Abstract
The human population is ever increasing while the quality and quantity of natural resources used for livestock production decline. This calls for improved product efficiency and the development of improved and sustainable cattle production methods to produce higher quality products to satisfy the demands of both the modern and transient world. The goal of this review was to summarize the interactions, challenges, and opportunities in cattle production relating to their endocrine system, and how reproductive hormones and others impact economically important traits, animal welfare, and human health. A comprehensive literature search was conducted with a focus on analysis of natural hormones and the use of exogenous hormone administration for reproduction, growth, and development of beef and dairy cattle. Hormones regulate homeostasis and enhance important traits in cattle, including fertility, growth and development, health, and the production of both meat and milk products. Reproductive hormones such as testosterone, estradiol, progesterone, and related synthetics like trenbolone acetate and zeranol can be strategically utilized in both beef and dairy cattle production systems to enhance their most valuable traits, but the impact of these substances must account for the welfare of the animal as well as the health of the consumer. This scientific review provides a comprehensive analysis of the bovine endocrine system’s impact on food animals and product quality which is vital for students, researchers, livestock producers, and consumers. Although important advances have been made in animal science and related technological fields, major gaps still exist in the knowledge base regarding the influence of hormones on the production and welfare of food animals as well as in the public perception of hormone use in food-producing animals. Filling these gaps through transformative and translational research will enhance both fundamental and applied animal science to feed a growing population.
Keywords: cattle, hormones, milk production, meat nutritive value, welfare
The understanding and use of hormones can enhance animal production sectors, particularly that of the cattle industry, in order to create a more productive, sustainable, and animal-centered system.
Introduction
Livestock systems are a principal component of global agriculture requiring a large fraction of the world’s agricultural land, which is valued at $1.4 trillion (Thornton, 2010). In 2017, U.S. agricultural exports totaled $138.2 billion, an increase of 2.6% above the previous year (Persaud, 2019). Additionally, U.S. agriculture and food–related industries contributed over $1 trillion in GDP with farms alone being $134.7 billion of this total (USDA, 2021). Cattle production is a cornerstone of livestock systems with beef and milk products being staples in the diets of consumers. As the global population grows and developing countries strive to consume more meat, there is an increased demand for animal protein and milk to maintain good nutrition and diets of consumers (FAO, 2006; Thornton, 2010). Increasing animal production requires efficiency and improvement of animal traits such as fertility, growth and development, carcass quality, and milk production.
To meet global demands, there must be a universal effort to improve cattle production and environmental sustainability. Farmland is becoming scarce, and it is difficult to find new lands for ranching systems, grazing fields, and row-cropping (i.e., corn and soybean). Between the years of 2001 and 2016, approximately 11 million acres of farmland was converted for other uses to include both urban and residential purposes (Freedgood et al., 2020). The valuation of arable U.S. farmland has increased to $3,160 per acre in 2019, nearly a 2% increase from the previous year. Pasture land was valued at $1,400 per acre with a similar 2.2% increase from the year previous (USDA/NASS, 2019). Overuse of pastures and/or overgrazing lowers the quality of pasture forage, which decreases the nutrient value of that forage. With increasing herd sizes, there is a concern for maintaining animal health, welfare, and concern for current consumer attitudes towards these factors. The climate and weather conditions can complicate production problems with new challenges such as heat stress, water supply limitations, insufficient nutrients, plant toxicity, and parasites, all of which increase costs for producers. Today’s research should aim to increase animal production while maintaining animal well-being and disease tolerance, while also minimizing land usage and accounting for environmental impacts.
The United States Department of Agriculture (USDA) states that although livestock production occurs in all U.S. states, Texas, California, Iowa, Kansas, and Nebraska maintain the highest sale values of their livestock and their products ((USDA, 2021). Leaders within government, private, and educational institutions have expressed the need for improved forage utilization, disease control, food safety, and improved management strategies. Moreover, there has been an emphasis on short-term production systems rather than improving long-term sustainability towards improved energy usage and climate control. Researchers often utilize biotechnological and chemical approaches to provide resolutions to these issues. Research in the areas of endocrine hormones and the concentrations of hormone secretion are appropriate with the current and future needs in cattle production. Animal health and welfare are additional avenues of research opportunities. These could determine better practices for improved production traits, examining stress and pain intensity and their relationship with immunity and energy balance in animals.
There is evidence that hormone usage improves animal production efficiency, management, and utilization of resources. An example would be the use of recombinant bovine somatotropin, rBST, to increase milk yield in dairy cattle (Flores et al., 2019). However, the general consumer associates negative connotations with the use of hormones in a production setting (McGlone, 2001). The public sector has communicated their concerns with the use of hormones in the livestock production industry (Stock et al., 2015). In response to satisfying misinformed public perceptions, there is a rise of organic production systems and non-hormone-promoted animal products, and these systems typically do not incorporate efficiency. Therefore, it is important to provide honest scientific knowledge to properly educate the public about hormone usage in cattle production, how they are produced, transported, their mode of action, and how they are utilized and removed from the animal’s body. This allows the consumer to gain factual knowledge and awareness of how food is raised then formulate their own opinion based on unbiased sources.
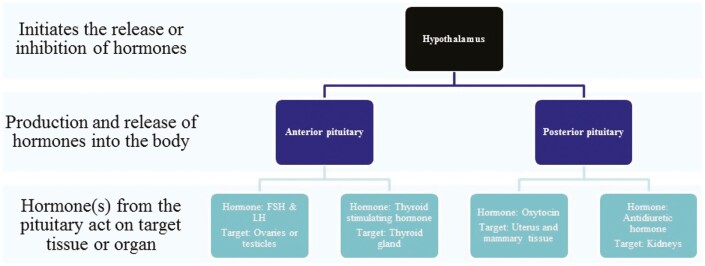
Hormones are signaling molecules that are secreted by endocrine glands to perform specific functions in cells and tissues (Figure 1). The principal endocrine organs are the hypothalamus, pituitary, gonads (ovary and testes), uterus, pancreas, thyroid, and adrenals (Hiller-Sturmhöfel and Bartke, 1998). Most hormones in mammals are triggered for release by the hypothalamus which also controls the feedback cascades of subsequent hormone secretions from the other endocrine glands (Hiller-Sturmhöfel and Bartke, 1998). The hypothalamus itself secretes a number of hormones: growth hormone-releasing hormone (GHRH) and gonadotropin-releasing hormone (GnRH) from the arcuate nucleus; and corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone from the paraventricular nucleus (Castro and Melmed, 2003; Malagon et al., 2006).
Figure 1.
Endocrine regulation. The hypothalamus controls the production and regulation of hormones by acting upon the anterior and posterior pituitary gland. Specific hormones are produced by the specialized cells in particular portions of the pituitary.
In the cattle production industry, several naturally occurring hormones are commercialized, including GH (rBST), prostaglandin (Lutalyse and Estromate), and Progestin (controlled internal drug release [CIDR]). All of these are widely utilized in estrous synchronization protocols. There are synthetic hormones available such as Zeranol, a non-steroidal form of estrogen and trenbolone acetate (TBA), which acts like testosterone (Jeong et al., 2010). Cloprostenol, or Estromate, is a synthetic form of PGF2α in which studies have shown that it can induce luteolysis in dairy heifers (Pfeifer et al., 2009; Leonardi et al., 2012).
There are three synthetic hormones that are currently approved by the Food and Drug Administration (FDA): zeranol (estrogenic), trenbolone acetate (androgenic), and melengestrol acetate (MGA) for the feedlot sector of the beef industry. The first two are primarily used as growth promoters and are administered as implants, whereas the MGA is a progestin (oral feed additive) that suppresses estrus and ovulation (used in feedlot heifers) and can be used in estrous synchronization protocols. Somatotropin or growth hormone (GH) is a protein hormone that acts on the liver to trigger production of insulin-like growth factor-1 (IGF-1), which stimulates the growth of bone and muscle by increasing growth efficiency through increased lean muscle accretion, but it also increases milk production in lactating females (Simmons et al., 1990). The toughness of meat, as measured by the Warner–Bratzler shear force test, appears to increase slightly in cattle that were implanted with hormonal growth promoters, but not enough to compromise tenderness (Lean et al., 2018). Recombinant bovine somatotropin not only improves milk production, but it also reduces the occurrence of mastitis-infected cows (Eppard et al., 1996). Uses of GnRH, P4, and PGF2α are extremely useful in estrous synchronization protocols such as Ovsynch and 7-day Cosynch + CIDR; these protocols increase the service rates of the herd and improve reproductive performance.
Hormone expertise and research are crucial for enhancing both the knowledge and application to real world problems in the industry. Hormone concentrations can be used to ascertain the current physiological challenges and welfare status of an animal. The evaluation of hormones through assays can provide indicators of reproductive performance and success among animals and their progeny (Post et al., 1987). Treatments can be introduced to improve reproductive performance and the efficiency of production. For example, GH and insulin are key indicators involved in fatty acid release in adipose tissues. Insulin can aid in diagnosing animals predisposed to conditions of insulin resistance, ketosis, and fatty liver disease in dairy cattle (Kirovski and Sladojevic, 2017). It has been demonstrated that early lactating cattle experience an increase in GH and hepatic function indicators such as albumin, in addition to a decrease in Triiodothyronine (T3), glucose, and urea (Djoković et al., 2015). Hormones like insulin can potentially provide therapeutic or medicinal benefits to metabolic disorders in dairy cattle (Sakai et al., 1993). A recent study using subcutaneous adipose tissue samples of dairy cattle demonstrated that the anti-lipolytic action of insulin increases insulin resistance of periparturient dairy cows during the first month after parturition (De Koster et al., 2018). The results showed that decreased free fatty acids (FFA) and glycerol levels coincide with increased insulin levels. This is explained by the presence of insulin binding to hormone sensitive lipase (HSL), which prevents glucagon from binding and therefore in the interruption of the FFA release mechanism caused by glycogen.
With regard to human medicine and its ties to animal hormones, insulin therapy initially was labor intensive. Hormone extraction from slaughtered animals is not efficient, but recent innovations in the technology have allowed for efficient and economic synthesis of recombinant insulin analogs. Other developments of new hormone technologies for such uses, as in vitro fertilization (IVF), have enhanced scientific knowledge and research by improving understanding of the mechanisms involved in embryogenesis and fetal growth and development for both humans and animals. These technologies have facilitated steps to upgrade quality of gametes, zygotes, and embryos, and use of surrogates to develop animals with improved muscling, growth, and productivity (Ventura-Juncá et al., 2015). This technology has significantly altered the methodology used for this scientific research for which its developer, Sir Robert Edwards was awarded the Nobel Prize in Physiology or Medicine in 2010. It is imperative that we advance hormone research further to improve management practices, ultimately leading to improved productivity, product quality, efficiency, sustainability, and welfare of cattle.
Hormones and Animal Welfare
Animal welfare is a crucial aspect of food safety and the desire to produce the best quality products. Welfare is a high priority issue and has led to changes in government regulations and consumer preferences over time. However, the definition of animal welfare is highly variable among producers, industry experts, veterinarians, and consumers. There are five basic freedoms that should be provided to animals: the freedom from thirst and hunger, freedom from discomfort, freedom from pain, injury, and disease, freedom from fear or distress, and freedom to express normal behavior (Brambell, 1965). According to the American Veterinary Medical Association, animal welfare is the human responsibility to provide proper housing, management, disease prevention and treatment, responsible care, humane handling, and, when necessary, humane euthanasia (AVMA, 2018). Animals must be provided adequate amounts of clean, fresh water and food. Shelter and care should be provided and should be free of potential hazards that could inflict injury and disease. All animals should be provided enough space to perform normal behaviors such as, lie down, stand up, and turn around. The living environment should be calm, provide adequate lighting, and avoid unnecessary stressors such as loud noises.
Combining these ideas helps create a holistic view of animal welfare. Variability in this area stems from how producers raise and manage their herds because every operation is managed differently. Despite on the farm practices consumers want to see “happy” cows and producers want their livestock to produce. Animal welfare is not only of value for ethical standards and beliefs, but also helps elevate production and provide healthier foods for human consumption. Hormones aid in the enhancement of farm productivity by improving breeding efficiency and increasing production and product quality (Lacasse and Ollier, 2015; Reineri et al., 2018). Happiness is not measurable in animals, so we must attain information about the animal’s well-being through other means such as biological or physiological indicators. Researchers are indeed capable of determining other factors such as stress levels by measuring hormone levels in their systems. New techniques are being created to improve the sensitivity and accuracy of these technologies to enhance our understanding of animal wellbeing.
Improvement of management and animal handling protocols can then be developed from learning what stressors intensify cortisol levels, for instance, and how to reduce them. The North American Meat Institute created audit criteria and recommendations to identify problems when it comes to animal handling, welfare, and harvesting plan procedures for turkey, chicken, cattle, swine, and sheep species. Audits that review rendering practices, facilities, and the handling of animals can encourage change and reduce instances of animal mistreatment and improve animal care (Grandin, 2000; Whaytt et al., 2003). Additionally, there are new adjustments on how hormone levels are detected, using factors such as increasing detection sensitivity, decreasing invasive procedures, and improving welfare-friendly approaches of data collection from the herd.
Animal welfare can be quantified using a combination of diverse, minimally invasive measurements including changes in heart and respiratory rates, pupil dilation, behavioral changes, and by detecting hormone levels in their biological system via blood, urine, and saliva. Hair samples are commonly utilized to assess the long-term stress levels of animals because of their reliability and the ease of acquisition of hair samples (Heimbürge et al., 2019). Biomarkers found on the hair samples can be indicative of a variety of potential stressors, to include pathological conditions, but variation occurs with the age, sex, and production level of the animal, i.e., pregnancy (Heimbürge et al., 2019), In addition, physiological changes in the animal can be evaluated to quantify stress. Animal care has been a topic of controversy for years and many techniques and studies have been performed to increase our understanding of animal husbandry and food production. Bruising of carcasses indicates inferior animal care pre-slaughter and decreases carcass value and profits at slaughter (Huertas et al., 2015). Commonly, bruising will occur during loading, transport, or unloading of the animals. A recent study showed that animal welfare training for truck drivers decreased the amount of deep/severe bruising (Huertas et al., 2018). Bruising caused in transportation increases cortisol in animals (De Freslon et al., 2014). Short-term transportation and holding systems elicit changes in stress response in cattle (Odore et al., 2010).
In a study performed in 2010, one group of cattle were raised in a tie-barn and another in a loose-housing system (Odore et al., 2010). In this study, hormone concentrations were evaluated by sampling the blood of the two groups to study the blood serum changes that were induced by stressors. Those in the tie-barn showed significant increases in blood cortisol levels, whereas the loose housed group did not. Both groups had significant increases in blood cortisol concentrations when transported on a double-decker truck for 45 min. In a later study, heifers were given oxytocin injections to observe endocrine system modulation of blood serum cortisol concentrations as compared to untreated groups (Barros et al., 2016). The use of exogenous oxytocin induced a reduction of serum cortisol levels in the treated heifers. Utilizing hormones as an indicator of animal welfare is valuable for the improvement of animal well-being and improving livestock rearing practices.
Within the United States, the FDA provides information for all approved hormonal products, implants specifically, which can enhance efficiency and productivity of the animal. Hormones used in the food animal sector undergo testing to demonstrate their safety. Manufacturers of these drugs are required to demonstrate that hormones are present in animal tissues at appropriate levels; appropriate meaning that it is present at a level that should not cause detrimental effects on human health after ingestion. Information for each of these implants is available in the Code of Federal Regulations, Title 21, in parts 522 and 556. In a survey study performed in 2013, more than 80% of veterinary practitioners agreed that hormones improve fertility and farm business profitability, but suggested that improvements in herd genetics and welfare could combat fertility issues that producers experience (Higgins et al., 2013). However, other countries do not share the same views as the United States when it comes to the use of hormones in animal production. Much of the European Union has placed a ban on hormone used for promoting growth (Stephany, 2001). The Joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) set global standards for food additives like contaminants, drugs, and toxins. They set limits to these hazards and risks such that producers can market their products world-wide if they fit within the given limits or standards. While we are always seeking to improve livestock management and care practices, welfare is always at question.
Castration and disbudding of calves are common practices among most livestock operations and they can be painful procedures (Rodriguez-Martinez et al., 1997). The efficacy of an anti-gonadotrophin-releasing factor vaccine vs. band castration methods has been explored. In one study, calves that were treated with the vaccine had a higher average daily gain and final body weight than that of the banded calves (Marti et al., 2015). It was also found that undesirable sexual and aggressive behaviors were appropriately reduced in the vaccinated group (Marti et al., 2015). These positive results make the animals easier to manage, improve growth, and thus enhance production. Disbudding reduces injury among the mature animals and others in day-to-day life making it easier to work with the cattle in a production setting. In a study performed in 2015, calves were given firocoxib, a non-steroidal anti-inflammatory, or placebo of saline solution to evaluate animal stress indicators after disbudding (Stock et al., 2015). Within the first 24-h period, no differences in cortisol were noted between the treatment and placebo groups. Calves in the placebo group showed increased levels of cortisol 48 h after the procedure had taken place. Utilization of hormones in the beef and dairy industry is growing and has proven to increase productivity and welfare in several areas. Adopting the best practices of animal welfare in cattle production and educating the consumer to these benefits will have a positive impact on the cattle industry.
Hormones and Economically Important Traits for Cattle Production
Fertility
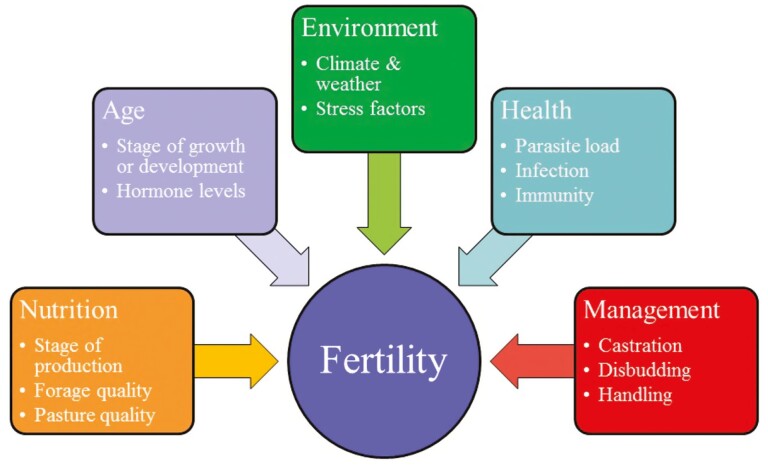
High fertility is defined as the ability to produce viable offspring and it is essential for reproductive efficiency in cattle production. Heritability of some reproductive traits is only 5%–10%, and they can be influenced by environmental factors such as housing, management, nutrition, transportation, and climate (Figure 2). Scrotal circumference can be an indicator of reproductive productivity and value when evaluating a bull for breeding purposes. Scrotal circumference is a highly heritable trait in Bos taurus cattle with a rage of 0.36 to 0.71 (Latimer et al., 1982; Bourdon and Brinks, 1986; Morris and Wilson, 1997). Scrotal circumference in sires is also correlated with earliest onset of puberty in that sire’s daughters (Smith et al., 1989). Bull fertility may be defined as the ability to produce viable, motile spermatozoa that can successfully fertilize and activate the ovum and support subsequent embryo development. In males, spermatogenesis, testosterone production, and testicular function play key roles in fertility. On average, blood serum testosterone levels in a healthy bull will range from approximately 0.47 to 4.04 ng/mL (Santos et al., 2004; Souza et al., 2011; Bolado-Sarabia et al., 2018). Ranges will vary among breed, season, and age. Bulls, for example, that have been immuno-castrated will have lower blood serum testosterone levels of approximately 0.22 ng/mL (Bolado-Sarabia et al., 2018). These factors can be analyzed via a breeding soundness exam. Bulls must have normal sperm morphology and motility, semen samples should be free of foreign materials such as blood or urine, and the animal should be healthy and free disease, injuries, or major conformational defects. When turned out with females, bulls must have the libido to service the herd and the ability to mount the females.
Figure 2.
Factors influencing fertility. A multitude of factors can affect the fertility of an individual animal. Nutrition, age, environment, health, and management play a role in contributing to the success or hindrance of fertility.
In cows and heifers, fertility is the ability to enter the estrous cycle and successfully maintain pregnancy until calving and maintain calving pattern. Calving intervals tend to fall within a 12- to 13-month timeframe (Arbel et al., 2001). For beef cattle, females begin to re-cycle and be rebred within 45–60 d of calving to maintain this calving interval. In dairy cattle, it is important for females to rebreed approximately 60 d postpartum to maintain production goals. However, this may take longer time in some situations such as high producing dairy cattle. The estrous cycle is composed of four phases: estrus, metestrus, diestrus, and proestrus. Proestrus and estrus comprise the follicular phase and are characterized by an increase in estrogen production by the granulosa cells in the follicles. Estrus, commonly known as “standing heat,” occurs when the female is receptive to breeding, estrogen levels are heightened, and a dominant follicle releases an oocyte. For breeding with artificial insemination (AI), the highest conception rates are obtained during the last half of standing heat (O’Connor, 2016). Metestrus is the period between ovulation and the formation of the corpus luteum (CL). Diestrus is known for the characteristic presence of a fully functional CL on the ovary, high levels of progesterone of approximately ≤ 4.5 ng/mL serum, and a lack of reproductive behavior (Snook et al., 1971). Metestrus and diestrus comprise the luteal phase, with diestrus being the longest. If the female is not bred, PGF2α causes the CL to regress, and the female enters proestrus which resets the estrous cycle (Senger, 2012). It is critical to properly manage estrous cycles and the beginning of puberty because with an earlier onset of puberty and proper management of heifers, females can be reproductively active and efficient for a longer productive life as a cow and provide greater profits.
Hormones play critical roles in the endocrine regulation of the reproductive system and overall fertility of mammals. Puberty is initiated by Kisspeptin, produced by the hypothalamus, which stimulates the production and secretion of GnRH which in turn signals the release follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary (Ahmed et al., 2009). These glycoproteins target the gonads to support gametogenesis and induce certain reproductive behaviors (Kumar and Sait, 2011). In females, FSH targets the ovary to initiate the development of the ovarian follicle and stimulates granulosa cells to produce estradiol (E2), which induces estrus. Additionally, the CL is responsible for producing progesterone (P4) which is responsible for allowing the endometrium to accommodate the developing fetus. The PGF2α is secreted by the endometrium and targets the CL, resulting in regression and thus decreasing P4 secretion if attachment of the hatched blastocyst after the elongation has not occurred (Papich, 2016). The LH targets the theca interna and luteal cells to regulate ovulation in females (Shoham, 2002). In males, FSH targets the testes, specifically the Sertoli cells, which support spermatogenesis. The LH targets the Leydig cells to produce testosterone in males, which is responsible for libido, development of secondary sexual traits associated with puberty, and support of spermatogenesis (Chen et al., 2009).
The dairy cattle industry relies heavily on the use of assisted reproductive technologies for estrous synchronization, AI, and embryo transfer (ET) to improve productivity and efficiency of their herds. A dairy cow, on average, stays in the milking herd for approximately 305 d a year and spends 60 d in the dry herd. This number can vary from farm to farm and there are documented advantages to shorter and longer dry periods (Bar-Anan and Soller, 1979; Holmann et al., 1984; Dijkhuizen et al., 1985). Estrous synchronization is a valuable tool in bringing non-cycling, anestrous, females into heat. The Ovsynch protocol series of hormonal injections is the preferred estrous synchronization protocol in the dairy industry (Carvalho et al., 2018). For this protocol, the producer administers an injection of GnRH and inserts a CIDR on day 0 and then removes the CIDR and gives an injection of PGF2α on day 7 (Pursley et al., 1997). Forty eight hours after the injection of PGF2α, the producer administers a second injection of GnRH, followed by the timed AI 8 to 18 h after the second injection of GnRH (Pursley et al., 1997).
The Ovsynch protocol can eliminate the need for heat detection. This is due in part to the reductions of labor inputs by synchronizing all the cows onto the same breeding schedule while also reducing missed heats. However, producers should observe their herd for signs of estrus even when utilizing an Ovsynch protocol to reduce the potential for missed services. The Ovsynch protocol yields 30%–40% pregnancy rates and variation in success can be contributed to factors such as heat stress and parity (Cartmill et al., 2001; Tenhagen et al., 2004). To further bolster the effectiveness, producers may choose to administer two treatments of PGF2α 2 wk apart to encourage a full luteal regression before the onset of the Ovsynch protocol, thus improving subsequent pregnancy rates (Borchardt et al., 2018). Timed AI yields a higher pregnancy rate per treatment than breeding on visual signs of estrus only (Celeghini et al., 2008).
The beef cattle industry has been slow to adopt assisted reproductive technologies, with only 7.6% of beef cattle producers utilizing these programs (Lamb and Mercadante, 2016). The reluctancy to use assisted reproductive technologies by this sector of the industry has been attributed to variable pregnancy rates among other factors. The average pregnancy rate from the 7-d Co-Synch + CIDR timed AI is around 50% (Larson et al., 2006). These variable rates deter beef producers from adopting these technologies because herd bulls can achieve greater conception rates. However, the 50% pregnancy rate is the percentage of the herd getting pregnant on a single day, early in the breeding season. Therefore, calves are weaned at heavier weights and females are more likely to calve early, allowing them more time to be bred in the next breeding season, increasing profitability of the herd. The 7-d Co-Synch timed AI is the most common estrous synchronization protocol used among beef cattle producers (Geary et al., 2001). In a traditional 7-d Co Synch + CIDR timed AI protocol, an injection of GnRH is administered and a CIDR that supplies progesterone is inserted on day 0. On day 7, the CIDR is removed and an injection of PGF2α is administered. Sixty to 66 h after the injection of PGF2α and CIDR removal, the producer can perform timed AI and administer GnRH to ensure the timing of ovulation. This protocol eliminates the need for heat detection requires the producer to work their cattle through the chute only 3 times, and yields pregnancy rates comparable to Ovsynch (Pursley et al., 1997; Geary et al., 2001).
Additionally, it is important to know that cattle that are predominantly Bos indicus or Bos indicus-influenced will not respond as well to hormone protocol compared to Bos taurus cattle. Other protocols have been developed for Bos indicus breeds and crosses (Sá Filho et al., 2009; Oliveira et al., 2019; Madureira et al., 2020). Beef cattle producers who have adopted assisted reproductive technological protocols are often elite seedstock and club calf producers. In other countries, assisted reproductive technologies such as estrous synchronization, timed AI, ET, and somatic cell nuclear transfer (SCNT) are more widely adopted compared to those in the United States.
Growth and development
Economically efficient cattle production is dependent on the rates of fetal development, growth, age at puberty, and development of desirable carcass traits; all of which are under the influence of the GH axis (Armstrong et al., 1995; Jenkinson et al., 1999). The loop of this axis starts at the hypothalamus, where GHRH is secreted and travels to the anterior pituitary to stimulate the release of GH (Carter-Su et al., 1996). When GH reaches the liver and other target tissues, it stimulates the secretion of IGF-I (Laron, 2001). The IGF-I signals its target tissues to promote bone proliferation, muscle development and growth, and the breakdown of adipose tissue (Carter-Su et al., 1996). The IGF-I is produced throughout life, but it is at its greatest synthesis and secretion levels during periods of growth. Once IGF-I reaches a threshold concentration in circulating blood, they signal the hypothalamus to secrete growth hormone-inhibiting hormone (GHIH; also called somatostatin) which decreases or stops the release of GH from the anterior pituitary (Jenkinson et al., 1999). During animal growth, cells in tissues and organs, including bones and muscles, proliferate and change the body weight, composition, and fat deposition in animals.
The maintenance and regulation of the GH axis is directly responsible for the process of growth in the body (Devesa et al., 2016). It has been previously demonstrated that growing cattle immunized with a GHRH antagonist experienced a decreased rate of gain, reduced skeletal muscle, increased fat deposition, and decreased feed efficiency (Simpson et al., 1991). In the cattle industry, producers expect cattle to consistently reach certain body weights at specific time points in the production process. For example, d205 weight, yearling weight, and average daily gain are common variables used by producers to determine input costs versus potential value of their cattle. At these time points, the concentrations of GH in the blood of these animals are greater due to GH being elevated in early life and puberty when rapid growth occurs. The influence of the GH axis during fetal development is still unclear. Exogenous GH has been demonstrated to increase fetal weights during late gestation, but placental size was not altered, suggesting that GH plays a vital role in late gestation fetal growth (Jenkinson et al., 1999). Cows supplemented with GH in the third trimester had increased levels of GH in their colostrum, which resulted in calves absorbing more growth factors and hormones (Phomvisith et al., 2017). The concentrations of GH in colostrum affect calf growth and development beyond puberty. GH is responsible for body composition, more specifically the bone: muscle: fat ratios which ultimately defines the value of an animal’s carcass at slaughter.
In the beef industry, hormonal implants are used in both yearling stocker operations and feedlots to increase the rate of growth and improve body composition in finishing steers and heifers (Stewart, 2013). Implants contain a mixture of synthetic estrogen and testosterone, such as zeranol and trenbolone acetate (Stewart, 2013). These exogenous hormones upregulate production of GH in implanted cattle, which leads to quicker closure of bone plates and therefore allows the animal to begin putting on muscle and formation of desirable fat deposits, such as intramuscular fat or marbling (Meinhardt and Ho, 2006). Cattle treated with these implants consistently have increased average daily weight gain and improved muscle to bone growth ratio, larger rib-eye area, and a leaner carcass (Huck et al., 1991). Androgenic implants are used in heifers, estrogenic products are used in steers to increase feed efficiency and daily gain, up to 5%–15% and 25%, respectively (Perry et al., 1991; Dunshea et al., 2005). Moreover, estrogenic or androgenic implants play a significant role on pre-weaning growth of cattle. Beta agonists manipulate the metabolism of fat by promoting the breakdown and usage of fat, therefore decreasing the fat composition of the carcass, leading to a leaner, more desirable cut (Muir, 1988). Growth promoters increase protein synthesis in the muscle and decrease rates of fat deposition in muscle. Additionally, growth hormones can impact meat palatability and tenderness score, positively or negatively. The manipulation of GH through alternate pathways can be beneficial in increasing the profitability of an animal. However, due to recent consumer concerns, less invasive and more natural mechanisms of altering growth need to be explored.
The demand for high quality food continues to rise but the amount of land for production is declining. This strain on the environment and limited resources also raises the concern of greenhouse gas emissions created by cattle production. Research has shown that cattle actually produce less greenhouse gas emissions as compared to the combustion of the feedstuffs or agricultural byproducts they consume (Russomano et al., 2012). This serves the environment well and demonstrates the ecological importance of supporting the livestock industry due to the utilization of by-products that would ultimately be wasted. Growth-promoting technologies have been used for many years in the cattle industry to enhance feed efficiency and weight gain with initial investigations in the efficacy of growth promotors, like somatostatins, beginning as early as the 1920s in rodent models and livestock models in the 1950s (Turman and Andrews, 1955; Brumby, 1959; Courtheyn et al., 2002). Use of exogenous hormones may aid in the reduction of greenhouse gas released by increasing productivity of livestock which would reduce herd sizes. Research is needed in this area to provide a greater insight into the potential for success of exogenous hormones on the reduction of greenhouse gas production by cattle.
Producers can increase the production efficiency of their cattle by targeting the GH axis. Currently, there are implants, feed additives, and exogenous GH injections available to cattle producers to increase animal growth. The rBST is an injectable synthetic form of GH that increases milk yields in dairy cattle by providing more nutrients to the mammary gland stemming from the elevated levels of circulating IGF-I (Bauman and Currie, 1980). Treating dams with rBST 124–220 d post-partum leads to increased milk yield and heavier calves being weaned without compromising reproductive efficiency (Armstrong et al., 1995). In 1993, the FDA approved Monsanto’s rBST, Posilac, for commercial use. In the first 20 yr of its approval, over 35 million dairy cows received rBST (Collier and Bauman, 2014). Due to consumer misconceptions, the usage of rBST in the dairy industry has become stigmatized, thus drastically decreasing usage in recent years (Kecinski et al., 2018). Contrary to the negative connotations, rBST could be of great value when it comes to the reduction of feed additives for dairy cattle (Capper et al., 2008). It has been demonstrated that the use of rBST can increase milk yields without hindering pregnancy rates in Holstein cattle under heat stress conditions (Jousan et al., 2007). This increased productivity means that there is potential for a reduction in herd size, thus reducing the carbon footprint of the herd.
Animal health
The health of cattle depends on a diverse array of environmental factors including nutrition, reproduction, and management styles. Animals become ill when the pathogen load exceeds what their immune system is capable of thwarting. The structure of the ruminant placenta restricts calves to minimal antibodies at birth, which makes it imperative that calves receive quality colostrum within 24 h of birth. The main immunoglobulin found in colostrum is immunoglobulin G1 (IgG1), along with other components such as cytokines and leukocytes (Chase et al., 2008). Once ingested, high levels of corticosteroid allow the colostrum to be absorbed by the calves’ intestinal cells, initiating the early developmental of their immune system (Sangild, 2003). Leukocytes work to develop antigen-presenting cells, important for immune responses to pathogens and vaccines. However, newborn calves do not receive all the immunoglobulins from maternal colostrum that are necessary to develop full immunity; therefore, vaccines are administered within specific time-frames to aid in immune system development (Weaver et al., 2000).
When cattle experience changes in their homeostatic balance, they become stressed. Stress is defined as behavioral and physiological changes caused by stimuli that affect an organism’s well-being (von Borell, 2001). Exposure to stressful environments triggers hormonal responses from the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis (Stewart et al., 2010). When stressed, the sympathetic nervous system, via the ANS, is activated and results in the “fight or flight” response. This response could be short or fast in its nature and is driven by the catecholamines (epinephrine, norepinephrine, and dopamine) synthesized in the adrenal medulla. Epinephrine and norepinephrine have a half-life of approximately 0.25 to 1.0 min and are responsible for increasing energy supplies, heart rate, respiratory rate, vasoconstriction, and vasodilation (Jones and Robinson, 1975; Stewart et al., 2010).
Detecting the presence of cortisol in animal products and secretion concentration in live animals can also determine whether animals were subjected to chronic levels of stress (Nemeth et al., 2016; Heimbürge et al., 2019). The parvocellular neurons in the hypothalamus secrete CRH and arginine vasopressin (VP) to initiate the HPA cascade (Whitnall, 1993). The CRH and VP cause the release of ACTH from the anterior pituitary that binds to the melanocortin receptors in the cortex of the adrenal glands to produce glucocorticoids. The main glucocorticoid is known as cortisol, and it acts on liver, muscles, and adipose tissue to supply the organism with fuel during stressful conditions. In comparison to the brief half-life of catecholamines, cortisol plasma clearance takes a longer amount of time. In one study, cows returned to basal cortisol concentrations 120 min after exposure to the milking machine (Negrão et al., 2004). This type of investigation strengthens the value of using salivary and plasma cortisol measurements as indicators of fear, stress, pain, or discomfort in cattle.
Adversely, an increase in glucocorticoids is related to a decrease in the immune system function. It has been found that calves that are suddenly weaned experience an increase in plasma cortisol and noradrenaline concentrations, along with a corresponding reduction of the immune function (Hickey et al., 2003). Calves exposed to early stress could experience compromised immune function; therefore, research is evaluating the use of non-steroidal anti-inflammatory drugs (NSAID) during stressful conditions to reduce pain or discomfort and support the immune system. For example, Meloxicam administration during knife castration or castration and branding was shown to mitigate the pain related to these management practices in cattle (Meléndez et al., 2018). In addition, pregnant dairy cows exposed to heat stress during late gestation had calves with lower birth weights. These offspring, when mature, produced less milk in the first lactation as compared to the offspring from non-heat stressed cows (Monteiro et al., 2016). These results suggest that even exposure to stress while in utero has detrimental consequences upon offspring which could be related to an increase in stress hormones during gestation.
Higher levels in stress hormones, such as cortisol, epinephrine, norepinephrine, and dopamine, influence cattle physiology and behavior, such as a temperament-display of a more nervous behavioral pattern as evaluated by their exit and pen scores (Sánchez-Rodríguez et al., 2013). Additionally, previous research has indicated that temperamental beef cows have higher cortisol levels and reduced pregnancy and calving rates as compared to less temperamental cows (Cooke et al., 2012). Previous studies have demonstrated an increase in fetal plasma cortisol concentrations caused by maternal uterine blood flow reduction (Bocking and Harding, 1986; Sue-Tang et al., 1992). An increase in this corticoid initiates parturition by enabling the placenta to synthesize PGF2α, leading to CL regression and myometrial contractions (Senger, 2012). Such an increase in fetal cortisol could cause pregnancy termination at any stage of gestation (Giri et al., 1990). Animals exposed to hot environments have increased cortisol concentrations, causing higher body temperatures (Dobson and Smith, 2000). Animals with higher body temperatures showed a reduction in uterine blood flow and an increase in peripheral blood flow in order to increase heat dissipation (West, 2003). Studies evaluating cow-calf separation have shown that calves tend to have higher cortisol levels than that of their dams when removed from one another (Acevedo et al., 2005; Newberry and Swanson, 2008). However, when restricted suckling protocols are utilized, calves can acclimate to their routines quickly and stress parameters decrease (Rasby, 2007; Orihuela and Galina, 2019). By using this technique in conjunction with the use of familiar surroundings and/or pen mates, calves are able to transition away from their dams in a less stressful manner (Færevik et al., 2007; Newberry and Swanson, 2008). Thus, an increase in plasma cortisol concentrations can have profound influences on reproductive health, performance, and behavior.
Production
Hormones play a vital role in the growth, development, reproduction, and social behavior of both beef and dairy cattle production sectors. Carcass traits are economically important and include quality grade, yield grade, ribeye area, and back fat thickness. Quality grade is determined by the amount of marbling on the surface of the ribeye between the 12th and 13th ribs (Hale et al., 2013). Marbling, or intramuscular fat, is the dispersion of fat within lean tissue (Griffin and Savell, 2018). The more marbling the ribeye contains, the higher quality grade the carcass receives. Yield grade is the estimate of trimmed retail cuts that a carcass is likely to yield. It is determined by external fat thickness, percentage of internal fat, ribeye size, and carcass weight. Yield grades are denoted on a 1 to 5 scale (Hale et al., 2013). The lesser the yield grade, the leaner the carcass. Carcasses with a yield grade higher than 3 are oftentimes discounted. Ribeye area is the surface area of the longissimus dorsi muscle which is sectioned between the 12th and 13th ribs. This area is the last location on the carcass where muscle and fat deposited. Backfat thickness is measured as the amount of fat between the 12th and 13th ribs. The muscle of beef cattle is composed of water, high-quality protein, carbohydrates, B vitamins, iron, zinc, phosphorous, selenium, omega-3 polyunsaturated fats, riboflavin, and pantothenic acid. Red meat generally is low in fat content, it is highly digestible protein, and it contains many vitamins and minerals that are essential for human health (Holt et al., 2007).
Composition of meat varies based on the animal’s breed, age, gender, plan of nutrition, and environment. However, carcass traits can be manipulated using hormones which may improve qualities for ever-changing consumer preferences. Growth hormone is essential for growth and development and ultimately impacts carcass traits because GH increases muscle mass and strength, bone density and cardiac output, and decreases adipose tissue accretion (Velloso, 2008; Bartke et al., 2013). Estrogen, progesterone, testosterone, zeranol, and trenbolone acetate are hormones that are approved for use in implants for cattle in the beef industry by the FDA. These hormones promote muscle growth at the expense of fat deposition with less feed consumed, thereby allowing cattle producers to produce leaner beef with fewer resources (Johnson et al., 2013). They reduce production cost and number of animals required, allowing for price competitiveness of beef with other forms of protein. Implants increase growth rates 8% to 20%, improve feed efficiency 5% to 20%, as well as increasing lean tissue mass by 3% to 10%. The hormones are metabolized and excreted by the animal, ensuring that there are no residues in edible tissues (Johnson et al., 2013). When the time comes for slaughter, there can be issues with dark cutting. When a carcass cuts dark, there has been a great amount of physical stress to that animal prior to slaughter. The muscles become depleted of glycogen. Under normal circumstances, glycogen would convert to lactic acid and allow for the pH to drop to a more acidic level. Without enough glycogen, the conversion cannot take place and allow for the pH to change. This alters the meat quality, making it unacceptable for human consumption.
Milk production begins with the growth of the mammary gland, which is stimulated by GH, prolactin, estrogen, and progesterone (Lacasse et al., 2016). GH and prolactin facilitate the rapid proliferation of mammary cells to prepare the gland for lactation and increases blood flow and glucose levels in the mammary tissue. Oxytocin facilitates the letdown of milk when the udder is stimulated by a calf suckling or by a producer that is cleaning and prepping the teats prior to milking (Akers and Lefcourt, 1984). Nutrients from non-mammary tissues are redirected to the mammary system by GH, prolactin, and leptin. Prolactin increases the absorption of calcium and facilitates the uptake of long-chain fatty acids for milk fat synthesis (Ajibade et al., 2010). Leptin plays a regulatory role in energy metabolism by stimulating energy expenditure and suppressing food intake (Block et al., 2001; Morrison et al., 2001; Liefers et al., 2003). Produced by adipose cells, high levels of leptin stimulate the hypothalamus to suppress hunger.
Hormones in Food Animals and Human Health
Since the early 1950s, exogenous anabolic promoters have been used to improve feed efficiency, weight gain, and milk yield of cattle in the U.S. Exogenous growth promoters including anabolic hormones (androgens), estrogens, glucocorticoids, progestogens, and synthetic compounds, such as TBA and MGA (Yang et al., 2009). For instance, MGA is a feed additive that is fed to suppress estrus cyclicity of heifers in a feedlot setting. Once these exogenous hormones are administered, the cow’s system utilizes the time-released effect by absorption through epithelial cells of the ear, or muscle tissue in the neck, thence to the blood where it is transported to various target tissues and organs. Once these exogenous hormones reach the target tissues or organs, the tissues respond to produce the outcome of increased growth, milk yield, or fat deposition in cattle. From the application of exogenous hormone promoters in the beef and dairy industries, consumers express growing concern that the hormones may be impacting the environment, quality of food products, and human health (Wandel and Bugge, 1997).
As a result of consumer concerns on the uses of exogenous hormones in the livestock industry, there has been a growing concern of residual hormones in beef and milk and their effects on human health and the environment. These health concerns include a slightly younger age of pubertal onset in girls and an increase in estrogen-related diseases attributed to the excessive consumption of animal proteins like milk and meat (Yermachenko and Dvornyk, 2014). However, research has demonstrated that the tissues of calves, steers, and heifers treated with exogenous growth hormone promoters, such as estradiol, testosterone, and progesterone, when given at proper administration levels, do not lead to toxic or harmful levels of hormonal residues in their tissues (Hartmann et al., 1998; Jeong et al., 2010). For example, the average man produces 210 to 480 μg/d of testosterone (Joint FAO/WHO Expert Committee on Food Additives, 1999). In nonhormone–treated animals, residues will range from about 0.006 to 0.029 μg/kg, whereas hormone-treated animals will have residues ranging from 0.031 to 0.360 (Paris et al., 2006). When treated meat is ingested, the present residues will range from about 0.0093 to 0.108 μg/d (Paris et al., 2006). These research findings indicate that effects of implanting, orally supplementing, or injecting beef and dairy cattle with these exogenous hormones apparently have no carry-over effects on human health. The use of these exogenous hormones in the livestock industry is not the cause of human disease or the effects on human health.
Due to the current market trend towards organic products, producers are having to eliminate the use of hormones in their herds to ensure the profitability of their milk and milk products (Kolodinsky, 2008). Milk yield is the most monitored trait in the dairy production sector. Hormones are necessary for milk production for a number of reasons. Milk is composed water, fats, proteins, lactose, vitamins, and minerals, including the most abundant ones of calcium and potassium. The diet of the animal greatly affects the composition of milk. The rBST positively affects mammary growth and increases milk production in dairy cattle. rBST has been deemed safe by the FDA both for the cattle receiving rBST and for human consumption; however, it is no longer widely used due to consumer misconceptions. In a survey conducted in 2012, researchers found that consumers were willing to pay more for milk that was rBST-free, but surprisingly the labeling of the milk did not cause a differential effect on how consumers valued the half-gallon size of milk (Wolf et al., 2011).
Use of exogenous hormones, particularly rBST, contributes little to no negative effects on human health. The way these exogenous hormones are administered, how the human body digests them, and the recommended withdrawal periods established by the USDA and the FDA for each of these growth hormone promoters prevents human health effects. Many these hormones are delivered in a pellet form which are implanted under the skin of the ear and are time-released. The highest concentrations of these hormones are in the ear and are slowly released throughout the animal’s body for 90 to 120 d. Once this time period is up, normally during the feedlot phase of the cattle industry, cattle must go through a withdrawal period to allow the residues to be metabolized and excreted from the animal’s system prior to harvest (NRC, 1999). In the dairy industry, the FDA has specified no withdrawal period for rBST or when human consumption of meat and milk can occur, thus indicating that there is no scientific evidence of detrimental effects on human health because it is not bioactive upon oral ingestion (WHO, 2013; FDA, 2019). The rBST is a bulky protein that is easily broken down by digestive enzymes in the human gastrointestinal system (FDA, 2019). Furthermore, the human system will not recognize rBST because of its bulky structure as a large protein, and that is distinctly different from human somatotropin (WHO, 2013; FDA, 2019). In conclusion, the human body can metabolize this protein and the residue is not utilized due to its foreign nature. Even as an exogenously supplemented hormone, it does not have any detrimental effects on human development and health.
The use of hormones in beef and milk production is specifically regulated by the FDA which approves an animal drug or hormone after studies have shown that the food products (milk and meat) from treated animals is safe for human consumption, and that the drug/hormone does not harm treated animals, nor the environment (FDA, 2019). Even further, the dairy industry concurring with consumer preferences has implemented a “zero tolerance level,” with residual artificial hormones or antibiotics in cow’s milk. Milk is regularly tested for minute amounts of artificial hormones and antibiotics before being pasteurized and homogenized to produce dairy consumables: milk, cheese, yogurt, butter, etc. This is not to say that there are no endogenous hormones secreted by the cow itself. Some of these naturally occurring hormones include bovine somatotrophin (bST, or otherwise referred to as GH), E2, P4, oxytocin, prolactin, and IGF-1. In 2018, the FDA determined that hormone concentrations are low in milk and the hormones present are easily digestible by humans. Additionally, milk contains very low concentrations of endogenous hormones, which are not at high enough concentration to elicit detrimental effects on human life or health. However, one of the most predominant estrogens found in cow’s milk, estrone, has been quantified in low quantities in milk as compared to other more concentrated products such as butter or yogurt (Hartmann et al., 1998; Ganmaa et al., 2004).
Conclusions
Sustainable, efficient, and profitable production of cattle, the key livestock for meat and milk production, is essential for feeding the globe. Synthesized within and secreted from the endocrine glands, hormones are critical for animal life in addition to the production of cattle with economically important traits and wholesome meat and milk. An increase of stress hormones in livestock production leads to a decrease in meat quality and milk yield and affects behavior and temperament resulting in problems in farm management and animal welfare. So, hormones can be measured to determine the status of animal welfare and to develop the best production systems. Also, hormones can be used to increase quantity and quality of meat and milk. For example, GH is an economically important hormone that aids in lean muscle accretion, decreases in adipose tissue deposition, and increases in overall efficiency of the animal. Further, GnRH, P4, and PGF2α can be used in combination to improve reproductive efficiency through synchronizing estrus. Commercialized forms of hormone products such as Factrel, CIDR, and Lutalyse can aid in decreasing labor costs. Through hormone biotechnology, efficiency of cattle reproduction and production as well as product quality can all be increased to improve food security on a global scale.
Acknowledgments
Erdogan Memili is a participant on Multistate Project NE1727: Influence of Ovary, Uterus, and Embryo on Pregnancy Success in Ruminants.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- VP
arginine vasopressin
- AI
artificial insemination
- ANS
autonomic nervous system
- CL
corpus luteum
- CRH
corticotropin-releasing hormone
- ET
embryo transfer
- E2
estradiol
- FSH
follicle stimulating hormone
- FFA
free fatty acids
- GnRH
gonadotropin releasing hormone
- GH
growth hormone
- GHIH
growth hormone-inhibiting hormone
- GHRH
growth hormone-releasing hormone
- HSL
hormone sensitive lipase
- HPA
hypothalamic–pituitary–adrenal axis
- IgG1
immunoglobulin G1
- IVF
in vitro fertilization
- IGF-1
insulin-like growth factor-1
- LH
luteinizing hormone
- MGA
melengestrol acetate
- NSAID
non-steroidal anti-inflammatory drugs
- P4
progesterone
- CIDR
progestin controlled internal drug release
- PGF2α
prostaglandin F-2α
- rBST
recombinant bovine somatotropin
- SCNT
somatic cell nuclear transfer
- TRH
thyrotropin-releasing hormone
- TBA
trenbolone acetate
- T3
triiodothyronine
- USDA
United States Department of Agriculture
- FDA
United States Food and Drug Administration
Contributor Information
Holly C Evans, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Elanie F Briggs, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Randy H Burnett, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Zully E Contreras-Correa, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Morgan A Duvic, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Lacey M Dysart, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Alicia A Gilmore, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Riley D Messman, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Dana Reid, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Muhammet Rasit Ugur, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Abdullah Kaya, Department of Reproduction and Artificial Insemination, Selcuk University, Konya, Turkey; Department of Animal and Dairy Sciences, University of Wisconsin-Madison, Madison, WI, USA.
Erdogan Memili, Department of Animal and Dairy Sciences, Mississippi State University, Mississippi State, MS 39762, USA.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Acevedo, N., Hernández C., Orihuela A., Lidfors L. M., and Berg C.. . 2005. Effect of restricted suckling or temporal weaning on some physiological and behavioural stress parameters in zebu cattle (Bos indicus). Asian-Australas. J. Anim. Sci. 18:1176–1181. doi: 10.5713/ajas.2005.1176. [DOI] [Google Scholar]
- Ahmed, A. E., Saito H., Sawada T., Yaegashi T., Yamashita T., Hirata T., Sawai K., and Hashizume T.. . 2009. Characteristics of the stimulatory effect of Kisspeptin-10 on the secretion of luteinizing hormone, follicle-stimulating hormone and growth hormone in prepubertal male and female cattle. J. Reprod. Dev. 55:650. doi: 10.1262/jrd.20255. [DOI] [PubMed] [Google Scholar]
- Ajibade, D. V., Dhawan P., Fechner A. J., Meyer M. B., Pike J. W., and Christakos S.. . 2010. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology 151:2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers, R. M., and Lefcourt A. M.. . 1984. Effect of presence of calf on milking-induced release of prolactin and oxytocin during early lactation of dairy cows. J. Dairy Sci. 67:115–122. doi: 10.3168/jds.S0022-0302(84)81274-6. [DOI] [PubMed] [Google Scholar]
- Arbel, R., Bigun Y., Ezra E., Sturman H., and Hojman D.. . 2001. The effect of extended calving intervals in high lactating cows on milk production and profitability. J. Dairy Sci. 84:600–608. doi: 10.3168/jds.S0022-0302(01)74513-4. [DOI] [PubMed] [Google Scholar]
- Armstrong, J. D., Harvey R. W., Poore M. A., Simpson R. B., Miller D. C., Gregory G. M., and Hartnell G. F.. . 1995. Recombinant bovine somatotropin increases milk yield and calf gain in diverse breeds of beef cattle: associated changes in hormones and indices of metabolism. J. Anim. Sci. 73:3051–3061. doi: 10.2527/1995.73103051x. [DOI] [PubMed] [Google Scholar]
- AVMA. 2018. Animal welfare: what is it? Available from: https://www.avma.org/resources/animal-health-welfare/animal-welfare-what-it
- Bar-Anan, R., and Soller M.. . 1979. The effects of days-open on milk yield and on breeding policy post partum. Anim. Sci. 29:109–119. doi: 10.1017/s0003356100012204. [DOI] [Google Scholar]
- Barros, J., Botteon R., Spíndola B., Oliveira E. B., Pereira J., and Botteon P.. . 2016. Action of exogenous oxytocin on stress modulation in crossbred Red Angus cows. Semin. Agrar. 37:3209–3213. doi: 10.5433/1679-0359.2016v37n5p3209. [DOI] [Google Scholar]
- Bartke, A., Sun L. Y., and Longo V.. . 2013. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman, D., and Currie W.. . 1980. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 63:1514–1529. doi: 10.3168/jds.S0022-0302(80)83111-0. [DOI] [PubMed] [Google Scholar]
- Block, S. S., Butler W. R., Ehrhardt R. A., Bell A. W., Van Amburgh M. E., and Boisclair Y. R.. . 2001. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance. J. Endocrinol. 171:339–348. doi: 10.1677/joe.0.1710339. [DOI] [PubMed] [Google Scholar]
- Bocking, A. D., and Harding R.. . 1986. Effects of reduced uterine blood flow on electrocortical activity, breathing, and skeletal muscle activity in fetal sheep. Am. J. Obstet. Gynecol. 154:655–662. doi: 10.1016/0002-9378(86)90625-3. [DOI] [PubMed] [Google Scholar]
- Bolado-Sarabia, J. L., Pérez-Linares C., Figueroa-Saavedra F., Tamayo-Sosa A. R., Barreras-Serrano A., Sánchez-López E., García-Reynoso I. C., Ríos-Rincón F. G., Rodríguez-Poché M. Y., García-Vega L. A., . et al. 2018. Effect of immunocastration on behaviour and blood parameters (cortisol and testosterone) of Holstein bulls. Aust. J. Vet. Sci. 50:77–81. doi: 10.4067/S0719-81322018000200077. [DOI] [Google Scholar]
- Borchardt, S., Pohl A., Carvalho P. D., Fricke P. M., and Heuwieser W.. . 2018. Short communication: effect of adding a second prostaglandin F 2α injection during the Ovsynch protocol on luteal regression and fertility in lactating dairy cows: a meta-analysis. J. Dairy Sci. 101:8566–8571. doi: 10.3168/jds.2017-14191. [DOI] [PubMed] [Google Scholar]
- von Borell, E. H. 2001. The biology of stress and its application to livestock housing and transportation assessment. J. Anim. Sci. 79:E260. doi: 10.2527/jas2001.79E-SupplE260x. [DOI] [Google Scholar]
- Bourdon, R., and Brinks J.. . 1986. Scrotal circumference in yearling Hereford bulls: adjustment factors, heritabilities and genetic, environmental and phenotypic relationships with growth traits. J. Anim. Sci. 62:958–967. doi: 10.2527/jas1986.624958x. [DOI] [PubMed] [Google Scholar]
- Brambell, F. 1965. Report of the technical committee to enquire into the welfare of animals kept under intensive livestock husbandry systems. Her Majesty’s Stationary Office, London. [Google Scholar]
- Brumby, P. J. 1959. The influence of growth hormone on growth in young cattle. New Zeal. J. Agric. Res. 2:683–689. doi: 10.1080/00288233.1959.10422827. [DOI] [Google Scholar]
- Capper, J. L., Castañeda-Gutiérrez E., Cady R. A., and Bauman D. E.. . 2008. The environmental impact of recombinant bovine somatotropin (rbST) use in dairy production. Proc. Natl. Acad. Sci. 105:9668 LP–9669673. doi: 10.1073/pnas.0802446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su, C., Schwartz J., and Smit L. J.. . 1996. Molecular mechanism of growth hormone action. Annu. Rev. Physiol. 58:187–207. doi: 10.1146/annurev.ph.58.030196.001155. [DOI] [PubMed] [Google Scholar]
- Cartmill, J. A., El-Zarkouny S. Z., Hensley B. A., Rozell T. G., Smith J. F., and Stevenson J. S.. . 2001. An alternative AI breeding protocol for dairy cows exposed to elevated ambient temperatures before or after calving or both. J. Dairy Sci. 84:799–806. doi: 10.3168/jds.S0022-0302(01)74536-5. [DOI] [PubMed] [Google Scholar]
- Carvalho, P. D., Santos V. G., Giordano J. O., Wiltbank M. C., and Fricke P. M.. . 2018. Development of fertility programs to achieve high 21-day pregnancy rates in high-producing dairy cows. Theriogenology 114:165–172. doi: 10.1016/j.theriogenology.2018.03.037. [DOI] [PubMed] [Google Scholar]
- Castro, A. V. B., and Melmed S.. . 2003. Growth regulation: clinical aspects of GHRH. In: Henry H. L. and A. W. B. T.-E. of H. Norman, editors. Encyclopeida of Hormones. Academic Press, New York. p. 226–234. Available from: http://www.sciencedirect.com/science/article/pii/B0123411033001340 [Google Scholar]
- Celeghini, E. C. C., de Arruda R. P., de Andrade A. F. C., Nascimento J., Raphael C. F., and Rodrigues P. H. M.. . 2008. Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim. Reprod. Sci. 104:119–131. doi: 10.1016/J.ANIREPROSCI.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chase, C. C. L., Hurley D. J., and Reber A. J.. . 2008. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. North Am. Food Anim. Pract. 24:87–104. doi: 10.1016/J.CVFA.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Ge R. -S., and Zirkin B. R.. . 2009. Leydig cells: from stem cells to aging. Mol. Cell. Endocrinol. 306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier, R. J., and Bauman D. E.. . 2014. Update on human health concerns of recombinant bovine somatotropin use in dairy cows. J. Anim. Sci. 92:1800–1807. doi: 10.2527/jas.2013-7383. [DOI] [PubMed] [Google Scholar]
- Cooke, R. F., Bohnert D. W., Cappellozza B. I., Mueller C. J., and Delcurto T.. . 2012. Effects of temperament and acclimation to handling on reproductive performance of Bos taurus beef females. J. Anim. Sci. 90:3547–3555. doi: 10.2527/jas.2011-4768. [DOI] [PubMed] [Google Scholar]
- Courtheyn, D., Le Bizec B., Brambilla G., De Brabander H. F., Cobbaert E., Van de Wiele M., Vercammen J., and De Wasch K.. . 2002. Recent developments in the use and abuse of growth promoters. Anal. Chim. Acta 473:71–82. doi: 10.1016/S0003-2670(02)00753-5. [DOI] [Google Scholar]
- De Freslon, I., Strappini A., Soto-Gamboa M., and Gallo C.. . 2014. Characterisation of behavioural reactivity in steers during handling and its relationship with blood cortisol, bruising and meat pH. Arch. Med. Vet. 46:229–237. doi: 10.4067/S0301-732X2014000200008. [DOI] [Google Scholar]
- De Koster, J., Nelli R. K., Strieder-Barboza C., de Souza J., Lock A. L., and Contreras G. A.. . 2018. The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows. Sci. Rep. 8:13378. doi: 10.1038/s41598-018-31582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devesa, J., Almengló C., and Devesa P.. . 2016. Multiple effects of growth hormone in the body: is it really the hormone for growth? Clin. Med. Insights Endocrinol. Diabetes. 9:CMED.S38201. doi: 10.4137/CMED.S38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen, A. A., Stelwagen J., and Renkema J. A.. . 1985. Economic aspects of reproductive failure in dairy cattle. I. Financial loss at farm level. Prev. Vet. Med. 3:251–263. doi: 10.1016/0167-5877(85)90020-0. [DOI] [Google Scholar]
- Djoković, R., Cincović M., Belić B., Toholj B., Davidov I., and Hristovska T.. . 2015. Relationship between blood metabolic hormones, metabolites, and energy balance in simmental dairy cows during peripartum period and lactation. Pak. Vet. J. 35:163–167. [Google Scholar]
- Dobson, H., and Smith R. F.. . 2000. What is stress, and how does it affect reproduction? Anim. Reprod. Sci. 60–61:743–752. doi: 10.1016/S0378-4320(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Dunshea, F. R., Souza D. N. D. Õ., Pethick D. W., Harper G. S., and Warner R. D.. . 2005. MEAT effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Sci. 71(1):8–38. doi: 10.1016/j.meatsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Eppard, P. J., Veenhuizen J. J., Cole W. J., Comens-Keller P. G., Hartnell G. F., Hintz R. L., Munyakazi L., Olsson P. K., Sorbet R. H., White T. C., . et al. 1996. Effect of bovine somatotropin administered to periparturient dairy cows on the incidence of metabolic disease. J. Dairy Sci. 79:2170–2181. doi: 10.3168/JDS.S0022-0302(96)76593-1. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (1999 : Rome, Italy), World Health Organization & Food and Agriculture Organization of the United Nations. 2000. Evaluation of certain veterinary drug residues in food: fifty-second report of the Joint FAO/WHO Expert Committee on Food Additives.World Health Organization. https://apps.who.int/iris/handle/10665/42251. ISBN: 9241208937; ISSN: 0512-3054. [Google Scholar]
- Færevik, G., Andersen I. L., Jensen M. B., and Bøe K. E.. . 2007. Increased group size reduces conflicts and strengthens the preference for familiar group mates after regrouping of weaned dairy calves (Bos taurus). Appl. Anim. Behav. Sci. 108:215–228. doi: 10.1016/j.applanim.2007.01.010. [DOI] [Google Scholar]
- FAO. 2006. World agriculture: towards 2030/2050. Interim report, Global Perspective Studies Unit. [Google Scholar]
- FDA. 2019. Bovine somatotrophin (BST). Available from: https://www.fda.gov/animal-veterinary/product-safety-information/bovine-somatotropin-bst
- Flores, J., García J. E., Mellado J., Gaytán L., de Santiago Á., and Mellado M.. . 2019. Effect of growth hormone on milk yield and reproductive performance of subfertile Holstein cows during extended lactations. Spanish J. Agric. Res. 17:11. doi: 10.5424/sjar/2019171-13842. [DOI] [Google Scholar]
- Freedgood, J., Hunter M., Dempsey J., and Sorenson A.. . 2020. Farms under threat: the state of the states. Available from: http://farmlandinfo.org/publications/farms-under-threat-the-state-of-the-states/
- Ganmaa, D., Qin L. -Q., Wang P. -Y., Tezuka H., Teramoto S., and Sato A.. . 2004. A two-generation reproduction study to assess the effects of cows’ milk on reproductive development in male and female rats. Fertil. Steril. 82:1106–1114. doi: 10.1016/j.fertnstert.2004.05.073. [DOI] [PubMed] [Google Scholar]
- Geary, T. W., Salverson R. R., and Whittier J. C.. . 2001. Synchronization of ovulation using GnRH or hCG with the CO-Synch protocol in suckled beef cows. J. Anim. Sci. 79:2536. doi: 10.2527/2001.79102536x. [DOI] [PubMed] [Google Scholar]
- Giri, S. N., Emau P., Cullor J. S., Stabenfeldt G. H., Bruss M. L., Bondurant R. H., and Osburn B. I.. . 1990. Effects of endotoxin infusion on circulating levels of eicosanoids, progesterone, cortisol, glucose and lactic acid, and abortion in pregnant cows. Vet. Microbiol. 21:211–231. doi: 10.1016/0378-1135(90)90033-R. [DOI] [PubMed] [Google Scholar]
- Grandin, T. 2000. Effect of animal welfare audits of slaughter plants by a major fast food company on cattle handling and stunning practices. J. Am. Vet. Med. Assoc. 216:848–851. doi: 10.2460/javma.2000.216.848. [DOI] [PubMed] [Google Scholar]
- Griffin, D., and Savell J.. . 2018. Understanding USDA beef quality grades. Texas A&M AgriLife Ext. Serv. Available from: https://meat.tamu.edu/files/2018/10/Understanding-USDA-beef-quality-grades.pdf [Google Scholar]
- Hale, D., Goodson K., and Savell J.. . 2013. USDA beef quality and yield grades. Texas A&M AgriLife Ext. Serv. Available from: https://meat.tamu.edu/beefgrading/ [Google Scholar]
- Hartmann, S., Lacorn M., and Steinhart H.. . 1998. Natural occurrence of steroid hormones in food. Food Chem. 62:7–20. doi: 10.1016/S0308-8146(97)00150-7. [DOI] [Google Scholar]
- Heimbürge, S., Kanitz E., and Otten W.. . 2019. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 270:10–17. doi: 10.1016/j.ygcen.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Hickey, M. C., Drennan M., and Earley B.. . 2003. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J. Anim. Sci. 81:2847–2855. doi: 10.2527/2003.81112847x. [DOI] [PubMed] [Google Scholar]
- Higgins, H. M., Ferguson E., Smith R. F., and Green M. J.. . 2013. Using hormones to manage dairy cow fertility: the clinical and ethical beliefs of veterinary practitioners. B. Kaltenboeck, editor. PLoS One 8:e62993. doi: 10.1371/journal.pone.0062993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller-Sturmhöfel, S., and Bartke A.. . 1998. The endocrine system: an overview. Alcohol Health Res. World 22:153–164. [PMC free article] [PubMed] [Google Scholar]
- Holmann, F. J., Shumway C. R., Blake R. W., Schwart R. B., and Sudweeks E. M.. . 1984. Economic value of days open for Holstein cows of alternative milk yields with varying calving intervals. J. Dairy Sci. 67:636–643. doi: 10.3168/jds.S0022-0302(84)81349-1. [DOI] [Google Scholar]
- Holt, W. V., O’Brien J., and Abaigar T.. . 2007. Applications and interpretation of computer-assisted sperm analyses and sperm sorting methods in assisted breeding and comparative research. Reprod. Fertil. Dev. 19:709. doi: 10.1071/RD07037. [DOI] [PubMed] [Google Scholar]
- Huck, G. L., Brandt R. T., Dikeman M. E., Simms D. D., and Kuhl G. L.. . 1991. Timing of trenbolone acetate implants on performance, carcass characteristics, and beef quality of finishing steer calves. Cattlemen’s Day, 1991, Kansas State University, Manhattan, KS. Kansas State University. Agricultural Experiment Station and Cooperative Extension Service; p. 90–92. [Google Scholar]
- Huertas, S., Kempener R., van Eerdenburg F., Huertas S. M., Kempener R. E. A. M., and Van Eerdenburg F. J. C. M.. . 2018. Relationship between methods of loading and unloading, carcass bruising, and animal welfare in the transportation of extensively reared beef cattle. Animals 8:119. doi: 10.3390/ani8070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas, S. M., van Eerdenburg F., Gil A., and Piaggio J.. . 2015. Prevalence of carcass bruises as an indicator of welfare in beef cattle and the relation to the economic impact. Vet. Med. Sci. 1:9–15. doi: 10.1002/vms3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, C. M. C., Min S. H., Mackenzie D. D. S., McCutcheon S. N., Breier B. H., and Gluckman P. D.. . 1999. Placental development and fetal growth in growth hormone-treated ewes. Growth Horm. IGF Res. 9:11–17. doi: 10.1054/GHIR.1998.0065. [DOI] [PubMed] [Google Scholar]
- Jeong, S. -H., Kang D., Lim M. -W., Kang C. S., and Sung H. J.. . 2010. Risk assessment of growth hormones and antimicrobial residues in meat. Toxicol. Res. 26:301–313. doi: 10.5487/TR.2010.26.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B. J., Ribeiro F. R. B., and Beckett J. L.. . 2013. Application of growth technologies in enhancing food security and sustainability. Anim. Front. 3:8–13. doi: 10.2527/af.2013-0018. [DOI] [Google Scholar]
- Jones, C. T., and Robinson R. O.. . 1975. Plasma catecholamines in foetal and adult sheep. J. Physiol. 248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousan, F. D., de Castro e Paula L. A., Block J., and Hansen P. J.. . 2007. Fertility of lactating dairy cows administered recombinant bovine somatotropin during heat stress. J. Dairy Sci. 90:341–351. doi: 10.3168/jds.S0022-0302(07)72635-8. [DOI] [PubMed] [Google Scholar]
- Kecinski, M., Keisner D. K., Messer K. D., and Schulze W. D.. . 2018. Measuring stigma: the behavioral implications of disgust. Environ. Resour. Econ. 70:131–146. doi: 10.1007/s10640-017-0113-z. [DOI] [Google Scholar]
- Kirovski, D., and Sladojevic Z.. . 2017. Prediction and diagnosis of fatty liver in dairy cows. SMJ. Gastroenterol. Hepatol. 3:1–7. https://www.jsmcentral.org/sm-gastroenterology/fulltext_smjgh-v3-1005.pdf. [Google Scholar]
- Kolodinsky, J. 2008. Affect or information? Labeling policy and consumer valuation of rBST free and organic characteristics of milk. Food Pol. 33:616–623. doi: 10.1016/J.FOODPOL.2008.07.002. [DOI] [Google Scholar]
- Kumar, P., and Sait S. F.. . 2011. Luteinizing hormone and its dilemma in ovulation induction. J. Hum. Reprod. Sci. 4:2–7. doi: 10.4103/0974-1208.82351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasse, P., and Ollier S.. . 2015. The dopamine antagonist domperidone increases prolactin concentration and enhances milk production in dairy cows. J. Dairy Sci. 98:7856–7864. doi: 10.3168/JDS.2015-9865. [DOI] [PubMed] [Google Scholar]
- Lacasse, P., Ollier S., Lollivier V., and Boutinaud M.. . 2016. New insights into the importance of prolactin in dairy ruminants. J. Dairy Sci. 99:864–874. doi: 10.3168/jds.2015-10035. [DOI] [PubMed] [Google Scholar]
- Lamb, G. C., and Mercadante V. R. G.. . 2016. Synchronization and artificial insemination strategies in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 32:335–347. doi: 10.1016/j.cvfa.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Laron, Z. 2001. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, J. E., Lamb G. C., Arseneau J. D., Stevenson J. S., Johnson S. K., Day M. L., Geary T. W., Kesler D. J., Dejamette J. M., Schrick F. N., . et al. 2006. Synchronization of estrus in suckled beef cows for detected estrus and artificial insemination and timed artificial insemination using gonadotropin-releasing hormone, prostaglandin F2α, and progesterone. J. Anim. Sci. 84:332–342. doi: 10.2527/2006.842332x. [DOI] [PubMed] [Google Scholar]
- Latimer, F., Wilson L., and Cain M.. . 1982. Scrotal measurements in beef bulls: heritability estimates, breed and test station effects. J. Anim. Sci. 54:473–479. doi: 10.2527/jas1982.543473x. [DOI] [PubMed] [Google Scholar]
- Lean, I. J., Golder H. M., Lees N. M., McGilchrist P., and Santos J. E. P.. . 2018. Effects of hormonal growth promotants on beef quality: a meta-analysis. J. Anim. Sci. 96:2675–2697. doi: 10.1093/jas/sky123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi, C. E. P., Pfeifer L. F. M., Rubin M. I. B., Singh J., Mapletoft R. J., Pessoa G. A., Bainy A. M., and Silva C. A. M.. . 2012. Prostaglandin F2α promotes ovulation in prepubertal heifers. Theriogenology 78:1578–1582. doi: 10.1016/j.theriogenology.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Liefers, S. C., Veerkamp R. F., te Pas M. F. W., Delavaud C., Chilliard Y., and van der Lende T.. . 2003. Leptin concentrations in relation to energy balance, milk yield, intake, live weight, and estrus in dairy cows. J. Dairy Sci. 86:799–807. doi: 10.3168/jds.S0022-0302(03)73662-5. [DOI] [PubMed] [Google Scholar]
- Madureira, G., Consentini C. E. C., Motta J. C. L., Drum J. N., Prata A. B., Monteiro P. L. J. J., Melo L. F., Gonçalves J. R. S., Wiltbank M. C., and Sartori R.. . 2020. Progesterone-based timed AI protocols for Bos indicus cattle II: reproductive outcomes of either EB or GnRH-type protocol, using or not GnRH at AI. Theriogenology 145:86–93. doi: 10.1016/j.theriogenology.2020.01.033. [DOI] [PubMed] [Google Scholar]
- Malagon, M. M., Vazquez-Martinez R., Martinez-Fuentes A. J., Gracia-Navarro F., and Castano J. P.. . 2006. Growth hormone-releasing hormone. In: A. J. B. T.-H. of B. A. P. Kastin, editor. Handbook of biologically active peptides. Academic Press, Burlington. p. 663–671. [Google Scholar]
- Marti, S., Devant M., Amatayakul-Chantler S., Jackson J. A., Lopez E., Janzen E. D., and Schwartzkopf-Genswein K. S.. . 2015. Effect of anti-gonadotropin-releasing factor vaccine and band castration on indicators of welfare in beef cattle. J. Anim. Sci. 93:1581–1591. doi: 10.2527/jas.2014-8346. [DOI] [PubMed] [Google Scholar]
- McGlone, J. J. 2001. Farm animal welfare in the context of other society issues: toward sustainable systems. Livest. Prod. Sci. 72:75–81. doi: 10.1016/S0301-6226(01)00268-8. [DOI] [Google Scholar]
- Meinhardt, U. J., and Ho K. K. Y.. . 2006. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. (Oxf). 65:413–422. doi: 10.1111/j.1365-2265.2006.02676.x. [DOI] [PubMed] [Google Scholar]
- Meléndez, D. M., Marti S., Pajor E. A., Moya D., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. . 2018. Effect of subcutaneous meloxicam on indicators of acute pain and distress after castration and branding in 2-mo-old beef calves. J. Anim. Sci. 96:3606–3621. doi: 10.1093/jas/sky245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro, A. P. A., Tao S., Thompson I. M. T., and Dahl G. E.. . 2016. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 99:8443–8450. doi: 10.3168/JDS.2016-11072. [DOI] [PubMed] [Google Scholar]
- Morris, C., and Wilson J.. . 1997. Progress with selection to change age at puberty and reproductive rate in Angus cattle. Proc. New Zeal. Soc. Anim. Prod 57:9–11. ISSN: 0370-2731. [Google Scholar]
- Morrison, C. D., Daniel J. A., Holmberg B. J., Djiane J., Raver N., Gertler A., and Keisler D. H.. . 2001. Central infusion of leptin into well-fed and undernourished ewe lambs: effects on feed intake and serum concentrations of growth hormone and luteinizing hormone. J. Endocrinol. 168:317–324. doi: 10.1677/joe.0.1680317. [DOI] [PubMed] [Google Scholar]
- Muir, L. 1988. Effects of beta-adrenergic agonists on growth and carcass characteristics of animals. In: Designing foods: animal product options in the marketplace. Available from: https://www.ncbi.nlm.nih.gov/books/NBK218165/ [Google Scholar]
- Negrão, J. A., Porcionato M. A., de Passillé A. M., and Rushen J.. . 2004. Cortisol in saliva and plasma of cattle after ACTH administration and milking. J. Dairy Sci. 87:1713–1718. doi: 10.3168/jds.S0022-0302(04)73324-X. [DOI] [PubMed] [Google Scholar]
- Nemeth, M., Pschernig E., Wallner B., and Millesi E.. . 2016. Non-invasive cortisol measurements as indicators of physiological stress responses in guinea pigs. PeerJ 4:e1590–e1590. doi: 10.7717/peerj.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry, R. C., and Swanson J. C.. . 2008. Implications of breaking mother–young social bonds. Appl. Anim. Behav. Sci. 110:3–23. doi: 10.1016/j.applanim.2007.03.021. [DOI] [Google Scholar]
- NRC. 1999. Food-animal production practices and drug use. In: The use of drugs in food animals: benefits and risks. National Research Council (US) Committee on Drug Use in Food Animals.Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- O’Connor, M. L. 2016. Heat detection and timing of insemination for cattle.PennState Ext. Available from: https://extension.psu.edu/heat-detection-and-timing-of-insemination-for-cattle [Google Scholar]
- Odore, R., Badino P., Re G., Barbero R., Cuniberti B., D’Angelo A., Girardi C., Fraccaro E., and Tarantola M.. . 2010. Effects of housing and short-term transportation on hormone and lymphocyte receptor concentrations in beef cattle. Res. Vet. Sci. 90:341–345. doi: 10.1016/J.RVSC.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Oliveira, F. A., de Almeida Í. C., Sena L. M., Penitente-Filho J. M., and Torres C. A. A.. . 2019. Recombinant bovine somatotropin in the synchronization of ovulation in crossbred dairy cows (Bos taurus indicus × Bos taurus taurus). Vet. World 13:746–750. doi: 10.14202/vetworld.2020.746-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela, A., and Galina C. S.. . 2019. Effects of separation of cows and calves on reproductive performance and animal welfare in tropical beef cattle. Animals 9:223. doi: 10.3390/ani9050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papich, M. G. 2016. Prostaglandin F2 alpha. In: M. G. B. T.-S. H. of V. D. (Fourth E. Papich), editor. Saunders handbook of veterinary drugs. W.B. Saunders, St. Louis. p. 689–690. [Google Scholar]
- Paris, A., Andre F., Antignac J. P., Le Bizec B., Bonneau M., Briant C., Caraty A., Chilliard Y., Cognie Y., Combarnous Y., . et al. 2006. Hormones and growth promoters in animal production: from physiology to risk assessment. INRA Prod. Animals 19:149–240. [Google Scholar]
- Perry, T. C., Fox D. G., and Beermann D. H.. . 1991. Effect of an implant of trenbolone acetate and estradiol on growth, feed efficiency, and carcass composition of Holstein and beef steers. J. Anim. Sci. 69:4696–4702. doi: 10.2527/1991.69124696x. [DOI] [PubMed] [Google Scholar]
- Persaud, S. 2019. Effects of trade on the U.S. economy. Available from: https://www.ers.usda.gov/data-products/agricultural-trade-multipliers/effects-of-trade-on-the-us-economy/
- Pfeifer, L. F. M., Siqueira L. G., Mapletoft R. J., Kastelic J. P., Adams G. P., Colazo M. G., and Singh J.. . 2009. Effects of exogenous progesterone and cloprostenol on ovarian follicular development and first ovulation in prepubertal heifers. Theriogenology 72:1054–1064. doi: 10.1016/j.theriogenology.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Phomvisith, O., Takahashi H., Mai H. T., Shiotsuka Y., Matsubara A., Sugino T., Mcmahon C. D., Etoh T., Fujino R., Furuse M., . et al. 2017. Effects of nutritional status on hormone concentrations of the somatotropin axis and metabolites in plasma and colostrum of Japanese Black cows. Anim. Sci. J. 88:643–652. doi: 10.1111/asj.12686. [DOI] [PubMed] [Google Scholar]
- Post, T. B., Christensen H. R., and Seifert G. W.. . 1987. Reproductive performance and productive traits of beef bulls selected for different levels of testosterone response to GnRH. Theriogenology 27:317–328. doi: 10.1016/0093-691X(87)90220-2. [DOI] [PubMed] [Google Scholar]
- Pursley, J. R., Kosorok M. R., and Wiltbank M. C.. . 1997. Reproductive management of lactating dairy cows using synchronization of ovulation. J. Dairy Sci. 80:301–306. doi: 10.3168/JDS.S0022-0302(97)75938-1. [DOI] [PubMed] [Google Scholar]
- Rasby, R. 2007. Early weaning beef calves. Vet. Clin. North Am. Food Anim. Pract. 23:29–40. doi: 10.1016/j.cvfa.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Reineri, P. S., Piccardi M. B., Arroquy J. I., Fumagalli A., Coria M. S., Hernández O., Bó G., and Palma G. A.. . 2018. Hormones and monensin use to improve pregnancy rates in grazing lactating beef cows in the semiarid region of Argentina. Anim. Reprod. 15:56–63. doi: 10.21451/1984-3143-2017-AR0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martinez, H., Larsson B., Zhang B., Soderquist L., and Zhang B. R.. . 1997. In vitro assessment of viability and fertilizing capacity of bull spermatozoa. J. Reprod. Dev. 43:1–11. [Google Scholar]
- Russomano, K. L., Van Amburgh M. E., and Higgs R. J.. . 2012. Utilization of byproducts from human food production as feedstuffs for dairy cattle and relationship to greenhouse gas emissions and environmental efficiency. Cornell Nutr. Conf. Available from: https://ecommons.cornell.edu/bitstream/handle/1813/36469/cnc2012_VAmburgh.txt.pdf?sequence=1 [Google Scholar]
- Sá Filho, O. G., Meneghetti M., Peres R. F. G., Lamb G. C., and Vasconcelos J. L. M.. . 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows. II. Strategies and factors affecting fertility. Theriogenology 72:210–218. doi: 10.1016/j.theriogenology.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Sakai, T., Hayakawa T., Hamakawa M., Ogura K., and Kubo S.. . 1993. Therapeutic effects of simultaneous use of glucose and insulin in ketotic dairy cows. J. Dairy Sci. 76:109–114. doi: 10.3168/jds.S0022-0302(93)77329-4. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rodríguez, H. L., Vann R. C., Youngblood R. C., Baravik-Munsell E., Christiansen D. L., Willard S., and Ryan P. L.. . 2013. Evaluation of pulsatility index and diameter of the jugular vein and superficial body temperature as physiological indices of temperament in weaned beef calves: relationship with serum cortisol concentrations, rectal temperature, and sex. Livest. Sci. 151:228–237. doi: 10.1016/j.livsci.2012.10.019. [DOI] [Google Scholar]
- Sangild, P. 2003. Uptake of colostral immunoglobulins by the compromised newborn farm animal. Acta Vet. Scand. 44:S105. doi: 10.1186/1751-0147-44-S1-S105. [DOI] [PubMed] [Google Scholar]
- Santos, M. D., Torres C. A. A., Ruas J. R. M., Guirmaraes J. D., and Silva Filho J. M.. . 2004. Reproductive potential of Nellore bulls submitted to different bull:cow proportions. Brazilian Arch. Vet. Med. Anim. Sci. 56:497–503. doi: 10.1590/S0102-09352004000400011. [DOI] [Google Scholar]
- Senger, P. L. 2012. Pathways to pregnancy and parturition. Redmond, OR: Current Conceptions; [2012]. Available from: https://search.ebscohost.com/login.aspx?direct=true&db=cat00043a&AN=mstate.2640080&authtype=sso&custid=magn1307&site=eds-live&scope=site&custid=magn1307 [Google Scholar]
- Shoham, Z. 2002. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil. Steril. 77:1170–1177. doi: 10.1016/s0015-0282(02)03157-6. [DOI] [PubMed] [Google Scholar]
- Simmons, D. M., Voss J. W., Ingraham H. A., Holloway J. M., Broide R. S., Rosenfeld L. W., and Swanson M. G.. . 1990. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 4:695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Simpson, R. B., Armstrong J. D., Harvey R. W., Miller D. C., Heimer E. P., and Campbell R. M.. . 1991. Effect of active immunization against growth hormone-releasing factor on growth and onset of puberty in beef heifers. J. Anim. Sci. 69:4914–4924. doi: 10.2527/1991.69124914x. [DOI] [PubMed] [Google Scholar]
- Smith, B. A., Brinks J. S., and Richardson G. V.. . 1989. Relationships of sire scrotal circumference to offspring reproduction and growth. J. Anim. Sci. 67:2881–2885. doi: 10.2527/jas1989.67112881x. [DOI] [PubMed] [Google Scholar]
- Snook, R. B., Saatman R. R., and Hansel W.. . 1971. Serum progesterone and luteinizing hormone levels during the bovine estrous cycle. Endocrinology 88:678–686. doi: 10.1210/endo-88-3-678. [DOI] [PubMed] [Google Scholar]
- Souza, L. W. de O., Andrade A. F. C., Celeghini E. C. C., Negrão J. A., and de Arruda R. P.. . 2011. Correlation between sperm characteristics and testosterone in bovine seminal plasma by direct radioimmunoassay. Rev. Bras. Zootec. 40(12):2721–2724. doi: 10.1590/S1516-35982011001200015. [DOI] [Google Scholar]
- Stephany, R. W. 2001. Hormones in meat: different approaches in the EU and in the USA. APMIS. Suppl. 109(S103):S357–S363; discussion S363-4. doi: 10.1111/j.1600-0463.2001.tb05787.x. [DOI] [PubMed] [Google Scholar]
- Stewart, L. 2013. Implanting beef cattle.Univ. Georg. Coop. Ext. Available from: https://secure.caes.uga.edu/extension/publications/files/pdf/B1302_3.PDF [Google Scholar]
- Stewart, M., Verkerk G. A., Stafford K. J., Schaefer A. L., and Webster J. R.. . 2010. Noninvasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 93:3602–3609. doi: 10.3168/JDS.2010-3114. [DOI] [PubMed] [Google Scholar]
- Stock, M. L., Millman S. T., Barth L. A., Van Engen N. K., Hsu W. H., Wang C., Gehring R., Parsons R. L., and Coetzee J. F.. . 2015. The effects of firocoxib on cautery disbudding pain and stress responses in preweaned dairy calves. J. Dairy Sci. 98:6058–6069. doi: 10.3168/JDS.2014-8877. [DOI] [PubMed] [Google Scholar]
- Sue-Tang, A., Booking A. D., Brooks A. N., Hooper S., White S. E., Jacobs R. A., Fraher L. J., and Challis J. R. G.. . 1992. Effects of restricting uteroplacental blood flow on concentrations of corticotrophin-releasing hormone, adrenocorticotrophin, cortisol, and prostaglandin E 2 in the sheep fetus during late pregnancy. Can. J. Physiol. Pharmacol. 70:1396–1402. doi: 10.1139/y92-196. [DOI] [PubMed] [Google Scholar]
- Tenhagen, B. -A., Surholt R., Wittke M., Vogel C., Drillich M., and Heuwieser W.. . 2004. Use of Ovsynch in dairy herds—differences between primiparous and multiparous cows. Anim. Reprod. Sci. 81:1–11. doi: 10.1016/j.anireprosci.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Thornton, P. K. 2010. Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci 365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turman, E. J., and Andrews F. N.. . 1955. Some effects of purified anterior pituitary growth hormone on swine. J. Anim. Sci. 14:7–18. doi: 10.2527/jas1955.1417. [DOI] [Google Scholar]
- USDA. 2021. Ag and food sectors and the economy. Available from: https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/ag-and-food-sectors-and-the-economy/
- USDA, N.A.S.S. 2019. Land values 2019 summary. Available from: https://www.nass.usda.gov/Publications/Todays_Reports/reports/land0819.pdf
- Velloso, C. P. 2008. Regulation of muscle mass by growth hormone and IGF-I. Br. J. Pharmacol. 154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Juncá, P., Irarrázaval I., Rolle A. J., Gutiérrez J. I., Moreno R. D., and Santos M. J.. . 2015. In vitro fertilization (IVF) in mammals: epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 48:68. doi: 10.1186/s40659-015-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandel, M., and Bugge A.. . 1997. Environmental concern in consumer evaluation of food quality. Food Qual. Prefer. 8:19–26. doi: 10.1016/S0950-3293(96)00004-3. [DOI] [Google Scholar]
- Weaver, D. M., Tyler J. W., VanMetre D. C., Hostetler D. E., and Barrington G. M.. . 2000. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 14:569–577. doi:. [DOI] [PubMed] [Google Scholar]
- West, J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/JDS.S0022-0302(03)73803-X. [DOI] [PubMed] [Google Scholar]
- Whaytt, H. R., Main D. C. J., Greent L. E., and Webster A.. . 2003. Animal-based measures for the assessment of welfare state of dairy cattle, pigs and laying hens: consensus of expert opinion. Anim. Welf. 12:205–217. [Google Scholar]
- Whitnall, M. H. 1993. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 40:573–629. doi: 10.1016/0301-0082(93)90035-Q. [DOI] [PubMed] [Google Scholar]
- WHO. 2013. Affirmation of the human food safety of recombinant bovine somatotropin by the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives. Available from: https://apps.who.int/iris/bitstream/handle/10665/127845/9789241209885_eng.pdf;jsessionid=F327FFB4A4F2E0C81D229755248F0BA8?sequence=1
- Wolf, C. A., Tonsor G. T., and Olynk N. J.. . 2011. Understanding U.S. consumer demand for milk production attributes. J. Agric. Resour. Econ 36(2):326–342. http://www.jstor.org/stable/23243084 [Google Scholar]
- Yang, Y., Shao B., Zhang J., Wu Y., and Duan H.. . 2009. Determination of the residues of 50 anabolic hormones in muscle, milk and liver by very-high-pressure liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. B 877:489–496. doi: 10.1016/J.JCHROMB.2008.12.054. [DOI] [PubMed] [Google Scholar]
- Yermachenko, A., and Dvornyk V.. . 2014. Nongenetic determinants of age at menarche: a systematic review. Biomed Res. Int. 2014:371583. doi: 10.1155/2014/371583. [DOI] [PMC free article] [PubMed] [Google Scholar]