Abstract
Objective
Neurodegenerative disease is a common neurodegenerative disorder. Lactobacillus pentosus (L. pentosus) plays a neuron-protective role. This study aimed to investigate the effects of L. pentosus on neurodegenerative diseases.
Methods
Cells were treated with lipopolysaccharide (LPS) to establish neurodegenerative diseases model in vivo and with L. pentosus strain S-PT84. Reverse transcription-quantitative PCR (RT-qPCR) was applied to determine mRNA levels. Western blot was performed to detect protein expression. Cellular behaviors were detected using Cell Counting Kit-8 (CCK-8), flow cytometry, and TdT-mediated dUTP nick-end labeling (TUNEL) assay. The interaction between baculoviral IAP repeat containing 3 (BIRC3) and NLR family CARD domain containing 4 (NLRC4) was predicted by STING and verified by western blot.
Result
L. pentosus suppressed LPS-induced pyroptosis and promoted the cell viability of neurons. Additionally, L. pentosus suppressed the release of proinflammatory cytokines (interleukin 1 beta (IL-1β) and IL-18) and the protein expression of pyroptosis biomarkers (cleaved caspase1 (CL-CASP1) and N-terminal fragment gasdermin D (GSDMD-N)). Moreover, L. pentosus upregulated BIRC3, which induced the inactivation of NLRC4. However, BIRC3 knockdown alleviated the effects of L. pentosus and induced neuronal degeneration.
Conclusion
L. pentosus may play a neuron-protective role via regulating BIRC3/NLRC4 signaling pathways. Therefore, L. pentosus may be a promising strategy for neurodegenerative diseases.
1. Introduction
Dementia is a common neurodegenerative disease, characterized by progressive cognitive degeneration, which induces huge burden on public health [1]. Elder neuron degeneration is the main cause of dementia [2]. Recently, Marchetti reveals that the changes in microenvironment, including inflammation, are a key factor for neural degeneration [3]. Hence, to develop a new strategy for suppressing inflammation-induced neurodegeneration is in urgent need.
Pyroptosis is a form of programmed cell death executed by Gasdermin D (GSDMD) [4] The activation of inflammasomes, such as NLR family pyrin domain containing 1 (NLRP1) [5], NLR family CARD domain containing 4 (NLRC4), NLRP3, NLRP6, and NLRP12 [6, 7], cleave caspase1 (CASP1) (canonical pathway) or CASP11 (noncanonical pathway), which promotes the N-terminal fragment GSDMD (GSDMD-N) assembling into cell membrane. The accumulation of GSDMD-N induces pore formation, the release of IL-1β and IL-18, and cell death [8, 9]. Previous studies evidence that the pyroptosis of neurons induces pathogenesis of optic nerve disorders and cerebral ischemia as well as Alzheimer's disease [10–12]. However, the molecular mechanisms underlying neuron pyroptosis have not been fully elucidated.
Probiotics are considered to be a safe alternative therapy for many diseases, including cancer and neural disorders [13, 14]. According to the Food and Drug administration (FDA), moderate-taken probiotics bring healthy benefits to the host [15]. Generally, probiotics can actively change the host's intestinal microbiota and improve health. Lactobacillus pentosus (L. pentosus), a member of probiotics from intestinal flora, plays a neuron-protective role and mitigates aging- and scopolamine-induced memory impairment [16, 17]. However, probiotic-based treatments for memory loss, especially for patients with neurodegenerative diseases, and their clinical outcomes are not fully documented.
Baculoviral IAP repeat containing 3 (BIRC3) is a member of inhibitor of apoptosis proteins (IAPs) [18]. BIRC3 suppresses cell apoptosis via inactivating CASPs cascades [19]. The abnormal levels of BIRC3 are closely associated with tumorgenesis [20, 21], inflammatory response [22], and immune disorders [23]. For instance, the activation of BIRC3 induces chemoresistance of colorectal cancer [24]. BIRC3 modulates canonical nuclear factor kappa B (NF-κB) target gene activation to attenuate inflammation, which promotes the development of splenic marginal zone lymphoma [25]. Moreover, BIRC3 promotes the cell survival of neurons [26]. However, the roles of BIRC3 in neurodegenerative diseases are still unclear. This study investigated the potentials of BIRC3. BIRC3 protected against neuronal degeneration via suppressing inflammation-induced pyroptosis.
2. Materials and Methods
2.1. Cell Culture
Human neuroblastoma cell lines SH-SY5Y were provided by American type culture collection (ATCC), USA. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) medium containing 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2.
Cells (2 × 105 cells) were incubated with 10 μg/L of lipopolysaccharide (LPS) and/or L. pentosus strain S-PT84 (L. pentosus, 5 × 107 cells/ml, Synbiotech Inc., Kaohsiung, China).
2.2. Cell Transfection
Sh-BIRC3 and its negative control were provided by GenePharm, Shanghai. Cells were transfected using Lipofectamine® 3000 for 48 h. After transfection, cells were used in the following experiments.
2.3. Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA was collected from SH-SY5Y cells. RNA was reversely transcribed into cDNA using a reverse transcription kit (Applied Biosystems, USA). Then, PCR was performed using SYBR Premix Ex Taq (Takara, Japan). GAPDH served as loading control. Relative mRNA levels were calculated using the 2−ΔΔCq method. Each independent experiment was conducted in triplicate.
2.4. Western Blot
Total protein was extracted from SH-SY5Y cells. Protein concentration was measured using a bicinchonininc acid (BCA) kit. The protein was separated using 12% SDS-PAGE. Afterwards, the separated protein was moved onto polyvinylidene difluoride (PVDF) membranes, which was then sealed by 5% skimmed milk. The membranes were incubated with primary antibodies against IL-1β, IL-18, procaspase1, cleaved caspase1, GSDMD-N, BIRC3, NLRP3, NLRC1, NLRP3, NLRC4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at 4°C overnight. Next day, the membranes were incubated with secondary antibodies. GAPDH was used as the loading control. Subsequently, the bands were captured using an efficient chemiluminescence (ECL) kit and analyzed using the ImageJ software.
2.5. Coimmunoprecipitation (Co-IP)
Cells were collected and lysed. Then, cell lysates were centrifuged at 12,000 × g and immunoprecipitated with specific antibodies. Afterwards, the proteins were coprecipitated and isolated using 12% SDS-PAGE. Immunoblotting was analyzed with antianalysis with the indicated primary antibodies against BIRC3, NLRC4, and GAPDH.
2.6. Cell Viability
Cells were seeded into 24-well plates (2 × 103 cells/well). After incubated with L. pentosus for 48 h, cells were cultured with Cell Counting Kit-8 (CCK-8) regents for 4 h. Subsequently, optic density was determined using a microplate at 450 nm.
2.7. Flow Cytometry
Cell pyroptosis was determined using an FAM-FLICA Caspase-1 Assay Kit. Cells were seeded in 6-well plates. After centrifugation at 1200 × g for 15 min, the supernatants were collected. Then, the cells were stained cultured with propidium iodide (PI). Subsequently, a flow cytometry was used to calculate the pyroptosis rates: active caspase1 + PI double positive cells/total cells × 100%.
2.8. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
Cells were collected and fixed with 4% paraformaldehyde. After washed with 5% PBS, cells were cultured with TUNEL solutions. Then, the cells were counterstained with DAPI. Finally, TUNEL positive cells were captured using a fluorescence microscope.
2.9. Statistical Analysis
All data were evaluated using GraphPad 6.0. and represented as mean ± SD. The comparison was performed using Student's t-test and one-way ANOVA. P < 0.05 dictated significant difference.
3. Results
3.1. The Cell Viability of Neuron Cells
To investigate the effects of Lactobacillus pentosus (L. pentosus) on neuronal degeneration, cells were cultured with L. pentosus. As shown in Figure 1, there were no significant changes in the cell viability of SH-SY5Y cells.
Figure 1.
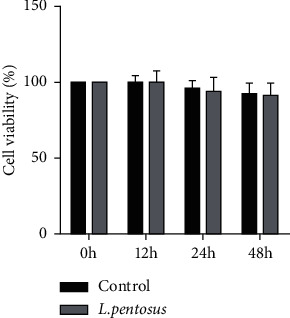
The cell viability of SH-SY5Y cells. The cell viability of SH-SY5Y cells were determined using CCK-8 assay.
3.2. L. pentosus Suppresses Inflammatory Response in SH-SY5Y Cells
Inflammation is a key factor for neuronal degeneration. To verify the effects of L. pentosus, SH-SY5Y cells were exposed to LPS. As shown in Figures 2(a)–2(c), LPS significantly increased the release of IL-1β and IL-18, which was antagonized by L. pentosus. These results were consistent with that from western blot. L. pentosus treatment markedly alleviated the effects of LPS and suppressed the protein expression of IL-1β and IL-18.
Figure 2.
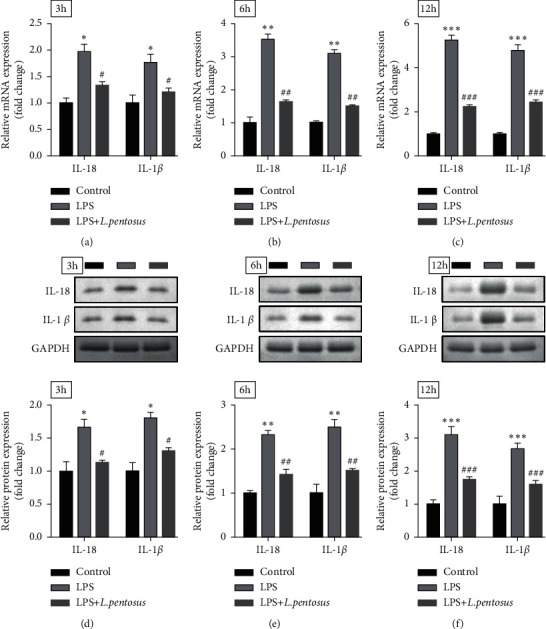
L. pentosus suppressed neuronal inflammatory response. The mRNA levels of IL-18 and IL-1β determined using RT-qPCR after treatment with L. pentosus for 3, 6, and 12 h. The protein expression of IL-18 and IL-1β detected by western blot after treatment with L. pentosus for 3, 6, and 12 h. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05, ##P < 0.01, ###P < 0.001.
3.3. L. pentosus Inhibits the Pyroptosis of SH-SY5Y Cells
Cellular functions were detected using flow cytometry and TUNEL assay. As shown in Figure 3(a), L. pentosus significantly decreased PI and active caspase1-positive cells induced by LPS. Additionally, LPS-mediated increase in TUNEL-positive cells was abated by L. pentosus treatment (Figure 3(b)). To further verify the effects of L. pentosus on LPS-induced neuronal cell death, we determined the protein expression of pyroptosis biomarkers. LPS exposure significantly increased the protein expression of cleaved caspase1 and GSDMD-N (Figure 3(c)), which was alleviated by L. pentosus.
Figure 3.
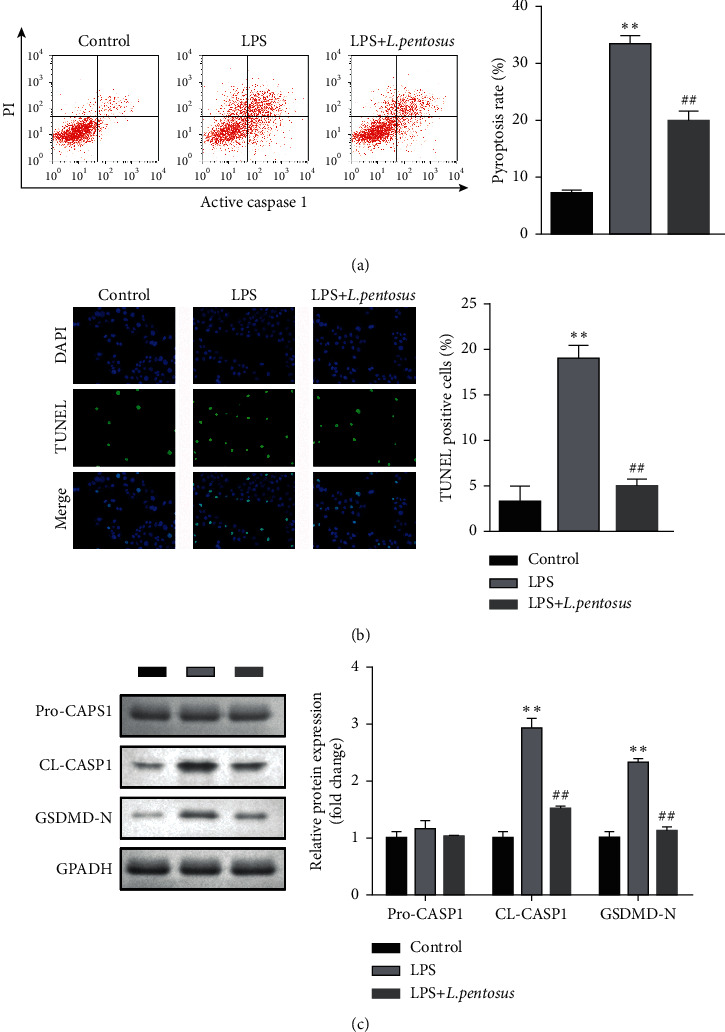
L. pentosus inhibited the pyroptosis of neurons. (a) The cell death of neurons detected using flow cytometry. (b) Neuronal cell death determined using the TUNEL assay. (c) The protein expression of pyroptosis biomarkers, cleaved caspase1, and GSDMD-N detected by western blot. ∗∗P < 0.01, ##P < 0.01.
3.4. L. pentosus Increased the Expression of BIRC3
Previous studies report that BIRC3 plays a neuron-protective role. We then determined the expression of BIRC3 in neurons. As shown in Figure 4(a), the mRNA expression of BIRC3 was significantly decreased in the LPS group; however, L. pentosus treatment significantly increased BIRC3 mRNA levels compared with the LPS group (Figure 4(a)). Moreover, L. pentosus also increased the protein expression of BIRC3 (Figure 4(b)).
Figure 4.
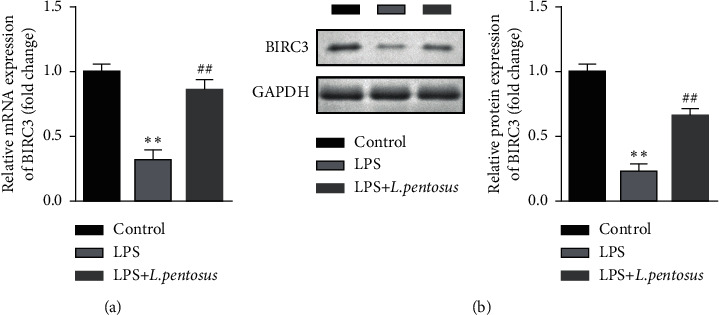
L. pentosus upregulated BIRC3. (a) The mRNA levels of BIRC3 determined using RT-qPCR. (b) The protein expression of BIRC3 detected using western blot. ∗∗P < 0.01, ##P < 0.01.
3.5. BIRC3 Knockdown Induces the Release of Proinflammatory Cytokines
The rescue assay was performed to verify the roles of BIRC3 in neuronal degeneration. As shown in Figure 5(a), sh-BIRC3 significantly suppressed the mRNA and protein expression of BIRC3 compared with LPS + L. pentosus + sh-NC group. BIRC3 knockdown significantly suppressed the cell viability of SH-SY5Y cells (Figure 5(c)). Additionally, BIRC3 markedly promoted the release and protein expression of proinflammatory cytokines, such as IL-1β and IL-18 (Figures 5(d) and 5(e)).
Figure 5.
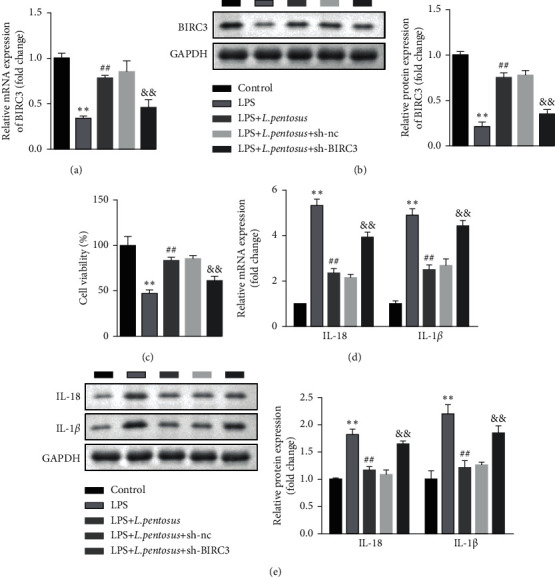
BIRC3 knockdown promoted inflammatory response and suppressed cell viability of neuronal cells. (a) The mRNA levels of BIRC3 determined using RT-qPCR. (b) The protein expression of BIRC3 detected using western blot. (c) The cell viability of neuronal cells detected using CCK-8. (d) The mRNA levels of IL-18 and IL-1β determined using RT-qPCR. (e) The mRNA levels of IL-18 and IL-1β detected by western blot. ∗∗P < 0.01, ##P < 0.01, &&P < 0.01.
3.6. BIRC3 Knockdown Induced the Pyroptosis of SH-SY5Y Cells
As shown in Figures 6(a) and 6(b), downregulated BIRC3 significantly increased the PI + active caspase1-positive cells as well as TUNEL-positive cells. Moreover, BIRC3 knockdown significantly increased the protein expression of cleaved caspase1 and GSDMD-N (Figure 6(c)).
Figure 6.
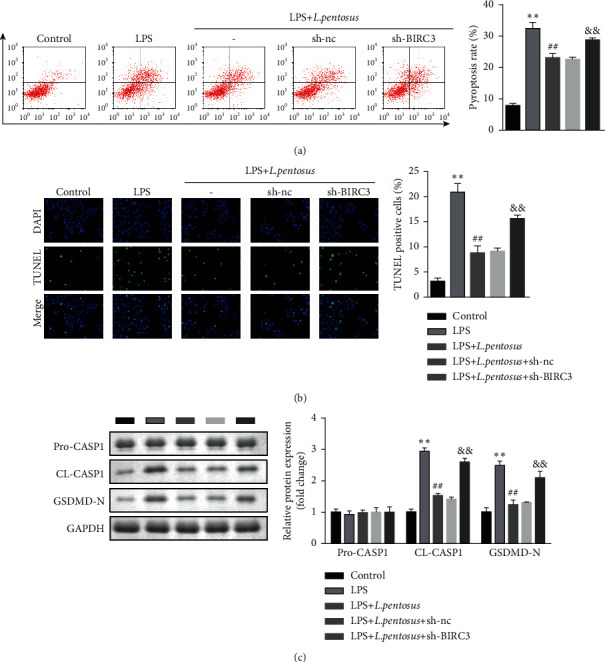
BIRC3 knockdown promoted the pyroptosis of neuronal cells. (a) The cell death of neurons detected using flow cytometry. (b) Neuronal cell death determined using the TUNEL assay. (c) The protein expression of pyroptosis biomarkers, cleaved caspase1, and GSDMD-N detected by western blot. ∗∗P < 0.01, ##P < 0.01, &&P < 0.01.
3.7. BIRC3 Inactivates NLRC4 Inflammasome
We further investigated the underlying molecular mechanisms that BIRC3 inhibited neuronal pyroptosis. The online database STING showed that the potential genes interacts with BIRC3 (Figure 7(a)). The results showed that BIRC3 could interact with NLRC4; however, the expression of NLRP1, NLRP3, NLRP4, and NLRP1 showed no significant changes after transfection with sh-BIRC3.
Figure 7.
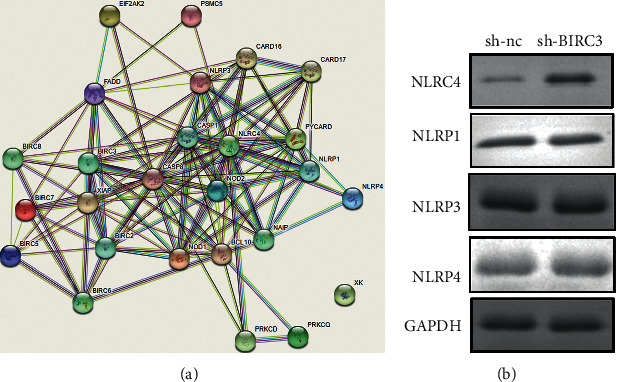
BIRC3 induced the inactivation of NLRC4 inflammasome. (a) The interaction between BIRC3 and inflammasome predicted by STING. (b) The protein expression of NLRP3, NLRP4, NLRP1, and NLRC4.
4. Discussion
In this study, L. pentosus play a neuron-protective role. L. pentosus suppressed inflammatory response and pyroptosis of neuron cells. Moreover, L. pentosus upregulated BIRC3, suppressing the inactivation of NLRC4 inflammasome. Hence, L. pentosus may be a promising therapy for neurodegenerative diseases.
The activation of inflammasomes increases cytotoxicity and contributes to the pyroptosis of neurons, which is a key factor for memory loss and cognitive impairment [27]. For instance, the activation of NLRC4 inflammasome interacts with CASP1, IL-1β, and p-Tau to contribute to neuroinflammation and memory impairment [28]. High levels of caspase1 induces human mild cognitive impairment and brain functions in patients with Alzheimer's disease, while depletion of NLRP3 promotes spatial memory and suppresses M2 polarization of microglia and deposition of amyloid-β [29]. NLRP1 deficiency suppresses the pyroptosis of neurons and improves cognitive capability [30]. These results suggested that inactivation of inflammasomes may be an effective strategy for neurodegenerative diseases. In this study, LPS exposure upregulated NLRC4 in neurons. The activation of NLRC4 inflammasome cleaved CASP1 and induced pore formation [31], which further contributed to neuronal pyroptosis. However, L. pentosus treatment restored neuronal cellular functions, manifested by the increase in cell viability and decrease in pyroptosis rates. Previous studies evidence that L. pentosus promotes cognitive capability and alleviates aging-dependent memory impairment [17, 32]. These results dictate the neuron-protective roles of L. pentosus, which is consistent with this study. Therefore, L. pentosus may be a promising strategy for neurodegenerative diseases.
BIRC3 plays a vital role in neuronal function. For instance, NPD1-mediated upregulation of BIRC3 promotes neural cell survival [26]. Additionally, cAMP responsive element binding protein interacting with brain derived neurotrophic factor and BIRC3 suppresses Aβ-induced neuronal apoptosis [33]. However, the roles of BIRC3 vary with diseases and cell types. It functions as a protective role in ischemic stroke, Alzheimer's disease, and brain injuries [34] as well as an oncogene [24]. Hence, identifying the roles of BIRC3 in neurodegenerative diseases is of vital importance. In this study, L. pentosus alleviated LPS-induced downregulation of BIRC3. However, BIRC3 knockdown alleviated the effects of L. pentosus and promoted inflammation and pyroptosis of neurons. These results suggested that BIRC3 may play a neuron-protective role, which is consistent with previous studies [26].
However, approximately 80% studies focused on the roles of BIRC3 cancer. The reports on its roles in neural disorders are limited. BIRC3 mainly exerts its neuron-protective functions via regulating caspases cascades, which suppresses the neuronal apoptosis [26]. Recently, the interplay between pyroptosis and apoptosis has attracted increasing attention. Rogers et al. report that pyroptosis may occur as secondary necrosis after apoptosis [35]. Additionally, pyroptosis and apoptosis may have common signal transduction pathways [36]. In this study, LPS stimulated the activation of NLRC4 inflammasome, which induced cleaved caspase1 and the assembly of N fragments of GSDMD. Additionally, GSDMD-mediated pore formation may orientate neuron to pyroptosis before the onset of apoptosis. However, L. pentosus-mediated upregulation of BIRC3 contributed to inactivation of NLRC4 inflammasome, which suppressed neuronal pyroptosis.
In conclusion, L. pentosus-induced upregulation of BIRC3 suppressed inflammatory response and pyroptosis of neurons via inactivating NLRC4 inflammasome. Therefore, L. pentosus may be an alternative strategy for neurodegenerative diseases.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Bellou V., Belbasis L., Tzoulaki I., Middleton L. T., Ioannidis J. P. A., Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta‐analyses. Alzheimer’s and Dementia . 2017;13(4):406–418. doi: 10.1016/j.jalz.2016.07.152. [DOI] [PubMed] [Google Scholar]
- 2.Karran E., De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nature Reviews Drug Discovery . 2022;21(4):306–318. doi: 10.1038/s41573-022-00391-w. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti B. Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson’s disease. Redox Biology . 2020;36 doi: 10.1016/j.redox.2020.101664.101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K., Sun Q., Zhong X., et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell . 2020;180(5):941–955. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Chavarría-Smith J., Vance R. E. The NLRP1 inflammasomes. Immunological Reviews . 2015;265(1):22–34. doi: 10.1111/imr.12283. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., Deng Y., Gan X., et al. NLRP12 collaborates with NLRP3 and NLRC4 to promote pyroptosis inducing ganglion cell death of acute glaucoma. Molecular Neurodegeneration . 2020;15(1):p. 26. doi: 10.1186/s13024-020-00372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Liu J., Wang W., Wang Y., Ouyang X. NLRP6 induces pyroptosis by activation of caspase-1 in gingival fibroblasts. Journal of Dental Research . 2018;97(12):1391–1398. doi: 10.1177/0022034518775036. [DOI] [PubMed] [Google Scholar]
- 8.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences . 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Burdette B. E., Esparza A. N., Zhu H., Wang S. Gasdermin D in pyroptosis. Acta Pharmaceutica Sinica B . 2021;11(9):2768–2782. doi: 10.1016/j.apsb.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammad A. E.-N. A. En-bloc resection versus resection after evacuation and suction of the content for orbital optic nerve glioma causing visual loss and disfiguring proptosis. Ophthalmic Plastic and Reconstructive Surgery . 2020;36(4):399–402. doi: 10.1097/iop.0000000000001577. [DOI] [PubMed] [Google Scholar]
- 11.Sun R., Peng M., Xu P., et al. Low-density lipoprotein receptor (LDLR) regulates NLRP3-mediated neuronal pyroptosis following cerebral ischemia/reperfusion injury. Journal of Neuroinflammation . 2020;17(1):p. 330. doi: 10.1186/s12974-020-01988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y.-S., Tan Z.-X., Wu L.-Y., Dong F., Zhang F. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Research Reviews . 2020;64 doi: 10.1016/j.arr.2020.101192.101192 [DOI] [PubMed] [Google Scholar]
- 13.Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nature Medicine . 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 14.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nature Reviews Gastroenterology & Hepatology . 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 15.Mergenhagen K. A., Wojciechowski A. L., Paladino J. A. A review of the economics of treating Clostridium difficile infection. Pharmacoeconomics . 2014;32(7):639–650. doi: 10.1007/s40273-014-0161-y. [DOI] [PubMed] [Google Scholar]
- 16.Jung I.-H., Jung M.-A., Kim E.-J., Han M. J., Kim D.-H. Lactobacillus pentosusvar.plantarumC29 protects scopolamine-induced memory deficit in mice. Journal of Applied Microbiology . 2012;113(6):1498–1506. doi: 10.1111/j.1365-2672.2012.05437.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeong J.-J., Woo J.-Y., Kim K.-A., Han M. J., Kim D.-H. Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent memory impairment in Fischer 344 rats. Letters in Applied Microbiology . 2015;60(4):307–314. doi: 10.1111/lam.12393. [DOI] [PubMed] [Google Scholar]
- 18.Frazzi R. BIRC3 and BIRC5: multi‐faceted inhibitors in cancer. Cell & Bioscience . 2021;11(1):p. 8. doi: 10.1186/s13578-020-00521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrish E., Brumatti G., Silke J. Future therapeutic directions for smac-mimetics. Cells . 2020;9(2):p. 406. doi: 10.3390/cells9020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quijada-Álamo M., Hernández-Sánchez M., Rodríguez-Vicente A. E., et al. Biological significance of monoallelic and biallelic BIRC3 loss in del (11q) chronic lymphocytic leukemia progression. Blood Cancer Journal . 2021;11(7):p. 127. doi: 10.1038/s41408-021-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Luan T., Zhou S., et al. LncRNA HCP5 promotes triple negative breast cancer progression as a ceRNA to regulate BIRC3 by sponging miR‐219a‐5p. Cancer Medicine . 2019;8(9):4389–4403. doi: 10.1002/cam4.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pradhan M., Baumgarten S. C., Bembinster L. A., Frasor J. CBP mediates NF-κB-Dependent histone acetylation and estrogen receptor recruitment to an estrogen response element in the BIRC3 promoter. Molecular and Cellular Biology . 2012;32(2):569–575. doi: 10.1128/mcb.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinogradova E. V., Zhang X., Remillard D., et al. An activity-guided map of electrophile-cysteine interactions in primary human T cells. Cell . 2020;182(4):1009–1026. doi: 10.1016/j.cell.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S., Yang Y., Weng W., et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. Journal of Experimental & Clinical Cancer Research . 2019;38(1):p. 14. doi: 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi D., Deaglio S., Dominguez-Sola D., et al. Alteration of BIRC3 and multiple other NF-κB pathway genes in splenic marginal zone lymphoma. Blood . 2011;118(18):4930–4934. doi: 10.1182/blood-2011-06-359166. [DOI] [PubMed] [Google Scholar]
- 26.Calandria J. M., Asatryan A., Balaszczuk V., et al. NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death & Differentiation . 2015;22(8):1363–1377. doi: 10.1038/cdd.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poh L., Fann D. Y., Wong P., et al. AIM2 inflammasome mediates hallmark neuropathological alterations and cognitive impairment in a mouse model of vascular dementia. Molecular Psychiatry . 2021;26(8):4544–4560. doi: 10.1038/s41380-020-00971-5. [DOI] [PubMed] [Google Scholar]
- 28.Gan H., Zhang L., Chen H., et al. The pivotal role of the NLRC4 inflammasome in neuroinflammation after intracerebral hemorrhage in rats. Experimental & Molecular Medicine . 2021;53(11):1807–1818. doi: 10.1038/s12276-021-00702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heneka M. T., Kummer M. P., Stutz A., et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature . 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan M.-S., Tan L., Jiang T., et al. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death & Disease . 2014;5(8) doi: 10.1038/cddis.2014.348.e1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schölwer I., Habib P., Voelz C., Rolfes L., Beyer C., Slowik A. NLRP3 depletion fails to mitigate inflammation but restores diminished phagocytosis in bv-2 cells after in vitro hypoxia. Molecular Neurobiology . 2020;57(6):2588–2599. doi: 10.1007/s12035-020-01909-2. [DOI] [PubMed] [Google Scholar]
- 32.Jo Y. M., Seo H., Kim G. Y., et al. Lactobacillus pentosus SMB718 as a probiotic starter producing allyl mercaptan in garlic and onion-enriched fermentation. Food & Function . 2020;11(12):10913–10924. doi: 10.1039/d0fo02000a. [DOI] [PubMed] [Google Scholar]
- 33.Pugazhenthi S., Wang M., Pham S., Sze C.-I., Eckman C. B. Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Molecular Neurodegeneration . 2011;6(1):p. 60. doi: 10.1186/1750-1326-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cartagena C. M., Schmid K. E., Phillips K. L., Tortella F. C., Dave J. R. Changes in apoptotic mechanisms following penetrating ballistic-like brain injury. Journal of Molecular Neuroscience . 2013;49(2):301–311. doi: 10.1007/s12031-012-9828-z. [DOI] [PubMed] [Google Scholar]
- 35.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E. S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature Communications . 2017;8(1) doi: 10.1038/ncomms14128.14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Gao W., Shi X., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature . 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


