Abstract
Objective: This study aimed to evaluate both device and functional outcomes of men who underwent initial artificial urinary sphincter (AUS) placement after pelvic radiation using the transcorporal versus the standard approach.
Methods: A retrospective review of patients who underwent first-time AUS placement after pelvic irradiation for prostate cancer was conducted between January 2008 and June 2020. Patients were grouped by transcorporal versus standard device placement. The primary outcomes of interest included major complications (revision or explant surgery) and functional outcomes (pads per day, International Prostate Symptom Score {IPSS}, quality of life {QOL} score).
Results: We identified 45 patients who underwent first-time AUS with a history of prior pelvic irradiation for prostate cancer, 27 underwent transcorporal placement and 18 underwent standard placement. Transcorporal AUS placement resulted in a significantly lower number of major complications (p=0.01), explants (p=0.02), and revisions (p=0.04) The transcorporal artificial urinary sphincter group had better postoperative pads per day (p=0.04), IPSS (p<0.01), and IPSS QOL score (p<0.01).
Conclusions: Initial transcorporal artificial urinary sphincter placement is a promising technique with lower rates of major complications in patients with a history of prior pelvic radiation and had better functional urinary outcomes.
Keywords: artificial urinary sphincter, urinary incontinence, urethra/radiation effects, quality of life, prostatectomy/adverse effects
Introduction
The artificial urethral sphincter is the gold standard treatment for male stress urinary incontinence which commonly results from prostatectomy or pelvic radiation for prostate cancer. Radiation therapy is a well-established risk factor for complications following artificial urinary sphincter placement including an increased risk of sphincter erosion and need for revision surgery [1,2]. This increased risk of erosion is thought to be due to a compromise to urethral blood supply caused by radiation-induced obliterative endarteritis [3-5]. This mechanism parallels several other identified risk factors including hypertension, cardiovascular disease, prior urethroplasty, and prior sphincter erosion in which the urethral blood supply is also compromised [3,6-8].
Despite significant risks associated with artificial urinary sphincter, specifically the need for future revision or device replacement, patient satisfaction rates remain high [9]. Several techniques are available to mitigate the risks of erosion in high-risk patients such as urethral wrapping with a xenograft or transcorporal artificial urinary sphincter placement. Such techniques provide an additional tissue barrier between the thinner dorsal corpora spongiosum and urethra. The transcorporal method specifically interposes ventral corporal cavernosa tunica albuginea between the cuff and urethra, and thereby theoretically reduces the risk of erosion [10]. However, the degree to which this technique reduces the future risk of erosion and its impact on functional outcomes remains an area of ongoing debate. One recent study by Redmond et. al. showed trends toward lower rates of erosion and need for revisions with the transcorporal approach [11]. However, a large study by Ortiz et. al. did not demonstrate the protective effect of transcorporal placement compared with standard sphincter placement [12].
Our objective was to study the impact on complications and functional outcomes of initial artificial urinary sphincter placement via the transcorporal approach. To our knowledge, this is one of the first studies evaluating initial transcorporal artificial urinary sphincter (AUS) placement versus the standard approach. We hypothesized that men with prostate cancer and prior pelvic irradiation who underwent initial placement of an artificial urinary sphincter via a transcorporal approach would have improved outcomes compared to men who underwent standard placement. The initial results of this study were previously presented at the Sexual Medicine Society of North America (SMSNA) annual meeting on November 14, 2020 [13].
Materials and methods
Study population
After institutional review board approval (STUDY19080339), we conducted a retrospective review of patients who underwent first-time artificial urinary sphincter placement between January 2008 and June 2020 after pelvic irradiation as part of their treatment for prostate cancer. Patients with a history of pelvic radiation for other reasons (e.g., rectal cancer) were excluded. Patients undergoing anything other than initial AUS surgery (e.g., dual cuffs) and men who underwent radiation after AUS placement were excluded. The decision to proceed with either transcorporal or standard AUS placement was based on history, risk factors, erectile function, plans for additional penile prosthesis surgery with shared decision making between surgeon and patient.
Surgical techniques
The artificial urinary sphincters (AMS 800TM; Minnetonka, MN: American Medical Systems) were implanted by two high-volume implant surgeons who are facile with both the standard and transcorporal techniques both of which used the traditional perineal approach. Patients were grouped by surgical approach for analysis.
Data collection and analysis
We collected patient demographic characteristics including age, body mass index (BMI), tobacco use, cardiovascular disease, diabetes mellitus, prior urethral dilation, urethrotomy or transurethral resection for bladder neck contracture, prior urethroplasty, prior urethral sling, prior bulking agent, radiation type (external beam radiotherapy, intensity-modulated radiation therapy, low dose rate brachytherapy, high dose rate brachytherapy), timing of radiation (salvage, adjuvant), radiation dose, timing of radiation to AUS implant, and American Society of Anesthesiologists (ASA) class. We collected device characteristics including cuff size and cuff pressure. Major complications and reasons for additional surgery were subdivided into mechanical, urethral atrophy, and infection/erosion. Functional outcomes including International Prostate Symptom Score (IPSS), quality of life (QOL), and pads per day were recorded preoperatively and postoperatively. Length of follow-up from date of surgery to last follow-up appointment was calculated.
Primary and secondary outcomes of interest
Our primary outcome of interest was occurrence of a major complication, defined as the need for revision or explant surgery, following AUS implant. We recorded the reasons for revision or explant surgery and categorized them as infection/erosion, mechanical device malfunction, or urethral atrophy. Our secondary outcomes of interest were patient functional outcomes measured by pads per day, IPSS with QOL score and need for urinary diversion after AUS placement.
Statistical analysis
All analyses were performed using RStudio (version 1.2.5025; Boston, MA: RStudio, Inc.) and a p-value of 0.05 was set for statistical significance. We used t-tests and Wilcoxon tests to check the differences in demographic characteristics based on the distribution assumption. Wilcoxon tests were used to compare the postoperative pads per day and IPSS scores between the transcorporal and standard approaches. To describe the probability of developing complication, we obtained Cox proportional hazard function over time with age, BMI, and cardiovascular disease and used the likelihood ratio test to compare the risk. Kaplan-Meier curve was used to visually show the device survival probability.
Results
Demographics
A total of 45 patients underwent first-time artificial urinary sphincter with a history of prior pelvic irradiation for prostate cancer. There were 27 patients who underwent transcorporal placement with a mean follow-up of 32 months and 18 patients who underwent standard placement with a mean follow-up of 39 months. There were no significant differences in the cohort with regards to age, BMI, tobacco use, androgen deprivation therapy, ASA class, except for cardiovascular disease (p=0.026). The difference in radiation dosage was significant between the groups with standard AUS receiving 69.8 Gy and the transcorporal group receiving 67 Gy (p=0.05). The majority of patients received salvage radiation. The median time from the last dose of radiation to artificial urinary sphincter implant was 86 months. Additionally, the standard group had a high percentage of history of prior urethral surgery 61% as compared to the transcorporal group 31% which was significant (p=0.05). The median size of AUS cuff was 4.5 cm (range: 3.5-5.5cm) for the transcorporal group and 4.0 cm (range: 3.5-4.5cm) for the standard group which was a significant difference (p=0.05). A pressure regulating balloon with a standard cuff pressure of 61-70 cm H2O was used in all except two cases which were done via the standard approach (Table 1).
Table 1. Patient characteristics: demographic information including comorbidities, radiation dosage received, history of prior urethral procedures.
ASA: American Society of Anesthesiologists; TUR: transurethral
| Characteristic | Standard AUS N=18 (%) | Transcorporal AUS N=27 (%) | p-Value | |
| Mean age ±SD (years) | 70.6±6.5 | 73.7 ±7.2 | 0.15 | |
| Median BMI (kg/m2) | 30.5 | 28.89 | 0.46 | |
| Median ASA score (range) | 3 (2-4) | 3 (2-3) | 0.91 | |
| Tobacco use | 12 | 13 | 0.36 | |
| Androgen deprivation therapy | 4 | 13 | 0.12 | |
| Cardiovascular disease | 15 (83) | 15 (56) | 0.026 | |
| Diabetes mellitus | 7 (39) | 7 (26) | 0.24 | |
| Prior urethral surgery (dilation, urethrotomy, or TUR for anastomotic contracture) | 11 (61) | 9 (33) | 0.05 | |
| Bulking agent | 0 (0) | 2 (7) | 0.27 | |
| Sling | 1 (6) | 3 (1) | 0.58 | |
| Urethroplasty | 0 (0) | 2 (7) | 0.27 | |
| Median radiation dose (Gy) (range) | 69.79 (66.6-78.0) | 67.0 (66.6-68.4) | 0.05 | |
| Type of radiation | Adjuvant | 2 (11) | 5 (19) | 0.68 |
| Salvage | 16 (89) | 20 (74) | 0.28 | |
| Primary | 0 (0) | 2 (7) | - | |
| Median time from radiation to device implant months (range) | 62 (13-170) | 108 (8-288) | 0.23 | |
| Median length of follow-up months (range) | 39 (2-135) | 32 (2-100) | 0.45 | |
| Cuff-size (cm) | 4.0 (3.5-4.5) | 4.5 (3.5-5.5) | 0.05 | |
| Cuff-pressure (cm H2O) | 61-70 | 61-70 | 1 | |
Primary outcome (major complication)
The major complication rate defined as revision or explant surgery was 56% in the standard group compared to 14.8% in the transcorporal group (p<0.01). Transcorporal AUS placement resulted in a significantly lower number of explants (p=0.02) with only a 7.4% explant rate in this group as opposed to 39% in the standard group. All explants were done for either infection or erosion. The revision rate was 33% in the standard group and 7.4% in the transcorporal group (p=0.04) (Table 2).
Table 2. Patient complications by surgical technique.
AUS: artificial urinary sphincter
| Standard AUS N (%) | Transcorporal AUS N (%) | p-Value | |
| Major complication: either AUS revision or explant | 10 (56) | 4 (14.8) | 0.01 |
| Median time to complication (AUS revision or explant) months | 46 | 19 | 0.01 |
| AUS explant | 7 (39) | 2 (7) | 0.02 |
| AUS revision | 6 (33) | 2 (7) | 0.04 |
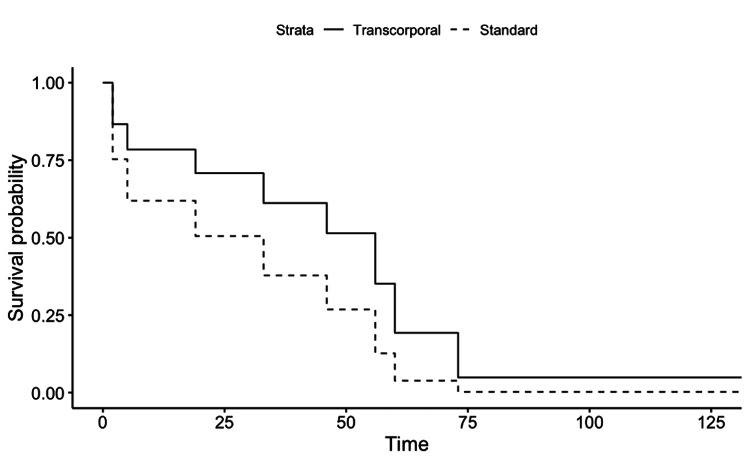
All AUS revisions were for either urethral atrophy or mechanical reasons, none were done for erosion. Cox proportional hazard function showed no significant device survival differences between the two groups (p=0.24). However, as seen on the Kaplan-Meier curves the transcorporal group trended toward having a lower risk for AUS complications (Figure 1).
Figure 1. Artificial urinary sphincter device complication free probability.
Kaplan-Meier curve showing time (months) to device complication (revision or explantation) probability for transcorporal AUS compared with standard AUS placement.
AUS: artificial urinary sphincter
Secondary outcome (patient functional outcomes)
Both transcorporal and standard approaches resulted in improvements in pads per day and IPSS after artificial urinary sphincter implantation, based on the Mann-Whitney test results. However, the transcorporal AUS group had superior outcomes in terms of postoperative pads per day (p=0.04), postoperative IPSS (p<0.01), and postoperative IPSS QOL score (p<0.01) (Table 3).
Table 3. Patient reported functional outcomes.
AUS: artificial urinary sphincter; IPSS: International Prostate Symptom Score; QOL: quality of life
| Standard AUS (N=18) | Transcorporal AUS (N=27) | p-Value | |
| Median pads per day last follow up (range) | 3 (0-7) (N=12) | 1 (0-7) (N=27) | 0.019 |
| Median IPSS postop (range) | 16 (8-24) (N=7) | 5 (1-15) (N=24) | 0.001 |
| IPSS postop QOL (range) | 3 (1-6) (N=8) | 1 (0-5) (N=24) | 0.01 |
Discussion
We report that transcorporal AUS placement resulted in a significantly lower number of major complications, explants, and revisions. Additionally, our study is important in that it captures a relatively long follow-up period of a median of 32 months for transcorporal AUS patients and 39 months for standard AUS patients. A recent large study reported that the median time to AUS explanation in irradiated patients is 26.4 months, thus we should be capturing the majority of potential complications within our cohort [2].
The increased risk of sphincter erosion and need for revision for patients with history of pelvic radiation has been well established [2,10-15]. A large meta-analysis found that 37% of men with a history of pelvic irradiation underwent surgical revision compared to 20% for non-irradiated men. Furthermore, the rates of surgical revision did not improve over a 25-year time period (1989-2014) of the study affirming the need for innovation [1]. Irradiated men may benefit from transcorporal placement of AUS, where a thick fibrous buttress between the cuff and urethra is created at the time of initial AUS placement. However, retrospective data does not show superiority of a transcorporal approach in cases of reimplantation after erosion with or without prior radiation. In these studies, the transcorporal approach was used for re-operative cases and revision surgery following sphincter erosion instead of first-time sphincter placement [2,10-15].
Functional outcomes after AUS placement in men with a history of radiation remain controversial in the literature. Some studies report equally high satisfaction rates of ~90% in men with and without a history of pelvic radiation despite an increase in complications such as need for revision surgery and equivalent functional outcomes in terms of incontinence, yet others report persistent urinary incontinence at rates of ~40% [15-18]. In this study, we utilized pads per day use to measure the degree of incontinence as this measure has been previously shown to correlate well with the degree of incontinence [19]. There was a statistically significant improvement in pad per day use after surgery regardless of surgical approach. The transcorporal AUS group had better outcomes in terms of postoperative pads per day, postoperative IPSS, and postoperative IPSS QoL score. These findings may be due to the much lower rate of explantation in the transcorporal group compared to those in the standard group resulting in more patients having an AUS on the last follow-up.
The most commonly cited pitfall of the transcorporal approach is violation of the tunica albuginea of the corporal bodies, which can result in new erectile dysfunction [7,8]. This complication has likely impacted the adoption of the transcorporal approach, particularly in the initial sphincter placement setting. However, successful penile prosthesis placement after transcorporal artificial urinary sphincter is well described [20]. Notably, none of the patients who underwent transcorporal AUS placement in our cohort have elected to undergo penile prosthesis placement despite preoperative counseling that this was an option for them should they wish to pursue it. The risk of new erectile dysfunction can be ameliorated with the newly described Gullwing modification to the transcorporal approach. In this technique, rectangular-shaped flaps are raised from the ventral corpora and wrapped circumferentially around the urethra for increased urethral buttressing. These flap defects are then repaired with xenograft (e.g., bovine pericardium) or allograft (e.g., cadaveric dermis) which prevents narrowing of the corporal bodies [21,22].
Our results must be interpreted in the context of several limitations. First, the small sample size may impact the statistical power to adequately assess for outcome differences. Second, the decision to proceed with either transcorporal or standard artificial urinary sphincter placement was made via shared decision-making. Thus, patients were not randomized which introduces a possible selection bias, although we found patient characteristics to be similar for both groups. Third, functional outcomes were assessed with pads per day in lieu of a standardized questionnaire. Fourth, due to the relatively recent adoption of transcorporal technique at our institution, we are not able to report on late complications. However, follow-up was greater than two years for both groups. Finally, our study was retrospective in nature and thus susceptible to the biases associated with such studies.
Conclusions
Initial transcorporal AUS placement is a promising technique with lower rates of major complications with a significantly lower number of explants due to erosion or infection compared with standard placement. Additionally, transcorporal AUS patients had better functional urinary outcomes with improved postoperative IPSS and QOL score as well as decreased number of pads per day compared to patients who underwent the standard approach. Initial transcorporal placement of AUS should be considered in patients who have fragile urethras due to history of pelvic radiation.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. University of Pittsburgh issued approval #STUDY19080339
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. Bates AS, Martin RM, Terry TR. BJU Int. 2015;116:623–633. doi: 10.1111/bju.13048. [DOI] [PubMed] [Google Scholar]
- 2.Outcomes and risk factors of revision and replacement artificial urinary sphincter implantation in radiated and nonradiated cases. Fuller TW, Ballon-Landa E, Gallo K, et al. J Urol. 2020;204:110–114. doi: 10.1097/JU.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 3.Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Brant WO, Erickson BA, Elliott SP, et al. Urology. 2014;84:934–938. doi: 10.1016/j.urology.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artificial urinary sphincter. Brant WO, Martins FE. Transl Androl Urol. 2017;6:682–694. doi: 10.21037/tau.2017.07.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Impact of adjuvant radiation on artificial urinary sphincter durability in postprostatectomy patients. Srivastava A, Joice GA, Patel HD, Manka MG, Sopko NA, Wright EJ. Urology. 2018;114:212–217. doi: 10.1016/j.urology.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Risk factors for artificial urinary sphincter failure. Kretschmer A, Buchner A, Grabbert M, Stief CG, Pavlicek M, Bauer RM. World J Urol. 2016;34:595–602. doi: 10.1007/s00345-015-1662-9. [DOI] [PubMed] [Google Scholar]
- 7.Artificial urinary sphincter outcomes in the “fragile urethra”. Hoy NY, Rourke KF. Urology. 2015;86:618–624. doi: 10.1016/j.urology.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. McGeady JB, McAninch JW, Truesdale MD, Blaschko SD, Kenfield S, Breyer BN. J Urol. 2014;192:1756–1761. doi: 10.1016/j.juro.2014.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artificial urinary sphincter: long-term results and patient satisfaction. Montague DK. Adv Urol. 2012;2012 doi: 10.1155/2012/835290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Impact of radiation and transcorporeal artificial sphincter placement in patients with prior urethral cuff erosion: results from a retrospective multicenter analysis. Moser DC, Kaufman MR, Milam DF, et al. J Urol. 2018;200:1338–1343. doi: 10.1016/j.juro.2018.06.069. [DOI] [PubMed] [Google Scholar]
- 11.Improved artificial urinary sphincter outcomes using a transcorporal cuff placement in patients with a "fragile urethra". Redmond E, Tong S, Zemp L, Hoy N, Rourke KF. Can Urol Assoc J. 2020;14:621–624. doi: 10.5489/cuaj.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artificial urinary sphincter cuff erosion heat map shows similar anatomical characteristics for transcorporal and standard approach. Ortiz NM, Wolfe AR, Baumgarten AS, Ward EE, VanDyke ME, Hudak SJ, Morey AF. J Urol. 2020;204:1027–1032. doi: 10.1097/JU.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 13.111 first-time transcorporal vs standard artificial urinary sphincter placement in patients with prior tadiation for prostate cancer: a comparison of outcomes. Pekala K, Miller D, Fuller TW, Orikogbo O, Rogers D, Maganty A, Rusilko P. J Sex Med. 2021;18:58–59. [Google Scholar]
- 14.Transcorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancer. Aaronson DS, Elliott SP, McAninch JW. Urology. 2008;72:825–827. doi: 10.1016/j.urology.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 15.Transcorporal artificial urinary sphincter in radiated and non - radiated compromised urethra. Assessment with a minimum 2 year follow-up. Le Long E, Rebibo JD, Nouhaud FX, Grise P. Int Braz J Urol. 2016;42:494–500. doi: 10.1590/S1677-5538.IBJU.2015.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artificial urinary sphincter implantation in the irradiated patient: safety, efficacy and satisfaction. Walsh IK, Williams SG, Mahendra V, Nambirajan T, Stone AR. BJU Int. 2002;89:364–368. doi: 10.1046/j.1464-4096.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- 17.Durability of artificial urinary sphincter with prior radiation therapy. Jhavar S, Swanson G, Deb N, et al. Clin Genitourin Cancer. 2017;15:175–180. doi: 10.1016/j.clgc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Radiotherapy is associated with reduced continence outcomes following implantation of the artificial urinary sphincter in men with post-radical prostatectomy incontinence. Guillaumier S, Solomon E, Jenks J, et al. Urol Ann. 2017;9:253–256. doi: 10.4103/UA.UA_25_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correlation of patient perception of pad use with objective degree of incontinence measured by pad test in men with post-prostatectomy incontinence: the SUFU Pad Test Study. Nitti VW, Mourtzinos A, Brucker BM. J Urol. 2014;192:836–842. doi: 10.1016/j.juro.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Penile prosthesis placement in men after transcorporal artificial urinary sphincter implantation. Donatucci CF, Buschemeyer WC, Webster GD. J Urol. 2008;179 [Google Scholar]
- 21.V05-07: transcorporal artificial urinary sphincter sphincter- the gullwing modification. Choudhan J. V05-07 TRANSCORPORAL. https://auau.auanet.org/content/v05-07-transcorporal-artificial-urinary-sphincter-sphincter-gullwing-modification Urological Association. 2018
- 22.A user’s guide for surgery involving the artificial urinary sphincter. Chouhan JD, Terlecki RP. Sex Med Rev. 2019;7:167–177. doi: 10.1016/j.sxmr.2018.10.004. [DOI] [PubMed] [Google Scholar]