Abstract
The biological activities of acetic acid bacteria (AAB) as Gram-negative bacteria have attracted our interests, especially in their inhibitory effects on allergic responses. To clarify the underlying mechanism that improves allergic symptoms by ingestion of the AAB Gluconacetobacter hansenii, we examined whether different extracts of heat-killed G. hansenii GK-1 could reduce the interleukin (IL)-4 production of immune cells from food-allergic model of OVA23-3, transgenic mice with ovalbumin (OVA)-specific T-cell-receptor genes. A hot-water extract fraction (FII) of G. hansenii GK-1 significantly decreased the in vitro IL-4 production of spleen cells of OVA23-3 mice compared with those stimulated with OVA alone. The IL-4 inhibitory effect was also observed for FIV (purified lipopolysaccharide (LPS) fraction), but the activity was lower than for FII or LPS from Escherichia coli. Unlike LPS from Escherichia coli, FIV significantly inhibited the LPS-induced IL-6 production of the spleen cells. The addition of FII or FIV to a Foxp3+T cell-inducing culture showed that FII significantly promoted the rate of Foxp3+CD4+T cells of OVA-stimulated mesenteric lymph node cells from recombination-activating-gene (RAG)-2-deficient food-allergic inflammatory OVA23-3 (R23-3) mice with suppression of IL-4 production, while FIV induced Foxp3+T cells from RAG-2-deficient DO11.10 non-inflammatory mice. Structure analysis showed a lack of O-antigen in FIV, which seemed to lead to the weak biological activities of FIV observed. The present study suggests that extracts of G. hansenii GK-1 to inhibit IL-4 production of immune cells and/or promote regulatory T cell differentiation synergistically play important roles in improving allergic symptoms safely as well as normal condition.
Keywords: Gluconacetobacter hanseniiGK-1, interleukin 4, Foxp3+CD4+T cells, food-allergic model mice, lipopolysaccharide, O-antigen
INTRODUCTION
Acetic acid bacteria (AAB), a group in the family Acetobacteraceae comprising gram-negative bacteria, are present everywhere in our environment [1]. The oxidization capability of ethanol to produce acetic acid has enabled us to create many kinds of traditional or industrial fermented foods all over the world, such as kombucha, vinegars, and nata de coco. It has been well known that foods fermented by using AAB have health-protecting or helth-improving effects (antibacterial, antioxidant, anti-diabetic or anti-tumor effects) [2] and people have recently been getting more and more conscious of the benefits. Among their benefits of AAB or their products, the anti-allergic effects have attracted our interests now. As chronic inflammatory diseases, allergic diseases such as food allergy, anaphylaxis, allergic rhinitis, and atopic dermatitis have spread worldwide, especially in westernized countries, but effective treatments have remained to be elucidated. On the other hand, early life microbial exposure to bacteria decreases the risks of children and infants being affected by allergic diseases. It was suggested by the significant increase in patients with allergic diseases, that has been thought to be the result of epigenetic changes derived from changes in lifestyles of present-day children, who have less chance to interact with diverse microbial environments [3]. There are several reports indicating the effects of AAB on amelioration of allergic inflammatory responses [4,5,6,7].
The administration of heat-killed Gluconacetobacter hansenii GK-1, a strain of AAB, has also been proven to improve nasal rubbing counts in a pollen allergy mice model [4]. The candidate G. hansenii GK-1 components exhibiting the effect has been thought to be the lipopolysaccharide (LPS) fraction binding to the TLR4 (Toll-like recepter 4) receptor on innate cells [5]. In addition, a randomized double-blind placebo-controlled study of ingestion of G. hansenii GK-1 itself suggested that G. hansenii GK-1 relieved daily nasal discomfort in Japanese subjects [6]. Lipopolysaccharides (LPS) major outer membrane components of the cell wall of gram-negative bacteria, have been reported to modulate allergic immune responses via LPS tolerance evoked by long-term exposure to LPS [7, 8]. However, in spite the reported benefits of AAB with respect to the improvement of allergic symptoms, the accurate effective component and underlying mechanism remains unknown, although AAB could play a role in ameliorating Th2 immune responses in allergic diseases.
Thus, we analyzed the capability of different extracts obtained by preparation process for LPS from G. hansenii GK-1 to inhibit the interleukin (IL) 4 production of immune cells purified from food-allergic enteropathy model mice [9], which produce excessively IL-4, when they are stimulated with an allergen, ovalbumin (OVA). The present study found that a hot water extracted fraction of G. hansenii GK-1, which had low LPS activity, was a major component in the AAB to show function in inhibiting the IL-4 production of innate immune cells stimulated with OVA, leading to the promotion of Foxp3+CD4+T cell induction. The LPS fraction lacked O-antigen and showed weaker biological activities.
MATERIALS AND METHODS
Mice
BALB/c mice (CLEA Japan, Inc., Tokyo, Japan), OVA23-3 mice, recombination-activating-gene (RAG)-2-deficient OVA23-3 mice (R23-3 mice), and RAG-2-deficient DO11.10 mice (RD10 mice) were used as experimental animals. OVA23-3 and R23-3 mice were donated by S. Habu (Tokai University School of Medicine) [10] and were bred by Sankyo Labo Service Corp. Inc. (Tokyo Japan). All mice were bred in a specific pathogen-free; SPF environment at The University of Tokyo and maintained using γ-ray irradiated feed (CE-2, CLEA Japan, Inc., Tokyo, Japan) and sterilized and deionized water. In the experiment, 7-week-old mice with matching genders were used. All experiments were performed in accordance with the guideline of The University of Tokyo. In the experiment, the mice ingested freely a feed comprising an egg-white diet which protein source consists of egg-white (an EW diet, Funabashi Farm, Chiba, Japan). A casein diet (CN diet, Funabashi Farm), the protein source of which was cow casein, was used as a control for the EW diet.
Preparation of extracts of G. hansenii GK-1
Extracts from G. hansenii GK-1 (FI, FII, FIII, and FIV) were prepared in accordance with a previously reported procedure to purify LPS from Gram-negative bacteria [11] and were provided by Kewpie Corporation, Tokyo, Japan. The procedure is shown in Fig. 1A and the preparation process for each sample and the resulting products were as follows: For FI, after culturing, the bacteria were centrifuged, washed, sterilized by heating at 80°C for 1 min, and freeze-dried (killed bacteria). For FII, FI was extracted with hot water at 90°C for 20 min (hot water extract). For FIII, FI was treated with the hot phenol-water method [11], and the aqueous layer obtained was ultra-filtrated and designated as FIII (phenol extract). For FIV, FIII was further purified by repeating extraction with the hot phenol-water method and ultrafiltration 3 times. LPS of Escherichia coli (0111:B4, Standard; Invivogen, Carlsbad, CA, USA) was used as a control. The LPS contents were measured with Limulus ES-II Single Test Wako (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan). The structure of LPS was analyzed by Tris-glycine SDS-PAGE analysis [12, 13]. The SDS-PAGE profile of density gradient fractions was visualized by periodic acid-silver staining (Silver Stain 2 Kit for electrophoresis, FUJIFILM Wako Pure Chemical Corp.) [14].
Fig. 1.
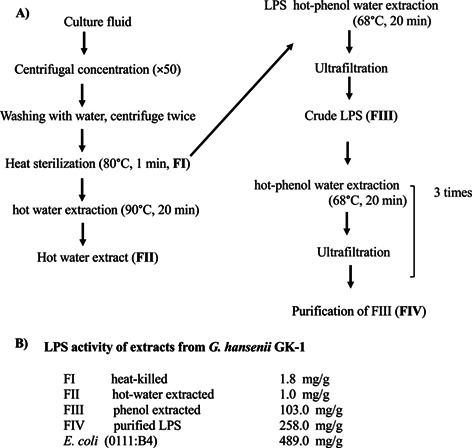
Preparation procedure of each fraction extracted from G. hansenii GK-1 acetic acid bacteria. (A) Preparation procedures for FI, FII, FIII, and FIV. (B) Endotoxin activity of each extract of G. hansenii GK-1 (FI, FII, FIII, and FIV) and that of E. coli (O111:B4) analyzed by Limulus test. Each value is indicated as the average of three independent tests. LPS: lipopolysaccharide.
Culture medium
To prepare complete RPMI medium, RPMI 1640 (10.2 g, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) was dissolved to 1 L of distilled water and sterilized. L(+)-glutamine (0.03%, FUJIFILM Wako Pure Chemical Corp.), sodium hydrogen carbonate (0.2%, FUJIFILM Wako Pure Chemical Corp.), penicillin G potassium (100 U/mL, Meiji Seika Pharma, Tokyo, Japan), streptomycin (100 µg/mL, Meiji Seika Pharma), 2-mercaptoethanol (50 µM, FUJIFILM Wako Pure Chemical Corp.) were further added (serum-free RPMI). Fetal calf serum (FCS; Gibco, Carlsbad, CA, USA) was added to the serum-free RPMI to 10% (complete RPMI).
Cell preparation and culture
Mice were euthanized by cervical dislocation by experts, and spleen and mesenteric lymph nodes (MLNs) were removed. The tissues were placed on dishes with complete RPMI on ice for further analysis. To examine the responses of MLN cells, spleen cells, and CD4+T cells to extracts of G. hansenii GK-1, MLN cells were isolated by treatment with 1 mg/mL collagenase (FUJIFILM Wako Pure Chemical Corp.) and 1 mg/mL DNase I (Roche, Mannheim, Germany) in a tube with complete RPMI at 37°C and stirred for 70 min. To prepare a single-cell suspension, the MLN cells were further passed through a 100 µm cell strainer (Falcon, Corning, Corning, NY, USA), washed twice with, and then re-suspended in complete RPMI. Spleen cells were isolated by hemolysis with ammonium-chloride-potassium lysis buffer and washed with complete RPMI twice. CD4+T cells (1×105 cells/well) prepared from MLNs or spleen cells with a MACS cell separation system (Miltenyi biotec, Bergisch Gladbach, Germany) and mitomycin C-treated spleen cells as antigen-presenting cells (APCs; 4×105 cells/well) were dispensed in a 96-well flat-bottomed plate (Falcon). To examine IL-4 production by spleen cells or CD4+T cells and APCs, OVA (Sigma-Aldrich, St. Louis, MO, USA) at final concentrations of 0, 0.25, and 1 mg/mL and each extract at final concentrations of 0, 0.05, 0.2, 1, and 5 µg/mL were added to the culture wells. The final liquid volume was adjusted to 200 µL/well, the cells were cultured at 37°C in a 5% CO2 incubator for 24 hr, and the supernatant was collected. To examine IL-6 production, spleen cells from BALB/c mice (2×106 cells/well) were incubated with FIV or E. coli LPS (final concentrations, 0, 0.5, 1, 2, 5, and 10 µg/mL). Polymyxin B (PMB, InvivoGen) was diluted to the appropriate concentration and added to the wells of the spleen cell culture with FIV (10 ng/mL) or E. coli LPS (200 ng/mL). After culture for 24 hr, the supernatant was collected to analyze IL-4 production.
Induction of regulatory T cells (Tregs)
The prepared MLNs cells from R23-3 or RD10 mice were dispensed into 96-well plates at 2.5×105 cells/well and incubated for 72 hr in 5% fetal calf serum (FCS)-RPMI medium in a 5% CO2 incubator at 37°C in the presence of the following materials at their final concentrations: OVA (0.25 mg/mL, Sigma-Aldrich), TGF-β (2 ng/mL, R&D Systems, Minneapolis, MN, USA), retinoic acid (1 μM, FUJIFILM Wako Pure Chemical Corp.), and rIL-2 (2 µg/mL, eBioscience, Waltham, MA, USA). After culturing, the collected cells were used for analysis by flow cytometry to analyze Foxp3 expression and the supernatant was collected to analyze IL-4 production.
Analysis of IL-4, IFN-γ, and IL-6 production
A sandwich ELISA was used for analysis of cytokine concentration in culture supernatants. Rat anti-mouse IL-4 (11B11, BD Pharmingen, San Jose, CA, USA), IFN-γ (R4-6A2, BD Pharmingen), or IL-6 (MP5-20F3, BD Pharmingen) antibodies in NaHPO3 buffer were coated onto 96-well polystyrene plates (Invitrogen/Thermo Fisher Scientific K.K., Tokyo, Japan) and incubated overnight at 4°C. After blocking with 3% BSA in phosphate-buffered saline (PBS) plus 0.05% Tween®20 (PBS–Tween, Sigma Aldrich), samples diluted with PBS–Tween were added to the wells and incubated for 2 hr at room temperature. After washing, biotinylated rat anti-mouse IL-4 (BVD6-24G2, BD Pharmingen), anti-mouse IFN-γ (XMG1.2, BD Pharmingen), or anti-mouse IL-6 (MP5-32C11, BD Pharmingen) antibodies were added, and the samples were incubated for further 2 hr at room temperature. After washing, alkaline phosphatase-labeled streptavidin (BD Pharmingen) diluted with PBS–Tween was added and the samples were incubated for 1 hr at room temperature. After a final washing, an enzyme substrate buffer solution, 0.1% p-nitrophenylphosphate, (Tokyo Chemical Industry, Tokyo, Japan) in a diethanolamine buffer at pH 9.8, was added to the plates, and the absorbance value of the solution in each well was recorded using an ELISA plate reader at 405 nm. The antibody titer was expressed as the optical density. The absorbance was calculated with reference to standard curves for each recombinant cytokine: mouse rIL-4 (BD Pharmingen, Cat No. 554434), mouse rIFN-γ (BD Pharmingen, Cat No. 551216), or mouse rIL-6 (R&D Systems).
Flow cytometry
PBS containing 1% FCS and 0.1% sodium azide (FUJIFILM Wako Pure Chemical Corp.) was used as the FACS buffer. After collecting the cultured cells for each well and washing them with FACS buffer, rat anti-mouse CD16/CD32 antibody (2.5 µg/mL of FACS buffer, BioLegend, San Diego, CA, USA) was added to the cells, and the mixture was incubated at 4°C for 15 min. After washing, 30 µL of FITC-labeled anti-CD4 antibody (H129.19, BD Pharmingen) was added, and the mixture was allowed to stand in a dark place at 4°C for 20 min. After washing with FACS buffer, Foxp3 molecule was stained by using Foxp3 Staining Buffer Set (Invitrogen) in according with the manufacturing procedure. Briefly, the cells were washed with 1×permeabilization buffer (PB, 10 times diluted with Milli-Q). Rat anti-mouse CD16/CD32 antibody diluted with PB (2.5 µg/mL) was added, and the mixture was reacted at 4°C for 15 min. After washing with PB, APC-labeled anti-Foxp3 antibody (FJK-16s, eBioscience) diluted with PB was added for staining, and the mixture was incubated in the dark at 4°C for 30 min. After washing, the cells were suspended in FACS buffer and measured using FACSVerse (BD Biosciences, Franklin Lakes, NJ, USA). FlowJo (Tree Star, Inc., Ashland, OR, USA) was used for the analysis.
Statistical analysis
Student’s t-test (Excel ver. 16.52) was used for comparisons between two groups in the statistical analysis. For the multigroup comparisons, Dunnett’s test was performed using the statistical analysis software R.
RESULTS
Lower LPS activities for each extract of G. hansenni than LPS of E. coli. (0111:B4).
Each extract from G. hansenii GK-1 (FI, FII, FIII, and FIV) was prepared in accordance with a procedure for purification of LPS from Gram-negative bacteria [11]. The procedure is shown in Fig. 1A, and the detailed preparation process for each sample and the resulting products are described in the MATERIALS AND METHODS section. The activity of the LPS of FIII (103.0 mg/one gram of biomass powder) was 40 to 300 times that of FI (1.8 mg/g) or FII (1.0 mg/g) (Fig. 1B). In FI and FII, the levels of LPS activity were extremely lower. To obtain FIV, the LPS of FIII was further purified by extraction with the hot phenol-water method and ultrafiltration 3 times. The purity of FIV (96.6%) was calculated as the ratio of the weight of the remaining component of the LPS fraction obtained as the FIV fraction, which was calculated by subtracting the amount of the other components (protein, 6.6 µg; nucleic acid, 27.7 µg) from the weight of the LPS fraction (1,000 µg) relative to the weight of the LPS fraction (1,000 µg). The LPS activity of FIV (258.0 mg/g) calculated by the Limulus test was higher than that of FIII (103.0 mg/one gram of biomass powder), but was lower than that of the LPS preparation from E. coli (489.0 mg/g).
Suppression of IL-4 production stimulated by OVA by the hot water extract fraction of G. hansenii GK-1 (FII) in spleen cells isolated from food-allergic model, OVA23-3 mice
We first analyzed the effect of each fraction (FI to FIII) extracted from G. hansenii GK-1 on Th2-type responses in food-allergic model mice, OVA23-3. Significant amounts of IL-4 and IFN-γ were produced by spleen cells purified from OVA23-3 mice, when they were stimulated with OVA (1 mg/mL). The IL-4 production was ameliorated by addition of 0.05–1 µg/mL of FII; the level of inhibition was highest and most stable at 0.05 µg/mL of FII; the inhibitory effect was lost at higher than 5 µg/mL (Fig. 2A). IFN-γ production seemed to be inhibited at lower concentrations of FII (Fig. 2B). However, although Fig. 2B shows representative data of several repetitions of our experiments, we could not reproduce the same results for the inhibition of IFN-γ production at the same concentration of FII. These results indicated that FII did not have the ability to suppress the IFN-γ production of spleen cells. A lower OVA level (0.25 mg/mL) also promoted the IL-4 production of spleen cells of OVA23-3 mice, and the production was inhibited by addition of FII without affecting IFN-γ, as shown in Supplementary Fig. 1; however, the inhibitory effect of FII was lower under culture condition in which spleen cells were stimulated with a low concentration of OVA (0.25 mg/mL), compared with when they were stimulated with a higher concentration of OVA (1 mg/mL). Therefore, we added OVA at a concentration of 1 mg/mL for further culture analyses.
Fig. 2.
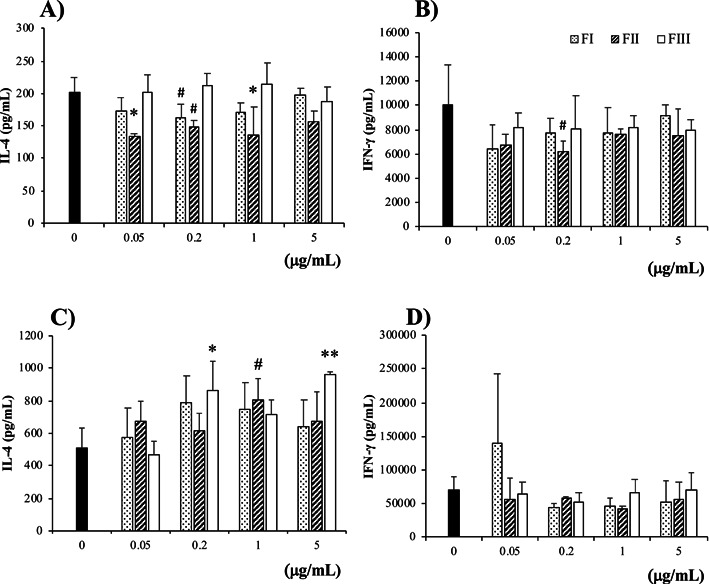
The hot water extract of G. hansenii GK-1 (FII) inhibited the IL-4 production of spleen cells, but not that of ovalbumin (OVA)-specific CD4+T cells, purified from food-allergic model mice. (A, B) Spleen cells of OVA23-3 mice or (C, D) spleen CD4+T cells of R23-3 mice and antigen-presenting cells prepared from spleens of BALB/c mice were stimulated with G. hansenii GK-1 fractions (FI, FII, and FIII; final concentrations 0.05, 0.2, 1, and 5 µg/mL) and OVA (final concentration 1 mg/mL). The cells were cultured for 24 hr, and (A, C) IL-4 and (B, D) IFN-γ production in the collected culture supernatant was measured by ELISA. The cells of 4 mice were pooled, cultured and measured for 3 wells under each condition. Significance was determined by Dunnett’s test for each G. hansenii GK-1 fraction vs. without each G. hansenii GK-1 fraction (*p<0.05, **p<0.01, #p<0.1). The data are representative of two independent experiments.
R23-3 mice have only OVA-specific CD4+T cells, not B cells nor CD8+T cells, as a responder to OVA stimulation [15]. In spleen cells of OVA23-3 mice, cells that play a role in the IL-4 inhibitory effect of FII were suggested to be either OVA-specific CD4+T cells or other innate immune cells (APCs), or both. To clarify which cells responded to FII in OVA23-3 mice, we used a CD4+T cell culture system in which OVA-specific CD4+T cells purified from R23-3 mice were cultured with mitomycin C-treated spleen cells from BALB/c mice as APCs and OVA as an antigen. In this culture system, APCs hardly responded to any stimulatory materials. None of the three extracts (FI, FII, FIII) showed significant inhibitory function with respect to IL-4 or IFN-γ production by immune cells. (Fig. 2C and 2D).
These results indicated that unlike the case of the spleen cell culture, FII extracted from G. hansenii GK-1 did not show an inhibitory effect on IL-4 production in CD4+T cell-culture system, suggesting that FII acted on innate immune cells as APCs. FII may affect OVA-activated CD4+T cells differently via APCs depending on its concentration, as indicated by lower levels (0.05–0.2 µg/mL) of it suppressing IL-4 production more effectively than higher levels (1–5 µg/mL).
The highly purified LPS fraction from G. hansenii GK-1 (FIV) biologically functioned but weakly affected spleen cells of OVA23-3 mice
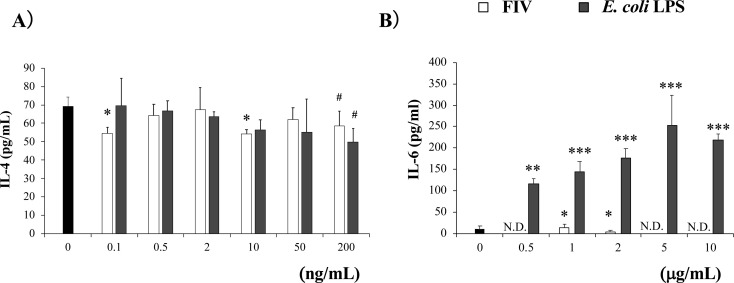
G. hansenii GK-1 has been reported to function in suppressing development of pollen allergy, and the LPS was suggested to function through TLR4 [4, 5]. However, the function of FIII, which corresponded to the crude LPS fraction, was lower than that of FII in inhibiting IL-4 production by spleen cells of OVA23-3 mice (Fig. 2A). This result might have been caused by insufficient purification of the LPS fraction (FIII). To confirm whether the LPS fraction of G. hansenii GK-1 showed an inhibitory effect on IL-4 production, we examined the activity of FIV in a spleen cell culture system. Because FIV inhibited the IL-4 production of spleen cells by preliminary investigation, we directly compared its effect with that of LPS purified from E. coli (InvivoGen). While the LPS from E. coli tended to ameliorate the IL-4 production of spleen cells from OVA23-3 mice at concentrations of more than 50 ng/mL when they were stimulated with OVA, similar results were obtained in the case of FIV, but at concentrations of 200 ng/mL and less than 10 ng/mL (Fig. 3A). The inhibitory effects on IL-4 of each LPS from E. coli (200 ng/mL) or G. hansenii GK-1(10 ng/mL) tended to be abrogated by the addition of the proper concentration of PMB (100 ng/mL, Supplementary Fig. 2).
Fig. 3.
Purified LPS from G. hansenii GK-1 (FIV) inhibited the IL-4 production of spleen cells from food-allergic model mice, but did not induce IL-6 production of BALB/c spleen cells.
(A) OVA23-3 spleen cells were stimulated with LPS fraction extracted from FIV or E. coli LPS (final concentrations, 0.1, 0.5, 2, 10, 50, and 200 ng/mL) and OVA (final concentration 1 mg/mL). The cells were cultured for 24 hr, and the production of IL-4 in the collected supernatant was measured by ELISA. (B) BALB/c mouse spleen cells were stimulated with FIV and E. coli LPS (final concentrations, 0.5, 1, 2, 5, and 10 µg/mL). The cells were cultured for 24 hr, and the production of IL-6 in the collected supernatant was measured by ELISA. The cells were pooled for 3 mice, cultured and measured in 3 wells per each condition. Significance was determined by Dunnett’s test for each sample concentration vs a sample concentration of 0 ng/mL or 0 µg/mL (*p<0.05, **p<0.01, ***p<0.001, #p<0.1). LPS: lipopolysaccharide; OVA: ovalbumin; N.D.: not detected. The data are representative of two independent experiments.
LPS from E. coli induced strong production of IL-6 from BALB/c spleen cells, while FIV induced only a minimal amount, with it rather inhibiting the IL-6 production of spleen cells from OVA23-3 mice (Fig. 3B). This may show another effect of FIV as a food compound to regulate immune responses. However, because the biological activity of G. hansenii GK-1 LPS was affected by the storage period and was easily lost, the IL-4-supressive activity of FIV was suggested to be less stable than that of FII. This shows that major components of G. hansenii GK-1 extracts inducing IL-4 inhibition via immune cells are possibly components other than the purified LPS fraction (FIV), although the LPS fraction itself does possess the biological activity. In addition, the differences in optimal concentration and stability in exhibiting IL-4 inhibitory effects between LPS from E. coli and the LPS fraction of G. hansenii GK-1 (FIV) were considered to be dependent on the differences in their structures.
Addition of FII to the Treg induction culture promotes Foxp3 molecule expression in CD4+T cells of food-allergic model, R23-3 model mice
We clarified that both FII and FIV extracts from G. hansenii GK-1 had the capability to inhibit the IL-4 production of OVA-specific spleen cells. This inhibitory effect on IL-4 production may play a role in inducing Tregs in allergies, because Foxp3+ expression is regulated by IL-4 treatment [16]. In R23-3 mice, an EW (egg-white) -diet significantly induced weight loss, a representative factor of the induction of enteropathy, and in this weight loss, IL-4-producing OVA-specific CD4+ T cells in MLNs have been reported to play a critical role in this weight loss and intestinal inflammation [17]. In contrast to EW-fed R23-3 mice, EW-fed RD10 mice (RAG-2-deficient OVA-T cell receptor genes transgenic mice similar to R23-3 mice but used as tolerance-inducing model mice) did not exhibit weight loss [15]. Furthermore, in R23-3 mice that were fed the EW diet and showed excessive IL-4 inflammatory responses, Treg induction was strongly obstructed for 7 days after the start of EW-feeding compared with RD10 mice [15]. In addition, MLNs play an important role in Treg induction [18].
Therefore, in this study, we analyzed whether the LPS fraction (FIV) from G. hansenii GK-1 could ameliorate obstructed Treg induction in MLNs of EW-fed R23-3 mice. Our FACS strategy is shown in Fig. 4A. EW-feeding induced significant weight loss in R23-3 mice compared with RD10 mice (Supplementary Fig. 3), as reported previously [15]. The lower level (5.27%) of Treg induction in MLNs cells purified from R23-3 mice fed with the EW diet for 7 days showed significant (p<0.05) recovery as a result of the addition of FII (6.24%; Fig. 4B, right), but not of FIV. Under the same culture conditions, the level of Tregs in MLNs CD4+Tcells of EW-diet fed RD10 mice was not inhibited (53. 3%), and the rate was not improved by the addition of FII (52.3%; Fig. 4B, left). While the addition of FII to the culture did not promote Treg development in CN-fed mice, FIV tended to increase the rate of Tregs in CN-fed RD10 mice (p<0.101) but not in CN-fed R23-3 mice, suggesting that FIV showed function in inducing Tregs under normal conditions that was independent of its IL-4-related function, when they were stimulated with OVA (1 mg/mL). Strong IL-4 production in the culture supernatant was observed in MLNs cells of EW-fed R23-3 mice, and the production tended to be inhibited by the addition of FII (Fig 4C, R23-3, p<0.191). On the other hand, no IL-4 was detected in the culture supernatants of MLNs cells from EW-fed RD10 mice and CN-fed the mice (Fig 4C, RD10), when they were stimulated with OVA (1 mg/mL).
Fig. 4.
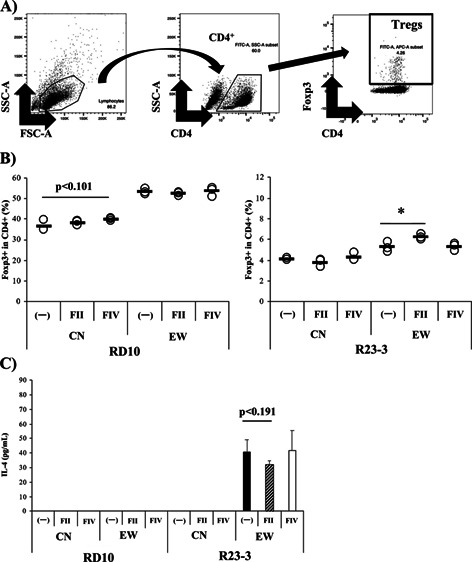
The hot water extract from G. hansenii GK-1 (FII) induced Foxp3+T cells in mesenteric lymph node (MLN) cells of R23-3 mice with severe inflammation caused by EW-feeding.
(A) Representative plots of a scheme examining the Foxp3+T cells. (B) Rates of Foxp3+T cells in CD4+T cells from MLN cells of RD10 (left) and R23-3 (right) mice fed with the EW-diet for 7 days (severe inflammation phase). MLN cells were incubated under Foxp3+T cell induction conditions stimulated with OVA (1 mg/mL), rIL-2, TGF-β, and retinoic acid, in the presence or absence of FII or FIV. The collected cells were analyzed by flow cytometry. (C) IL-4 production in the culture supernatant was analyzed by ELISA. 〇 indicates the value of each well, and the horizontal line indicates the mean of each group. The cells of 3 mice were pooled, cultured and analyzed for 3 wells for each condition. Significance was determined by Student’s t-test (*p<0.05, #p<0.1). The data are representative of two independent experiments.
Lack of O-antigen in the LPS fraction (FIV) of G. hansenii GK-1
Because the biological activity of FIV was shown to be lower than that of E. coli (0111:B4; Fig. 3), to clarify structural differences in LPS between G. hansenii GK-1 and E. coli (0111:B4), we conducted a Tris-glycine SDS-PAGE analysis and visualized the SDS-PAGE profile of density gradient fractions by periodic acid oxidized silver staining (Supplementary Fig. 4). The SDS-PAGE profile from LPS of E. coli showed a clear ladder pattern of O-antigens, whereas that from G. hansenii GK-1 lacked the pattern of O-antigens, although lipooligosaccharide (LOS) was confirmed by a clear band, suggesting that the LPS of G. hansenii GK-1 was comprised by only the LOS structure without O-antigen. Endotoxin activity was two times stronger in LPS from E. coli (489.0 mg/g) compared with that of FIV (258.0 mg/g; Fig. 1B). Therefore, cytotoxic effects of the LPS fraction of G. hansenii GK-1 might be lower than that of LPS from E. coli, because without O-antigen, cognate interaction and stimulation of signal transduction with innate immune cells may be weakened in the LPS of G. hansenii GK-1 [19, 20].
DISCUSSION
In this study, we found that the hot water extract (FII) of G. hansenii GK-1, not the LPS fraction (FIV) had the ability to inhibit the IL-4 production of spleen cells from food-allergic model mice, leading to the promotion of Foxp3+CD4+T cell induction in MLNs, which has a substantial role in food-allergic intestinal inflammation [15]. In the LPS fraction from G. hansenii GK-1 (FIV), the ability to induce Foxp3+T cells was proven to be exhibited under normal conditions. Although FIV could inhibit the IL-4 production of spleen cells, the biological activity was suggested to be weaker than those of FII and the LPS from E. coli. used in our study. The ability of FII from G. hansenii GK-1 suggested the possibility of using the extracts in ameliorating food-allergic reactions. It is possible to provide some scientific and underlying evidence for its effects from clinical observations of G. hansenii GK-1: it reduced nasal discomfort in Japanese subjects [6], and it decreased rubbing counts in pollen-allergic rhinitis model mice [4].
The existence of effector-memory T cells and its strong Th2 responses has been known to prevent the differentiation of naïve T cells into Foxp3+T cells. [16, 21]. Therefore, under severe allergic conditions, as in EW-fed R23-3 mice, the induction and activation of Foxp3+T cells is suggested to be restricted. In EW-fed R23-3 mice, EW-feeding for 28 days fully induced Foxp3+T cells, but they were observed to be only slightly induced by day 7 of feeding [15]. However, FII promoted Foxp3+T cells induction from MLNs cells purified from the model mice under the severe conditions of 7 days of feeding with the EW diet. Therefore, even if the increase in the rate of Foxp3+T cells seemed to be small (5.27→6.24%, Fig. 4), we thought that the potential of FII to ameliorate allergic responses was rather high. The target cells of FII from G. hansenii GK-1 were suggested to be innate immune cells, not CD4+T cells, because the IL-4 levels in the supernatants of CD4+T cells cultured with inactivated APCs plus OVA were not affected by the addition of FII. However, it remains unknown whether IL-4 was produced by innate immune cells as well as CD4+T cells in the spleen cells and how the extracts inhibit IL-4 production via innate immune cells in the model mice.
Examination of the FII used in the analysis for Supplementary Fig. 4, which was another lot of the FII used in the analyses for Fig. 2 and Supplementary Fig. 1, revealed that FII was mainly consisted of nucleic acids (65.5 mg / gram of biomass powder; QuantiFluor dsDNA system, Promega, Madison, WI, USA) and other components suggested to be proteins (29.3 mg/g), in addition to a small LPS fraction (5.2 mg/g activity as measured by Limulus ES-2 Single Test WAKO). These results suggested that contrary to our prediction that LPS, as a major component of AAB, has activity that suppresses allergic symptoms, components other than the LPS fraction of G. hansenii GK-1 functioned in vitro as major and stable components that inhibited the IL-4 production of spleen cells from food-allergic model mice, although in vivo, they may affect immune cells in cooperation with each other.
LPS constitutes the outer membrane of Gram-negative bacteria and is composed of three units: a hydrophilic polysaccharide, O-antigen, and lipid A, which known as the hydrophobic domain. It generally functions as a strong immunomodulatory component by causing proinflammatory responses in target cells. In the present study, we focused on LPS of AAB in reducing allergic responses [3, 6] and examined the effects of the LPS fraction of G. hansenii GK-1 on the regulation of IL-4 responses by using food-allergic model mice. Although the LPS fraction of G. hansenii GK-1 (FIV) had the ability to ameliorate the IL-4 production of OVA-activated spleen cells from OVA23-3 mice (Fig. 3), we thought that the activity was less stable than for FII or LPS from E. coli. This is because the addition of PMB only weakly abrogated the LPS activity inhibiting IL-4 production (Supplementary Fig. 2) and the activity of FIV declined 1–2 months after preparation, even when preserved at −20°C. Unlike the LPS from E. coli used in this study (Fig. 3, Supplementary Fig. 2), the LPS fraction of G. hansenii GK-1 (FIV) was shown to be a form lacking O-antigen as a structure confirmed by the SDS-PAGE profile in this experiment (Supplementary Fig. 4). LPS without O-antigen is one of the mutant forms of LPS classified based on the absence or presence of O-antigen. The absence of O-antigen in the LPS of G. hansenii GK-1 may the cause of the unstable inhibitory effect on IL-4 production by spleen cells. Actually, the structure of the O-antigen sugar moiety has been reported to affect proinflammatory cytokine production (IL-6) and to determine the course and severity of urinary tract infections [19], and it has also been reported to affect IFN-γ production in human NK cells [20]. Regarding relationships between function of FIV and its structure, the endotoxin activities of FIV were lower compared with those of LPS from E. coli. We also observed significant IL-6 production in spleen cells from BALB/c mice stimulated with LPS from E. coli, but the IL-6 production in these cells was inhibited by FIV. Therefore, the present study suggests that the difference between the severe inflammatory activities of LPS and its inhibitory function may be due to structural characteristics related to the absence of O-antigen in the LPS of G. hansenii GK-1. This characteristic of the LPS would enable us to use extracts of G. hansenii GK-1 for improving allergic immune responses more safely than other Gram-negative bacteria, even if other Gram-negative bacteria, such as E. coli, have similar abilities to inhibit IL-4 production (Fig. 3A). Reports indicating the improvement of pollen-allergic rhinitis by injection with G. hansenii GK-1 suggested that a main source of the activity may be the LPS fraction [4, 5]. Although our results suggest a weak effect of the LPS fraction (FIV) of G. hansenii GK-1 in inducing Foxp3+T cells under strong IL-4 conditions, the possibility remains that the G. hansenii GK-1 LPS fraction may function in the mitigation of the symptoms under the appropriate conditions [22].
Excess IL-4 production has been reported to prevent the expression of Foxp3 on CD4+T cells [16]. In this study, it remains to be clarified in detail whether the inhibitory effect of FII of G. hansenii GK-1 directly promotes the differentiation of CD4+T cells into regulatory T cells. Compared with spleen cells of EW-fed R23-3 mice, the spleen cells of the RD10 mice, which exhibit tolerant characteristics when fed the EW diet, maintained a higher level of Foxp3 expression in CD4+T cells (>52% in FII/RD10 mice) under lower IL-4 conditions in the culture supernatant (Fig. 4). Therefore, we thought that the increase in the rate of Foxp3-expressing CD4+T cells was caused by the addition of FII of G. hansenii GK-1 and its IL-4 inhibitory effect, although further studies are needed. However, in the EW-fed RD10 mice, we should have analyzed Foxp3+CD4+T cell induction by suppressing IL-4 production during an earlier period of EW-feeding, in which spleen cells would positively proliferate and produce IL-4 by stimulation with OVA. This is because we previously reported that RD10 mice fed an EW diet for 7 days acquired tolerance [15], and this might have prevented us from observeing the inhibitory activity of FII clearly.
Inhibition of IL-4 production has been thought to play an important role in the regulation of different allergic symptoms. The cytotoxicity of FII would be low, because CD4+T cells of RD10 mice could differentiate into Foxp3+CD4+T cells for 72 hr of incubation without dying, as shown in Fig. 4. Therefore, although we could not observe any effect of FII on Th1 responses, we think that G. hansenii GK-1, mainly through FII, can possibly contribute to the suppression of IL-4 production in different clinical allergic cases. Furthermore, the structural analysis using FIV urged us to suggest that G. hansenii GK-1 and its extracts will contribute to improving allergic responses as a safe and new types of food components from Gram-negative bacteria.
Supplementary Material
REFERENCES
- 1.Gomes RJ, Borges MF, Rosa MF, Castro-Gómez RJH, Spinosa WA. 2018. Acetic acid bacteria in the food industry: systematics, characteristics and applications. Food Technol Biotechnol 56: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. 2014. Functional properties of vinegar. J Food Sci 79: R757–R764. [DOI] [PubMed] [Google Scholar]
- 3.Renz H, Skevaki C. 2021. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol 21: 177–191. [DOI] [PubMed] [Google Scholar]
- 4.Inagawa H, Nishizawa T, Kochi C, Amano S, Soma GI. 2019. Pollen allergy suppression effect by oral administration of acetic acid bacteria (Gluconacetobactor hansenii). Anticancer Res 39: 4511–4516. [DOI] [PubMed] [Google Scholar]
- 5.Kimura M, Oe M, Okuyama Y, Yoshioka S. 2019. TLR4 reactivity of Gluconacetobacter (Gluconacetobacter hansenii GK-1) and their synergistic action with Lactobacillus in producing antiallergic effects. Jpn Pharmacol Ther 47: 2001–2006. [Google Scholar]
- 6.Yoshioka S, Oe M, Kamijyo F, Shimada K, Okuyama Y, Kishiyama H, Matsuoka R, Masuda Y, Kanemitsu T, Enomoto M. 2019. Acetic acid bacteria (Gluconacetobacter hansenii GK-1) relieves nasal discomforts—a randomized double-blinded placebo-controlled study. Jpn Pharmacol Ther 47: 461–467. [Google Scholar]
- 7.Amano S, Inagawa H, Nakata Y, Ohmori M, Kohchi C, Soma G. 2015. Oral administration of lipopolysaccharide of acetic acid bacteria protects pollen allergy in a murine model. Anticancer Res 35: 4509–4514. [PubMed] [Google Scholar]
- 8.Seeley JJ, Ghosh S. 2017. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol 101: 107–119. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima-Adachi H, Ebihara A, Kikuchi A, Ishida T, Sasaki K, Hirano K, Watanabe H, Asai K, Takahashi Y, Kanamori Y, Shimojo N, Matsuda H, Kohno Y, Hachimura S, Kaminogawa S. 2006. Food antigen causes TH2-dependent enteropathy followed by tissue repair in T-cell receptor transgenic mice. J Allergy Clin Immunol 117: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Sasahara T, Nakamura Y, Osaki T, Hasegawa T, Tadakuma T, Arata Y, Kumagai Y, Katsuki M, Habu S. 1994. Naive T cells can mediate delayed-type hypersensitivity response in T cell receptor transgenic mice. Eur J Immunol 24: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 11.Westphal O, Jann K, Himmelspach K. 1983. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy 33: 9–39. [PubMed] [Google Scholar]
- 12.Schägger H. 2006. Tricine-SDS-PAGE. Nat Protoc 1: 16–22. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CM, Frasch CE. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119: 115–119. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima-Adachi H, Shibahara K, Fujimura Y, Takeyama J, Hiraide E, Kikuchi A, Murakami H, Hosono A, Nochi T, Wakatsuki Y, Shimojo N, Kaminogawa S, Sato R, Kiyono H, Hachimura S. 2017. Critical role of intestinal interleukin-4 modulating regulatory T cells for desensitization, tolerance, and inflammation of food allergy. PLoS One 12: e0172795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. 2008. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 9: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima-Adachi H, Kikuchi A, Fujimura Y, Shibahara K, Makino T, Goseki-Sone M, Kihara-Fujioka M, Nochi T, Kurashima Y, Igarashi O, Yamamoto M, Kunisawa J, Toda M, Kaminogawa S, Sato R, Kiyono H, Hachimura S. 2014. Peyer’s patches and mesenteric lymph nodes cooperatively promote enteropathy in a mouse model of food allergy. PLoS One 9: e107492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiokawa A, Kotaki R, Takano T, Nakajima-Adachi H, Hachimura S. 2017. Mesenteric lymph node CD11b- CD103+ PD-L1High dendritic cells highly induce regulatory T cells. Immunology 152: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath DJ, Jr, Patel AS, Mohamed A, Storm DW, Singh C, Li B, Zhang J, Koff SA, Jayanthi VR, Mason KM, Justice SS. 2016. Association of O-antigen serotype with the magnitude of initial svstemic cytokine responses and persistence in the urinary tract. J Bacteriol 198: 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanevskiy LM, Erokhina SA, Streltsova MA, Ziganshin RH, Telford WG, Sapozhnikov AM, Kovalenko EI. 2019. The role of O-antigen in LPS-induced activation of human NK cells. J Immunol Res 2019: 3062754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. 2008. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity 29: 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Védrine M, Berthault C, Leroux C, Répérant-Ferter M, Gitton C, Barbey S, Rainard P, Gilbert FB, Germon P. 2018. Sensing of Escherichia coli and LPS by mammary epithelial cells is modulated by O-antigen chain and CD14. PLoS One 13: e0202664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.