Abstract
The preovulatory intrafollicular environment plays a major role in determining oocyte competence. The basis of this review is to highlight the importance of the preovulatory follicle’s physiological status prior to the preovulatory luteinizing hormone (LH) surge and onset of oocyte maturation to promote an optimal follicular microenvironment and optimal oocyte developmental competence in cattle. While the underlying mechanisms remain unclear, and are likely multifactorial, the preovulatory follicle’s physiological status prior to the preovulatory LH surge is highly influential on the oocyte’s capacity to undergo postfertilization embryo development. Changes in the intrafollicular environment of the preovulatory follicle including steroid hormone production, metabolome profiles, and proteome profiles likely support the oocyte’s developmental and metabolic competency. This review focuses on the relationship between bovine oocyte developmental competency and antral follicle progression to the preovulatory phase, the role of the preovulatory follicle in improving oocyte developmental competence in cattle, and the importance of the ever-evolving preovulatory intrafollicular environment for optimal fertility.
Keywords: cattle, intrafollicular environment, oocyte competence, preovulatory follicle
The preovulatory follicle’s physiological maturity influences pregnancy rates and oocyte competence in cattle. This review highlights critical intrafollicular changes that occur during the preovulatory follicle and discusses how such changes support oocyte competence.
Introduction
The production of a developmentally competent oocyte is essential for successful pregnancy establishment because the oocyte plays a key role in successful fertilization, embryo cleavage, and cell divisions in the early embryo (Sirard and Blondin, 1996; Rizos et al., 2002; Sirard et al., 2006). The intrafollicular environment of the antral follicle contributes to oocyte developmental competency by providing a suitable microenvironment for oocyte growth and maturation and providing metabolic substrates required for oocyte metabolic competency. The follicle contains somatic cells that either directly associate with the oocyte to regulate meiotic resumption and provide metabolic support or are located around the follicle’s periphery and produce steroid hormones and extracellular vesicle packaged mRNA, DNA, proteins, and lipids that influence follicular trajectory toward ovulation or mediate gene regulatory action and function of the cumulus–oocyte-complex, respectively (Eppig, 1991; Gilchrist et al., 2004; Thompson et al., 2007; Kidder and Vanderhyden, 2010; Conti et al., 2012; Di Pietro, 2016).
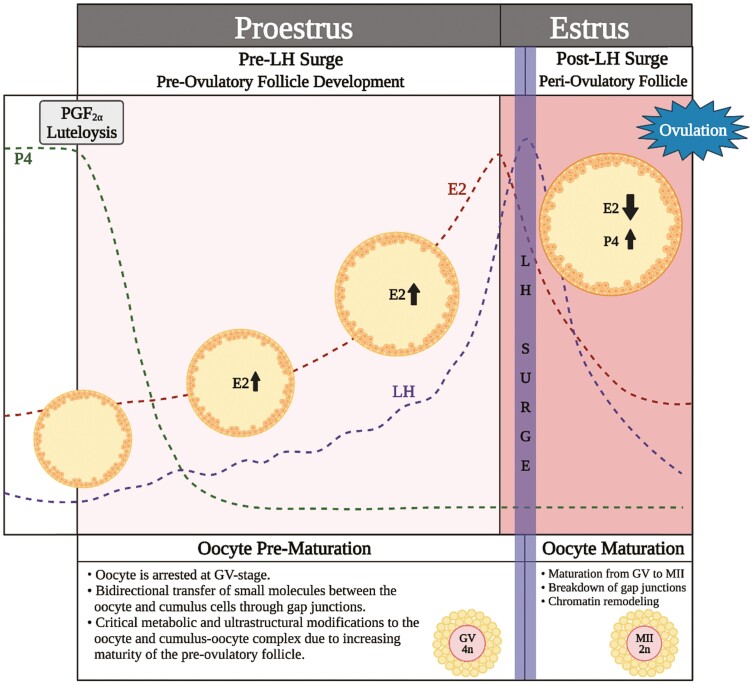
Interestingly, maximal oocyte developmental competency is only achieved if the oocyte is allowed to remain in the follicle during the later stages of antral follicle development, especially through the preovulatory stage, and undergo subsequent in vivo maturation (Leemput van de et al., 1999; Dieleman et al., 2002; Rizos et al., 2002; Humblot et al., 2005). The preovulatory time period (time after luteolysis up to but just before the luteinizing hormone [LH] surge; Figure 1) is critical for both the developing follicle and oocyte within, as it includes the progression of the follicle from dominance to the preovulatory stage of folliculogenesis and offers a time period of final stages of oocyte capacitation or prematuration prior to oocyte nuclear maturation from germinal vesicle (GV) to metaphase two (MII). The preovulatory follicle’s progression through proestrus, toward estrous expression and an endogenous LH surge influences preparation of both the intrafollicular environment and the oocyte for conditions that lead to optimal oocyte competence for successful maturation and postfertilization embryo development (Figure 1).
Figure 1.
Schematic depicting the important hormonal and cumulus–oocyte complex changes during proestrus and estrus. After luteolysis, the follicular phase of the estrous cycle begins, starting with proestrus. A decline in progesterone allows for an increase in LH pulsatility and a rise in estradiol both in circulation as well as within the follicle itself. During proestrus, the oocyte is completing critical prematuration developmental processes and is arrested at the germinal vesicle stage. At this time, there is bidirectional communication between the oocyte and cumulus cells allowing for critical metabolic modifications to occur. Once the LH surge occurs, the cross-talk between the oocyte and cumulus cells ceases and maturation to the MII stage begins. The intrafollicular hormone profile changes from predominantly estrogen to progesterone. The preovulatory intrafollicular environment influences oocyte developmental competency and the microenvironment of the periovulatory follicle in which the oocyte matures (Created with Biorender.com).
The physiological maturity of the preovulatory follicle prior to the LH surge influences pregnancy rates, early pregnancy maintenance, and oocyte developmental competence for embryo cleavage and production of a high-quality blastocyst, as such parameters were reported to improve with increasing future preovulatory follicle diameter and estradiol production in animals undergoing fixed-time artificial insemination (FTAI; Lamb et al., 2001; Vasconcelos et al., 2001; Perry et al., 2005, 2007; Sá Filho et al., 2010; Atkins et al., 2013; Jinks et al., 2013). Recent studies by our lab and others have indicated that the impact of preovulatory follicle physiological status before an induced LH surge on oocyte developmental competence may be strongly related to the preovulatory follicle’s influence on the metabolic capacity of the oocyte and the follicular environment to support oocyte metabolism (Moorey et al., 2021; Read et al., 2021, 2022). Though the preovulatory time period is relatively short compared with the entirety of the estrous cycle and the time period of antral follicle development, the preovulatory period is essential in preparing both the oocyte and intrafollicular environment for optimal in vivo oocyte maturation. The purpose of this review article is to highlight the importance of the preovulatory follicle’s physiological status prior to the preovulatory LH surge and onset of oocyte maturation to promote an optimal follicular microenvironment and optimal oocyte developmental competence.
Impact of Antral Follicle Progression on Oocyte Competence
The oocyte acquires developmental competence throughout antral follicle development, and in vitro studies have long highlighted the importance of the follicular origin of the oocyte for successful downstream development. Though this is not the focus of the current review, it is critical to mention that antral follicle size strongly influences oocyte competence in 2 to 10 mm follicles collected for in vitro embryo production (IVP), with greater meiotic competency, fertilization, cleavage, and blastocyst rates reported in larger follicles (Pavlok et al., 1992; Lonergan et al., 1994; Blondin and Sirard, 1995; Otoi et al., 1997; Hagemann et al., 1999). Oocytes contain the intrinsic ability to undergo nuclear maturation after they have completed their growth phase, and thus bovine oocytes collected from follicles as small as 2 to 3 mm are able to complete nuclear maturation and yield a blastocyst (Hyttel et al., 1997). Despite the ability to resume meiosis beyond this timepoint, oocytes do not complete nuclear maturation until exposure to the preovulatory gonadotropin surge in vivo or removal from the follicle to be utilized for in vitro maturation (Edwards, 1965). Continued exposure of the oocyte to the progressing follicle’s microenvironment allows the opportunity to improve embryo development capacity, and full progression to the preovulatory stage of folliculogenesis provides the highest oocyte competency (Arlotto et al., 1996; Hyttel et al., 1997; Leemput van de et al., 1999; Atkins et al., 2013; Jinks et al., 2013).
The idea that the progression of a follicle through the events of a natural estrous cycle for optimal fertility has been leveraged previously to improve outcomes during IVP. Because of the molecular and chromatin changes, an oocyte undergoes following exposure and then starvation from physiologically relevant follicle-stimulating hormone (FSH) levels during follicular wave recruitment, selection, and dominance, procedures of FSH coasting were developed that demonstrated improved blastocyst rate when oocytes were collected via ovum pick up (OPU) from super-stimulated ovaries ~54 h after the conclusion of FSH priming (Nivet et al., 2012). The early atresia or plateau stage of follicular development following such treatments is not unlike intrafollicular conditions during the final stages of preovulatory and periovulatory follicle development, and such conditions appear to improve oocyte developmental capacity (Blondin and Sirard, 1995; Nivet et al., 2012).
The positive relationship between oocyte developmental competence and the follicle’s physiological progression toward estrus and ovulation remains true even though proestrus and the preovulatory time period (Atkins et al., 2013; Jinks et al., 2013). Though much less emphasis has been placed on the impact of the preovulatory follicles’ physiological status on oocyte developmental capacity, critical changes in the intrafollicular milieu and oocyte infrastructure occur during the proestrus and preovulatory period that improve oocyte competency in estrual animals (Figure 1). The remainder of this review will highlight such changes and summarize what is known of the physiological impacts preovulatory follicular progression toward estrus has on the preovulatory follicle’s microenvironment and the ability to support optimal oocyte competence.
Preovulatory Hormone Profiles and Follicular Dynamics
The period from luteolysis to estrus or the onset of the preovulatory LH surge is associated with unique hormone profiles within the developing preovulatory follicle (Figure 1). Dynamic changes in circulating and intrafollicular steroid hormone concentrations occur during the period of proestrus and from the onset of estrus to ovulation. Prior to luteolysis, which occurs approximately 4 d before estrus, circulating progesterone concentrations are at full luteal phase levels of approximately 5 to 12 ng/mL (Henricks et al., 1971; Ginther et al., 2013). Once luteolysis is initiated, circulating progesterone concentration markedly drops to below 2 ng/mL by approximately 1.5 to 3.5 d before estrus, and continues to decrease to concentrations below 1 ng/mL prior to the onset of estrus (Henricks et al., 1971; Ginther et al., 2013). As progesterone levels decrease due to luteolysis, the dominant follicle increases estradiol production due to increased pulse frequency of LH (Rahe et al., 1980; Walters and Schallenberger, 1984; Peters, 1985). Thus, the period of proestrus is characterized by a rise in estradiol concentration observed both in circulation as well as within the follicle itself.
Within the follicle, estradiol concentration rises during proestrus reaching a peak of 1,040 to 1,100 ng/mL prior to estrus while progesterone levels remain low during this period (Dieleman et al., 1983; Fortune and Hansel, 1985). Serum estradiol concentration also increases over the proestrus period with rises beginning ~3 d before estrus and a peak in circulating estradiol concentrations reported to occur ~30 h prior to ovulation. Circulating estradiol concentrations reach between 3 and 25 pg/mL prior to estrus (Henricks et al., 1971; Walters and Schallenberger, 1984; Lucy and Stevenson, 1986). The rapid rise in circulating estradiol concentrations toward peak concentrations begins approximately 12 to 36 h before the onset of estrus, and such levels reach the peak and remain at high concentration until ~2 to 5 h after the onset of estrus, which corresponds to the timing of the preovulatory gonadotropin surge (Henricks et al., 1971; Wettemann et al., 1972; Ginther et al., 2013).
The preovulatory gonadotropin surge will occur shortly after the peak in estradiol with a LH surge magnitude ranging from 5.3 to 42.4 ng/mL (Chenault et al., 1975; Walters and Schallenberger, 1984; Lucy and Stevenson, 1986). The first elevation of circulating LH concentrations that was followed by higher LH values in the endogenously stimulated LH surge was observed to occur approximated 2 h after the onset of estrus, whereas the LH surge peak was recorded at approximately 4.5 h after the onset of estrus (Dieleman et al., 1983). Interestingly, circulating LH levels can remain elevated up to 17 h after the LH surge (Chenault et al., 1975; Lucy and Stevenson, 1986). LH concentration in the follicular fluid has been observed to reach a peak at about 4 h after the peak concentration of LH in circulation, with LH concentrations in follicular fluid (FF) significantly increased from 2 to 6 h post circulating LH peak compared with before the circulating LH peak or 10 to 25 h after peak concentration in circulation was observed (Dieleman et al., 1983). Similarly, FSH concentration in the FF before the preovulatory gonadotropin surge was lower than from the time of the peak LH surge until 18 h post surge, whereas intrafollicular prolactin concentrations were reported to fluctuate but not significantly change in relation to the LH surge (Dieleman et al., 1983).
The LH surge is a pivotal and critically important milestone for the preovulatory follicle to complete the final stages of follicle development and ovulate. Toward this end, LH-binding receptors on mural granulosa induce a myriad of changes for induction of ovulation ~24 to 30 h thereafter (Malhi et al., 2005; Ginther et al., 2013), initiates corpus luteum formation, and facilitates changes important for meiotic resumption of the oocyte. A major hallmark of the beginning of corpus luteum formation is the shift from estrogen to progesterone production. By 6 h post estrus, intrafollicular hormone concentrations of estradiol and progesterone have abruptly shifted to favor progesterone production. Within the follicle, estradiol concentration remains constant until 6 h after the LH surge where concentrations will then decrease up to 12-fold by 24 h after the surge and prior to ovulation (Dieleman et al., 1983; Fortune and Hansel, 1985). The drop in estradiol concentration can be attributed to the inhibition of aromatase activity within the follicle (Dieleman and Blankenstein, 1984). While estradiol production decreases, progesterone production within the follicle will begin to increase and reach its highest level since luteolysis (Dieleman et al., 1983; Fortune and Hansel, 1985). This rapid change from estrogenic activity to progesterone production is observed in intrafollicular estradiol to progesterone concentration ratios of approximately 45:1 during the latter stages of proestrus prior to estrus, 2.6:1 by 15 h post estrus, and <1:1 by 24 h post estrus (Fortune and Hansel, 1985). These dynamic changes in steroid hormone concentrations occur during oocyte maturation and may influence the maturing oocyte. We recently observed that follicular fluid progesterone concentration influences the metabolome of preovulatory follicular fluid collected approximately 18 h after an induced gonadotropin surge such that numerous glucogenic amino acids and citric acid cycle intermediates were higher abundant in the follicular fluid of preovulatory follicles with higher progesterone concentrations (Read et al., 2022).
Follicle dynamics closely resemble the increased estradiol concentrations during proestrus that are noted above, with the future preovulatory follicle growing about 0.5 mm every 8 h during proestrus to eventually reach its maximum diameter (Ginther et al., 2013). Interestingly, developing preovulatory follicles from ~10 to >15 mm in size are capable of estradiol production necessary to stimulate the preovulatory gonadotropin surge, and physiologically less mature dominant follicles that have not yet produced adequate estradiol concentrations to stimulate an endogenous gonadotropin surge to respond to a pharmacologically induced gonadotropin surge and undergo induced ovulation if they have reached ~9 mm and acquired LH receptors in their granulosa cells (Perry et al., 2005; Atkins et al., 2013). Thus, during procedures that employ pharmacological administration to induce a preovulatory gonadotropin surge in non-estrual animals, all preovulatory follicles have not equally progressed to the same physiological maturity that is acquired throughout proestrus.
The physiological status or progression of the preovulatory follicle toward stimulation of estrus appears to be critical for both pregnancy outcomes and oocyte developmental competency in cattle. If a developing follicle is of sufficient physiological maturity to stimulate estrus, its size likely has no impact on the downstream development potential of the enclosed oocyte. Therefore, it is essential to understand that the preovulatory follicle’s size at the time of the gonadotropin surge is not a perfect roadmap to the follicle’s progression through proestrus to ovulation. It is, however, a reasonable estimator of follicular maturity and is closely tied to the steroidogenic capacity of the follicle and oocyte developmental competency in animals that do not reach the onset of estrus before pharmacological induction of the preovulatory gonadotropin surge (Perry et al., 2005; Atkins et al., 2013; Jinks et al., 2013).
Importance of the Preovulatory Follicle and In Vivo Oocyte Maturation
The period of preovulatory follicle development is often associated with oocyte prematuration, or the final stages of oocyte capacitation. Though the timespan of ~ 96 h from luteolysis to the preovulatory gonadotropin surge is brief in relation to the entirety of folliculogenesis, this period is essential for optimal downstream oocyte maturation, fertilization, cleavage, and embryo development because it serves as a critical opportunity for the oocyte to accrue necessary modifications required for optimal fertility before the onset of nuclear and cytoplasmic maturation (Dieleman et al., 2002).
Assisted reproductive technologies (ART) of superovulation, FTAI and IVP reduce or eliminate the time period that the oocyte is exposed to the preovulatory follicle, preovulatory follicle developmental progression, and the length of oocyte prematuration period, thereby causing oocyte maturation to occur in vitro or a potentially inferior follicular environment (Figures 2 and 3). This often results in reduced oocyte developmental competence in subsets of animals or oocytes compared with single, naturally stimulated ovulations.
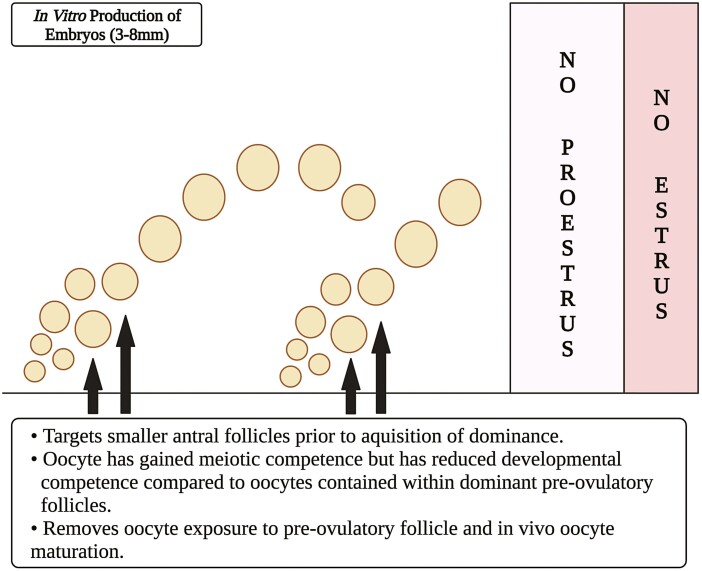
Figure 2.
Schematic depicting possible antral pool of follicles ranging in size of 3 to 8 mm (predominant) from which cumulus–oocytes are collected for use with in vitro production (IVP) of embryos. Because larger follicles are limited in number and may pose issues with clotting depending on collection approaches, cumulus–oocytes utilized for IVP are largely from a pool of estrogen-active (nondominant) antral follicles that have not been exposed to the preovulatory follicular environment (Created with Biorender.com).
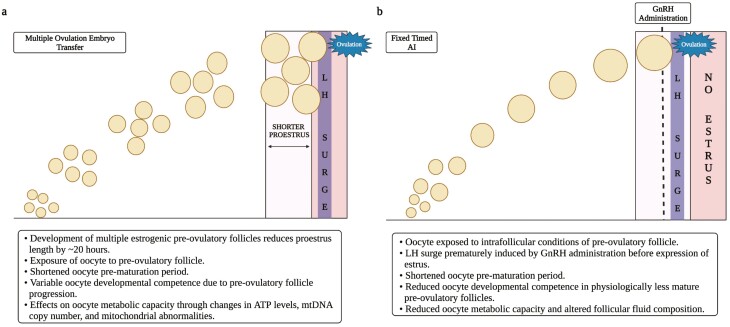
Figure 3.
Schematic depicting the development of multiple dominant follicles after FSH administration (A) for multiple ovulation embryo transfer (MOET) and preovulatory follicle development where the LH surge was pharmacologically induced in animals before they expressed estrus (B). Regarding MOET procedure depicted in (A), super stimulation of the ovary using FSH effectively recruits multiple follicles to grow and attain dominance. Greater levels of circulating estradiol cause the LH surge to occur sooner, thereby shortening the proestrus time period by ~20 h. The cumulus–oocyte complexes resident within said follicles are exposed to a shorter prematuration period which may affect developmental competence (i.e., after fertilization, cleavage, and blastocyst development). Ovulated cumulus–oocyte complexes resulting from hyperstimulation with FSH, have diminished metabolic capacity (Created with Biorender.com). Regarding the preovulatory follicle in (B), in cows that do not exhibit estrus before a fixed timed AI (FTAI), exogenous GnRH administration induces the LH surge prematurely (well before the time when it would have occurred spontaneously if expression of estrus had occurred) thereby shorting the cumulus–oocyte’s prematuration time period. Pharmacological induction of the LH surge has been related to reduced oocyte competence, reduced metabolic capacity of the oocyte, as well as altered follicular fluid composition (Created with Biorender.com).
IVP commonly utilizes in vitro culture for oocyte maturation, fertilization, and early embryo development. Thus, most IVP procedures eliminate intrafollicular prematuration of the oocyte and the critical oocyte–follicular interactions that occur within the preovulatory follicle (Figure 2). As such, oocyte developmental competence during IVP is lower than that observed during a natural estrous cycle or ART that utilize in vivo oocyte maturation. While rates of successful fertilization remain 70% to 80% during IVP, the percentage of oocytes entering IVP that form a blastocyst is reduced to 14% to 70% as compared with 43% to 93% blastocyst rates reported during in vivo maturation (Diskin and Sreenan, 1980; Roche et al., 1981; Pavlok et al., 1992; Breuel et al., 1993; Lonergan et al., 1994; Blondin and Sirard, 1995; Hyttel et al., 1997; Otoi et al., 1997; Hagemann et al., 1999; Leemput van de et al., 1999; Dieleman et al., 2002; Iwata et al., 2004; Lequarre et al., 2005; Carter et al., 2008; Reese et al., 2020). Though interactions between the oocyte and intrafollicular environment are not recovered, an in vitro prematuration period can be induced during IVP by the inclusion of specific compounds which inhibit GV breakdown into the media. This process delays oocyte nuclear maturation and allows added time for the cumulus–oocyte complexes (COCs) to acquire necessary metabolic, nuclear, and cytoplasmic modifications before oocyte nuclear maturation begins and the breakdown of COC bidirectional communication begins. Exposure to a prematuration period has been demonstrated to improve oocyte metabolic capacity and embryo development outcomes (Hashimoto et al., 2002; Coy et al., 2005). Utilization of this technique, however, has failed to consistently provide improvements in oocyte developmental competence, as oocyte developmental competence is reduced when oocyte maturation occurs ex vivo.
Such results, however, eliminate the ability to examine the impacts of variable preovulatory follicle maturity or physiological status throughout the proestrus period on oocyte developmental competency. There is a strong evidence that the status of the preovulatory follicle prior to an endogenous or exogenously triggered gonadotropin surge influences oocyte developmental competency either in a direct mechanism or by altering the intrafollicular conditions of the post LH surge, the periovulatory follicle which houses the oocyte during in vivo maturation (Greve et al., 1995; Atkins et al., 2013; Jinks et al., 2013; Afedi et al., 2021; Moorey et al., 2021; Read et al., 2021). Reduced or variable fertility during FTAI and superovulation support the notion that in vivo maturation within a physiologically compromised or immature periovulatory follicle does not yield the same oocyte developmental outcomes as in vivo maturation within a physiologically mature periovulatory follicle that naturally progressed through proestrus and stimulated an endogenous gonadotropin surge.
Oocyte Developmental Competence during the Preovulatory Time Period
Though oocyte maturation occurs in vivo during superovulation and FTAI, the onset of the preovulatory LH surge often occurs earlier than in a natural estrous cycle (Figure 3). Such circumstances may alter both the oocyte’s prematuration or capacitation and the periovulatory follicle’s composition during oocyte maturation, as the physiological status of the preovulatory follicle at the onset of the LH surge, as well as the length of proestrus, impact pregnancy success, and oocyte developmental competence. The onset of the LH surge was reported ~20 h sooner after prostaglandin F2α injection to induce luteolysis in cattle undergoing superovulation compared with non-stimulated animals (Figure 3A; Assey et al., 1994a, 1994b; Greve et al., 1995). Estradiol production within the super-stimulated follicles is variable in nature (Fortune and Hansel, 1985; Berisha et al., 2019). The amount of estradiol produced by individual follicles is lower than that observed in follicular fluid from a typical preovulatory follicle which results in a lower estradiol to progesterone ratio. The estradiol to progesterone ratio prior to an LH surge in super-stimulated cattle has been shown to be approximately 10:1, which is significantly lower than observed in single preovulatory follicles (Fortune and Hansel, 1985; Berisha et al., 2019). The ratio, expectedly, continues to decrease after the LH surge and has been observed to approximately be 3:1 by 4 h after exogenous gonadotropin-releasing hormone (GnRH) administration, <1.4:1 by 10 h natural variation in preovulatory follicle status in super-stimulated cattle may explain the equally variable levels of oocyte competence during such procedures (Greve et al., 1995).
Further, ~40% to 60% of beef cattle undergoing FTAI rely on the administration of GnRH to induce the preovulatory gonadotropin surge near the time of insemination (Richardson et al., 2016). Such animals are denied the final hours or days of proestrus that would have been required for preovulatory follicle development to stimulate estrus and an endogenous gonadotropin surge (Figure 3B). Multiple studies have indicated a strong, positive relationship between preovulatory follicle diameter or preovulatory serum estradiol concentrations and pregnancy attainment and/or late embryonic survival when animals undergo FTAI (Lamb et al., 2001; Perry et al., 2005, 2007; Sá Filho et al., 2010). Both follicle size and estradiol production serve as measures of follicular progression toward the essential onset of estrus, and comprehensive reviews and literature focus toward the impact of preovulatory follicle status on the maternal environment (Perry et al., 2005; Pohler et al., 2012; Atkins et al., 2013; Geary et al., 2013; Jinks et al., 2013; Ciernia et al., 2021). The current review is focused on literature that suggests the preovulatory follicle’s status prior to an induced LH surge affects oocyte developmental competence. Both preovulatory follicle diameter and serum estradiol concentration at the time of GnRH injection to induce the preovulatory gonadotropin surge positively impacted embryo cleavage rate (reported as fertilization rate) and embryo quality grade in a reciprocal embryo transfer study (Atkins et al., 2013; Jinks et al., 2013). The mean size of preovulatory follicles in which a cleaved embryo was recovered was ~12.6 mm whereas the mean follicle diameter that resulted in the recovery of an uncleaved embryo was ~11.7 mm (Atkins et al., 2013). A similar trend was observed for serum estradiol concentrations, in which mean serum estradiol concentrations were ~8.8 pg/mL in cows that produced a cleaved embryo and only ~6.7 pg/mL in cows that produced an unfertilized oocyte (Jinks et al., 2013). Though no animals that displayed estrus were utilized in this pair of studies, increasing follicle diameter and estradiol production prior to an exogenously stimulated gonadotropin surge indicate further follicle progression toward the stimulation of estrus and an endogenous gonadotropin surge improves oocyte developmental competence.
Impact of Preovulatory Follicle Status on the Intrafollicular Environment and Oocyte Metabolic Capacity
To further understand why preovulatory follicle developmental status influenced oocyte cleavage and embryo quality, we performed RNA-sequencing on pools of four oocytes or surrounding cumulus cell (CC) collected from small (≤11.7mm) and large (≥12.7mm) follicles ~18 h after GnRH injection to induce the preovulatory gonadotropin surge (Moorey et al., 2021). We also collected samples from preovulatory follicles of cows who displayed estrus and would have experienced an endogenously stimulated gonadotropin surge. We discovered numerous differentially expressed gene (DEG) transcripts among oocyte or CC pools originating from the three follicle classifications. Functional analysis of DEGs between CC from small and large follicles revealed significant enrichment in KEGG pathways related to metabolism (“glycolysis,” “metabolic pathways,” “fructose and mannose metabolism,” “carbon metabolism,” and “biosynthesis of amino acids”), while the biological pathway “oxidative phosphorylation” was significantly enriched with DEG from small vs. estrous follicle oocyte pools (Moorey et al., 2021). Such results suggest that CC from small follicles may have reduced ability to convert glucose to metabolic substrates for use by the oocyte and that oocytes from small follicles may have altered mitochondrial function or number. Lowered production and transfer of pyruvate, lactate, or phosphoenolpyruvate by the CC of small, developmentally immature follicles, and reduced mitochondrial function in the oocyte would lead to reduced ATP availability for oocyte maturation, fertilization, and downstream embryo development. We have since performed complementary analyses of intraoocyte ATP levels, mitochondrial DNA copy number, and metabolic capacity per mitochondrial DNA copy number. Serum estradiol concentration at the time of an induced preovulatory gonadotropin surge was positively related to ATP levels in oocytes nearing completion of maturation (Read et al., 2022). Serum estradiol is a strong indicator of the preovulatory follicle’s progression through proestrus, toward the expression of estrus, and the endogenous gonadotropin surge. Oocytes collected from preovulatory follicles of animals with higher serum estradiol were likely allowed exposure to the follicular conditions during prematuration and maturation that more closely resembled an estrual animal. Though modulated follicular maturity at the onset of an exogenously stimulated gonadotropin surge likely impacts the follicular microenvironment during the critical hours of oocyte maturation and subsequently reduced developmental and metabolic competency of the oocyte, there may also be a direct effect of estradiol on COC metabolic dichotomy, as estradiol has been demonstrated to improve gap junctional communication in somatic cells (Firestone and Kapadia, 2012).
Interestingly, superovulation protocols also can have effects on the metabolic capacity of the oocyte, also affecting downstream embryo development. Murine oocytes derived from super-stimulated follicles have lower mitochondrial DNA copy numbers, ATP levels, and mitochondrial membrane potential (Shu et al., 2015). Additionally, deformities in the mitochondria of murine oocytes retrieved from super-stimulated follicles have been observed by increases in vacuolated mitochondria and multivesicular body formation murine oocytes (Lee et al., 2017). Similarly, rabbit oocytes collected from follicles following a superovulation protocol had significantly lower ATP levels and lower blastocyst rates compared with oocytes from a single dominant follicle (Cortell et al., 2015).
Processes of oocyte maturation, fertilization, cleavage, and embryo development to the blastocyst stage require adequate storage of energetic precursors and production of ATP by the oocyte or early embryo (Biggers et al., 1967; Cetica et al., 2002; Johnson et al., 2007; Chappel, 2013). Interestingly, the pre-blastocyst stage embryo and oocyte both rely on oxidative phosphorylation as a primary energetic pathway (Chappel, 2013). Before oocyte maturation, the CC perform glycolysis and transfer pyruvate and metabolic intermediates to the oocyte for ATP production or storage (Gilchrist et al., 2004; Thompson et al., 2007; Sutton-McDowall et al., 2010). Once oocyte maturation begins, and gap junctions break down, the metabolic transfer between the oocyte and CC ceases. The oocyte and early embryo must drive necessary metabolic processes with the metabolic compounds accrued until this timepoint until the blastocyst embryo gains the ability to utilize glucose as a primary energetic compound through glycolysis.
The preovulatory follicular fluid contains metabolites and proteins that support COC metabolism and oocyte preparation for maturation. Both the metabolome of the post LH surge, periovulatory follicle and the proteome of the preovulatory follicle have been demonstrated to be impacted by preovulatory follicle physiological status (Afedi et al., 2021; Read et al., 2021, 2022). The metabolome of periovulatory follicular fluid collected ~18 to 20 h post-GnRH administration was related to both preovulatory follicle diameter and serum estradiol concentrations at GnRH (Read et al., 2021, 2022). In both studies, metabolites involved in glucose metabolism, the citric acid cycle, and proteinogenesis were elevated in larger or higher estrogen-producing preovulatory follicles. The proteome of preovulatory follicular fluid also appears to be different from that of less progressed follicles and modulated by follicular maturity as observed by circulating and intrafollicular estradiol levels (Afedi et al., 2021). Indeed, intrafollicular levels of proteins relevant to metabolism were highly impacted based on preovulatory estradiol production at ~36 h post prostaglandin F2 alpha administration. Such observations clearly demonstrate that the physiological status of the preovulatory follicle strongly influences the metabolic composition of the follicular fluid that likely leads to reduced metabolic competency in oocytes within physiologically immature preovulatory follicles that undergo an induced LH surge.
Conclusion
The importance of the follicle’s full progression through dominance and the preovulatory stage of development is critical for the creation of intrafollicular conditions that support optimal oocyte developmental competence. Preovulatory follicle size and estradiol production during this stage of folliculogenesis serve as markers not only for follicular progression to physiological maturity to induce estrus and the LH surge but also for oocyte developmental competence. Animals induced to ovulate a physiologically immature follicle experience reduced day 7 embryo quality and poorer embryo developmental rates than those that ovulate a physiologically more advanced follicle. This reduction in fertility appears to be strongly related to the reduced metabolic capacity of the COC and a metabolically altered intrafollicular environment. Though oocytes within preovulatory follicles have been exposed to the intrafollicular conditions of selection, dominance, and the positive gonadotropin environment post luteolysis, full progression of the preovulatory follicle to endogenously stimulate the LH surge is required to produce an intrafollicular environment that best supports oocyte competence. Such changes in the ever-evolving preovulatory intrafollicular environment serve as essential building blocks to improve conditions for both in vivo and in vitro oocyte prematuration and maturation and thereby improve fertility in agriculturally relevant livestock species and humans.
Acknowledgments
The funding for this work was provided by the USDA National Institute of Food and Agriculture Multistate Project NE1727: Influence of Ovary, Uterus, and Embryo on Pregnancy Success in Ruminants (Participants: S.E.M. and J.L.E.), the University of Tennessee AgResearch, and the University of Tennessee Department of Animal Science. We would like to thank Neal Schrick, Michael Smith, and Tom Geary for their intellectual contribution over the years and great discussions about various aspects of this topic. We would also like to thank Casey Read and Emma Horn for their assistance in compiling the information presented in this review.
Glossary
Abbreviations
- ART
assisted reproductive technologies
- CC
cumulus cell
- COC
cumulus–oocyte complex
- DEG
differentially expressed gene
- FSH
follicle-stimulating hormone
- FTAI
fixed-time artificial insemination
- GnRH
gonadotropin-releasing hormone
- GV
germinal vesicle
- IVP
in vitro embryo production
- LH
luteinizing hormone
- MII
metaphase II
- OPU
ovum pick up
Contributor Information
Sarah E Moorey, Department of Animal Science, University of Tennessee, Knoxville, TN 37996, USA.
Emma A Hessock, Department of Animal Science, University of Tennessee, Knoxville, TN 37996, USA.
J Lannett Edwards, Department of Animal Science, University of Tennessee, Knoxville, TN 37996, USA.
Conflict of interest statement
The authors do not have any actual or potential conflicts of interest that may affect their ability to objectively present or review research or data.
Literature Cited
- Afedi, P. A., Larimore E. L., Cushman R. A., Raynie D., and Perry G. A.. . 2021. iTRAQ-based proteomic analysis of bovine pre-ovulatory plasma and follicular fluid. Domest. Anim. Endocrinol. 76:106606. doi: 10.1016/j.domaniend.2021.106606 [DOI] [PubMed] [Google Scholar]
- Arlotto, T., Schwartz J. L., First N. L., and Leibfried-Rutledge M. L.. . 1996. Aspects of follicle and oocyte stage that affect in vitro maturation and development of bovine oocytes. Theriogenology 45:943–956. doi: 10.1016/0093-691X(96)00024-6 [DOI] [PubMed] [Google Scholar]
- Assey, R. J., Hyttel P., Greve T., and Purwantara B.. . 1994a. Oocyte morphology in dominant and subordinate follicles. Mol. Reprod. Dev. 37:335–344. doi: 10.1002/mrd.1080370313 [DOI] [PubMed] [Google Scholar]
- Assey, R. J., Hyttel P., Roche J. F., and Boland M.. . 1994b. Oocyte structure and follicular steroid concentrations in superovulated versus unstimulated heifers. Mol. Reprod. Dev. 39:8–16. doi: 10.1002/mrd.1080390103 [DOI] [PubMed] [Google Scholar]
- Atkins, J. A., Smith M. F., MacNeil M. D., Jinks E. M., Abreu F. M., Alexander L. J., and Geary T. W.. . 2013. Pregnancy establishment and maintenance in cattle. J. Anim. Sci. 91:722–733. doi: 10.2527/jas.2012-5368 [DOI] [PubMed] [Google Scholar]
- Berisha, B., Rodler D., Schams D., Sinowatz F., and Pfaffl M. W.. . 2019. Prostaglandins in superovulation induced bovine follicles during the preovulatory period and early corpus luteum. Front. Endocrinol. 10:467. doi: 10.3389/fendo.2019.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers, J., Whittingham D., and Donahue R.. . 1967. The pattern of energy metabolism in the mouse oocyte and zygote. Zoology 58:560–567. doi: 10.1073/pnas.58.2.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin, P., and Sirard M.-A.. . 1995. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol. Reprod. Dev. 41:54–62. doi: 10.1002/mrd.1080410109 [DOI] [PubMed] [Google Scholar]
- Breuel, K. F., Lewis P. E., Schrick F. N., Lishman A. W., Inskeep E. K., and Butcher R. L.. . 1993. Factors affecting fertility in the postpartum cow: Role of the oocyte and follicle in conception rate. Biol. Reprod. 48(3):655–661. doi: 10.1095/biolreprod48.3.655 [DOI] [PubMed] [Google Scholar]
- Carter, F., Forde N., Duffy P., Wade M., Fair T., Crowe M. A., Evans A. C., Kenny D. A., Roche J. F., Lonergan P.. . 2008. Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod. Fertil. Dev. 20(3):368–375. doi: 10.1071/rd07204 [DOI] [PubMed] [Google Scholar]
- Cetica, P., Pintos L., Dalvit G., and Beconi M.. . 2002. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 124:675–681. doi: 10.1530/rep.0.1240675 [DOI] [PubMed] [Google Scholar]
- Chappel, S. 2013. The role of mitochondria from mature oocyte to viable blastocyst. Obstet. Gynecol. Int. 2013:183024. doi: 10.1155/2013/183024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenault, J. R., Thatcher W. W., Kalra P. S., Abrams R. M., and Wilcox C. J.. . 1975. Transitory changes in plasma progestins, estradiol, and luteinizing hormone approaching ovulation in the bovine. J. Dairy. Sci. 58(5):709–717. doi: 10.3168/jds.S0022-0302(75)84632-7 [DOI] [PubMed] [Google Scholar]
- Ciernia, L. A., Perry G. A., Smith M. F., Rich J. J., Northrop E. J., Perkins S. D., Green J. A., Zezeski A. L., and Geary T. W.. . 2021. Effect of estradiol preceding and progesterone subsequent to ovulation on proportion of postpartum beef cows pregnant. Anim. Reprod. Sci. 227:106723. doi: 10.1016/j.anireprosci.2021.106723 [DOI] [PubMed] [Google Scholar]
- Conti, M., Hsieh M., Musa Zamah A., and Oh J. S.. . 2012. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell Endo. 356:65–73. doi: 10.1016/j.mce.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortell, C., Salvetti P., Joly T., and Viudes-de-Castro M. P.. . 2015. Effect of different superovulation stimulation protocols on adenosine triphosphate concentration in rabbit oocytes. Zygote 23(4):507–513. doi: 10.1017/S0967199414000112 [DOI] [PubMed] [Google Scholar]
- Coy, P., Romar R., Payton R. R., McCann L., Saxton A. M., and Edwards J. L.. . 2005. Maintenance of meiotic arrest in bovine oocytes using the S-enantiomer of roscovitine: effects on maturation, fertilization and subsequent embryo development in vitro. Reproduction 129:19–26. doi: 10.1530/rep.1.00299 [DOI] [PubMed] [Google Scholar]
- Di Pietro, C. 2016. Exosome-mediated communication in the ovarian follicle. J. Assist. Reprod. Genet. 33:303–311. doi: 10.1007/s10815-016-0657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman, S. J., Bevers M. M., Poortman J., and van Tol H. T.. . 1983. Steroid and pituitary hormone concentrations in the fluid of preovulatory bovine follicles relative to the peak of LH in the peripheral blood. J. Reprod. Fertil. 69:641–649. doi: 10.1530/jrf.0.0690641 [DOI] [PubMed] [Google Scholar]
- Dieleman, S. J. and Blankenstein D. M.. . 1984. Changes in oestrogen-synthesizing ability of preovulatory bovine follicles relative to the peak of LH. J. Reprod. Fertil. 72(2):487–494. doi: 10.1530/jrf.0.0720487 [DOI] [PubMed] [Google Scholar]
- Dieleman, S. J., Hendriksen P. J., Viuff D., Thomsen P. D., Hyttel P., Knijn H. M., Wrenzycki C., Kruip T. A., Niemann H., Gadella B. M., . et al. 2002. Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos. Theriogenology 57:5–20. doi: 10.1016/s0093-691x(01)00655-0 [DOI] [PubMed] [Google Scholar]
- Diskin, M. G., and Sreenan J. M.. . 1980. Fertilization and embryonic mortality rates in beef heifers after artificial insemination. J. Reprod. Fertil. 59:463–468. doi: 10.1530/jrf.0.0590463 [DOI] [PubMed] [Google Scholar]
- Edwards, R. G. 1965. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208:349–351. doi: 10.1038/208349a0 [DOI] [PubMed] [Google Scholar]
- Eppig, J. 1991. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays 13:569–574. doi: 10.1002/bies.950131105 [DOI] [PubMed] [Google Scholar]
- Firestone, G. L., and Kapadia B. J.. . 2012. Minireview: regulation of gap junction dynamics by nuclear hormone receptors and their ligands. Mol. Endocrinol. 26:1798–1807. doi: 10.1210/me.2012-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune, J. E., and Hansel W.. . 1985. Concentrations of steroids and gonadotropins in follicular fluid from normal heifers and heifers primed for superovulation. Biol. Reprod. 32(5):1069–1079. doi: 10.1095/biolreprod32.5.1069 [DOI] [PubMed] [Google Scholar]
- Geary, T. W., Smith M. F., MacNeil M. D., Day M. L., Bridges G. A., Perry G. A., Abreu F. M., Atkins J. A., Pohler K. G., Jinks E. M., . et al. 2013. Triennial Reproduction Symposium: influence of follicular characteristics at ovulation on early embryonic survival. J. Anim. Sci. 91:3014–3021. doi: 10.2527/jas.2012-5887 [DOI] [PubMed] [Google Scholar]
- Gilchrist, R. B., Ritter L. J., and Armstrong D. T.. . 2004. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 8:431–446. doi: 10.1016/j.anireprosci.2004.05.017 [DOI] [PubMed] [Google Scholar]
- Ginther, O. J., Pinaffi F. L., Khan F. A., Duarte L. F., and Beg M. A.. . 2013. Follicular-phase concentrations of progesterone, estradiol-17β, LH, FSH, and a PGF2α metabolite and daily clustering of prolactin pulses, based on hourly blood sampling and hourly detection of ovulation in heifers. Theriogenology 79:918–928. doi: 10.1016/j.theriogenology.2012.12.015 [DOI] [PubMed] [Google Scholar]
- Greve, T., Callesen H., Hyttel P., Høier R., and Assey R.. . 1995. The effects of exogenous gonadotropins on oocyte and embryo quality in cattle. Theriogenology 43:41–50. doi: 10.1016/0093-691X(94)00013-K [DOI] [Google Scholar]
- Hagemann, L. J., Beaumont S. E., Berg M., Donnison M. J., Ledgard A., Peterson A. J., Schurmann A., and Tervit H. R.. . 1999. Development during single IVP of bovine oocytes from dissected follicles: interactive effects of estrous cycle stage, follicle size and atresia. Mol. Reprod. Dev. 53(4):451–458. doi: [DOI] [PubMed] [Google Scholar]
- Hashimoto, S., Minami N., Takakura R., and Imai H.. . 2002. Bovine immature oocytes acquire developmental competence during meiotic arrest in vitro. Biol. Reprod. 66:1696–1701. doi: 10.1095/biolreprod66.6.1696 [DOI] [PubMed] [Google Scholar]
- Henricks, D. M., Dickey J. F., and Hill J. R.. . 1971. Plasma estrogen and progesterone levels in cows prior to and during estrus. Endocrinology 89:1350–1355. doi: 10.1210/endo-89-6-1350 [DOI] [PubMed] [Google Scholar]
- Humblot, P., Holm P., Lonergan P., Wrenzycki C., Lequarré A. S., Joly C. G., Herrmann D., Lopes A., Rizos D., Niemann H., . et al. 2005. Effect of stage of follicular growth during superovulation on developmental competence of bovine oocytes. Theriogenology 63:1149–1166. doi: 10.1016/j.theriogenology.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Hyttel, P., Fair T., Callesen H., and Greve T.. . 1997. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 47:23–32. doi: 10.1016/S0093-691X(96)00336-6 [DOI] [Google Scholar]
- Iwata, H., Hashimoto S., Ohota M., Kimura K., Shibano K., and Miyake M.. . 2004. Effects of follicle size and electrolytes and glucose in maturation medium on nuclear maturation and developmental competence of bovine oocytes. Reproduction 127(2):159–164. doi: 10.1530/rep.1.00084 [DOI] [PubMed] [Google Scholar]
- Jinks, E. M., Smith M. F., Atkins J. A., Pohler K. G., Perry G. A., Macneil M. D., Roberts A. J., Waterman R. C., Alexander L. J., and Geary T. W.. . 2013. Preovulatory estradiol and the establishment and maintenance of pregnancy in suckled beef cows. J. Anim. Sci. 91:1176–1185. doi: 10.2527/jas.2012-5611 [DOI] [PubMed] [Google Scholar]
- Johnson, M. T., Freeman E. A., Gardner D. K., and Hunt P. A.. . 2007. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol. Reprod. 77:2–8. doi: 10.1095/biolreprod.106.059899 [DOI] [PubMed] [Google Scholar]
- Kidder, G. M., and Vanderhyden B. C.. . 2010. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 88:399–413. doi: 10.1139/y10-009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, G. C., Stevenson J. S., Kesler D. J., Garverick H. A., Brown D. R., and Salfen B. E.. . 2001. Inclusion of an intravaginal progesterone insert plus GnRH and prostaglandin F2α for ovulation control in postpartum suckled beef cows. J. Anim. Sci. 79:2253–2259. doi: 10.2527/2001.7992253x [DOI] [PubMed] [Google Scholar]
- Lee, M., Ahn J. I., Lee A. R., Ko D. W., Yang W. S., Lee G., Ahn J. Y., and Lim J. M.. . 2017. Adverse effect of superovulation treatment on maturation, function and ultrastructural integrity of murine oocytes. Mol. Cells. 40(8):558– 566. doi: 10.14348/molcells.2017.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemput van de, E. E., Vos P. L., Zeinstra E. C., Bevers M. M., van der Weijden G. C., and Dieleman S. J.. . 1999. Improved in vitro embryo development using in vivo matured oocytes from heifers superovulated with a controlled preovulatory LH surge. Theriogenology 52:335–349. doi: 10.1016/s0093-691x(99)00133-8 [DOI] [PubMed] [Google Scholar]
- Lequarre, A.-S., Vigneron C., Ribaucour F., Holm P., Donnay I., Dalbiès-Tran R., Callesen H., and Mermillod P.. . 2005. Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology 63(3):841–859. doi: 10.1016/j.theriogenology.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Lonergan, P., Monaghan P., Rizos D., Boland M. P., and Gordon I.. . 1994. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 37:48–53. doi: 10.1002/mrd.1080370107 [DOI] [PubMed] [Google Scholar]
- Lucy, M. C., and Stevenson J. S.. . 1986. Gonadotropin-releasing hormone at estrus: Luteinizing hormone, estradiol, and progesterone during the periestrual and postinsemination periods in dairy cattle. Biol. Reprod. 35(2):300–311. doi: 10.1095/biolreprod35.2.300 [DOI] [PubMed] [Google Scholar]
- Malhi, P. S., Adams G. P., and Singh J.. . 2005. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol. Reprod. 73:45–53. doi: 10.1095/biolreprod.104.038745 [DOI] [PubMed] [Google Scholar]
- Moorey, S. E., Monnig J. M., Smith M. F., Ortega M. S., Green J. A., Pohler K. G., Bridges G. A., Behura S. K., and Geary T. W.. . 2021. Differential transcript profiles in cumulus-oocyte complexes originating from pre-ovulatory follicles of varied physiological maturity in beef cows. Genes (Basel) 12:893. doi: 10.3390/genes12060893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivet, A. L., Bunel A., Labrecque R., Belanger J., Vigneault C., Blondin P., and Sirard M. A.. . 2012. FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction 143:165–171. doi: 10.1530/rep-11-0391 [DOI] [PubMed] [Google Scholar]
- Otoi, T., Yamamoto K., Koyama N., Tachikawa S., and Suzuki T.. . 1997. Bovine oocyte diameter in relation to developmental competence. Theriogenology 48:769–774. doi: 10.1016/S0093-691X(97)00300-2 [DOI] [PubMed] [Google Scholar]
- Pavlok, A., Lucas-Hahn A., and Niemann H.. . 1992. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol. Reprod. Dev. 31:63–67. doi: 10.1002/mrd.1080310111 [DOI] [PubMed] [Google Scholar]
- Perry, G. A., Smith M. F., Lucy M. C., Green J. A., Parks T. E., MacNeil M. D., Roberts A. J., and Geary T. W.. . 2005. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. U. S. A. 102:5268–5273. doi: 10.1073/pnas.0501700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, G. A., Smith M. F., Roberts A. J., MacNeil M. D., and Geary T. W.. . 2007. Relationship between size of the ovulatory follicle and pregnancy success in beef heifers. J. Anim. Sci. 85:684–689. doi: 10.2527/jas.2006-519 [DOI] [PubMed] [Google Scholar]
- Peters, A. R. 1985. Hormonal control of the bovine oestrous cycle I. The natural cycle. Br. Vet. J. 141(6):564–575. doi: 10.1016/0007-1935(85)90003-X [DOI] [PubMed] [Google Scholar]
- Pohler, K. G., Geary T. W., Atkins J. A., Perry G. A., Jinks E. M., and Smith M. F.. . 2012. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 349:649–664. doi: 10.1007/s00441-012-1386-8 [DOI] [PubMed] [Google Scholar]
- Rahe, C. H., Owens R. E., Fleeger J. L., Newton H. J., and Harms P. G.. . 1980. Pattern of plasma luteinizing hormone in the cyclic cow: dependence upon the period of the cycle. Endocrinology 107(2):498–503. doi: 10.1210/endo-107-2-498 [DOI] [PubMed] [Google Scholar]
- Read, C. C., Edwards J. L., Schrick F. N., Rhinehart J. D., Payton R. R., Campagna S. R., Castro H. F., Klabnik J. L., Horn E. J., and Moorey S. E.. . 2021. Correlation between pre-ovulatory follicle diameter and follicular fluid metabolome profiles in lactating beef cows. Metabolites 11:623. doi: 10.3390/metabo11090623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, C. C., Edwards J. L., Schrick F. N., Rhinehart J. D., Payton R. R., Campagna S. R., Castro H. F., Klabnik J. L., and Moorey S. E.. . 2022. Pre-ovulatory serum estradiol concentration is positively associated with oocyte ATP and follicular fluid metabolite abundance in lactating beef cattle. J. Anim. Sci. doi: 10.1093/jas/skac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese, S. T., Franco G. A., Poole R. K., Hood R., Montero L. F., Filho R. V., Cooke R. F., and Pohler K. G.. . 2020. Pregnancy loss in beef cattle: A meta-analysis. Anim. Reprod. Sci. 212:106251. doi: 10.1016/j.anireprosci.2019.106251 [DOI] [PubMed] [Google Scholar]
- Richardson, B. N., Hill S. L., Stevenson J. S., Djira G. D., and Perry G. A.. . 2016. Expression of estrus before fixed-time AI affects conception rates and factors that impact expression of estrus and the repeatability of expression of estrus in sequential breeding seasons. An. Reprod. Sci. 166:133–140. doi: 10.1016/j.anireprosci.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Rizos, D., Ward F., Duffy P., Boland M. P., and Lonergan P.. . 2002. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 61:234–248. doi: 10.1002/mrd.1153 [DOI] [PubMed] [Google Scholar]
- Roche, J. F., Bolandl M. P., and McGeady T. A.. . 1981. Reproductive wastage following artificial insemination of heifers. Vet. Rec. 109(18):401–404. doi: 10.1136/vr.109.18.401 [DOI] [PubMed] [Google Scholar]
- Sá Filho, M. F., Crespilho A. M., Santos J. E. P., Perry G. A., and Baruselli P. S.. . 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. An. Reprod. Sci. 120:23–30. doi: 10.1016/j.anireprosci.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Shu, J., Xing L. L., Ding G. L., Liu X. M., Yan Q. F., and Huang H. F.. . 2015. Effects of ovarian hyperstimulation on mitochondria in oocytes and early embryos. Reprod. Fertil. Dev. 28(8):1214. doi: 10.1071/rd14300 [DOI] [PubMed] [Google Scholar]
- Sirard, M. A., and Blondin P.. . 1996. Oocyte maturation and IVF in cattle. An. Reprod. Sci. 42:417–426. doi: 10.1016/0378-4320(96)01518-7 [DOI] [Google Scholar]
- Sirard, M.-A., Richard F., Blondin P., and Robert C.. . 2006. Contribution of the oocyte to embryo quality. Theriogenology 65:126–136. doi: 10.1016/j.theriogenology.2005.09.020 [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall, M. L., Gilchrist R. B., and Thompson J. G.. . 2010. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139:685. doi: 10.1530/rep-09-0345 [DOI] [PubMed] [Google Scholar]
- Thompson, J. G., Lane M., and Gilchrist R. B.. . 2007. Metabolism of the bovine cumulus-oocyte complex and influence on subsequent developmental competence. Soc. Reprod. Fertil. Suppl. 64:179–190. doi: 10.5661/rdr-vi-179 [DOI] [PubMed] [Google Scholar]
- Vasconcelos, J. L. M., Sartori R., Oliveira H. N., Guenther J. G., and Wiltbank M. C.. . 2001. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology 56:307–314. doi: 10.1016/S0093-691X(01)00565-9 [DOI] [PubMed] [Google Scholar]
- Walters, D. L., and Schallenberger E.. . 1984. Pulsatile secretion of gonadotrophins, ovarian steroids and ovarian oxytocin during the periovualtory phase of the oestrus cycle in the cow. Reproduction 71(2):503–512. doi: 10.1530/jrf.0.0710503 [DOI] [PubMed] [Google Scholar]
- Wettemann, R. P., Hafs H. D., Edgerton L. A., and Swanson L. V.. . 1972. Estradiol and progesterone in blood serum during the bovine estrous cycle. J. Anim. Sci. 34:1020–1024. doi: 10.2527/jas1972.3461020x [DOI] [PubMed] [Google Scholar]