Abstract
The impact of low pathogenicity avian influenza (LPAI) has been confirmed mainly in farms. Unlike apparent losses caused by the high pathogenicity avian influenza (HPAI), the LPAI impact has been hardly evaluated due to underestimating its spread and damage. In 2019, a questionnaire study was conducted in southern Vietnam to identify the specific risk factors of LPAI virus (LPAIV) circulation and to find associations between husbandry activities and LPAI prevalence. A multilevel regression analysis indicated that keeping Muscovy ducks during farming contributed to LPAIV positivity [Odds ratio=208.2 (95% confidence interval: 13.4–1.1 × 104)]. In cluster analysis, farmers willing to report avian influenza (AI) events and who agreed with the local AI control policy had a slightly lower risk for LPAIV infection although there was no significance in the correlation between farmer characteristics and LPAI occurrence. These findings indicated that keeping Muscovy ducks without appropriate countermeasures might increase the risk of LPAIV infection. Furthermore, specific control measures at the local level are effective for LPAIV circulation, and the improvement of knowledge about biosecurity and attitude contributes to reducing LPAI damage.
Keywords: knowledge attitude and practice survey, low pathogenicity avian influenza, risk factor, surveillance, Vietnam
Avian influenza (AI), caused by an AI virus (AIV) infection, is a contagious disease worldwide in poultry. Based on the antigenic characteristics of glycoproteins on the surface of the virus particle, AIVs are categorized into 16 hemagglutinin and 9 neuraminidase subtypes [13]. Based on their pathogenicity in chickens, AIVs are classified into high pathogenicity AIVs (HPAIVs) and low pathogenicity AIVs (LPAIVs). Unlike HPAIV, which causes extremely high mortality of up to 100%, LPAIV usually causes mild or low pathogenicity with minor or atypical clinical signs [2]. The natural reservoir of AIVs is wild waterfowl that could harbor all AIV subtypes but rarely show any clinical signs [1]. Originally, natural reservoirs, such as ducks, are not infected with HPAIV. However, HPAIVs have recently been reported to cause mild or high pathogenicity even in wild waterfowl. By circulating in the migration of wild waterfowl flocks, HPAIVs could be transferred worldwide [8, 28]. HPAIV infection outbreaks in poultry, following wild birds, due to the spillover of contagious pathogens are frequently reported [9]. Compared to high mortality in chickens, HPAIV infection in ducks with minor or atypical clinical signs is hardly detected in the flock; this silent infection might lead to widespread infection and huge damage in the poultry industry [20]. Substantial poultry production losses caused by LPAIV infection could be recognized through increased mortality and decreased egg production [16].
The Vietnamese government has officially developed systemic documents to support the strategy to control AI, particularly targeting HPAI. Active and passive surveillance in poultry is important in AI control because the numbers of H5N1 HPAI outbreaks in Vietnam have markedly decreased by maintaining these activities since 2004 [9]. Many studies have been established based on the output of active surveillance to improve knowledge on AIV ecology in birds [4, 5, 20, 22, 23]. Despite remarkable improvements in AI control, especially in reducing HPAIV outbreaks, substantial economic losses in the poultry industry probably due to AIV infection are continuously suspected. The current diagnosis system in Vietnam is focused on HPAI only; therefore, there are remaining concerns about how LPAIV circulation in the flock would influence the ecology of AI in poultry and the resulting economic losses. However, there are no reports on the potential damage by LPAIV infection and circulation in Vietnamese poultry.
Long-term surveillance has been conducted in poultry to characterize AIV ecology in different geographic regions in Vietnam. From 2009 to 2019, a total of 26,347 samples were collected from poultry and environments: 6,262 samples from two provinces in northern Vietnam, 5,085 samples from one province in central Vietnam, and 15,000 samples from five provinces in southern Vietnam (Fig. 1; Supplementary Table 1) [4, 5, 20, 22, 23]. In Vietnam, AIVs are suspected to be maintained in the poultry population and spread by farming or trading activities. In detail, a high AIV prevalence was identified in live bird markets (LBMs), followed by backyard farms [5, 22, 23]. Unfortunately, the control measure applied in LBMs was ineffective in reducing the AIV prevalence [4]. An intensive investigation was conducted during this surveillance to determine the hidden contributor to the high AIV prevalence in LBMs. Poultry delivery stations (PDSs) are private businesses that usually receive birds from large catchment areas (up to 100 km) and mix several poultry species under relatively poor biosecurity conditions. Therefore, PDS was a hot spot for AIV circulation, with AIV prevalence even higher than LBMs.
Fig. 1.
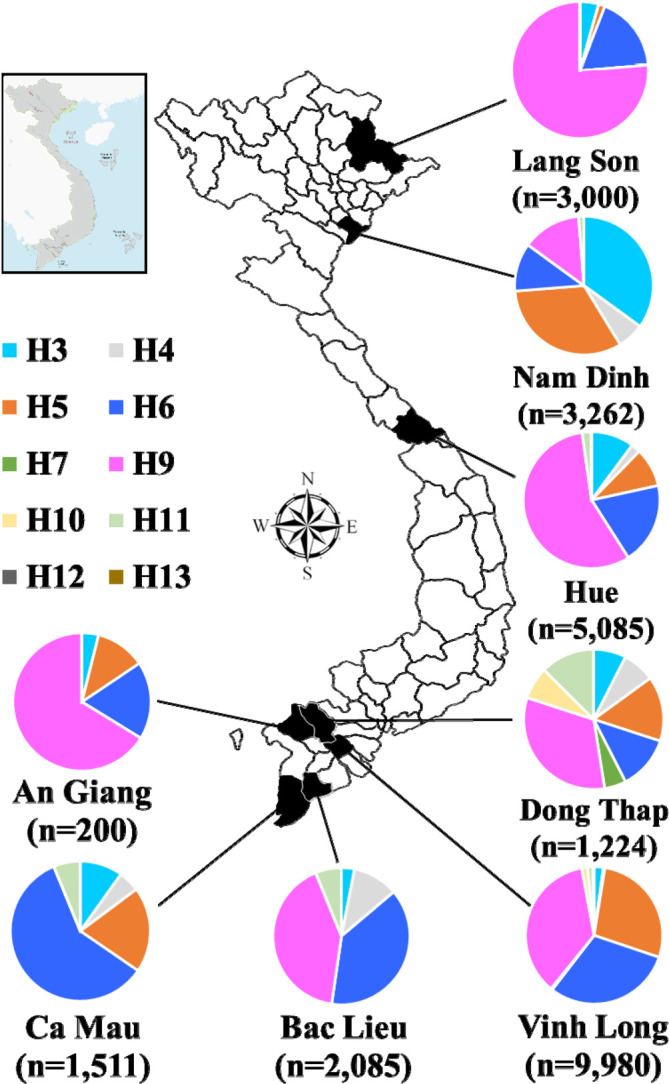
Diversity of avian influenza virus isolates in each sampling area, Vietnam. The geographical location of Vietnam in Southeast Asia is indicated by the small rectangle. The map of Vietnam shows the location of eight provinces where sampling was indicated in black and the number of samples in the parentheses. The proportions of AIV hemagglutinin subtypes in each location are indicated in the pie charts.
The introduction of new AIVs in northern Vietnam was supposedly related to cross-border transmission, whereas southern Vietnam maintained predominant AIVs [21]. A cross-sectional study on AI focusing on LPAIV was conducted in Vinh Long Province to identify the characteristics of farmers associated with LPAIV infection in poultry. A direct multivariable analysis might identify the individual risk factors contributing to LPAIV infection, but these factors might not strongly influence the field situation. Therefore, analyzing a group of co-occur factors might provide more details on the relationship between the farmer’s response tendency and LPAIV infection.
MATERIALS AND METHODS
Study design and area
Based on the evaluation of the Department of Animal Health, Vietnam (DAH), the provinces at risk for AI were selected over the years. A questionnaire survey was conducted to collect epidemiological data from the participants in August 2019. The eligible participants were farmers of backyard and high biosecurity farms randomly selected from a sampling frame provided by the provincial DAH. All participants were asked if they consented to take part in the study before collecting samples from birds and implementing direct interviews by the first author and staff from the Sub-Departments of Animal Health (SDAH) of Vinh Long Province. A backyard farm was likely to lack prevention measures following local authority guidelines, such as disinfection, vaccination, keeping poultry in a separate place, and raising poultry for self-consumption. A farm applying at least two prevention measures and raising poultry mainly for trading was defined as a high biosecurity farm in this study.
Swab sample collection and laboratory procedures
Swab samples (oropharyngeal, cloacal, and fecal) from chickens, ducks, and Muscovy ducks and environment swab samples from the sampling sites were stocked in the transport medium used in the previous study [17]. A total of 1,634 swab samples were collected for AIV isolation from birds in backyard farms, high biosecurity farms, LBMs, and PDSs in Vinh Long Province. The samples were transported to the Regional Animal Health Office No. 7 (RAHO7; Can Tho, Vietnam). Aliquots of up to 10 samples collected from the same flock were pooled to investigate the presence of influenza type A virus using real-time reverse transcription-polymerase chain reaction (RT-PCR) targeting the matrix (M) gene [6]. After screening by real-time RT-PCR in Vietnam, all samples were transferred to Japan for virus isolation at the Laboratory of Microbiology, Faculty of Veterinary Medicine, Hokkaido University.
Virus isolation
Each sample in an M-gene-positive pooled sample was resuspended with the transport medium and inoculated into the allantoic cavity of 10-day-old embryonated chicken eggs. Inoculated eggs were incubated for 48 hr at 35°C, and allantoic fluid was collected to confirm the presence of AIV by the hemagglutination assay. Antisera against the reference influenza A virus strains were used for subtyping influenza A virus isolates by the hemagglutination inhibition and neuraminidase inhibition tests [15].
Questionnaire study and data management
The adapted questionnaire was developed by referring to previous studies conducted by the DAH, with specific questions related to LPAIV epidemiology and ecology [4]. In detail, the questionnaire comprising 150 questions (Supplementary Fig. 1) regarding knowledge, attitudes, practices, and economic losses associated with LPAIV infection or prevention in farms was developed in partnership with the provincial DAH staff to collect details on (1) demographic characteristics, (2) general knowledge regarding LPAIV, (3) attitudes on the control measures of LPAI and AI, (4) routine biosecurity measures applied, and (5) suspected economic losses. Sixty-two face-to-face interviews were conducted by the SDAH staff of Vinh Long Province after training. The sampling schedule was announced to participants and local veterinarians in advance. Questionnaire responses from the participants and the AIV isolation results were recorded in two tables. A unique identification code was assigned for each respondent enrolled in this study to the link between tables for making a relational database.
Univariable analysis
LPAIV prevalence at the bird level was defined as the proportion of the number of individual birds with LPAIV-positive samples per the total number of birds sampled. Unconditional associations between the responses on the questionnaire (explanatory variables) and the laboratory results (numbers of the presence or absence of LPAIV per bird) were expressed as the odds ratio (OR). Analysis of variance (ANOVA) was performed to determine the effects of independent variables on the dependent variable in a regression study to determine the potential factors related to LPAIV infection. Any explanatory variables with P≤0.2 (two-sided) of unconditional association were applied in the multilevel model.
Multiple correspondence analysis (MCA)
Sixty-one farms were retained in the final dataset because one H5 HPAI-positive farm did not satisfy the enrollment criteria for risk factor analysis of LPAIV infection. The relationships between responses in each of the four sections of the questionnaire (demographic details, knowledge, attitude, and practice) were graphically represented using MCA. MCA is a generalization of principal component analysis suitable for categorical variables and was performed according to the protocol in the previous study [18]. Briefly, a pairwise cross-tabulation of the individual variable was used to construct an I×J indicator matrix, where I is the set of i individual records, and J is the set of j variable categories [25]. Each variable represented a mark on the MCA two-dimensional graph, and the association of the categories was expressed as the distance of these marks. The squares of the distance between the representative marks and the relative frequency of each category were introduced as described in the previous study [18]. The sum of all square distances between each set of the two representative records was calculated using the sum of squared distance between individuals i and i′ and a set of all variables to introduce the squared distance between categories j and j′.
The responses to each question in four sections were used to develop MCA scatterplots. Hierarchical clustering on principal components for each of the four plots was performed using Ward’s method. Based on the output, the respondents were aggregated into relatively homogeneous subgroups (‘clusters’) in each section. The assigned clusters of demographic details, knowledge, attitude, and practice were used as explanatory variables in a multivariable logistic regression model of LPAIV infection risk at the individual bird level. All MCA analyzes were performed in R version 4.0.5 [24] with the FactoMineR package [14].
Mixed-effects logistic regression
According to the previous study, the probability of LPAIV infection in a bird was parameterized as a function of m explanatory variables in a fixed-effects multiple logistic regression model [18]. The variable with an unconditional association to the outcome at the P≤0.2 level of significance was selected as the fixed-effect model’s potential variable. The backward stepwise approach was adopted to select explanatory variables until all remaining variables were associated with the outcome at α≤0.05. The effects of excluding explanatory variables in univariable analyzes were reconfirmed in the final model by reincluding them. If any of the estimated regression coefficients changed more than 20% by including these explanatory variables, they were retained in the final model. Biologically plausible two-way interactions between explanatory variables were assessed: none were found to be significant at α≤0.05. The model was then extended by including the effect of herd level (farm level) and explanatory variables were retained in the mixed-effects model regardless of their statistical significance according to the previous study [18].
The assumptions of normality and homogeneity of variance were investigated by constructing the histograms of the residuals in the multilevel model and plots between the residuals and predicted values, respectively. In the multilevel model, extrabinomial variation was not included in the individual bird variance. Estimates of variance at each of the two levels (farms and birds) were regarded as the lowest level of variance on the logit scale of π2/3, where π=3.1416 [25].
The LPAIV positivity status at the bird level predicted by the model was evaluated to construct the Receiver Operating Characteristic (ROC) curve. The ability to discriminate between LPAIV-positive and LPAIV-negative birds in the model was assessed by the area under the ROC curve (AUC). Unconditional measures of association analyzes in the data were carried out using the epiR package [26]. The mixed-effects logistic regression model was developed under the contribution of the lme4 package [3]. All analyzes were conducted in R version 4.0.5.
RESULTS
Descriptive statistics
Based on the results of the active surveillance since 2009, Vinh Long Province was selected to conduct a cross-sectional study in 2019 to assess the risk factors associated with LPAIV infection. Because Vinh Long Province had the highest number of samples among the eight provinces, poultry value chains among PDSs, LBMs, backyard farms, and high biosecurity farms in the province were assessed [18]. A total of 1,634 samples were collected (Table 1) from 45 backyard farms (10 samples per farm; range 9–20; median=10; total 459 samples), 17 high biosecurity farms (29 samples per farm; range, 20–30; median=30; total 500 samples), 31 sellers in 10 LBMs (14 samples per seller; range 1–36; median=10; total 420 samples), and 7 PDSs (36 samples per PDS; range 13–61; median=40; total 255 samples). Because the LPAIV impact should be assessed only in farms as the cumulative impact in a long time, backyard and high biosecurity farms were selected for further analysis. AIVs were isolated only in backyard farms with a prevalence of 3.9% [95% confidence interval (CI): 2.3–6.1%] in which HPAIVs were isolated in a single farm, returning in LPAIV prevalence to 2.4% (95% CI: 1.2–4.2%). LPAIVs were isolated in 4 of 62 farms, returning in LPAIV prevalence at farm level to 6.5% (95% CI: 1.8–15.7%).
Table 1. Avian influenza viruses isolated in Vinh Long province in Vietnam in 2019.
| Model | Species | No. of samples | AIV positive | Prevalence (95% CI) | Subtype (no. of isolates) |
|---|---|---|---|---|---|
| Backyard | Chicken | 220 | 17 | 3.9 (2.3‒6.1) | H5N1 (7), H9N2 (10) |
| Duck | 189 | 1 | H10N3 (1) | ||
| Muscovy duck | 50 | 0 | |||
| Biosecurity | Chicken | 330 | 0 | 0.0 | |
| Duck | 140 | 0 | |||
| Muscovy duck | 30 | 0 | |||
| LBM | Chicken | 212 | 16 | 10.7 (7.9‒14.1) | H9N2 (16) |
| Duck | 140 | 28 | H5N1 (2), H5N6 (20), H6N6 (4), H9N2 (2) | ||
| Muscovy duck | 68 | 1 | H10N3 (1) | ||
| PDS | Chicken | 106 | 14 | 18.0 (23.5‒23.3) | H9N2 (14) |
| Duck | 117 | 32 | H5N1 (3), H5N6 (2), H6N6 (27) | ||
| Muscovy duck | 32 | 0 | |||
| Total | 1,634 | 109 | 6.7 (5.5‒8.0) | ||
AIV: avian influenza virus, CI: confidence interval, LBM: live bird market, PDS: poultry delivery station. High pathogenicity avian influenza viruses are highlighted in bold.
Univariable analysis
Twenty-one explanatory variables showed the association with virus isolation positivity at P≤0.2 in ANOVA (Supplementary Table 2). Most birds were raised by male farmers [708 of 928 (76%)], and the odds of birds raised by male farmers being LPAIV positive was 0.03 (95% CI: 0.00–0.16) times higher than that of birds raised by female farmers. The odds of birds being LPAIV positive in farms with Muscovy ducks during poultry farming was 19.92 (95% CI: 5.08–131.44) times higher than farms without Muscovy ducks. The odds of birds being LPAIV positive in farms that bought hatchlings from the same commune was 0.18 (95% CI: 0.03–0.69) times higher than farms that imported hatchlings from other communes. Moreover, buying hatchlings due to low price had 11.11 (95% CI: 1.49–92.19) times higher risk in birds with LPAIV positive than that due to accessibility. Farmers unwilling to report an AI event were 27.51 (95% CI: 7.79–127.45) times at higher risk of possessing LPAIV positive birds than those reporting an AI event. Interestingly, reporting to local officers, veterinarians, or the local government could reduce the risk of birds with LPAIV positive [OR=0.04 (95% CI: 0.01–0.13)] compared with not reporting the event anywhere.
Multivariable logistic regression analysis for individual variables
The mixed-effects logistic regression model with estimated regression coefficients of the five variables is retained in the final model (Table 2). Keeping Muscovy ducks could significantly exacerbate the risk of LPAIV infection at 208 times more than not raising Muscovy ducks. In the current model, 66 (44%) variables were excluded due to the zero count of LPAIV in the exposure or nonexposure group, meaning that the effect of these variables would not be fully included regardless of their potential contribution to LPAIV positivity. Although a significant difference was observed, the standard error of the mean of coefficient in this model was high (1,643), suggesting that other variables might contribute to the risk of LPAIV infection.
Table 2. A mixed-effects logistic regression model quantifying the association between factors and low pathogenicity avian influenza virus positivity.
| Explanatory variable | Samples | LPAIV positive | Coefficient (SEM) | z | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Intercept | 939 | 11 | 16.04 (1643.00) | |||
| Sampling species | ||||||
| Chicken | 520 | 10 | Ref | Ref | Ref | 1.0 |
| Duck | 328 | 1 | 1.06 (1.66) | 0.64 | 0.52 | 2.9 (0.1‒98.9)a |
| Muscovy duck | 80 | 0 | −1.08 (4.07 × 106) | 0.00 | 1.00 | NA |
| Age | ||||||
| Under 20 year-old | 59 | 1 | Ref | Ref | Ref | 1.0 |
| 20–30 year-old | 280 | 10 | −20.48 (1643.00) | 0.00 | 0.99 | 0.0 (0.0‒3.6) |
| 31–40 year-old | 280 | 0 | −51.77 (3.12 × 106) | 0.00 | 1.00 | NA |
| 41–50 year-old | 279 | 0 | −49.50 (1.87 × 106) | 0.00 | 1.00 | NA |
| Upper 50 year-old | 30 | 0 | −44.60 (1.31 × 107) | 0.00 | 1.00 | NA |
| Gender | ||||||
| Female | 220 | 10 | Ref | Ref | Ref | 1.0 |
| Male | 708 | 1 | −23.72 (1.64 × 104) | 0.00 | 0.99 | 0.0 (0.0‒3.0) |
| Experience | ||||||
| Under 1 year | 49 | 1 | Ref | Ref | Ref | 1.0 |
| 1–5 years | 431 | 9 | 55.92 (2.34) | 0.24 | 0.81 | 1.8 (0.0‒2.3) |
| 6–10 years | 288 | 1 | −0.03 (2.59) | −0.01 | 0.99 | 1.0 (0.0‒7.6) |
| More 10 years | 160 | 0 | −1.37 (4.62 × 106) | 0.00 | 1.00 | NA |
| Keep Muscovy duck | ||||||
| No | 757 | 2 | Ref | Ref | Ref | 1.0 |
| Yes | 171 | 9 | 5.34 (1.66) | 3.21 | <0.01 | 208.2 (13.4‒1.11 × 104) |
| Random effects | Variance | SE | ||||
| Individual farm | 1.46 × 10−15 | 3.82 × 10−8 |
LPAIV: low pathogenicity avian influenza virus, SEM: standard error of the mean, OR: odds ratio, CI: confidence interval, Ref: reference, NA: not assessable. a Interpretation: after adjusting for the effect of sampling in chicken the odds of a bird being LPAIV positive if it was from a ‘Chicken’ was 2.9 (95% CI: 0.1‒98.9) times the odds of a bird from a ‘Duck’ being LPAIV positive.
MCA
In the demographic section, two clusters were identified (Supplementary Fig. 2A; Supplementary Table 3). The first (n=17) comprised predominantly large-scale farms with a poultry population of >500 heads (‘Big farm’; median=2,000; mean=2,072) that paid more attention to the quality of hatchlings by buying hatchlings from hatcheries they knew previously. The second (n=44) was mostly small-scale farms with ≤500 heads of poultry (‘Small farm’; median=215; mean=347) that preferred to buy hatchlings from traders at a lower price.
The questionnaire survey demonstrated that none of the respondents knew about LPAIV, so they could not differentiate between HPAI and LPAI cases. Two clusters were identified in the knowledge section (Supplementary Fig. 2B; Supplementary Table 4) by confirmation of their general knowledge about AI. In the first cluster (n=51; ‘Correct knowledge’), most respondents obtained information about AI from local radio stations and believed that the separation of newly imported poultry could reduce AI risk. All respondents in this cluster agreed that eating sick birds might cause AIV infection in humans. All respondents in this cluster understood AI clinical signs, and most (54.9%) believed that AI clinical signs might not be observed in infected ducks. In the second cluster (n=10; ‘Mixed knowledge’), the respondents rarely believed that the separation of newly imported poultry could reduce AI risk. Eighty percent of this cluster thought that AIV might not infect humans by eating sick birds. Most responders in this cluster were unsure about AI clinical signs but believed clinical signs in infected ducks.
Three clusters were identified in the attitude section (Supplementary Fig. 2C; Supplementary Table 5). For the first cluster (n=8; ‘Report AI but disagree with policy’), although all respondents were willing to report a disease notification to local veterinarians or officials when detected, they were not satisfied yet with the control measures applied in their husbandry area. Furthermore, it was thought that AI control was not their responsibility, which did not benefit their farming in this cluster. The members of the second cluster (n=46; ‘Report AI and agree with policy’) were relatively good attitude respondents because of their willingness to report a disease notification to both local veterinarians and officials when detected. They also agreed to the control measures applied in their area and understood that AI control was under their responsibility, which might benefit their farming. In contrast, all members of the third cluster (n=7; ‘Don’t report AI’) declared unwillingness to report a disease notification even when detected. Although they agreed to the control measures applied in their area and understood that AI control was under their responsibility, they were unsure about the AI situation in their husbandry area.
For the practice section, two clusters were identified (Supplementary Fig. 2D; Supplementary Table 6). Respondents comprising the first cluster (n=17; ‘High biosecurity’) mostly used personal protective equipment (PPE) when slaughtering or handling dead birds. This cluster also disinfected equipment, vehicle, and the barn at high frequency. Furthermore, none of the respondents shared their vehicles for other purposes, except farming. Respondents comprising the second cluster (n=44; ‘Low biosecurity’) seemed to be more careless with a lower frequency of PPE use for slaughtering or handling dead birds. They commonly shared vehicles for other purposes without disinfection and disinfected barn with less frequency.
Multivariable logistic regression analysis for variables identified by MCA
The mixed-effects logistic regression model using clustering data was developed to assess all variables and reduce the bias from eliminating the variables in the screening process. The mixed-effects logistic regression model with estimated regression coefficients of the knowledge and attitude clusters is shown in Table 4. One knowledge cluster (‘Correct knowledge’) and two attitude clusters (‘Report AI but disagree with policy’ and ‘Report AI and agree with policy’) showed the potential to reduce the risk of LPAIV infection, although a significant difference was not observed. The proportions of unexplained variance at the farm and bird levels after adjustment for including the fixed effects in the model were 0.42 and 0.58, respectively. The AUC for the fixed-effects model was 0.91, indicating that the model possessed sufficient power to discriminate between AIV-positive and -negative birds. In the mixed-effects model, the AUC was 0.99.
Table 4. A mixed-effects logistic regression model quantifying the association between clusters and low pathogenicity avian influenza virus positivity.
| Explanatory variable | Samples | LPAIV positive | Coefficient (SEM) | z | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Intercept | 939 | 11 | −3.48 (1.63) | |||
| Knowledge | ||||||
| Mixed knowledge | 100 | 1 | Ref | Ref | Ref | 1.0 |
| Correct knowledge | 839 | 10 | −1.67 (2.11) | −0.79 | 0.42 | 0.2 (0.0‒11.8)a |
| Attitude | ||||||
| Report AI but disagree with policy | 79 | 2 | Ref | Ref | Ref | 1.0 |
| Report AI and agree with policy | 770 | 1 | −3.61 (2.08) | −1.74 | 0.08 | 0.0 (0.0‒1.6) |
| Report AI no | 90 | 8 | 0.28 (2.07) | 0.14 | 0.89 | 1.3 (0.0‒76.1) |
| Random effects | Variance | SEM | ||||
| Individual farm | 7.34 | 2.71 |
LPAIV: low pathogenicity avian influenza virus, SEM: standard error of mean, OR: odds ratio, CI: confidence interval, Ref: reference, AI: avian influenza. a Interpretation: After adjusting for the effect of respondent knowledge category and attitude category the odds of a bird being LPAIV positive if it was from a ‘Correct knowledge’ cluster was 0.2 (95% CI: 0.0‒11.8) times the odds of a bird from a ‘Mixed knowledge’ cluster being LPAIV positive.
DISCUSSION
Application of multiapproach countermeasures, such as strengthening active and passive surveillance, mass vaccination, and education campaigns, reduced HPAI cases in Vietnam, leading to minimized substantial losses in the domestic poultry sector. However, field reports of AI typical signs but not due to H5 HPAIV infection should raise the concern about the damage caused by LPAI. The prevalence of AIV positivity in poultry in backyard farms in this study was 3.9% (95% CI: 2.3–6.1%), where HPAIVs were isolated in a single farm, returning LPAIV prevalence to 2.4% (95% CI: 1.2–4.2%). This result was comparable to previous studies in southern Vietnam, where LPAIV prevalence in backyard farms ranged from 0.6% to 5.0% (0.6%, 95% CI: 0.1–1.7% in 2011; 1.7%, 95% CI: 0.8–3.0% in 2012 [4, 5, 20, 22, 23]; 1.4%, 95% CI: 0.7–2.3% in 2016; 5.0%, 95% CI: 3.4–7.2% in 2017 [18] and 0.8%, 95% CI: 0.2–2.0% in 2018 [17]). These results indicated that LPAIV prevalence varied among sample collection times and locations. Overall, LPAIV prevalence in backyard farms (0.6–5.0%) was higher than that in high biosecurity farms (0.0–2.3%) [17, 18]. Although HPAIV detection in farms during active surveillance was sporadic, the low biosecurity condition in backyard/small-scale farms might promote the occurrence of outbreaks [20]. In addition, the circulation of multisubtypes was also confirmed in Vietnam. This situation promoted the reassortment event that led to the antigenic shift. The report on the reassortment of LPAIV with H5N6 HPAIV was released in Vietnam [19, 21]. Furthermore, mixing many species and a free-grazing farming model might enhance the frequency of reassortment events [7]. Based on these results, countermeasures focused more on backyard farms should be established to reduce the risk of AIV infection in the region.
Considering the effect of individual birds, the unmeasured effect existed at the individual bird level because not all the poultry are positive for LPAIV infection under the same condition on a farm, meaning LPAI positivity at the individual bird level comprised the measured effect at herd level and unmeasured effect at individual bird level. In the mixed-effects model, the effects at herd level (or farm level) and individual bird level were included to explain the unmeasured effects operating at both levels that influenced the proportions of variance in LPAIV positivity. The multilevel analysis model provided the opportunity to separate the influence of the herd and individual bird on the risk of being LPAIV positive.
The strong correlation between the population size of aquatic birds and the circulation of AIVs, including LPAIVs, was confirmed in this study (Table 2). In the mixed model of the individual factors, keeping Muscovy ducks during farming had significantly higher risk of LPAIV positivity, consistent with a previous study that identified the role of Muscovy ducks as the promoter for AIV spreading [20]. However, only 84 of 150 variables were assessed in this model due to the zero count of positive birds. Based on the mixed model results of the individual factors, the range of odds and the standard error implied that it might contain unrevealed factors, and keeping Muscovy ducks might not be a sole variable contributing to LPAIV positivity in the total dataset. MCA analysis was performed to identify the clusters of responses for each section and be used as explanatory variables in the multivariable analysis to incorporate variables as much as possible. The profiles of each cluster showed the effect on LPAIV positivity among clusters in each section. Large-scale farms were more likely to consider the safety of the farming process through the quality of imported poultry, whereas small-scale farms prefer to buy low price hatchlings (Supplementary Table 3). Whereas most large-scale farms run poultry farming as the main business, the main business in most small-scale farms was rice padding, cattle farming, worker, etc. Therefore, small-scale farms pay less attention to poultry farming than large-scale farms. This would support that most small-scale farms lacked resources and were unlikely to pay money for infrastructure for raising poultry, which is a side business [10, 12]. Good practice by applying biosecurity measures can minimize the risk of LPAIV infection. In previous studies in the Mekong River Delta, good biosecurity practices, vaccination, and separation of poultry species significantly reduced AI risk in farms [11, 27]. In the previous study, countermeasures applied in LBMs might not appropriately prevent AIV introduction because infection in birds might occur before their introduction to LBMs [4].
In the fixed-effects logistic regression model, knowledge explanatory variable (‘Correct knowledge’ and ‘Mixed knowledge’) and attitude explanatory variable (‘Report AI but disagree with policy’, ‘Report AI and agree with policy’, and ‘Report AI no’) showed a significant correlation with LPAIV infection at the bird level (Table 3 ). In detail, farmers classified in the ‘Correct knowledge’ cluster had lower odds of their birds being LPAIV positive than farmers classified as having ‘Mixed knowledge’. Opposite trends were also confirmed in the attitude variable when the cluster of farmers was likely to report an outbreak of AI but disagreed with countermeasures against AIV by local authorities (‘Report AI but disagree with policy’), which was classified as the reference category. Farmers who provided consistent responses in their willingness to report an AI outbreak and support for the countermeasures applied by the local authorities (‘Report AI and agree with policy’) had lower odds of their birds being LPAIV positive. In contrast, farmers unwilling to report an AI outbreak to authorities (‘Report AI no’) had higher odds. Although none showed a significant association with LPAIV positivity status, the outcome of the mixed-effects regression model at the farm level, including a random effect, was similar to one of the fixed-effects regression models in the tendency and magnitude of the point estimates of the regression coefficients. A good attitude by reporting the outbreak might mitigate the risk of AI infection in the future was confirmed on farms in the Mekong River Delta in the previous study [11], meaning LPAI control should focus on improving the specific knowledge of LPAI to enhance the awareness of LPAI, leading to change the attitude at the farm level.
Table 3. A fixed-effects logistic regression model quantifying the association between clusters and low pathogenicity avian influenza virus positivity.
| Explanatory variable | Samples | LPAIV positive | Coefficient (SEM) | z | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|
| Intercept | 939 | 11 | −3.04 (0.73) | |||
| Knowledge | ||||||
| Mixed knowledge | 100 | 1 | Ref | Ref | Ref | 1.0 |
| Correct knowledge | 839 | 10 | −2.15 (1.07) | −2.00 | 0.04 | 0.1 (0.0‒0.6)a |
| Attitude | ||||||
| Report AI but disagree with policy | 79 | 2 | Ref | Ref | Ref | 1.0 |
| Report AI and agree with policy | 770 | 1 | −3.58 (1.24) | −2.89 | <0.01 | 0.0 (0.0‒0.3) |
| Report AI no | 90 | 8 | 1.26 (0.82) | 1.55 | 0.12 | 3.5 (0.8‒24.2) |
LPAIV: low pathogenicity avian influenza virus, SEM: standard error of the mean, OR: odds ratio, CI: confidence interval, Ref: reference, AI: avian influenza. a Interpretation: In the knowledge category, the odds of a birds being LPAIV positive if it was from a farm in ‘Correct knowledge’ cluster was 0.1 (95% CI: 0.0‒0.6) times the odds of a birds from a farm in ‘Mixed knowledge’ cluster being LPAIV positive.
This epidemiological investigation would be categorized as a trial study to estimate the prevalence of LPAIV and assume the risk factors relating to LPAIV infection, and the prevalence both at herd level and individual level was lower than our expectation. Given the low prevalence and epidemiology of LPAIV infection in poultry, monitoring and sampling methods should be improved to assess the true disease dynamics in population efficiently, like as antibody survey. Furthermore, because the questionnaire used in this study was developed based on past AIV surveillance, especially targeting HPAIV, specific and critical factors for LPAIV infection might not be fully covered. Further investigation, such as an unstructured survey covering specific knowledge of LPAI, would contribute to overcoming this issue by combining data from the community that might not be included in this questionnaire study. Furthermore, this study did not measure actual loss due to LPAIV infection, such as low egg or growth rates. By focusing on the potential risk factors of LPAIV, future studies should evaluate these outcomes to perform clearer and more direct epidemiological investigations to elucidate the economic loss of LPAIV infection.
The difference in farming systems and value chains might affect the ecology characteristics of AI. Therefore, effective AI control measures should consider the characteristics of the farmers in the specific region. Good knowledge is the key to controlling LPAI, and the local authority is the best candidate to transfer the correct knowledge about LPAI (100% of farmers got AI information from the local veterinarian or local officer). This phenomenon was suited for not only LPAI but also HPAI [18]. Long-term and intensive monitoring is the key to a deeper understanding of LPAI. The appropriate policy for LPAI control should be established based on the above scientific evidence.
CONFLICT OF INTEREST
The authors declare that no competing interests exist.
Supplementary Material
Acknowledgments
We thank the Vietnamese DAH. We thank Dr. Pham Van Dong from the DAH and Drs. Diep Quoc Truong, Nguyen Thi Thanh Thao, Tran Quoc Phong, and Ha Tan An and the RAHO7 staff for their kind support. We highly appreciate the great support of Prof. Mark A. Stevenson (Asia-Pacific Centre for Animal Health, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, Victoria, Australia) in modeling the analysis in R. We also thank the staff of the Vinh Long SDAH for field activities. This work was supported by the Program for Leading Graduate Schools (F01) from the Japan Society for the Promotion of Science, funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Japan Agency for Medical Research and Development (AMED; grant no. JP18fm0108008). This work was partially supported by the e-ASIA Joint Research Program funded by AMED (grant no. JP20jm0210054h0004). This work was partially supported by the Doctoral Program for World-Leading Innovative & Smart Education (WISE), by MEXT. This research was also supported by the International Priority Graduate Programs, Faculty of Veterinary and Medicine, Hokkaido University, powered by MEXT.
REFERENCES
- 1.Alexander D. J.2007. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002-2006. Avian Dis. 51 Suppl: 161–166. doi: 10.1637/7602-041306R.1 [DOI] [PubMed] [Google Scholar]
- 2.Alexander D. J., Brown I. H.2009. History of highly pathogenic avian influenza. Rev. Sci. Tech. 28: 19–38. [DOI] [PubMed] [Google Scholar]
- 3.Bates D., Mächler M., Bolker B., Walker S.2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 4.Chu D. H., Stevenson M. A., Nguyen L. V., Isoda N., Firestone S. M., Nguyen T. N., Nguyen L. T., Matsuno K., Okamatsu M., Kida H., Sakoda Y.2017. A cross-sectional study to quantify the prevalence of avian influenza viruses in poultry at intervention and non-intervention live bird markets in central Vietnam, 2014. Transbound. Emerg. Dis. 64: 1991–1999. doi: 10.1111/tbed.12605 [DOI] [PubMed] [Google Scholar]
- 5.Chu D. H., Okamatsu M., Matsuno K., Hiono T., Ogasawara K., Nguyen L. T., Van Nguyen L., Nguyen T. N., Nguyen T. T., Van Pham D., Nguyen D. H., Nguyen T. D., To T. L., Van Nguyen H., Kida H., Sakoda Y.2016. Genetic and antigenic characterization of H5, H6 and H9 avian influenza viruses circulating in live bird markets with intervention in the center part of Vietnam. Vet. Microbiol. 192: 194–203. doi: 10.1016/j.vetmic.2016.07.016 [DOI] [PubMed] [Google Scholar]
- 6.Das A., Spackman E., Senne D., Pedersen J., Suarez D. L.2006. Development of an internal positive control for rapid diagnosis of avian influenza virus infections by real-time reverse transcription-PCR with lyophilized reagents. J. Clin. Microbiol. 44: 3065–3073. doi: 10.1128/JCM.00639-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delabouglise A., Nguyen-Van-Yen B., Thanh N. T. L., Xuyen H. T. A., Tuyet P. N., Lam H. M., Boni M. F.2019. Poultry population dynamics and mortality risks in smallholder farms of the Mekong river delta region. BMC Vet. Res. 15: 205–218. doi: 10.1186/s12917-019-1949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducatez M. F., Webster R. G., Webby R. J.2008. Animal influenza epidemiology. Vaccine 26Suppl 4: D67–D69. doi: 10.1016/j.vaccine.2008.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FAO2021. Global animal disease information system (Empres-i). Food and Agriculture Organization of the United Nations, Rome. https://empres-i.review.fao.org/#/ [accessed on October 15, 2021].
- 10.Henning J., Henning K. A., Long N. T., Ha N. T., Vu T., Meers J.2013. Characteristics of two duck farming systems in the Mekong Delta of Viet Nam: stationary flocks and moving flocks, and their potential relevance to the spread of highly pathogenic avian influenza. Trop. Anim. Health Prod. 45: 837–848. doi: 10.1007/s11250-012-0296-9 [DOI] [PubMed] [Google Scholar]
- 11.Henning K. A., Henning J., Morton J., Long N. T., Ha N. T., Meers J.2009. Farm- and flock-level risk factors associated with Highly Pathogenic Avian Influenza outbreaks on small holder duck and chicken farms in the Mekong Delta of Viet Nam. Prev. Vet. Med. 91: 179–188. doi: 10.1016/j.prevetmed.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 12.Hong Hanh P. T., Burgos S., Roland-Holst D.2007. The poultry sector in Viet Nam: Prospects for smallholder producers in the aftermath of the HPAI crisis. Pro-poor livestock policy initiative research report. Food and Agriculture Organisation of the United Nations. https://www.fao.org/3/bp284e/bp284e.pdf [assessed on October 16, 2021].
- 13.Howley M., Knipe D.2020. Orthomyxoriruses. pp. 649–673. In: Fields Virology: Emerging Viruses, 7th ed., Wolters Kluwer, Philadelphia. [Google Scholar]
- 14.Husson F., Josse J., Lê S.2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25: 31460–31478. [Google Scholar]
- 15.Kida H., Yanagawa R.1979. Isolation and characterization of influenza a viruses from wild free-flying ducks in Hokkaido, Japan. Zentralbl. Bakteriol. Orig. A 244: 135–143. [PubMed] [Google Scholar]
- 16.Kinde H., Read D. H., Daft B. M., Hammarlund M., Moore J., Uzal F., Mukai J., Woolcock P.2003. The occurrence of avian influenza A subtype H6N2 in commercial layer flocks in Southern California (2000–02): clinicopathologic findings. Avian Dis. 47 Suppl: 1214–1218. doi: 10.1637/0005-2086-47.s3.1214 [DOI] [PubMed] [Google Scholar]
- 17.Le K. T., Okamatsu M., Nguyen L. T., Matsuno K., Chu D. H., Tien T. N., Le T. T., Kida H., Sakoda Y.2020. Genetic and antigenic characterization of the first H7N7 low pathogenic avian influenza viruses isolated in Vietnam. Infect. Genet. Evol. 78: 104117–104125. doi: 10.1016/j.meegid.2019.104117 [DOI] [PubMed] [Google Scholar]
- 18.Le K. T., Stevenson M. A., Isoda N., Nguyen L. T., Chu D. H., Nguyen T. N., Nguyen L. V., Tien T. N., Le T. T., Matsuno K., Okamatsu M., Sakoda Y.2021. A systematic approach to illuminate a new hot spot of avian influenza virus circulation in South Vietnam, 2016–2017. Transbound. Emerg. Dis. (in press). [DOI] [PubMed] [Google Scholar]
- 19.Le T. B., Le V. P., Lee J. E., Kang J. A., Trinh T. B. N., Lee H. W., Jeong D. G., Yoon S. W.2021. Reassortant highly pathogenic H5N6 avian influenza virus containing low pathogenic viral genes in a local live poultry market, Vietnam. Curr. Microbiol. 78: 3835–3842. doi: 10.1007/s00284-021-02661-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen L. T., Stevenson M. A., Firestone S. M., Sims L. D., Chu D. H., Nguyen L. V., Nguyen T. N., Le K. T., Isoda N., Matsuno K., Okamatsu M., Kida H., Sakoda Y.2020. Spatiotemporal and risk analysis of H5 highly pathogenic avian influenza in Vietnam, 2014–2017. Prev. Vet. Med. 178: 104678–104688. doi: 10.1016/j.prevetmed.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen L. T., Firestone S. M., Stevenson M. A., Young N. D., Sims L. D., Chu D. H., Nguyen T. N., Nguyen V. L., Lee T. T., Nguyen V. H., Nguyen H. N., Tien T. N., Nguyen D. T., Tran B. N., Matsuno K, Okamatsu M., Kida H., Sakoda Y. 2019. A systematic study towards evolutionary and epidemiological dynamics of currently predominant H5 highly pathogenic avian influenza viruses in Vietnam. Sci. Rep. 9: 7723–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura N., Sakoda Y., Endo M., Yoshida H., Yamamoto N., Okamatsu M., Sakurai K., Hoang N. V., Nguyen L. V., Chu H. D., Tien T. N., Kida H.2012. Characterization of avian influenza viruses isolated from domestic ducks in Vietnam in 2009 and 2010. Arch. Virol. 157: 247–257. doi: 10.1007/s00705-011-1152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamatsu M., Nishi T., Nomura N., Yamamoto N., Sakoda Y., Sakurai K., Chu H. D., Thanh L. P., Van Nguyen L., Van Hoang N., Tien T. N., Yoshida R., Takada A., Kida H.2013. The genetic and antigenic diversity of avian influenza viruses isolated from domestic ducks, muscovy ducks, and chickens in northern and southern Vietnam, 2010–2012. Virus Genes 47: 317–329. doi: 10.1007/s11262-013-0954-7 [DOI] [PubMed] [Google Scholar]
- 24.R Core Team 2021. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna. https://www.r-project.org/ [assessed on May 16, 2021].
- 25.Snijders T., Bosker R.1999. Multilevel analysis: an introduction to basic and advanced multilevel modeling, SAGE Publications, New Delhi. [Google Scholar]
- 26.Stevenson M. A., Sergeant E., Nunes T., Heuer C., Marshall J., Sanchez J., Thornton R., Reiczigel J., Robison-Cox J., Sebastiani P., Solymos P., Yoshida K., Jones G., Pirikahu S., Firestone S. M., Kyle R., Popp J., Jay M., Reynard C.2021. epiR: Tools for the analysis of epidemiological data. R package version 2.0.19, https://fvas.unimelb.edu.au/research/groups/veterinary-epidemiology-melbourne [assessed on August 16, 2021].
- 27.Tung D. X., Costales A.2007. Market participation of smallholder poultry producers in northern Viet Nam, Food and Agriculture Organisation of the United Nations. https://www.fao.org/documents/card/en/c/bb4a8d66-6ece-44c4-a745-305e536a11ff/ [assessed on October 18, 2021].
- 28.Webster R. G., Bean W. J., Gorman O. T., Chambers T. M., Kawaoka Y.1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56: 152–179. doi: 10.1128/mr.56.1.152-179.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


