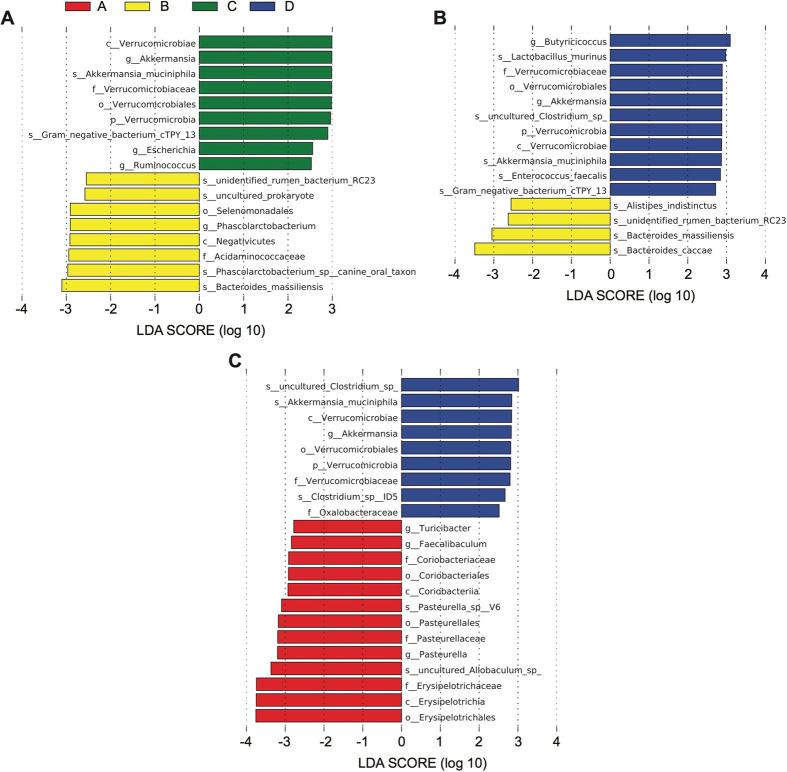
LEfSe analysis was used to identify the differences in intestinal flora composition among the different groups (Fig. 4). All the groups displayed distinctive differences in the compositions of their intestinal flora. It was found that a common set of organisms saw their relative populations increased by exercise, in particular the family Verrucomicrobiaceae, genus Akkermansia, and species Akkermansia muciniphila. Meanwhile, the Kruskal–Wallis nonparametric test was used to analyze the relative abundances of A. muciniphila in the four groups, and statistical differences were found among the four groups (p<0.01; Supplementary Fig. 2).
Fig. 4.
Exercise regulates abundances and composition of intestinal microbiota in rats administered a high-fat diet (A to C). Determinations were performed 5 weeks after treatment. An linear discriminant analysis Effect Size (LEfSe) analysis was used for the calculations, and we defined the linear discriminant analysis (LAD) threshold as 2.0. A p value lower than 0.05 was defined as indicating statistical significance. A: control group; B: statins group; C: exercise and statins group; D: exercise group.
Furthermore, we used the Kruskal-Wallis nonparametric test to identify differences in intestinal flora composition between the non-exercising rats (groups A and B) and exercising rats (groups C and D) at different taxonomic levels (Fig. 5A, B). At the genus level, we found that the abundances of Akkermansia, Prevotella, and Ruminococcus were significantly increased in rats receiving exercise training. Meanwhile, at the species level, the abundance of A. muciniphila was significantly increased in exercising rats (groups C and D). We performed an LEfSe analysis of the composite groups (Fig. 5C) and observed that the exercising rats showed an increase in the populations of the species A. muciniphila, the genus Ruminococcus, and the phylum Verrucomicrobia.
Fig. 5.
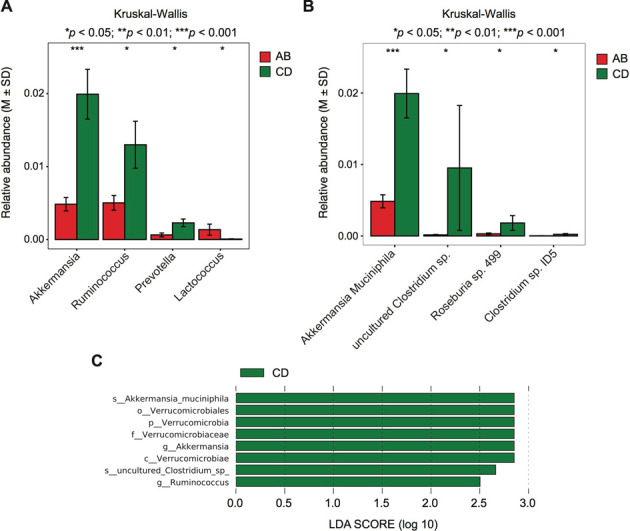
Statistical analysis of the intestinal flora in the exercising and non-exercising groups. Analyses were performed with the Kruskal–Wallis rank sum test. (A) At the genus level. (B) At the species level. (C) linear discriminant analysis Effect Size (LEfSe) analysis of the intestinal microbiota in exercising rats. AB denotes the non-exercising groups: group A (control group) and group B (statins group). CD denotes the exercising groups: group C (exercise and statins group) and group D (exercise group).
Finally, we analyzed the changes in intestinal flora composition of the rats in group C after the exercise training program was discontinued for 5 weeks (group CA). Using LEfSe, we observed significant changes in intestinal flora composition in the rats (Supplementary Fig. 3), as the relative abundance of A. muciniphila significantly decreased after the exercise training program was discontinued.
DISCUSSION
Statins have been identified as the first-line drugs for the prevention and treatment of CHD. Recently, there has been widespread concern about statin therapy related to the risk of type 2 diabetes. Therefore, exploring effective strategies to prevent an increase in blood glucose level due to statin treatment are critically important. In the present study, we conducted a clinical study in CHD patients and an in vivo study in a high-fat rat model. We ultimately found that exercise results in changes in the intestinal flora. Based on previous evidence, the changes in intestinal flora composition after exercise were considered to contribute to the improvement of glucose and lipid metabolism.
Our retrospective clinical study involved 501 patients with CHD receiving PCI. All patients were treated with statins and adhered to different exercise programs. We observed that the amount of exercise was negatively correlated with the level of glycosylated hemoglobin. Both the blood glucose and glycosylated hemoglobin levels of the patients who reached the recommended standard for the amount of exercise were significantly lower than those of the patients in the no exercise group, suggesting that qualified exercise can reduce the risk of hyperglycemia related to statin treatment. Although we observed a clear but nonsignificant negative correlation between the incidence of new-onset diabetes and the amount of exercise, we considered this result to have been limited by the sample size. The correlation may be significant with a larger sample size and/or longer follow-up period. We also observed that the reduction in lipid levels could be amplified after qualified exercise, as the NS and RS groups showed reductions in TC and LDL-C levels compared with the NE group. Additionally, we found that exercise significantly decreased the incidence of cardiovascular events. We considered the reduction in the recurrence of primary cardiovascular events with exercise to be closely related to improvement of glucose and lipid metabolism.
The results of our animal study are consistent with our clinical observations. In the high-fat rat model, we found that exercise with or without statin effectively reduced plasma glucose levels compared with statin administration without exercise. Additionally, when we discontinued exercise in rats for 5 weeks in the exercise and stain administration group (group C), we observed a significant increase in the plasma FPG and LDL-C blood levels. These results further support that exercise can counteract hyperglycemia induced by statin administration. Furthermore, the results of the animal experiments revealed that the combination of exercise and statin administration effectively improves dyslipidemia.
Although the combination of both physical activity and statin treatment reduced cardiovascular risk, SAMS are the most reported adverse effect of statin treatment [25]. As high-intensity physical exercise may promote SAMS, moderate-intensity physical activity may counteract statin-induced myocellular changes by improving mitochondrial function and attenuate SAMS [26]. In our study, we measured the CK levels of patients after exercise and found no significant change in CK levels. These results suggested that moderate-intensity exercise did not promote SAMS.
Multiple studies have shown there is close relationship between gut flora and cardiovascular diseases. Dysregulation of intestinal flora leads to metabolic disorders such as obesity and diabetes. A previous study indicated that qualified exercise influenced the composition of gut flora, which contributes to improvement of the cardiac function of rats after MI [16]. In our high-fat model study, we analyzed 16S rRNA high-throughput sequencing data for the intestinal flora of rat cohorts. Calculation of the Shannon diversity showed that there was no significant difference in alpha diversity among the different groups. However, the combination of exercise and statin therapy seems to promote the highest microbial diversity (Supplementary Fig. 1). The LEfSe differential analysis of the intestinal flora for the rats with and without exercise revealed that the main difference in the flora of the different groups resided in the relative abundance of the bacterium A. muciniphila and that increasing exercise led to an increased abundance of A. muciniphila. This increase seemed to be strictly dependent on exercise, since the discontinuation of exercise for the rats in group C resulted in the relative abundance of A. muciniphila in their intestinal flora decreasing while their FPG and LDL-C levels increased to varying degrees.
Taking our results and the previous literature together, we consider the changes in blood glucose and blood lipid levels to be closely related to the changes in A. muciniphila content, as the relationship between exercise and Akkermansia has already been reported repeatedly and it is abundantly clear that exercise results in an increase the abundance of Akkermansia in the intestinal flora via the secretion of mucins by the intestine [16, 27, 28]. Akkermansia can increase insulin sensitivity and reduce the TC levels. These beneficial effects further lead to reduced incidences of obesity and diabetes. The positive effects of Akkermansia on cardiac function have also been reported by Liu et al. [16, 29,30,31].
On the other hand, the human study we performed was not randomized, and confounding factors such as medications, bowel movements, and mood cannot be completely excluded, although there were no significant differences in major clinical factors among the groups. In future studies, we will pay attention to these issues.
In conclusion, our results support previous studies about the beneficial effects of moderate exercise on the composition of the gut microbiome and its links to cardiac metabolism. We further suggest that exercise can stabilize plasma glucose and lipid levels by increasing the effect of statins and counteracting their side effects. To the best of our knowledge and based on existing evidence, the underlying mechanism is closely related to the exercise-induced increase in the abundance of A. muciniphila. Further study by conducting a randomized human clinical trial over an extended period of time is essential.
AUTHOR’S CONTRIBUTION
Concept and design: LW, JM and YW. Analysis and interpretation of data: LW and HW. Acquisition or data collection: LW, HJ, JY, XY and BZ. Drafting and revising of the manuscript: LW, BZ and JM.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 81600327), China Postdoctoral Science Foundation funded project (2018M631177), and Fundamental Research Funds for the Central Universities (Grant Number: xjj2018103).
DATA AVAILABILITY
Data are available through reasonable request to the corresponding author.
CONFLICT OF INTEREST
The authors declare that there are no potential conflicts of interest in this study.
Supplementary Material
Acknowledgments
We thank Shanghai Weiji Technologies for helping to perform the analyses.
REFERENCES
- 1.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, Li X, Wang L, Wang L, Liu Y, Liu J, Zhang M, Qi J, Yu S, Afshin A, Gakidou E, Glenn S, Krish VS, Miller-Petrie MK, Mountjoy-Venning WC, Mullany EC, Redford SB, Liu H, Naghavi M, Hay SI, Wang L, Murray CJL, Liang X. 2019. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 394: 1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK. 2019. Lipoprotein(a): an independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J 71: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SJ, Park SH, Sin HS, Jang SH, Lee SW, Kim SY, Kwon B, Yu KY, Kim SY, Yang DK. 2017. Hypocholesterolemic effects of probiotic mixture on diet-induced hypercholesterolemic rats. Nutrients 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. 1997. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 278: 313–321. [PubMed] [Google Scholar]
- 5.Han X, Zhang Y, Yin L, Zhang L, Wang Y, Zhang H, Li B. 2018. Statin in the treatment of patients with myocardial infarction: a meta-analysis. Medicine (Baltimore) 97: e0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Li N, Zhou Y, Xiao Z, Tian H, Hu M, Li S. 2020. Cost-effectiveness analysis of ezetimibe as the add-on treatment to moderate-dose rosuvastatin versus high-dose rosuvastatin in the secondary prevention of cardiovascular diseases in China: a Markov model analysis. Drug Des Devel Ther 14: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akyea RK, Kai J, Qureshi N, Iyen B, Weng SF. 2019. Sub-optimal cholesterol response to initiation of statins and future risk of cardiovascular disease. Heart 105: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwala A, Kulkarni S, Maddox T. 2018. The association of statin therapy with incident diabetes: evidence, mechanisms, and recommendations. Curr Cardiol Rep 20: 50. [DOI] [PubMed] [Google Scholar]
- 9.Maki KC, Diwadkar-Navsariwala V, Kramer MW. 2018. Statin use and risk for type 2 diabetes: what clinicians should know. Postgrad Med 130: 166–172. [DOI] [PubMed] [Google Scholar]
- 10.Raun SH, Henriquez-Olguín C, Karavaeva I, Ali M, Møller LLV, Kot W, Castro-Mejía JL, Nielsen DS, Gerhart-Hines Z, Richter EA, Sylow L. 2020. Housing temperature influences exercise training adaptations in mice. Nat Commun 11: 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Jr, Virani SS, Williams KA, Sr, Yeboah J, Ziaeian B. 2019. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 74: e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemoto S, Kasahara Y, Izawa KP, Watanabe S, Yoshizawa K, Takeichi N, Kamiya K, Suzuki N, Omiya K, Matsunaga A, Akashi YJ. 2019. Effects of αβ-blocker versus β1-blocker treatment on heart rate response during incremental cardiopulmonary exercise in Japanese male patients with subacute myocardial infarction. Int J Environ Res Public Health 16: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Chang R. 2019. Effects of exercise after percutaneous coronary intervention on cardiac function and cardiovascular adverse events in patients with coronary heart disease: systematic review and meta-analysis. J Sports Sci Med 18: 213–222. [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhakaran D, Chandrasekaran AM, Singh K, Mohan B, Chattopadhyay K, Chadha DS, Negi PC, Bhat P, Sadananda KS, Ajay VS, Singh K, Praveen PA, Devarajan R, Kondal D, Soni D, Mallinson P, Manchanda SC, Madan K, Hughes AD, Chathurvedi N, Roberts I, Ebrahim S, Reddy KS, Tandon N, Pocock S, Roy A, Kinra S, Yoga-CaRe Trial Investigators2020. Yoga-based cardiac rehabilitation after acute myocardial infarction: a randomized trial. J Am Coll Cardiol 75: 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C, Sung J, Lee JH, Kim WS, Lee GJ, Jee S, Jung IY, Rah UW, Kim BO, Choi KH, Kwon BS, Yoo SD, Bang HJ, Shin HI, Kim YW, Jung H, Kim EJ, Lee JH, Jung IH, Jung JS, Lee JY, Han JY, Han EY, Won YH, Han W, Baek S, Joa KL, Lee SJ, Kim AR, Lee SY, Kim J, Choi HE, Lee BJ, Kim S. 2019. Clinical practice guideline for cardiac rehabilitation in Korea: recommendations for cardiac rehabilitation and secondary prevention after acute coronary syndrome. Korean Circ J 49: 1066–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Liu HY, Zhou H, Zhan Q, Lai W, Zeng Q, Ren H, Xu D. 2017. Moderate-intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine 2011. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 18.Godlasky E, Hoffman T, Weber-Peters S, Bradford R, Miller N, Kunselman AR, Lott MEJ. 2018. Effects of β-blockers on maximal heart rate prediction equations in a cardiac population. J Cardiopulm Rehabil Prev 38: 111–117. [DOI] [PubMed] [Google Scholar]
- 19.Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. 2013. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 113: 147–155. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Cao H, Jiang P, Tang H. 2018. Cardiac rehabilitation in acute myocardial infarction patients after percutaneous coronary intervention: a community-based study. Medicine (Baltimore) 97: e9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Lane DA, Chen Y, Lip GYH. 2020. Mobile health technology facilitates population screening and integrated care management in patients with atrial fibrillation. Eur Heart J 41: 1617–1619. [DOI] [PubMed] [Google Scholar]
- 22.Knopp RH, Zhu X, Bonet B. 1994. Effects of estrogens on lipoprotein metabolism and cardiovascular disease in women. Atherosclerosis 110 Suppl: S83–S91. [DOI] [PubMed] [Google Scholar]
- 23.Ericson U, Brunkwall L, Hellstrand S, Nilsson PM, Orho-Melander M. 2020. A health-conscious food pattern is associated with prediabetes and gut microbiota in the Malmö offspring study. J Nutr 150: 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allard NAE, Hopman MTE, Timmers S. 2020. Letter to the editor: “Exercise training adaptations in metabolic syndrome individuals on chronic statin treatment”. J Clin Endocrinol Metab 105: e3484–e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfim MR, Oliveira AS, do Amaral SL, Monteiro HL. 2015. Treatment of dyslipidemia with statins and physical exercises: recent findings of skeletal muscle responses. Arq Bras Cardiol 104: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O’Reilly M, Jeffery IB, Wood-Martin R, Kerins DM, Quigley E, Ross RP, O’Toole PW, Molloy MG, Falvey E, Shanahan F, Cotter PD. 2014. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 28.Munukka E, Ahtiainen JP, Puigbó P, Jalkanen S, Pahkala K, Keskitalo A, Kujala UM, Pietilä S, Hollmén M, Elo L, Huovinen P, D’Auria G, Pekkala S. 2018. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol 9: 2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Buschard K, Hansen AK. 2012. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 55: 2285–2294. [DOI] [PubMed] [Google Scholar]
- 30.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available through reasonable request to the corresponding author.