Abstract
Chihuahuas are one of the most common small breed dogs in Japan, and this breed is known to be predisposed to myxomatous mitral valve disease (MMVD). Echocardiographic assessment is essential for definitive diagnosis of MMVD in dogs as well as determining the stage. Because the Chihuahua’s body size is the smallest among the dog breeds and the widely used echocardiographic reference range is established on the basis of larger dogs, it is unclear whether the existing reference range can be applied to the Chihuahua breed as well. Therefore, this study aimed to determine an echocardiographic reference range for Chihuahua dogs. The left atrial short-axis diameter (LA), aortic valve short-axis diameter, interventricular septum thickness at end-diastole, left ventricular internal dimension at end-diastole (LVIDd), and left ventricular posterior wall thickness at end-diastole from 47 healthy Chihuahuas were analyzed. These parameters increased logarithmically as body weight increased. Furthermore, LA and LVIDd were within the previously established reference range generated primarily from multiple breeds of moderate to large breed dogs.
Keywords: canine, cardiology, Chihuahua, echocardiography, reference interval
Chihuahuas are one of the most common small breed dogs in Japan. According to the Japan Kennel Club, fifty thousand or more Chihuahuas are raised and living in Japan [29]. Myxomatous mitral valve disease (MMVD) is the most common heart disease in dogs [10], and is particularly prevalent in small dogs, including Chihuahuas [4].
Echocardiography is an essential modality for diagnosing and assessing MMVD. Echocardiography can identify the abnormal morphology of mitral valve such as thickened mitral valve, ruptured cordae tendineae, which is needed to suspect the etiology of mitral valve regurgitation and allow for chamber size measurements, which are necessary to determine the disease stage and predict a development of congestive heart failure and cardiac-related death [6, 7, 11, 18]. Chamber size assessment of the left atrium (LA) and left ventricle is important in asymptomatic dogs with MMVD when diagnosing Stage B1 or B2; it can determine if early medical intervention is indicated or not. Echocardiographic parameters often depend on body weight (BW), body surface area, gender, age, heart rate (HR), and daily activity level [2, 16, 19,20,21, 23, 28]. It is difficult to estimate normal reference ranges for echocardiographic parameters using a simple linear regression model, even within the same breed [22]. Because there is a wide variety of body sizes and thoracic anatomy among dog breeds, some have established breed-specific reference ranges to accurately assess echocardiographic parameters, such as for English bulldogs [24], Dachshunds [13], and Cavalier King Charles Spaniels [21].
The echocardiographic reference range determined by Cornell et al. incorporated many dog breeds with a minimum BW of 2 kg but mainly included large breed dogs [3, 9]. Thus, it is unclear whether this reference range applies to Chihuahuas whose BW is often less than 2 kg. Small dogs have a larger heart volume relative to the thoracic cavity volume than that in large dogs [31]. On thoracic radiography, a Chihuahua’s heart silhouette is larger than that of a typical reference [25]. Therefore, this study aimed to determine the echocardiographic reference range in Chihuahuas and investigate whether the previously reported echocardiographic reference range is suitable for Chihuahuas.
MATERIALS AND METHODS
This study was approved by the Azabu University Experimental Animal Committee (approval number 201124-2). Client-owned or breeder-owned clinically healthy volunteer Chihuahuas aged between ten months to seven years were prospectively recruited. All dogs were reportedly healthy according to their owners and received a physical examination by a board-certified veterinary cardiologist (YF) and eight-year experienced veterinarian specializing in cardiology (SN). The body temperature, HR, and respiratory rate were measured. Careful thoracic auscultation was performed, and dogs with no obvious abnormal findings were included in the study. Dogs without heart murmur or with a very soft heart murmur (grade 1/6) or mid-systolic clicks were also included. Dogs under ten months or over seven years old, dogs with soft to loud heart murmurs (grade 2/6 or more) on auscultation, systemic chronic disease, or disease requiring treatment were excluded. Furthermore, dogs with a body condition score of 1 or 5 on a 5 point scaling system, and dehydration or overhydration on physical examination were also excluded. Dogs with abnormal echocardiographic findings such as significant valvular regurgitation, prolapse or thickening of mitral valve, or congenital heart disease, were also excluded.
Echocardiography
All transthoracic echocardiographic examinations were performed using an ultrasound system (Vivid E9; GE Healthcare, Boston, MA, USA) with phased-array transducers at nominal frequencies of 6–12 mHz. Echocardiography was performed by a board-certified cardiologist (YF) or experienced veterinarian (SN) supervised by a cardiologist. Each dog was gently restrained in lateral recumbency without sedation during echocardiographic examination. A single trained observer reviewed and measured each study, and all parameters were recorded as the average of three or more measurements from consecutive cardiac cycles.
Left ventricular internal dimension at end-diastole (LVIDd) and end-systole (LVIDs), and left ventricular and interventricular wall thickness at end-diastole (LVPWd and IVSd, respectively) were measured from the right-side parasternal short-axis view at the chordae tendineae level using the M-mode method. The normalized left ventricular internal diameter in diastole (LVIDDN) was calculated according to the following formula: LVIDDN=LVIDd (cm)/(BW (kg)0.294) [9]. The percentage of change in the left ventricular diameter during systole (i.e., the fractional shortening [FS]) was calculated as FS (%)=100 ×(LVIDd−LVIDs)/LVIDd. Left atrial and aortic diameters were measured using the right parasternal short-axis view at the heart base level during end-systole, as previously described [17]. Briefly, the length of the line extending from and parallel to the commissure of the non-coronary and left coronary aortic valve cusps to the dorsal left atrial wall was recorded as the left atrial diameter. The aortic valve short-axis diameter (Ao) was measured along the commissure of the non-coronary and left coronary aortic valve cusps, bisecting the right coronary cusp. Then, the left atrial-to-aortic ratio (LA/Ao) was calculated. In addition to the dimensional measurements, pulsed-wave Doppler was used to measure the blood velocity of left ventricular early filling wave (E-wave) using the left parasternal apical four-chamber view.
Ten prospective echocardiographic studies were randomly selected to evaluate intra- and interobserver variability. These ten studies were analyzed twice, one month apart, first by one author for quantification of intraobserver variability, and then by another author for quantification of interobserver variability; the results of each were unknown to the other.
Statistical analyses
Statistical analyses were performed using commercial software (SPSS Statistics version 27.0; IBM Corp., Tokyo, Japan). Statistical significance was set at P<0.05.
All variables were tested for normality using the Shapiro-Wilk normality test. The mean and standard deviation for each variable were calculated for normally distributed data, whereas data that failed tests were presented as medians and ranges.
The median, upper and lower reference limits and 90% confidence intervals of the limits were calculated using an open-source application (Reference Value Advisor version 2.1) [15]. The non-parametric method to determine reference intervals was used with the entire eligible dataset.
The effects of age, sex, BW, and HR on echocardiographic variables were evaluated using an analysis of covariance, and regression coefficients were estimated. For echocardiographic variables influenced by BW (LA, Ao, LVIDd, LVPWd, and IVSd), allometric scaling was performed. The coefficients of the allometric equation Y=aMb were calculated as previously described by Cornell et al. [9], where Y is the echocardiographic variable in question, M is BW, ‘a’ is the proportionality constant, and ‘b’ is the scaling exponent. These coefficients were used to reconstruct the predicted reference intervals for BW-dependent echocardiographic variables. LA size and LVIDd are important parameters for determining Stage B1 or B2, and MMVD guidelines recommend medical intervention for B2 [18]. Therefore, plots of these two parameters from our data with 95% prediction interval and the previously published prediction interval created by Cornell et al. were drawn in the same graphs and manually inspected.
Intra- and interobserver variability was quantified by the coefficient of variation (CV), where CV=the mean difference between the measurements/the average of the measurements × 100%.
RESULTS
In total, 56 apparently healthy Chihuahuas underwent echocardiographic evaluation. Nine dogs were excluded due to auscultatory and/or echocardiographic findings: murmur grade >2/6 (n=7) and obvious mitral regurgitation (n=9). No dog was obese, lean or inadequately hydrated. Three dogs had a very soft murmur (grade 1/6) on careful auscultation in a quiet room, and echocardiography revealed trivial mitral regurgitation, which was considered hemodynamically insignificant. Thus, they were included in the study. Although careful auscultation did not detect a heart murmur, 38 dogs had trivial mitral regurgitation (such as a few Color-flow pixels) close to the mitral valve level. These findings were also considered insignificant, and the dogs were included in the study.
Overall, 47 dogs (31 females and 16 males; 37 intact and 10 neutered) were included in the analysis. The median age was 29 months (range, 10–80 months), and the median BW was 2.08 kg (range, 1.60–4.75 kg). The mean HR was 115 beats per min (bpm) (range, 76–184 bpm).
All echocardiographic variables were obtained from the entire study population, and all except IVSd had a normal distribution. Table 1 presents the results of the echocardiographic variables.
Table 1. Echocardiographic values in healthy Chihuahuas (n=47).
| Variable | Median | Mean | SD | Min-Max | Lower RI | 90% CI | Upper RI | 90% CI |
|---|---|---|---|---|---|---|---|---|
| LA (mm) | 10.8 | 11.0 | 1.5 | 7.9–14.6 | 8.0 | 7.4–8.7 | 14.0 | 13.4–14.6 |
| Ao (mm) | 8.9 | 9.2 | 1.0 | 6.9–11.2 | 7.2 | 6.8–7.6 | 11.2 | 10.7–11.6 |
| LA/Ao | 1.20 | 1.20 | 0.10 | 0.90–1.49 | 0.99 | 0.95–1.03 | 1.41 | 1.37–1.46 |
| IVSd (mm) | 4.5 | 4.8 | 1.2 | 2.9–10.0 | 3.0 | 2.9–3.3 | 9.4 | 6.4–10.0 |
| LVIDd (mm) | 18.6 | 18.4 | 1.8 | 13.6–23.9 | 14.7 | 14.0–15.6 | 22.1 | 21.3–22.9 |
| LVPWd (mm) | 4.6 | 4.5 | 0.8 | 3.0–6.6 | 3.0 | 2.7–3.3 | 6.1 | 5.7–6.4 |
| LVIDs (mm) | 10.6 | 10.5 | 1.7 | 6.5–13.4 | 7.1 | 6.4–7.8 | 13.9 | 13.1–14.6 |
| FS (%) | 42.6 | 43.3 | 6.8 | 32.2–57.6 | 29.4 | 26.6–32.2 | 57.3 | 54.5–60.4 |
| E-wave (m/sec) | 0.65 | 0.67 | 0.10 | 0.40–0.93 | 0.47 | 0.43–0.51 | 0.86 | 0.82–0.90 |
| LVIDDN | 1.46 | 1.46 | 0.13 | 1.15–1.72 | 1.18 | 1.13–1.25 | 1.73 | 1.67–1.78 |
LA, left atrial short-axis diameter; Ao, aortic valve short axis diameter; LA/Ao, left atrial-to-aortic ratio; IVSd, interventricular septum thickness at end-diastole; LVIDd, left ventricular internal dimension at end-diastole; LVPWd, left ventricular posterior wall thickness at end-diastole; LVIDs, left ventricular internal dimension at end-systole; FS, fractional shortening; E-wave, peak velocity of early diastolic transmitral flow; LVIDDN, normalized left ventricular internal diameter in diastole; SD, standard deviation; Min-Max, minimum maximum reference intervals; RI, reference interval; CI, confidence interval.
Covariance analysis revealed that LA (b=1.528; P<0.001), Ao (b=0.895; P<0.001), LVIDd (b=1.618; P<0.001), LVPWd (b=0.615; P<0.001), and IVSd (b=0.89; P=0.002) were positively correlated with BW. Allometric scales were calculated using the equation derived by Cornell et al. to generate BW reference intervals for LA, Ao, LVIDd, LVPWd, and IVSd (Figs. 1 and 2, Table 2). Age positively affected LVPWd (b=0.012; P=0.019), whereas HR negatively affected LVIDd (b= −0.027; P=0.021) and LVIDDN (b= −0.002; P=0.03). Sex did not influence the echocardiographic variables.
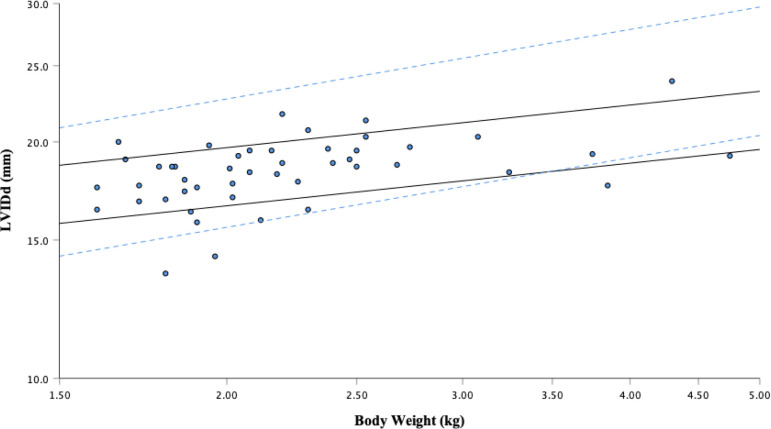
Fig. 1.
Left ventricular end-diastolic diameter (LVIDd) versus body weight after logarithmic transformation. The solid regression lines represent the 95% prediction interval from this study: Y=14.64 to 17.36 × M0.18. The dotted lines are the 95% prediction interval determined by the regression equation reported by Cornell et al.: Y=12.7 to 18.5 × M0.294.
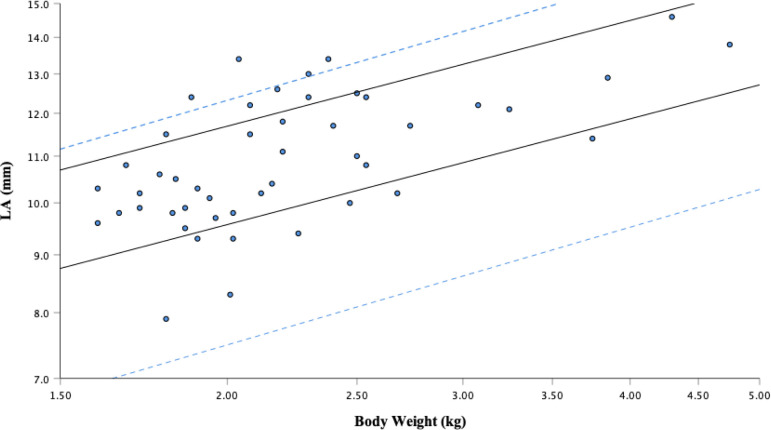
Fig. 2.
Left atrium (LA) diameter versus body weight after logarithmic transformation. The solid regression lines represent the 95% prediction interval from this study: Y=7.72 to 9.43 × M0.31. The dotted lines are the 95% prediction interval determined by the regression equation reported by Cornell et al.: Y=5.9 to 9.7 × M0.345.
Table 2. Predicted reference intervals of body weight-dependent echocardiographic variables (mean and 95% prediction intervals).
| BW (kg) | LAa (mm) | Aob (mm) | IVSdc (mm) | LVIDdd (mm) | LVPWde (mm) |
|---|---|---|---|---|---|
| 1.5 | 9.7 (8.8–10.7) | 8.2 (7.6–8.9) | 4.0 (3.2–4.8) | 17.2 (15.7–18.7) | 3.9 (3.4–4.3) |
| 2.0 | 10.6 (9.6–11.7) | 8.9 (8.2–9.6) | 4.5 (3.6–5.5) | 18.1 (16.6–19.7) | 4.3 (3.8–4.8) |
| 2.5 | 11.4 (10.3–12.5) | 9.4 (8.7–10.1) | 5.1 (4.1–6.1) | 18.9 (17.3–20.5) | 4.7 (4.2–5.3) |
| 3.0 | 12.0 (10.9–13.2) | 9.8 (9.1–10.6) | 5.5 (4.4–6.6) | 19.5 (17.8–21.2) | 5.1 (4.5–5.7) |
| 3.5 | 12.6 (11.4–13.9) | 10.2 (9.4–11.0) | 5.9 (4.8–7.1) | 20.0 (18.3–21.8) | 5.4 (4.7–6.0) |
| 4.0 | 13.2 (11.9–14.5) | 10.5 (9.7–11.4) | 6.3 (5.1–7.6) | 20.5 (18.8–22.3) | 5.7 (5.0–6.4) |
| 4.5 | 13.7 (12.3–15.0) | 10.9 (10.0–11.8) | 6.7 (5.4–8.0) | 21.0 (19.2–22.8) | 6.0 (5.2–6.7) |
| 5.0 | 14.1 (12.7–15.5) | 11.1 (10.3–12.1) | 7.1 (5.7–8.5) | 21.4 (19.6–23.2) | 6.2 (5.5–6.9) |
BW, body weight; LA, left atrial short-axis diameter; Ao, aortic valve short-axis diameter; IVSd, interventricular septum thickness at end-diastole; LVIDd, left ventricular internal dimension at end-diastole; LVPWd, left ventricular posterior wall thickness at end-diastole. Allometric equation with 95% prediction intervals: Y=aMb [9]; Y=echocardiographic variable; M=BW; ‘a’=proportionality constant; ‘b’=scaling exponent. a Allometric equation with 95% prediction intervals: Y=7.72 to 9.43 × M0.31. b Allometric equation with 95% prediction intervals: Y=6.89 to 8.07 × M0.25. c Allometric equation with 95% prediction intervals: Y=6.89 to 8.07 × M0.25. d Allometric equation with 95% prediction intervals: Y=14.64 to 17.36 ×M0.18. e Allometric equation with 95% prediction intervals: Y=2.91 to 3.70 ×M0.39.
Table 3 presents the intra- and interobserver measurement variability results. Nearly all CVs were less than 6%. The coefficient of variation was <10% for all variables.
Table 3. The intra- and interobserver variability of echocardiographic measurements from healthy Chihuahua echocardiograms (n=10).
| Intraobserver CV | Interobserver CV | |||
|---|---|---|---|---|
| Mean | (%) | Mean | (%) | |
| LA (mm) | 10.84 | 5.6 | 11.10 | 0.9 |
| Ao (mm) | 9.15 | 1.4 | 9.31 | 1.9 |
| LA/Ao | 1.18 | 4.1 | 1.19 | 3.3 |
| IVSd (mm) | 4.93 | 2.3 | 4.95 | 1.7 |
| LVIDd (mm) | 18.08 | 1.2 | 18.27 | 0.8 |
| LVPWd (mm) | 4.64 | 10.0 | 4.72 | 7.0 |
| LVIDs (mm) | 11.08 | 0.5 | 11.01 | 0.8 |
| FS (%) | 38.51 | 3.1 | 39.75 | 3.2 |
| E-wave (m/sec) | 0.64 | 9.3 | 0.66 | 6.0 |
LA, left atrial short-axis diameter; Ao, aortic valve short-axis diameter; LA/Ao, left atrial-to-aortic ratio; IVSd, interventricular septum thickness at end-diastole; LVIDd, left ventricular internal dimension at end-diastole; LVPWd, left ventricular posterior wall thickness at end-diastole; LVIDs, left ventricular internal dimension at end-systole; FS, fractional shortening; E-wave, peak velocity of early diastolic transmitral flow; CV, coefficient of variation.
DISCUSSION
This study revealed that the heart size of the Chihuahua increases logarithmically with BW. Gender had no effect on heart size. In certain breeds, echocardiographic values differ between males and females [20]. Previous studies have revealed that age influences myocardial wall thickness and the lumen diameter [1, 27]. However, in our study, age slightly affected LVPWd in Chihuahuas, but it was considered to be of low clinical importance. This is probably because our study population was composed mainly of young dogs.
It has been reported that Chihuahuas’ vertebral heart score is higher than that of the conventional reference values [25]. In small breeds, the ratio of the heart volume to the thoracic cavity volume is small compared to that of large dogs [31]. However, the Chihuahua reference range calculated in this study was generally within the reference range obtained from multiple breeds reported by Cornell et al. [9]. Moreover, the reference intervals found for the Chihuahua breed appeared to be narrow in range.
The LA/Ao and LVIDDN eliminate the effects of BW, and these are commonly used to evaluate the size of the LA and left ventricle. In dog breeds such as boxers and beagles, the aortic diameter is relatively small, so the LA/Ao is large, and 1.73 is considered the upper limit of the normal range [26]; Visser et al. reported that the normal upper limit for LA/Ao was 1.7 [32]. Rishniw et al. investigated LA/Ao by dog type, reporting that, although the number of Chihuahuas was small, the LA/Ao of Chihuahuas was less than 1.6, similar to our results [26]. For LVIDDN, no Chihuahuas in this study exceeded the upper reference range of 1.85, determined by Cornell et al. [9]. Furthermore, sex, age, and BW did not affect LA/Ao or LVIDDN in our study.
The E-wave velocity was partially reflected by left ventricular filling pressure; therefore it has been used as a brief parameter of left atrial pressure in dogs with MMVD. Because previous studies have shown that elevated E-waves are associated with the onset of heart failure and cardiac death in MMVD [5], this parameter was included in this study. E-wave values of 1.2 m/sec or higher in dogs with MMVD are considered to indicate an increased risk of cardiogenic pulmonary edema [5]. The E-wave of healthy dogs has been reported to be 0.87 ± 0.13 m/sec (mean ± standard deviation) [8], which is approximately the same as our results.
Since Chihuahuas are predisposed to MMVD, data were collected from relatively young individuals, but, interestingly, many dogs included in this study already had trivial mitral regurgitation. Cavalier King Charles Spaniels have been reported to develop MMVD at a younger age than other breeds [12]. Chihuahuas may have the same tendency of early development of MMVD, although further investigation is warranted. Mitral regurgitations confirmed on echocardiography in the current study were not accompanied by a heart murmur in most cases and is similar to that reported in Dachshund and Norfolk terriers [14, 30].
There were some limitations to this study. First, the sample size was small for creating a reference range. In addition, since our study population was primarily young individuals, this result may not represent the reference range for the entire population. Further, of the 47 dogs, 34 dogs were included from a solo breeder; therefore, relatively genetically close individuals might be included, and genetic characteristics may be reflected in the results. Finally, mitral regurgitation on echocardiography was present in more dogs than expected. It was difficult to determine whether this mitral regurgitation was pathologically associated with MMVD, or whether it was a physiological feature in Chihuahua. No Chihuahua included in this study had obvious mitral valve thickening or prolapse. In addition, dogs with obvious mitral regurgitation were excluded from this study, so the effect on heart size was considered negligible.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
We thank Eijiro Hanaoka and Mutsuki Umezawa for the sample collection.
REFERENCES
- 1.Akasheva D. U., Plokhova E. V., Tkacheva O. N., Strazhesko I. D., Dudinskaya E. N., Kruglikova A. S., Pykhtina V. S., Brailova N. V., Pokshubina I. A., Sharashkina N. V., Agaltsov M. V., Skvortsov D., Boytsov S. A.2015. Age-related left ventricular changes and their association with leukocyte telomere length in healthy people. PLoS One 10: e0135883. doi: 10.1371/journal.pone.0135883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavegems V., Duchateau L., Sys S. U., De Rick A.2007. Echocardiographic reference values in whippets. Vet. Radiol. Ultrasound 48: 230–238. doi: 10.1111/j.1740-8261.2007.00234.x [DOI] [PubMed] [Google Scholar]
- 3.Boon J., Wingfield W. E., Miller C. W.1983. Echocardiographic indices in the normal dog. Vet. Radiol. 24: 214–221. doi: 10.1111/j.1740-8261.1983.tb00718.x [DOI] [Google Scholar]
- 4.Borgarelli M., Buchanan J. W.2012. Historical review, epidemiology and natural history of degenerative mitral valve disease. J. Vet. Cardiol. 14: 93–101. doi: 10.1016/j.jvc.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 5.Borgarelli M., Crosara S., Lamb K., Savarino P., La Rosa G., Tarducci A., Haggstrom J.2012. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J. Vet. Intern. Med. 26: 69–75. doi: 10.1111/j.1939-1676.2011.00860.x [DOI] [PubMed] [Google Scholar]
- 6.Borgarelli M., Savarino P., Crosara S., Santilli R. A., Chiavegato D., Poggi M., Bellino C., La Rosa G., Zanatta R., Haggstrom J., Tarducci A.2008. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J. Vet. Intern. Med. 22: 120–128. doi: 10.1111/j.1939-1676.2007.0008.x [DOI] [PubMed] [Google Scholar]
- 7.Boswood A., Häggström J., Gordon S. G., Wess G., Stepien R. L., Oyama M. A., Keene B. W., Bonagura J., MacDonald K. A., Patteson M., Smith S., Fox P. R., Sanderson K., Woolley R., Szatmári V., Menaut P., Church W. M., O’Sullivan M. L., Jaudon J. P., Kresken J. G., Rush J., Barrett K. A., Rosenthal S. L., Saunders A. B., Ljungvall I., Deinert M., Bomassi E., Estrada A. H., Fernandez Del Palacio M. J., Moise N. S., Abbott J. A., Fujii Y., Spier A., Luethy M. W., Santilli R. A., Uechi M., Tidholm A., Watson P.2016. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: The EPIC study-a randomized clinical trial. J. Vet. Intern. Med. 30: 1765–1779. doi: 10.1111/jvim.14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetboul V., Sampedrano C. C., Concordet D., Tissier R., Lamour T., Ginesta J., Gouni V., Nicolle A. P., Pouchelon J. L., Lefebvre H. P.2005. Use of quantitative two-dimensional color tissue Doppler imaging for assessment of left ventricular radial and longitudinal myocardial velocities in dogs. Am. J. Vet. Res. 66: 953–961. doi: 10.2460/ajvr.2005.66.953 [DOI] [PubMed] [Google Scholar]
- 9.Cornell C. C., Kittleson M. D., Della Torre P., Häggström J., Lombard C. W., Pedersen H. D., Vollmar A., Wey A.2004. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 18: 311–321. doi: 10.1111/j.1939-1676.2004.tb02551.x [DOI] [PubMed] [Google Scholar]
- 10.Detweiler D. K., Patterson D. F.1965. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 127: 481–516. doi: 10.1111/j.1749-6632.1965.tb49421.x [DOI] [PubMed] [Google Scholar]
- 11.Franchini A., Abbott J. A., Tyrrell W., Rosenthal S., Lahmers S., Menciotti G., Crosara S., Häggström J., Borgarelli M.2021. Predictors of reoccurrence of congestive signs within 180 days after successful treatment of the first episode of congestive heart failure in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 34: 112–119. doi: 10.1016/j.jvc.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 12.Franchini A., Borgarelli M., Abbott J. A., Menciotti G., Crosara S., Häggström J., Lahmers S., Rosenthal S., Tyrrell W.2021. The Longitudinal Outcome Of Canine (K9) myxomatous mitral valve disease (LOOK-mitral registry): baseline characteristics. J. Vet. Cardiol. 36: 32–47. doi: 10.1016/j.jvc.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Garncarz M., Parzeniecka-Jaworska M., Czopowicz M., Hulanicka M., Jank M., Szaluś-Jordanow O.2018. Reference intervals for transthoracic echocardiographic measurements in adult Dachshunds. Pol. J. Vet. Sci. 21: 779–788. [DOI] [PubMed] [Google Scholar]
- 14.Garncarz M., Parzeniecka-Jaworska M., Hulanicka M., Jank M., Szaluś-Jordanow O., Kurek A.2017. Mitral regurgitation in Dachshund dogs without heart murmurs. J. Vet. Res. (Pulawy) 61: 363–366. doi: 10.1515/jvetres-2017-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geffré A., Concordet D., Braun J. P., Trumel C.2011. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 40: 107–112. doi: 10.1111/j.1939-165X.2011.00287.x [DOI] [PubMed] [Google Scholar]
- 16.Giraut S., Häggström J., Koskinen L. L. E., Lohi H., Wiberg M.2019. Breed-specific reference ranges for standard echocardiographic measurements in salukis. J. Small Anim. Pract. 60: 374–378. doi: 10.1111/jsap.12975 [DOI] [PubMed] [Google Scholar]
- 17.Hansson K., Häggström J., Kvart C., Lord P.2002. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound 43: 568–575. doi: 10.1111/j.1740-8261.2002.tb01051.x [DOI] [PubMed] [Google Scholar]
- 18.Keene B. W., Atkins C. E., Bonagura J. D., Fox P. R., Häggström J., Fuentes V. L., Oyama M. A., Rush J. E., Stepien R., Uechi M.2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 33: 1127–1140. doi: 10.1111/jvim.15488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo L., Canada N., Bussadori C., Gomes J. L., Carvalheira J.2008. Transthoracic echocardiography in Estrela Mountain dogs: reference values for the breed. Vet. J. 177: 250–259. doi: 10.1016/j.tvjl.2007.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Locatelli C., Santini A., Bonometti G. A., Palermo V., Scarpa P., Sala E., Brambilla P. G.2011. Echocardiographic values in clinically healthy adult dogue de Bordeaux dogs. J. Small Anim. Pract. 52: 246–253. doi: 10.1111/j.1748-5827.2011.01055.x [DOI] [PubMed] [Google Scholar]
- 21.Misbach C., Lefebvre H. P., Concordet D., Gouni V., Trehiou-Sechi E., Petit A. M., Damoiseaux C., Leverrier A., Pouchelon J. L., Chetboul V.2014. Echocardiography and conventional Doppler examination in clinically healthy adult Cavalier King Charles Spaniels: effect of body weight, age, and gender, and establishment of reference intervals. J. Vet. Cardiol. 16: 91–100. doi: 10.1016/j.jvc.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Morrison S. A., Moise N. S., Scarlett J., Mohammed H., Yeager A. E.1992. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J. Vet. Intern. Med. 6: 220–224. doi: 10.1111/j.1939-1676.1992.tb00342.x [DOI] [PubMed] [Google Scholar]
- 23.Muzzi R. A., Muzzi L. A., de Araújo R. B., Cherem M.2006. Echocardiographic indices in normal German shepherd dogs. J. Vet. Sci. 7: 193–198. doi: 10.4142/jvs.2006.7.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patata V., Vezzosi T., Marchesotti F., Domenech O.2021. Echocardiographic parameters in 50 healthy English bulldogs: preliminary reference intervals. J. Vet. Cardiol. 36: 55–63. doi: 10.1016/j.jvc.2021.04.010 [DOI] [PubMed] [Google Scholar]
- 25.Puccinelli C., Citi S., Vezzosi T., Garibaldi S., Tognetti R.2021. A radiographic study of breed-specific vertebral heart score and vertebral left atrial size in Chihuahuas. Vet. Radiol. Ultrasound 62: 20–26. doi: 10.1111/vru.12919 [DOI] [PubMed] [Google Scholar]
- 26.Rishniw M., Caivano D., Dickson D., Vatne L., Harris J., Matos J. N.2019. Two-dimensional echocardiographic left- atrial-to-aortic ratio in healthy adult dogs: a reexamination of reference intervals. J. Vet. Cardiol. 26: 29–38. doi: 10.1016/j.jvc.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Spasojević Kosić L., Trailović D. R., Krstić N.2017. Age-dependent electrocardiographic and echocardiographic changes in German Shepherd dogs. Majallah-i Tahqiqat-i Dampizishki-i Iran 18: 43–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson H. M., Fonfara S., López-Alvarez J., Cripps P., Dukes-McEwan J.2012. Screening for dilated cardiomyopathy in Great Danes in the United Kingdom. J. Vet. Intern. Med. 26: 1140–1147. doi: 10.1111/j.1939-1676.2012.00987.x [DOI] [PubMed] [Google Scholar]
- 29.The Kennel Club (Japan)2020. Number of Dog Registration in Japan. https://www.jkc.or.jp/archives/enrollment/14222 [accessed on October 26, 2021].
- 30.Trafny D. J., Freeman L. M., Bulmer B. J., MacGregor J. M., Rush J. E., Meurs K. M., Oyama M. A.2012. Auscultatory, echocardiographic, biochemical, nutritional, and environmental characteristics of mitral valve disease in Norfolk terriers. J. Vet. Cardiol. 14: 261–267. doi: 10.1016/j.jvc.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 31.Uehara T., Orito K., Fujii Y.2019. CT-based anatomical features of large airway and heart volume in dogs of different body size. Vet. J. 246: 21–26. doi: 10.1016/j.tvjl.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Visser L. C., Ciccozzi M. M., Sintov D. J., Sharpe A. N.2019. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: a prospective study proposing reference intervals and assessing repeatability. J. Vet. Intern. Med. 33: 1909–1920. doi: 10.1111/jvim.15562 [DOI] [PMC free article] [PubMed] [Google Scholar]