Abstract
Introduction
Chyle leak is an uncommon yet potentially fatal complication of oesophagectomy for oesophageal cancer. The management of chyle leak is a debated, controversial topic and to date there is no standardised approach or validated algorithm for its management. This review aims to summarise current treatment algorithms for chyle leak post-oesophagectomy and their outcomes.
Methods
A systematic search of Embase, MEDLINE, UpToDate and Cochrane was conducted to identify studies reporting on the management of chyle leak following oesophagectomy for oesophageal cancer. Data on interventional success rate and mortality are reported.
Findings
Twenty-one studies met the inclusion criteria including over 23,254 oesophagectomies and identifying 838 chyle leaks (incidence <3.6%). The majority of cases were initially managed conservatively (95.3%), with a failure rate of 50.4%. Immediate surgical or radiological management resolved chylothorax in the majority of cases (97.3%), however the numbers were small. Death occurred in 54 cases (6.6%), all of whom underwent conservative management initially.
Conclusions
Owing to the heterogeneity of treatment algorithms, timings and indications for interventions, the optimal strategy for managing chyle leak remains unclear. This review has identified an unmet need for prospective multicentre studies assessing the efficacy of predefined algorithms.
Keywords: Oesophagectomy, Chyle, Oesophageal neoplasms, General surgery, Thoracic duct, Oesophagus
Introduction
Oesophageal cancer is the seventh most common malignancy worldwide and is responsible for approximately 450,000 deaths per year.1 Radical oesophagectomy is a curative treatment and in the UK is offered to patients with locally or regionally advanced adeno- or squamous cell carcinoma of the oesophagus (National Institute for Health and Care Excellence 2020)2.
Oesophagectomies are anatomically challenging and usually necessitate an aggressive two- or three-field resectional approach.3 The procedure has the highest morbidity and mortality of any elective gastrointestinal operation.4
The thoracic duct is responsible for transporting enriched lymph (chyle) from the cisterna chyli into the venous system at the level of the subclavian and internal jugular veins. The duct lies posterior to the oesophagus throughout the majority of its intrathoracic course and is therefore susceptible to injury and transection during oesophagectomy, with injury occurring in 3.7–7.2% cases.5 Elective thoracic duct resection occurs in the case of radical lymphadenectomy.
The body circulates 2.4 litres of chyle through the thoracic duct per day;6 chyle is comprised of lipids, albumin and inflammatory material.7,8 Loss of chyle into the thorax is not only nutritionally draining, but also increases the risk of severe infection and sepsis due to loss of immune material.5,7 Chylous leak is usually identified as a milky chest drain output9 and the majority of cases are identified within the first week although has been identified up to 13 days postoperatively.5
The Esophagectomy Complications Consensus Group categorised chyle leak into three types depending on treatment (I, enteric dietary modifications; II, total parenteral nutrition; and III, interventional or surgical therapy) and two types depending on severity (A, <1 litre output per day; and B, >1 litre output per day).10 Cases are often initially managed conservatively by discontinuation of enteral feeding with bridging total parenteral nutrition (TPN).11 Some studies advocate the adjuvant use of somatostatin analogues, such as octreotide.12,13 More recently, radiological intervention such as embolisation has been reported.14,15 Surgical management either immediately or after failed conservative intervention involves re-thoracotomy or thoracoscopy (video-assisted thoracoscopic surgery (VATS)) with thoracic duct ligation.16,17
Overall, the management of chyle leak remains controversial, with some advocating immediate surgical intervention,18,19 whereas others prefer conservative approaches.13,20,21 As chyle leak is a potentially life-threatening complication of oesophagectomy, it is important to establish best practice and promote a consistent and evidence-based management strategy.
This systematic review aims to summarise the management of chyle leak in patients who have undergone oesophagectomy for oesophageal carcinoma, comparing outcomes from both interventional and conservative approaches.
Methods
This review was designed and written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.22 This review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42020210819).
A literature search was conducted to identify studies reporting on the management of chyle leak following oesophagectomy for oesophageal cancer. Embase, MEDLINE, UpToDate and Cochrane Library databases were interrogated using the search criteria (Supplementary file 1) for English language studies with no specific date criteria (TR). The search results were screened by two independent authors (AVR and LK) to identify appropriate studies for inclusion. Case reports and case series were excluded if not all cases of chyle leak at the institution were accounted for because these may have been subject to selective reporting and selection bias. The number of cases in the study was not limited if all possible cases of chylothorax were reported. Conference abstracts were excluded.
Conservative management is classified as ward-based treatment approaches, including simple nil by mouth (NBM), chest drain output monitoring, dietary modifications (ie, low-fat diet or total parenteral nutrition) or pleurodesis. Radiological management includes the use of lymphangiography, with either immediate embolisation or for treatment stratification. Surgical management includes formal return to theatre for a VATS or open procedure.
Owing to heterogeneity of patient management, patient cross-over and inadequate reporting, a meta-analysis was deemed inappropriate. Instead, we followed the Cochrane Synthesis Without Meta-analysis guidance23 where possible. Studies were grouped by interventional method (ie, conservative, radiological and surgical), with most studies falling into more than one category depending on the management algorithm. The standardised metric for outcomes were success rate of intervention (proportion of total cases which resolved following treatment), death (number of deaths attributable to complications following chyle leak despite management) and the need for further intervention (number of patients). This was because the majority of studies were observational and reporting on small numbers. Overall numbers and rates were pooled for each intervention and summarised in each table. Where possible, studies were also pooled into a flow chart. Trials of conservative management were grouped into <7 days and >7 days because this was the median time before further management across all patients in all studies and therefore deemed a reasonable cut-off.
The definition of ‘success rate’ between studies was often heterogenous; as such, where possible we have extracted the raw data from the study to minimise the risk of reporting bias.
Findings
Study characteristics
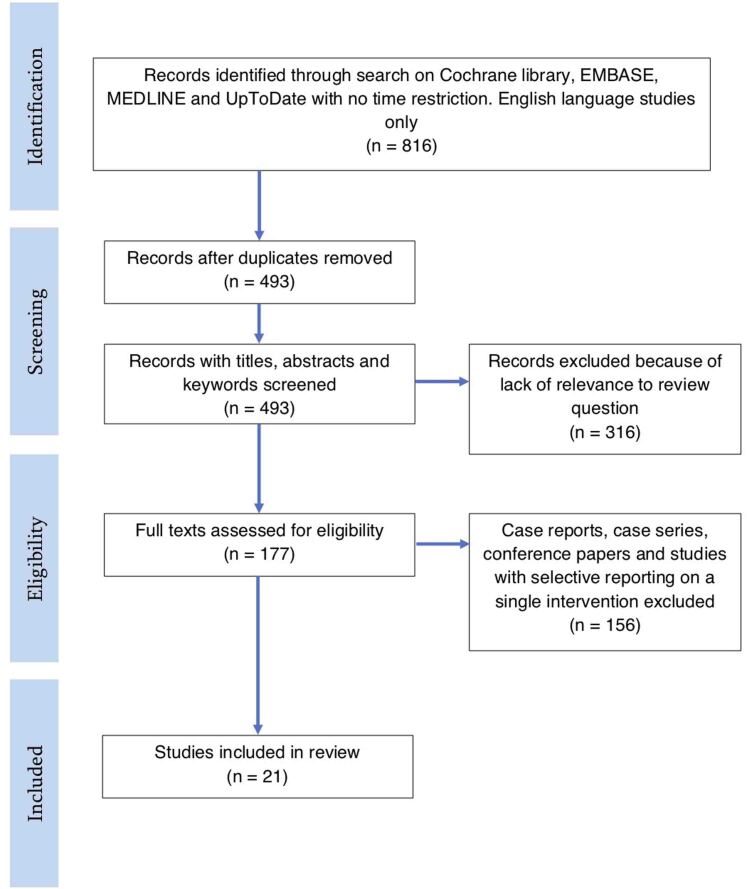
The systematic search strategy identified 816 records. After deduplication and per-protocol exclusions, 21 were included in the review (Figure 1). The characteristics of the included studies are shown in Table 1; the majority of studies had a retrospective observational design, with only one randomised control trial meeting the inclusion criteria.
Figure 1 .
Systematic search flow diagram
Table 1 .
Characteristics of included studies
| Study | Design | Operation type | Total index operations | Total chyle leak | Mean age (years) | Management |
|---|---|---|---|---|---|---|
| Milito et al 202031 | RCS | TTE | 992 | 50 | 68 | Surgical management if >1,000ml in 24h for >48h despite maximal conservative management |
| Alamdari et al 201825 | RCT | THE | Not stated | 98 | 59 | Two weeks conservative and then randomised to surgery or platelet-rich fibrin glue pleurodesis |
| Weijs et al 201720 | RCS | MIE, robotic and open | 371 | 76 | 64 | Volume-guided step-up management pathway |
| Brinkmann et al 201633 | RCS | Ivor Lewis | 906 | 17 | 68.7 | Two days’ conservative management then early re-thoracotomy |
| Abe et al 201626 | RCS | Open subtotal | 542 | 9 | 68 | Pedal or intranodal lymphangiography followed by conservative, pleurodesis or VATS |
| Gupta et al 201534 | RCS | THE, MIE, TTE | 45 | 4 | 56.25 | Conservative management then reoperation at a minimum of 3 days postoperatively |
| Miao et al 201532 | RCS | Ivor Lewis | 1,290 | 34 | 60 | Conservative management followed by re-thoracotomy at minimum 3 days postoperatively |
| Marthaller et al 201524 | RCS | Ivor Lewis, THE, Distal | Not stated | 5 | 66.6 | Percutaneous thoracic duct embolisation |
| Kim et al 201429 | RCS | Ivor Lewis | 1,514 | 57 | 62.7 | Conservative management, then octreotide or pleurodesis, then surgery |
| Fujita and Daiko 201413 | RCS | 521 | 20 | Before-and-after study of normal conservative management vs octreotide enhanced management. Surgery for treatment failure | ||
| Li et al 201321 | RCS | Open | 10,574 | 306 | 58 | 2-day vs 2-week conservative management protocol followed by surgery if not resolved |
| Shah et al 201228 | RCS | Multiple | 892 | 34 | 67.5 | Variable conservative management followed by surgery |
| Hayden et al 200727 | RCS | Multiple | 129 | 6 | 58 | Immediate minimally invasive thoracoscopy |
| Schumacher et al 200735 | RCS | Ivor Lewis, THE | 409 | 10 | 61 | Conservative or immediate surgical |
| Lagarde et al 20055 | RCS | TTE, THE | 536 | 21 | 62 | Conservative management followed by surgery |
| Rao et al 200430 | RCS | Multiple | 520 | 14 | 49 | Conservative followed by surgery |
| Bonavina et al 200136 | RCS | Ivor Lewis | 316 | 3 | 56-63 | Conservative followed by surgery |
| Merigliano et al 200019 | RCS | Multiple | 1,787 | 19 | 57 | Pre- and post study of conservative management vs immediate operative |
| Alexiou et al 199837 | RCS | 523 | 21 | 64.7 | Conservative management and then clinical decision to operate | |
| Dugue et al 199838 | RCS | Ivor Lewis | 850 | 23 | 54 | Conservative management for 12 days and reoperate if output >500ml |
| Bolger et al 199139 | RCS | Multiple | 537 | 11 | Conservative management with clinical decision to operate | |
| 23,254 | 838 (<3.6%) |
MIE, minimally invasive oesophagectomy; RCS, retrospective cohort study; RCT, randomised controlled trial; THE, transhiatal oesophagectomy; TTE, transthoracic oesophagectomy; VATS, video-assisted thoracoscopic surgery.
Approximately 23,254 oesophagectomies were undertaken (two studies did not report the total number in the cohort24,25), with chyle leak occurring in 814 (3.5%) cases. Of these, the vast majority were managed conservatively in the first instance (776, 95.3%), with only four studies advocating immediate surgical or radiological intervention.19,24,26,27
Conservative management
Conservative management involved a non-standardised approach, with the least-restrictive option being a low-fat feed only (Table 2). Weijs et al20 suggest a success rate of 65.6% (40 of 61) with a 7-day low-fat feed in chyle leaks <500ml/day. Two studies with 148 patients specified a medium-chain triglyceride feed in combination with a number of different treatment approaches. Timings, patient numbers and success rates for different aspects of conservative management were not defined. Another common approach was maintaining nutrition with TPN, which was used in at least 378 cases (47.5%). Octreotide use was variable; some studies advocated administering it to all patients (n=181, ∼23% of cases), whereas others used octreotide in the case of increasing drain output (n=57) without specifying the exact indications. Pleurodesis was a relatively common procedure; however, the indications and patient numbers were seldom specified, and practice varied in terms of timing. One study trialled platelet-rich fibrin glue pleurodesis in 26 patients following conservative management with eventual 100% success after up to two administrations.25
Table 2 .
Outcomes after conservative management
| Author | Regime | Success rate | Outcomes | |||
|---|---|---|---|---|---|---|
| Death | Surgery | Other | ||||
| Milito et al31 | MCT feed, rarely TPN, cotrimoxazole if lymphocyte count <1,000 per μl. Surgery if drain output >1,000ml in 24h for >48h | 22/50 | 26 | 2 (pleuroperitoneal shunt) | ||
| Alamdari et al25 | Two-week NBM or fat-restricted feed supplemented with MCT; TPN; tube thoracostomy; octreotide 100μg three times daily | 46/98 | 26 | 26 (PRFG pleurodesis) | ||
| Weijs et al20 | Low-fat feed for 7 days (if drain output <500ml) | 40/61 | 1 | 20 (TPN) | ||
| TPN for 7 days (if drain output >500ml) | 11/15 | 4 | ||||
| TPN and low-fat feed for 7 days | 1/1 | |||||
| Brinkmann et al33 | TPN, chest drain for >48h | 2/17 | 15 | |||
| Miao et al32 | NBM, TPN and octreotide | 23/34 | 11 | |||
| Kim et al29 | NBM, TPN; ± pleurodesis and octreotide if clinically not improving | 43/54 | 11 | |||
| Fujita and Daiko13 | TPN only | 2/5 | 3 | |||
| TPN and octreotide 100μg TDS | 13/15 | 2 | ||||
| Li et al21 | Low-fat feed, chest drain, protein supplementation if needed | 48-h trial | 45/186 | 6 | 135 | |
| 2-week trial | 77/120 | 3 | 36 | 4 (recurrence) | ||
| Shah et al28 | NBM, ± additional chest drain ± TPN ± elemental feeds ± octreotide ± pleurodesis | 13/34 | (6) | 21 | ||
| Schumacher et al35 | TPN and albumin infusions | 1/2 | 1 | |||
| Lagarde et al5 | NBM, TPN. If drain <500ml per day, started on low-fat diet | 16/20 | 4 | |||
| Rao et al30 | NBM, TPN, chest drain, octreotide in one patient | 5/14 | 2 | 7 | ||
| Bonavina et al36 | 2-week trial of NBM, TPN and chest drain. Octreotide and ethylephrine given in 2 | 0/3 | 3 | |||
| Merigliano et al19 | NBM, TPN, chest drain | 4/11 | 7 | |||
| Alexiou et al37 | NBM, TPN, chest drain | 13/21 | 4 | 4 | ||
| Dugue et al38 | 12-day trial of TPN, chest drain | 14/23 | 9 | |||
| Bolger et al39 | TPN, chest drain | 3/11 | 4 | 3 | 1 (recurrence) | |
| Total | 394/795 (49.6%) | 20 (2.5%) | 328 (41.3%) | 53 (6.6%) | ||
MCT, medium chain triglycerides; NBM, nil by mouth; PRFG, platelet-rich fibrin glue; TPN, total parenteral nutrition.
Radiological management
Only two studies opted for radiological intervention (Table 3).24,26 Abe et al26 used either pedal lymphangiography or intranodal lymphangiography in nine patients to visualise the chyle leak, which was unsuccessful in one case. This investigation guided subsequent management, with five successfully undergoing pleurodesis, two continuing with conservative management and two cases having a VATS procedure. All patients recovered without complications. Conversely, Marthaller et al24 performed coil embolisation during the lymphangiography in five cases. Although one patient required a two-staged procedure all cases were successful overall.
Table 3 .
Outcomes after radiological management
| Author | Technique | Procedure success rate | Management | Overall success rate of intervention |
|---|---|---|---|---|
| Abe et al26 | Pedal lipidiol lymphangiography | 6/6 | Pleurodesis 4/4 Conservative 1/1 VATS 1/1 |
6/6 |
| Intranodal lipidiol lymphangiography | 2/3 | VATS 1/1 Pleurodesis 1/1 Conservative (not identified) 1/1 |
3/3 | |
| Marthaller et al24 | Pedal ethiodol lymphangiography | 2/3 | Coil embolisation 3/3 (one patient required 2-stage CT-guided embolisation) | 3/3 |
| Intranodal ethiodol lymphangiography | 2/2 | Coil embolisation 2/2 | 2/2 | |
| 15/15 |
CT, computerised tomography scan; VATS, video-assisted thoracoscopic surgery.
Surgical management
Overall, 362 cases (49.2% chyle leaks) underwent surgical intervention with the majority occurring within 14 days of diagnosis of chyle leak (n=220, 60.8%; Table 4). The surgical management of chyle leak was similar between studies. The most common procedure was re-thoracotomy and mass ligation of the tissues between the aorta and vertebral bodies (n=302, 83.4%). However, thoracoscopy (n>8) and laparotomy (n=9) were also used.5,26–28 From the included studies, earlier intervention (ie, within 14 days) yielded a higher success rate (83.4% vs 76.7%) but seemingly more deaths occurred in this group (11.4% vs 8.5% after 14 days). However, this should be interpreted cautiously, as surgical indications were not consistently objective in different studies and a patient requiring early surgical intervention may have had a greater clinical need and thus have had poor physiological reserve.
Table 4 .
Outcomes after surgical intervention
| Author | Technique | Average time from oesophagectomy to procedure (days) | Success rate | Outcomes | ||
|---|---|---|---|---|---|---|
| Death | Repeat surgery | Other | ||||
| <14 days | ||||||
| Milito et al31 | Not specified | 6 | 23/26 | 3 | ||
| Weijs et al20 | Not specified | 12 | 6/8 | 2 | ||
| Gupta et al34 | Transabdominal masse ligation of tissue between aorta and azygous vein with Ethibond | 3.7 | 3/3 | |||
| Miao et al32 | Re-thoracotomy and masse ligation between aorta and vertebral bodies | 5 | 11/11 | |||
| Li et al21 | Right- or left-sided thoracotomy and thoracic duct ligation with silk | 2 | 109/135 | 20 | 6 (recurrence) | |
| Hayden et al27 | Right-sided thoracoscopy and ligaclip ± fibrin glue ± suture applied to thoracic duct | 5 | 5/6 | 1 | ||
| Schumacher et al35 | Laparotomy, adhesiolysis, mass double-ligation using Vicryl | 10 | 8/9 | 1 | ||
| Lagarde et al5 | Thoracotomy or thoracoscopy with thoracic duct ligation or clipping | 4 | 3/4 | |||
| Merigliano et al19 | Right thoracotomy and mass ligation of thoracic duct and surrounding tissues | 12 | 6/7 | 1 | ||
| Right thoracotomy and mass ligation of thoracic duct and surrounding tissues | 2 | 8/8 | ||||
| Bolger et al39 | Thoracotomy and thoracic duct ligation | 9–10 | 2/3 | 1 | ||
| 184/220 (83%) | 25 (11.4%) | |||||
| >14 days | ||||||
| Alamdari et al25 | Right posterolateral thoracotomy ad duct ligation between T8-T12 | 17.5 | 12/26 | (4) | 14 | |
| Brinkmann et al33 | Right-sided thoracotomy, pleural adhesiolysis, ligation, pleural lavage | 17.2 | 14/15 | 1 | ||
| Abe et al26 | VATS-assisted clipping or ligation of thoracic duct | 18 | 2/2 | |||
| Kim et al29 | Thoracic duct ligation | 18.7 | 14/14 | 1 | ||
| Fujita and Daiko13 | Unspecified | Not provided | 5/5 | |||
| Li et al21 | Right- or left-sided thoracotomy and thoracic duct ligation with silk | 14 | 31/36 | 2 | 3 (recurrence) | |
| Shah et al28 | Thoracoscopy or thoracotomy and mass ligation of tissue between aorta and vertebral bodies | 13.5 | 14/21 | 2 | 5a | |
| Rao et al30 | Right-sided thoracotomy and mass ligation of thoracic duct and soft tissue | Not provided | 5/7 | 2 | ||
| Bonavina et al36 | Right thoracotomy or thoracoscopy and thoracic duct ligation (one patient underwent mass ligation of tissues) | 14 | 3/3 | |||
| Alexiou et al37 | Thoracotomy and thoracic duct ligation | 20 | 3/4 | 1 | ||
| Dugue et al38 | Right thoracotomy and thoracic duct ligation ± surrounding tissues ± pleural decortication | 18 | 7/9 | 2 | ||
| 110/142 (77.5%) | 12 (8.5%) | |||||
aThoracocentesis = 1, pleurodesis = 1, chest drain = 3.
Criteria for treatment failure
The majority of studies did not specify objective criteria for treatment failure or indications for treatment escalation. However, in the studies that either followed predefined criteria or determined the trend from their cohort, there was a strong association between a high or persistent drain output and surgical intervention (Table 5). Some studies advocated a weight-based quantification of chyle output (eg, ml/kg), whereas others applied the same threshold to all patients (eg, >1,000ml).
Table 5 .
Suggested thresholds for treatment escalation
| Author | Intervention | Indication | Comments |
|---|---|---|---|
| Milito et al31 | Surgery | Chest drain output >1,000ml in 24h for >48h | Prospectively used in study |
| Alamdari et al25 | Surgery | Chest drain output >500ml/day; or 250–500ml/day after 1 week; or 100–250 ml/day after 2 weeks | Prospectively used in study |
| Miao et al32 | Surgery | Chest drain output >13.5ml/kg on day 3 more likely to fail medical management (sensitivity 100%, specificity 83%) | Study observation |
| Kim et al29 | Surgery | Chest drain output >1,000ml despite conservative management, pleurodesis and octreotide | Prospectively used in study |
| Fujita and Daiko13 | Surgery or TDE | Chest drain output >1,000ml with Octreotide after 2 days | Recommended, not tested |
| Li et al21 | Surgery | Chest drain output >1,000ml at any timepoint | Prospectively used in study |
| Shah et al28 | Surgery | Chest drain output >11.6ml/kg 11× more likely to fail medical management | Study observation |
| Lagarde et al5 | Continue conservative | Chest drain output <10ml/kg on day 5 predicts successful conservative management | Study observation |
| Surgery | Recommend if chest drain output >2,000ml after 2 days of optimal conservative therapy | Recommended, not tested | |
| Rao et al30 | Surgery | Chest drain output >1,000ml after 48h or if output increasing after 5 days Or if chylothorax not resolved after 2 weeks |
Prospectively used in study |
| Dugue et al38 | Surgery | Drain output >500ml after 12 days | Prospectively used in study |
| Chyle leak >10mg/kg at day 5 | Study observation |
Pooled outcomes
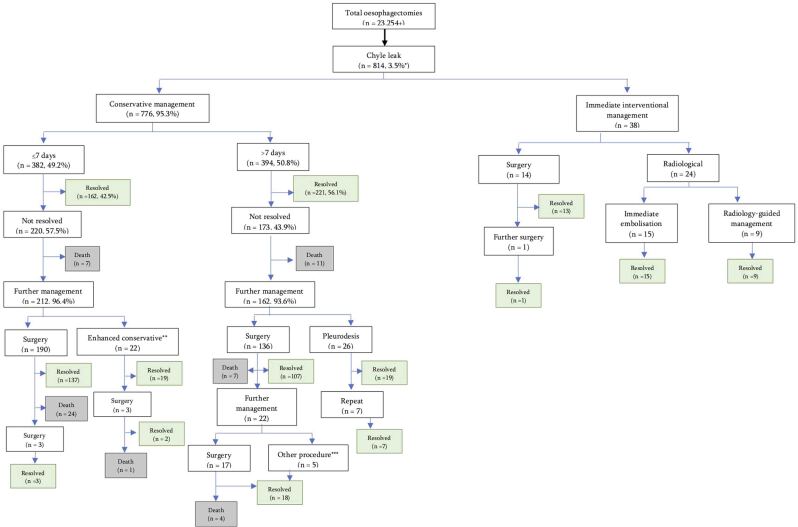
The outcomes of treatment algorithms for each study were pooled to produce Figure 2. Conservative management resolved the chyle leak in 162 (42.5%) of those who received a trial for <7 days, and 221 (56.1%) in patients who received a trial for >7 days (Figure 2). Eventually, 50.5% patients required surgery in the <7-day arm and 34.5% in the >7-day arm. The rates of death were 8.4% in those who were initially trialled with <7 days’ conservative management vs 5.6% in those who initially received >7 days’ conservative management. There were no deaths in those who underwent immediate surgical or radiological intervention, although the numbers are very small.
Figure 2 .
Pooled data from included studies
*Approximate percentage, as two studies failed to report total number of oesophagectomies undertaken.
**Regimen escalated to total parenteral nutrition (n=20), pleuroperitoneal shunt placed (n=2).
***Thoracocentesis = 1, pleurodesis = 1 or prolonged chest drain = 3.
Discussion
This systematic review has summarised the best evidence available on the management of chyle leak after oesophagectomy for oesophageal cancer. Overall, the incidence of chyle leak is low (∼3.5%, range 0.9–20.5%) and the overall mortality from the complication averaged 6.6% (range 0–45.5%).
Importantly, the management of chyle leak is highly variable and currently it is challenging to determine the preferred management strategy to optimise patient outcomes. Much of the treatment escalation was clinically guided with only very few studies using a predefined algorithm. This may be due to the observational nature of the studies, and also the low incidence of chyle leak overall. Some studies had only a few cases over a 5–10-year period.
Only three trialled a fat-restricted feed as the least restrictive option.20,21,25 This may be appropriate in patients with a low drain output (ie, <500ml or ∼7ml/kg per day based on a 70kg patient). In the instance of increasing output, or an initial output >500ml, TPN may be more appropriate. Somewhere between 10 and 12ml/kg in 24 hours may represent a reasonable threshold for surgical management. Given the majority of patients are initially managed conservatively, and the number of different algorithms, feeds and pleurodesis compounds available, this review has identified a significant paucity of reporting and good quality data comparing such approaches. Further, the additional influence of biochemical markers and physiological parameters needs to be formally investigated because these were not reported in the identified studies.
It may be that longer trials of conservative treatment are more efficacious, as the pooled data demonstrates that the success rate is higher (56.1% vs 42.5%) and fewer patients died overall. However, this needs to be interpreted with caution because many of the conservative trials were ended owing to clinical concerns, which implies many of the patients who underwent <7 days’ conservative treatment were critically unwell and demanded earlier intervention.
The Esophagectomy Complications Consensus Group has defined the major postoperative complications following oesophagectomy: anastomotic leak, conduit necrosis, chyle leak and vocal cord injury.10 The classification of chyle leak is based on the required management and drain output (<1,000 or >1,000ml per day); however, there is no recommendation as to how patients should be prospectively managed. Weijs et al20 allocate patients based on drain output, whereby <500ml in 24 hours is managed with a low-fat feed for 7 days, 500–1,000ml can be allocated to either low-fat feed or TPN depending on ‘clinical condition’ and whether this output is increasing or decreasing, and patients with an output >1,000ml have TPN for 7 days. Patients on TPN with persistent leak proceed to surgery. No other study specified the conservative management pathways in this much detail.
Drain output of >1,000ml was commonly deemed an indication for escalation to surgical intervention at any time point.13,21,29–31 Three studies found an association between weight-adjusted threshold (ie, 13.5ml/kg,32 11.6ml/kg28 or 10ml/kg5) and risk of failing medical management. Weight-adjusted thresholds may be preferential to standard, as presumably the nutritional and immunological consequences of losing 1,000ml chyle per day in a 50kg patient are more significant than in a 90kg patient.
Radiological management appears to be a promising solution to diagnose, classify and treat chyle leak. Because the majority of studies available are case series and reports, we did not include them in this systematic synthesis due to concerns over selection bias. However, the two studies that were included offered 100% success.24,26 A systematic review and meta-analysis of lymphangiography for chylothorax reports a pooled technical success rate of 94.2%,40 with an overall complication rate of 1.6% and the pooled clinical success rate (of thoracic duct dilation or embolisation) was 56.9%. Overall, larger studies are required to prospectively determine the efficacy of radiological interventions.
Limitations
This review is limited by the level of evidence available. Of the 21 studies, 20 were retrospective cohort studies (level II) and only one study was a prospective trial,25 but randomisation methods and blinding were not described which questions its validity.
Risk of bias
Because the studies were not comparing interventions but describing cohorts of patients over time, a formal risk of bias assessment could not be performed. However, using the ROBINS-I tool we have identified a number of sources of bias.41 Preintervention bias includes the potential for confounding, as management was often dictated by clinical judgement and not objective measures. Similarly, the conservative approaches often varied within studies without justification (ie, the administration of octreotide in select patients). The decision to operate on patients after a prolonged period of unsuccessful conservative management introduces selection bias because these patients were likely less able to undergo an operation once nutritionally deplete and, in some cases, septic. As mentioned previously, reporting bias was present where studies deemed a treatment success even when more than one of the interventions was required (eg, Alamdari et al25 report a success rate of 100% for pleurodesis in their study, when in fact seven patients required two administrations so we have deemed these as unsuccessful initial management).
Some studies were subject to missing data due to their retrospective nature; for example, not all studies reported the time from oesophagectomy to repeat thoracotomy. Selective reporting bias was a common issue, but we excluded any case series or studies that failed to account for all cases of chyle leak. The definition of chyle leak differed between studies, which may explain the variation in incidence.
Conclusion
Because chyle leak is a rare complication, we propose a Delphi approach42 in the first instance to understand the variation in practices among surgical units worldwide, with a subsequent international, multicentre cohort study to review conservative, surgical and radiological management of chylous complications following oesophagectomy.
Topics for a Delphi study could include, but not be limited to: clinical and biochemical definitions of chylothorax; indications for a low-fat diet vs NBM and TPN; indications for octreotide or whether all cases should receive it; timing of pleurodesis; the length of conservative trial; classification of treatment failure (ie, volume guided, physiological or biochemical); weight-adjusted vs standard drain output volumes; indications for radiological investigation and embolisation; and indication for surgical intervention plus preferred methods (ie, VATS vs open).
References
- 1.Institute of Health Metrics. Global health data exchange. http://ghdx.healthdata.org/gbd-results-tool (cited January 2021).
- 2.National Institute for Health and Care Excellence UK. Oesophago-gastric cancer: Assessment and management in adults. https://www.nice.org.uk/guidance/ng83 (cited January 2021).
- 3.Swanson S. Surgical management of resectable esophageal and esophagogastric junction cancers. UpToDate 2020. https://www.uptodate.com/contents/surgical-management-of-resectable-esophageal-and-esophagogastric-junction-cancers (cited January 2021).
- 4.Schwarze ML, Barnato AE, Rathouz PJet al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg 2015; 150: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagarde SM, Omloo JMT, De Jong Ket al. Incidence and management of chyle leakage after esophagectomy. Ann Thorac Surg 2005; 80: 449–454. [DOI] [PubMed] [Google Scholar]
- 6.Macfarlane JR, Holman CW. Chylothorax. Am Rev Respir Dis 1972; 105: 287–291. [DOI] [PubMed] [Google Scholar]
- 7.McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010; 104: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest 1965; 44: 1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis 2014; 6: S355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low DE, Alderson D, Cecconello Iet al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 2015; 262: 286–294. [DOI] [PubMed] [Google Scholar]
- 11.Wemyss-Holden SA, Launois B, Maddern GJ. Management of thoracic duct injuries after oesophagectomy. British Journal of Surgery 2001; 88: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 12.Kalomenidis I. Octreotide and chylothorax. Curr Opin Pulm Med 2006; 12: 264–267. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T, Daiko H. Efficacy and predictor of octreotide treatment for postoperative chylothorax after thoracic esophagectomy. World J Surg 2014; 38: 2039–2045. [DOI] [PubMed] [Google Scholar]
- 14.Lambertz R, Chang DH, Hickethier Tet al. Ultrasound-guided lymphangiography and interventional embolization of chylous leaks following esophagectomy. Innov Surg Sci 2019; 4: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun H, Hur S. Interventional radiology treatment for postoperative chylothorax. Korean J Thorac Cardiovasc Surg 2020; 53: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devulapalli C, Anderson J, Llore NNPet al. Thoracic duct ligation: right video-assisted thoracoscopic surgery approach. Oper Tech Thorac Cardiovasc Surg 2016; 21: 152–159. [Google Scholar]
- 17.Meguid RA. Chylothorax: surgical ligation of the thoracic duct through thoracotomy. Oper Tech Thorac Cardiovasc Surg 2016; 21: 139–151. [Google Scholar]
- 18.Choh CTP, Rychlik IJ, McManus K, Khan OA. Is early surgical management of chylothorax following oesophagectomy beneficial? Interact Cardiovasc Thorac Surg 2014; 19: 117–119. [DOI] [PubMed] [Google Scholar]
- 19.Merigliano S, Molena D, Ruol Aet al. Chylothorax complicating esophagectomy for cancer: A plea for early thoracic duct ligation. J Thorac Cardiovasc Surg 2000; 119: 453–457. [DOI] [PubMed] [Google Scholar]
- 20.Weijs TJ, Ruurda JP, Broekhuizen MEet al. Outcome of a step-Up treatment strategy for chyle leakage after esophagectomy. Annals of Thoracic Surgery 2017; 104: 477–484. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Dan G, Jiang Jet al. A 2-wk conservative treatment regimen preceding thoracic duct ligation is effective and safe for treating post-esophagectomy chylothorax. J Surg Res 2013; 185: 784–789. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8(5):336–341. [DOI] [PubMed] [Google Scholar]
- 23.Campbell M, McKenzie JE, Sowden Aet al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020; 368: 16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marthaller KJ, Johnson SP, Pride RMet al. Percutaneous embolization of thoracic duct injury post-esophagectomy should be considered initial treatment for chylothorax before proceeding with open re-exploration. Am J Surg 2015; 209: 235–239. [DOI] [PubMed] [Google Scholar]
- 25.Alamdari DH, Asadi M, Rahim ANet al. Efficacy and safety of pleurodesis using platelet-rich plasma and fibrin glue in management of postoperative chylothorax after esophagectomy. World J Surg 2018; 42: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, Kawakami J, Uemura Net al. Therapeutic strategy for chylous leakage after esophagectomy: focusing on lymphangiography using lipiodol. Esophagus 2016; 13: 237–244. [Google Scholar]
- 27.Hayden JD, Sue-Ling HM, Sarela AI, Dexter SPL. Minimally invasive management of chylous fistula after esophagectomy. Dis Esophagus 2007; 20: 251–255. [DOI] [PubMed] [Google Scholar]
- 28.Shah RD, Luketich JD, Schuchert MJet al. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012; 93: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Cho J, Kim K, Shim YM. Chyle leakage patterns and management after oncologic esophagectomy: A retrospective cohort study. Thorac Cancer 2014; 5: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao DVLN, Chava SP, Sahni P, Chattopadhyay TK. Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus 2004; 17: 141–145. [DOI] [PubMed] [Google Scholar]
- 31.Milito P, Chmelo J, Dunn Let al. Chyle leak following radical En bloc esophagectomy with TwoField nodal dissection: predisposing factors, management, and outcomes. Ann Surg Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao L, Zhang Y, Hu Het al. Incidence and management of chylothorax after esophagectomy. Thorac Cancer 2015; 6: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann S, Schroeder W, Junggeburth Ket al. Incidence and management of chylothorax after Ivor Lewis esophagectomy for cancer of the esophagus. J Thorac Cardiovasc Surg 2016; 151: 1398–1404. [DOI] [PubMed] [Google Scholar]
- 34.Gupta R, Singh H, Kalia Set al. Chylothorax after esophagectomy for esophageal cancer: risk factors and management. Indian J Gastroenterol 2015; 34: 240–244. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher G, Weidemann H, Langrehr JMet al. Transabdominal ligation of the thoracic duct as treatment of choice for postoperative chylothorax after esophagectomy. Dis Esophagus 2007; 20: 19–23. [DOI] [PubMed] [Google Scholar]
- 36.Bonavina L, Saino G, Bona Det al. Thoracoscopic management of chylothorax complicating esophagectomy. J Laparoendosc Adv Surg Tech - Part A 2001; 11: 367–369. [DOI] [PubMed] [Google Scholar]
- 37.Alexiou C, Watson M, Beggs Det al. Chylothorax following oesophagogastrectomy for malignant disease. Eur J Cardio-thoracic Surg 1998; 14: 460–466. [DOI] [PubMed] [Google Scholar]
- 38.Dugue L, Sauvanet A, Farges Oet al. Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg 1998; 85: 1147–1149. [DOI] [PubMed] [Google Scholar]
- 39.Bolger C, Walsh TN, Tanner WAet al. Chylothorax after oesophagectomy. Br J Surg 1991; 78: 587–588. [DOI] [PubMed] [Google Scholar]
- 40.Kim PH, Tsauo J, Shin JH. Lymphatic interventions for chylothorax: A systematic review and meta-analysis. J Vasc Interv Radiol 2018; 29: 194–202. [DOI] [PubMed] [Google Scholar]
- 41.Sterne JA, Hernán MA, Reeves BCet al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore PG, Lingstone HA, Turoff M.. The Delphi Method: Techniques and Applications. Reading, MA: Addison-Wesley, 1975. [Google Scholar]