Abstract
The yeast Schwanniomyces occidentalis produces a killer toxin lethal to sensitive strains of Saccharomyces cerevisiae. Killer activity is lost after pepsin and papain treatment, suggesting that the toxin is a protein. We purified the killer protein and found that it was composed of two subunits with molecular masses of approximately 7.4 and 4.9 kDa, respectively, but was not detectable with periodic acid-Schiff staining. A BLAST search revealed that residues 3 to 14 of the 4.9-kDa subunit had 75% identity and 83% similarity with killer toxin K2 from S. cerevisiae at positions 271 to 283. Maximum killer activity was between pH 4.2 and 4.8. The protein was stable between pH 2.0 and 5.0 and inactivated at temperatures above 40°C. The killer protein was chromosomally encoded. Mannan, but not β-glucan or laminarin, prevented sensitive yeast cells from being killed by the killer protein, suggesting that mannan may bind to the killer protein. Identification and characterization of a killer strain of S. occidentalis may help reduce the risk of contamination by undesirable yeast strains during commercial fermentations.
Killer yeasts secrete toxins lethal to sensitive yeasts but are immune to their own toxins. Since first discovered in Saccharomyces cerevisiae (2), killer strains have been isolated from several yeast genera, including Candida (46), Cryptococcus (10), Hanseniaspora (33), Kluyveromyces (14), Pichia (27), Torulopsis (7), Ustilago (30), Williopsis (45), and Zygosaccharomyces (32). Based on killing and immunity interactions among killer yeasts, killer phenotypes are classified into at least 10 groups (48) and the responsible genes may be carried on a chromosome (S. cerevisiae KHS, KHR, Williopsis mrakii) (11, 12, 21), on a double-stranded RNA (dsRNA) (S. cerevisiae K1, K2, K28, Ustilage maydis, Hanseniaspora uvarum) (5, 15, 22, 35, 49), or on a linear double-stranded DNA (dsDNA) (Kluyveromyces lactis, Pichia inositovora, Pichia acaciae) (14, 16, 44).
Schwanniomyces occidentalis produces amylolytic enzymes, including α-amylase and glucoamylase (8). It is one of the few yeasts capable of completely hydrolyzing soluble starch. Moreover, it can grow to high cell mass by utilizing cheap starch from plants such as cassava, corn, potato, sorghum, and wheat as the carbon source (40). S. occidentalis has been used to produce ethanol and single cell protein from starch fermentation (19, 42). S. occidentalis has no detectable extracellular proteases and can secrete large proteins (40). It also has an established transformation system and available inducible promoters, which could make it a commercially important system for the production of heterologous proteins (40). For example, endoglucanase D recently has been successfully expressed and secreted in this system (31).
Wild killer yeasts sometimes contaminate cultures of industrial yeasts, resulting in lagging or stopped fermentation and poor product quality (39). To avoid such complications, the use of industrial killer strains as starters has been suggested (17). Commercially interesting killer strains for sake brewing, wine making, alcohol fermentation, and lager, beer, and ale production have been constructed (29, 36, 39, 47). Furthermore, the W. mrakii mycocin expressed by Aspergillus niger can reduce silage and yogurt spoilage caused by yeasts (25).
The killer phenotype of S. occidentalis has not been described previously. Thus, our objectives in this study were as follows: (i) to screen killer strains from S. occidentalis for a killer phenotype; (ii) to purify and partially characterize this killer toxin, including the effect of pH and temperature on its stability and activity; (iii) to identify whether this killer protein is related to other yeast killer proteins by N-terminal amino acid sequencing; (iv) to determine the location of the killer protein gene; and (v) to identify possible toxin binding sites in the cell wall of a sensitive yeast. From our studies, we will determine the relationship between the killer strain from S. occidentalis and other killer yeasts and whether the killer toxin in this yeast could be used in an industrial fermentation.
MATERIALS AND METHODS
Yeast strains and media.
All yeast strains were obtained from the American Type Culture Collection (ATCC). S. cerevisiae ATCC 26609 was used as the sensitive strain. According to the cross-interaction assay of Young and Yagiu (48), we classified S. occidentalis ATCC 44252 by interaction between killer yeast strains, which included S. cerevisiae ATCC 60733 (K1), S. cerevisiae ATCC 36900 (K2), Saccharomyces capensis ATCC 36899 (K3), Torulopsis glabrata ATCC 36909 (K4), Hansenula subpelliculosa ATCC 36905 (K5), Kluyveromyces fragilis ATCC 36907 (K6), Candida valida ATCC 36897 (K7), Hansenula anomala ATCC 36904 (K8), W. mrakii ATCC 10743 (K9), Candida glabrata ATCC 15126 (K11) and K. lactis ATCC 8585. All yeast strains were grown on yeast extract-peptone-dextrose (YEPD)–agar slants (1% yeast extract, 2% peptone, 2% glucose, and 2% agar) at 24°C and were maintained at 4°C. For the killer activity assay, YEPD-agar medium was dissolved with 0.1 M citrate phosphate buffer and adjusted to pH 4.6, and methylene blue was added to 0.03% as an indicator. Unless otherwise specified, all strains were incubated for 2 days at 24°C with shaking at 120 rpm on a rotary shaker.
Killer activity assay.
We determined killer toxin activity with a well test (43). The plate was seeded with the sensitive strain at a final concentration of 6 × 105 cells per ml of the assay medium. The inhibition zones were measured after 48 h of incubation at 24°C. A linear relationship was observed between the diameter of the clear zone (in millimeters; x axis) and the logarithm of the amount of killer protein (in nanograms; y axis). By the linear regression equation (y = 0.30x − 0.36; R2 = 0.999), the amount of the killer protein of samples was calculated. We defined one arbitrary unit (aU) as the amount of the killer protein that caused a clear zone of 1 mm. One arbitrary unit corresponded to about 0.9 ng of killer protein. Thus, the killer activity was quantified from the bioassay by converting the diameter of the clear zone into the arbitrary unit. For the cross-interaction assay, S. occidentalis was streaked on assay plates that were seeded with other killer yeast strains.
Preparation and concentration of crude killer protein.
S. occidentalis ATCC 44252 was cultivated in YEPD medium with 20 mM citrate phosphate buffer (pH 4.4) in a 5-liter fermenter (Mini-JAR Fermentor KMJ; Mituwa Co., Osaka, Japan) with a 2.5-liter working volume and was stirred at 120 rpm. The temperature was maintained at 24°C. After 18 h, the cultures were centrifuged at 5,000 × g for 15 min to remove the cells. The supernatant was concentrated with spiral-wound membrane cartridge S1Y3 (MWCO, 3 kDa; Amicon, Beverly, Mass.).
Purification of killer protein.
The concentrate was applied to a QAE-Sephadex A-25 column (5.5 by 11 cm; Pharmacia Biotech, Uppsala, Sweden) to which the killer protein did not bind when equilibrated with 20 mM citrate phosphate buffer (pH 4.4). The eluate was collected and applied to a butyl-Toyopearl 650M column (2.5 by 27 cm; Tosoh Corporation, Tokyo, Japan) preequilibrated with 20 mM citrate phosphate buffer (pH 4.4) containing 0.7 M ammonium sulfate. The column was first washed with 200 ml of 0.7 M ammonium sulfate in the same buffer, followed by 1 liter of 20 mM citrate phosphate buffer (pH 4.4). The killer protein was eluted with 1.2 liter of 20 mM citrate phosphate buffer (pH 4.4) containing 10% ethylene glycol (Nacalai Tesque, Inc., Kyoto, Japan). The fractions containing the killer activity were pooled and loaded onto a Fractogel EMD COO− 650(S) column (1 by 2 cm; E. Merck, Darmstadt, Germany) equilibrated with 20 mM citrate phosphate buffer (pH 4.4). The killer protein was eluted at 0.3 M NaCl in the same buffer. To determine if the killer protein was a heterodimer protein, the purified killer protein, insulin, cytochrome c, ovalbumin, and blue dextran were subjected, separately, to a Fractogel TSK HW50 (F) column (1.5 by 95 cm; E. Merck).
Electrophoresis.
In native electrophoresis, a continuous acid polyacrylamide gel was prepared in a 0.375 M acetic acid–KOH solution (pH 4.2) (3). After samples were loaded, electrophoresis was carried out at a constant voltage of 70 V at 4°C overnight, using methylene blue as the tracking dye. In Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 20), the purified killer protein was subsequently analyzed with or without β-mercaptoethanol. Tricine was substituted for glycine to enhance the resolution of small proteins (34). The protein bands were visualized by staining the gel with Coomassie blue R 250 (E. Merck).
To determine if the separated subunits have the killing effect, the purified killer protein was incubated with 10% mercaptoethanol in 20 mM citrate phosphate buffer (pH 4.4) for 24 h at room temperature (24 to 28°C). Because β-mercaptoethanol interferes with the growth of sensitive yeast, an aliquot of β-mercaptoethanol-treated killer protein was dialyzed (Spectra/Por membrane; MWCO, 1,000; Spectrum Medical Industries, Inc., Gardena, Calif.) with 20 mM citrate phosphate buffer (pH 4.4) for 18 h and then was tested for its killer activity by a well test. Samples with or without dialysis were analyzed in Tricine SDS-PAGE under nonreducing conditions (no β-mercaptoethanol).
NH2-terminal amino acid sequencing.
After electrophoresis, the purified killer protein was transferred to a polyvinylidene fluoride membrane and stained with Coomassie blue (26). Two protein bands were cut out and subjected to 15 cycles of sequence analysis in an Applied Biosystems model 477A protein sequencer (Foster City, Calif.).
Extraction of dsRNA plasmids.
dsRNA was prepared as described by Fried and Fink (9) and was purified by using CF11 cellulose (Whatman International Ltd., Maidstone, England) (41). The eluate from the CF11 cellulose pellet was precipitated with ethanol, washed once with 70% ethanol, and resuspended in TE buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA [pH 8.0]). Samples were analyzed by 1% agarose gel electrophoresis.
Extraction of dsDNA plasmids.
The dsDNA plasmids were isolated by using an osmotic lysis protocol (38). The protoplasts of yeast cells were prepared as described by Skala and Kotylak (37). After osmotic lysis of protoplasts in TM buffer (10 mM Tris-HCl [pH 8.0], 10 mM MgCl2), the lysate was centrifuged for 15 min at 10,000 × g. After treatment with RNase (300 μg/ml for 1 h at 37°C) and proteinase K (50 μg/ml for 1 h at 37°C), samples were extracted with phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with ethanol, and washed once with 70% ethanol. The DNA pellets were dissolved in TE buffer and analyzed by 0.7% agarose gel electrophoresis.
Plasmid nomenclature.
We named the linear plasmids in this study according to the system set forth in Ligon et al. (24). The larger plasmid was designated pSocl-1 and the smaller one pSocl-2.
Curing.
The killer yeast was spread on YEPD-agar plates at a density of 4.5 × 103 cells/plate and was subjected to UV irradiation (254 nm) at a dose of 20,000 μJ/cm2 for 9 s from a UV cross-linker (Stratalinker UV Crosslinker 1800; La Jolla, Calif.) (13). UV-irradiated plates were incubated at 24°C for 4 days in the dark to prevent photoreactivation of DNA repair. The surviving colonies were transferred with toothpicks to assay plates for killer activity measurement. The presence of DNA plasmids was confirmed by agarose gel electrophoresis.
Determination of the binding of killer protein by polysaccharides.
Various amounts of polysaccharides (glucan, laminarin, and mannan; Sigma), which are components of the yeast cell wall, were mixed with 4.3 × 105 cells of the susceptible S. cerevisiae strain in YEPD medium. Because β-glucan could not be fully dissolved in 20 mM citrate phosphate buffer (pH 4.4), it was initially dissolved in an alkaline solution and then adjusted to a final pH of 4.4. Killer protein (2,500 aU) or buffer was added and incubated at 24°C for 24 h. Following dilution, the cells were plated on YEPD-agar plates and the number of viable cells was determined.
RESULTS
Killer activity of S. occidentalis.
We screened 33 strains of S. occidentalis for the ability to kill S. cerevisiae ATCC 26609. Eight strains had killer activity, of which ATCC 44252 had the highest. The S. cerevisiae (K1 and K2), S. capensis (K3), T. glabrata (K4), H. subpelliculosa (K5), H. anomala (K8), and C. glabrata (K11) killer yeasts were sensitive to the killer toxin of S. occidentalis ATCC 44252, while K. fragilis (K6), C. valida (K7), W. mrakii (K9), and K. lactis were resistant. This killing pattern was similar to that of toxin K9, which is produced by W. mrakii (48).
Purification of the killer protein of S. occidentalis ATCC 44252.
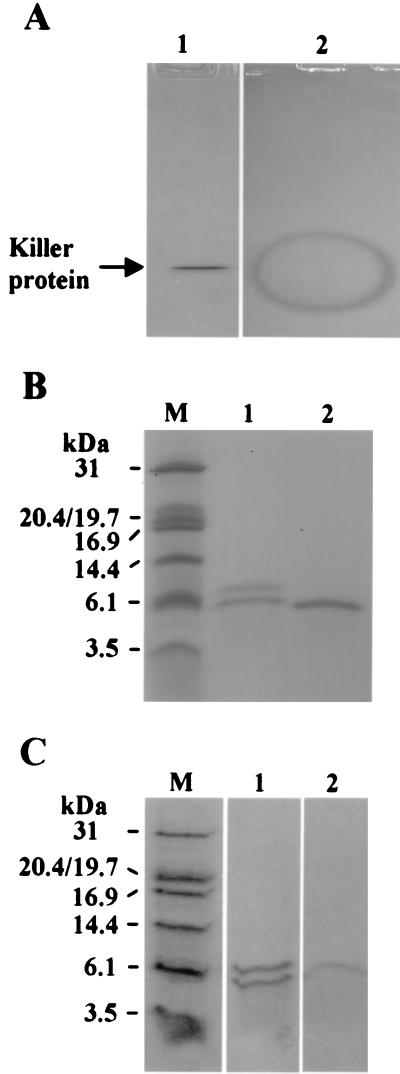
To determine if the killer toxin was a protein, we incubated the killer toxin with pepsin and papain. The killer activity was completely destroyed by both enzymes (data not shown), suggesting that the killer toxin is a protein. This killer protein was concentrated by ultrafiltration and purified by quaternary aminoethyl, butyl, and COO− chromatography (Table 1). The killer activity of the purified toxin increased 13,000-fold, and the yield was 13%. In native gel electrophoresis, only a single protein band was found that produced a clear inhibition zone when the gel was overlaid on an assay plate for killer activity measurement (Fig. 1A). In contrast, no distinct band could be observed by periodic acid-Schiff staining (data not shown). Two protein bands with apparent molecular masses of 7.4 and 4.9 kDa were detected when separated by Tricine SDS-PAGE, but only one protein band was detected if β-mercaptoethanol was omitted from the sample buffer (Fig. 1B). In the native Fractogel TSK HW50 (F) chromatography, the killer activity comigrated with cytochrome c, which has a molecular mass of 12.3 kDa (data not shown). These results are consistent with the hypothesis that the killer protein of S. occidentalis is a heterodimer composed of two disulfide-linked subunits.
TABLE 1.
Purification of the killer protein from S. occidentalisa
| Stepb | Total vol (ml) | Total protein (mg) | Total activity (107 aU) | Sp act (102 aU/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Culture broth | 10,000 | 160,000 | 1.6 | 1.0 | 1.0 | 100 |
| Ultrafiltration | 880 | 44,000 | 1.1 | 2.5 | 2.5 | 69 |
| QAE-Sephadex | 1,000 | 9,200 | 1.0 | 11 | 11 | 63 |
| Butyl-Toyopearl | 2,400 | 31 | 0.6 | 1,900 | 1,900 | 38 |
| Fractogel EMD COO− | 44 | 1.5 | 0.2 | 13,000 | 13,000 | 13 |
Purification procedures are described in Materials and Methods; 1 aU, the amount of killer protein that caused a clear zone of 1 mm.
QAE, quaternary aminoethyl.
FIG. 1.
(A) Native PAGE analysis of the killer protein stained with Coomassie blue. Lane 1, purified killer protein in an 18% acidic native polyacrylamide gel (pH 4.2); lane 2, an inhibition zone on methylene blue agar plate overlaid with a native gel containing the purified killer protein. (B) Tricine SDS-PAGE of the killer protein stained with Coomassie blue. M, protein molecular mass markers (Promega Corporation, Madison, Wis.), including carbonic anhydrase (31 kDa), soybean trypsin inhibitor doublet (20.4/19.7 kDa), horse heart myoglobin (16.9 kDa), lysozyme (14.4 kDa), aprotinin (6.1 kDa), and insulin β chain (3.5 kDa); lane 1, with β-mercaptoethanol; lane 2, without β-mercaptoethanol. Lanes 1 and 2 contain 1.4 μg of protein. (C) Nonreducing Tricine SDS-PAGE of the killer protein stained with Coomassie blue. M, protein marker. Lanes 1 (1.6 μg) and 2 (0.7 μg) are 10% mercaptoethanol-treated killer protein with and without dialysis, respectively.
We also treated the killer protein and its separated subunits with 10% β-mercaptoethanol and resolved them by Tricine SDS-PAGE in the absence of β-mercaptoethanol. The killer protein without dialysis separated into two protein bands, and that with dialysis yielded only a single protein band (Fig. 1C) that had no killer activity. Thus, β-mercaptoethanol separated the killer protein into two subunits. Moreover, β-mercaptoethanol influenced the conformation of the killer protein, resulting in the loss of the killer activity even after it was removed.
Properties of the purified killer protein.
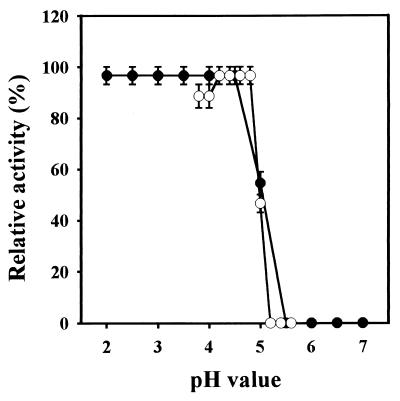
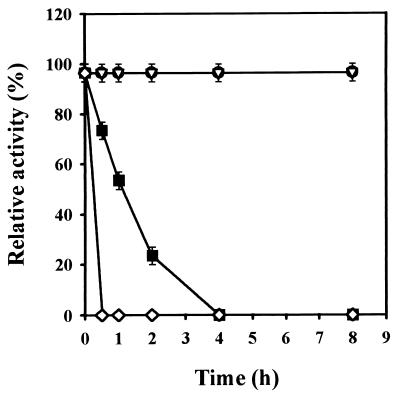
The killer protein was active only at an acidic pH (Fig. 2), with the optimal pH between 4.2 and 4.8. The killer protein was stable in the range of pH 2.0 to 5.0 for at least 8 h. When maintained at pH 5.0 for 8 h at 24°C, the killer protein lost half of the activity, and the activity was completely lost at pH 6.0 (Fig. 2). The protein was stable in 20 mM citrate phosphate (pH 4.4) at both 30 and 20°C for at least 8 h but had a half life of 1 h at 40°C (Fig. 3).
FIG. 2.
Effect of pH on the killer activity (○) or on the stability (●) of the purified killer protein from S. occidentalis. The change of pH values was adjusted with 0.1 M citrate phosphate buffer. The 100% killer activity is 500 aU, under conditions containing 0.4 μg of killer protein. To determine the optimal pH, the killer protein solution was adjusted to various pH values, and the killer activity of samples was then determined with the assay plate that had identical pH values. For pH stability, after incubation in different pH values at 24°C for 8 h, the pH value of samples was adjusted to a final pH of 4.4, and the residual killer activity was determined. Error bars represent the mean ± the standard deviation of the mean for duplicate samples.
FIG. 3.
Effect of temperature on the stability of killer protein from S. occidentalis. The 100% killer activity is 500 aU, under conditions containing 0.4 μg of killer protein. ●, 20°C; ▿, 30°C; ■, 40°C; ◊, 50°C. Error bars represent the mean ± the standard deviation of the mean for duplicate samples.
NH2-terminal amino acid sequences of killer protein.
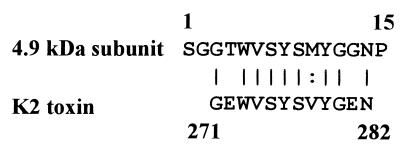
We sequenced the 15 NH2-terminal amino acids. No proteins in the databases examined (nonredundant GenBank, including coding sequence translations, Brookhaven protein data bank, SwissProt, Spupdate, and protein information resource) had the same sequence as the killer protein using position-speciifc iterated BLAST analysis (1). The NH2-terminal region of the 4.9-kDa subunit (residues 3 to 14) has 75% identity and 83% similarity to the K2 toxin at positions 271 to 282 (Fig. 4).
FIG. 4.
N-terminal amino acid sequences of a 4.9-kDa subunit of the S. occidentalis killer protein. Fifteen NH2-terminal amino acid residues were displayed in a one-letter code. Residues 3 to 14 from the 4.9-kDa subunit were aligned with residues 271 to 282 from the K2 toxin. The solid lines indicate identical residue; the dotted line indicates similar residue.
Plasmid isolation and curing.
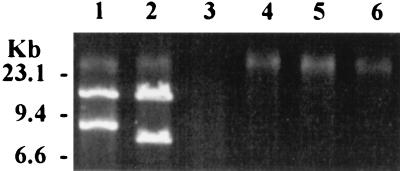
Yeast killer protein genes may be located on either plasmids or chromosomes. We did not isolate any dsRNA plasmids. We did isolate two dsDNA plasmids of 13.4 and 8.1 kb designated pSocl-1 and pSocl-2, respectively (Fig. 5, lane 2). These plasmids were sensitive to DNase but not to RNase. They could be digested with exonuclease III, suggesting that pSocl-1 and pSocl-2 are linear (Fig. 5, lane 3).
FIG. 5.
Agarose gel electrophoresis of dsDNA plasmids extracted from killer yeasts. Lane 1 (125 ng), K. lactis; lane 2 (215 ng), S. occidentalis; lane 3 (215 ng), the plasmids of S. occidentalis with ExoIII digestion; lanes 4 to 6 (15, 15, and 10 ng, respectively), UV irradiation-cured S. occidentalis isolates.
The survival rate of the 20,000 μJ/cm2 UV-treated cells was 2.4%. All 108 surviving isolates retained the killer activity. Three isolates arbitrarily selected from the 108 colonies lost both dsDNA plasmids (Fig. 5, lanes 4 to 6) but retained both killer activity and immunity to their own toxin. These data strongly suggest that the production of killer protein and immunity to it are not associated with the dsDNA plasmids and that these functions probably are coded by genes on the chromosomal DNA.
Competitive inhibition of killer protein action by polysaccharides.
The primary components of the yeast cell wall are glucans and mannoproteins. We tested β-glucan, laminarin (β-1,3-glucan), and mannan as competitive inhibitors of the killing process. When 4.3 × 105 cells of the sensitive yeast S. cerevisiae were incubated with the killer toxin for 24 h, less than 100 cells could survive. In the absence of killer toxin, the cells grew to a total of 5.2 × 107 cells. Of the polysaccharides tested, 10 mg of β-glucan and laminarin/ml could not rescue cells, and the number of surviving cells was less than 100. Only mannan competitively inhibited the killing and enhanced cell survival. At concentrations of 5 and 10 mg of mannan/ml, the number of surviving cells reached 6.1 × 103 and 3.0 × 107, respectively. We think that the mannan in the cell wall might provide a binding site for killer protein.
DISCUSSION
Yeast killer strains are characterized by a killing spectrum based on the sensitivity and resistance of other yeasts to the killer toxin. We used the killing spectrum to identify the killer phenotype of this S. occidentalis strain. Ten different groups, K1 to K10 (48), are recognized with respect to killing and resistance phenotypes. In the cross-interaction assay, the spectrum of the killer activity of S. occidentalis is similar to that of W. mrakii (K9), but other biochemical properties are different, including pH stability, thermostability, and molecular mass (45). The killer protein of S. occidentalis is a dimer and is active only at moderate temperature (30°C for 8 h) and at acidic pH (pH 2 to 5), whereas the killer protein of W. mrakii (designated HM-1 or HMK) is a monomer and is stable at higher temperatures (100°C for 10 min) and across a wider range of pHs (2 to 11). Thus, the killer protein of S. occidentalis is different from those of the K1 to K10 classes. Although portions of the N-terminal amino acid sequences of the killer protein from S. occidentalis are similar to portions of the S. cerevisiae K2 toxin (Fig. 4), S. cerevisiae K2 is sensitive to the killer strain of S. occidentalis, while S. occidentalis is resistant to K2 toxin. These results are consistent with the hypothesis that the killer protein of S. occidentalis is in a new class.
Linear DNA plasmids are present in a wide range of yeast species. We identified two endogenous plasmids (pSocl-1 and pSocl-2) from S. occidentalis. In the killer yeast system, the linear dsDNA plasmids of K. lactis and P. acaciae associated with killer phenotype and immunity were highly sensitive to UV irradiation (28, 44). When we cured S. occidentalis isolates of their plasmids, the cured strains still produced toxin and possessed immunity, so we conclude that the killer protein and immunity of S. occidentalis are probably chromosomally encoded.
Analysis by Tricine SDS-PAGE under denaturing or nondenaturing conditions supports the hypothesis that the killer protein of S. occidentalis is composed of two subunits linked by disulfide bonds (Fig. 1B). Furthermore, removal of the β-mercaptoethanol causes the two β-mercaptoethanol-separated subunits to form a single ineffective killer protein band (Fig. 1C). These data suggest that the conformation of the killer protein is essential for killer activity.
The cytocidal action of the S. occidentalis killer toxin was prevented by the addition of mannan. Mannoproteins may be the primary receptors for the S. occidentalis toxin. In preliminary experiments, we found that bromocresol purple could enter the cytoplasm of cells after treatment with S. occidentalis killer toxin (data not shown). Bromocresol purple staining has been described for detecting yeast cells with plasma membrane damage and is used to determine the activity of S. cerevisiae pore-forming killer toxin K1 (6, 23). We hypothesize that the S. occidentalis toxin binds to mannoproteins on the cell wall of sensitive yeasts. Consequently, this toxin damages the plasma membrane, resulting in the leakage of cytoplasmic components and cell death.
The killer phenotype has been transferred to commercial strains to combat wild strains during the production of beer (47), wine (36), and bread (4) or to prevent yeast spoilage during food preservation (18, 25). S. occidentalis has been used to produce ethanol and single cell protein from starch by fermentation (19, 42) and is a promising host for the production of heterologous proteins (40). Through breeding or genetic engineering, the killer system from S. occidentalis may be made available for industrial fermentations to reduce the risk of contamination by wild yeast strains.
ACKNOWLEDGMENTS
This work was supported by a grant (NSC 85-2331-B-010-019) from the National Science Council of the Republic of China.
Shenq-Chyi Chang thanks the Medical Research and Advancement Foundation for a research award in memory of Chi-Shuen Tsou during the course of this study.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan E A, Makower M. Proceedings of the XIth International Congress on Genetics. 1963. The physiological basis of the killer character in yeast; pp. 202–203. [Google Scholar]
- 3.Bollag D M, Rozycki M D, Edelstein S J. Gel electrophoresis under nondenaturing conditions. In: Bollag D M, Rozycki M D, Edelstein S J, editors. Protein methods. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 155–172. [Google Scholar]
- 4.Bortol A, Nudel C, Fraille E, De Torres R, Giuletti A, Spencer J F T, Spencer D. Isolation of yeast with killer activity and its breeding with an industrial baking strain by protoplast fusion. Appl Microbiol Biotechnol. 1986;24:414–416. [Google Scholar]
- 5.Bostian K A, Hopper J E, Rogers D T, Tipper D J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980;19:403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- 6.Bussey H. K1 killer toxin, a pore-forming protein from yeast. Mol Microbiol. 1991;5:2339–2343. doi: 10.1111/j.1365-2958.1991.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 7.Bussey H, Skipper N. Membrane-mediated killing of Saccharomyces cerevisiae by glycoproteins from Torulopsis glabrata. J Bacteriol. 1975;124:476–483. doi: 10.1128/jb.124.1.476-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deibel M R, Jr, Hiebsch R R, Klein R D. Secreted amylolytic enzymes from Schwanniomyces occidentalis: purification by fast protein liquid chromatography (FPLC) and preliminary characterization. Prep Biochem. 1988;18:77–120. doi: 10.1080/00327488808062514. [DOI] [PubMed] [Google Scholar]
- 9.Fried H M, Fink G R. Electron microscopic heteroduplex analysis of “killer” double-stranded RNA species from yeast. Proc Natl Acad Sci USA. 1978;75:4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golubev W, Shabalin Y. Microcin production by the yeast Cryptococcus humicola. FEMS Microbiol Lett. 1994;119:105–110. doi: 10.1111/j.1574-6968.1994.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 11.Goto K, Fukuda H, Kichise K, Kitano K, Hara S. Cloning and nucleotide sequence of the KHS killer gene of Saccharomyces cerevisiae. Agric Biol Chem. 1991;55:1953–1958. [PubMed] [Google Scholar]
- 12.Goto K, Iwatuki Y, Kitano K, Obata T, Hara S. Cloning and nucleotide sequence of the KHR killer gene of Saccharomyces cerevisiae. Agric Biol Chem. 1990;54:979–984. [PubMed] [Google Scholar]
- 13.Gunge N, Takahashi S, Fukuda K, Ohnishi T, Meinhardt F. UV hypersensitivity of yeast linear plasmids. Curr Genet. 1994;26:369–373. doi: 10.1007/BF00310503. [DOI] [PubMed] [Google Scholar]
- 14.Gunge N, Tamaru A, Ozawa F, Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981;145:382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannig E M, Leibowitz M J. Structure and expression of the M2 genomic segment of a type 2 killer virus of yeast. Nucleic Acids Res. 1985;13:4379–4400. doi: 10.1093/nar/13.12.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayman G T, Bolen P L. Linear DNA plasmids of Pichia inositovora are associated with a novel killer toxin activity. Curr Genet. 1991;19:389–393. doi: 10.1007/BF00309600. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs C J, Van Vuuren H J J. Effects of different killer yeasts on wine fermentations. Am J Enol Vitic. 1991;42:295–300. [Google Scholar]
- 18.Jakobsen M, Narvhus J. Yeasts and their possible beneficial and negative effects on the quality of dairy products. Int Dairy J. 1996;6:755–768. [Google Scholar]
- 19.Jamuna R, Ramakrishna S V. SCP production and removal of organic load from cassava starch industry waste by yeasts. J Ferment Bioeng. 1989;67:126–131. [Google Scholar]
- 20.Khalkhali-Ellis Z. An improved SDS-polyacrylamide gel electrophoresis for resolution of peptides in the range of 3.5-200 kDa. Prep Biochem. 1995;25:1–9. doi: 10.1080/10826069508010103. [DOI] [PubMed] [Google Scholar]
- 21.Kimura T, Kitamoto N, Matsuoka K, Nakamura K, Iimura Y, Kito Y. Isolation and nucleotide sequences of the genes encoding killer toxins from Hansenula mrakii and H. saturnus. Gene. 1993;137:265–270. doi: 10.1016/0378-1119(93)90018-x. [DOI] [PubMed] [Google Scholar]
- 22.Koltin Y, Day P R. Inheritance of killer phenotypes and double-stranded RNA in Ustilago maydis. Proc Natl Acad Sci USA. 1976;73:594–598. doi: 10.1073/pnas.73.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurzweilova H, Sigler K. Fluorescent staining with bromocresol purple: a rapid method for determining yeast cell dead count developed as an assay of killer toxin activity. Yeast. 1993;9:1207–1211. doi: 10.1002/yea.320091107. [DOI] [PubMed] [Google Scholar]
- 24.Ligon J M, Bolen P L, Hill D S, Bothast R J, Kurtzman C P. Physical and biological characterization of linear DNA plasmids of the yeast Pichia inositovora. Plasmid. 1989;21:185–194. doi: 10.1016/0147-619x(89)90042-5. [DOI] [PubMed] [Google Scholar]
- 25.Lowes K F, Shearman C A, Payne J, MacKenzie D, Archer D B, Merry R J, Gasson M J. Prevention of yeast spoilage in feed and food by the yeast mycocin HMK. Appl Environ Microbiol. 2000;66:1066–1076. doi: 10.1128/aem.66.3.1066-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 27.Middelbeek E J, Hermans J M, Stumm C. Production, purification and properties of a Pichia kluyveri killer toxin. Antonie Leeuwenhoek. 1979;45:437–450. doi: 10.1007/BF00443282. [DOI] [PubMed] [Google Scholar]
- 28.Niwa O, Sakaguchi K, Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981;148:988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouchi K, Wickner R B, Toh-e A, Akiyama H. Breeding of killer yeasts for sake brewing by cytoduction. J Ferment Technol. 1979;57:483–487. [Google Scholar]
- 30.Peery T, Shabat-Brand T, Steinlauf R, Koltin Y, Bruenn J. Virus-encoded toxin of Ustilago maydis: two polypeptides are essential for activity. Mol Cell Biol. 1987;7:470–477. doi: 10.1128/mcb.7.1.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piontek M, Hagedorn J, Hollenberg C P, Gellissen G, Strasser A W. Two novel gene expression systems based on the yeasts Schwanniomyces occidentalis and Pichia stipitis. Appl Microbiol Biotechnol. 1998;50:331–338. doi: 10.1007/s002530051300. [DOI] [PubMed] [Google Scholar]
- 32.Radler F, Herzberger S, Schonig I, Schwarz P. Investigation of a killer strain of Zygosaccharomyces bailii. J Gen Microbiol. 1993;139:495–500. doi: 10.1099/00221287-139-3-495. [DOI] [PubMed] [Google Scholar]
- 33.Radler F, Schmitt M J, Meyer B. Killer toxin of Hanseniaspora uvarum. Arch Microbiol. 1990;154:175–178. doi: 10.1007/BF00423329. [DOI] [PubMed] [Google Scholar]
- 34.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt M J, Tipper D J. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4807–4815. doi: 10.1128/mcb.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki T, Choi E, Ryu D. Construction of killer yeast strain. Appl Environ Microbiol. 1985;49:1211–1215. doi: 10.1128/aem.49.5.1211-1215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skala J, Kotylak Z. Protoplast fusion in Saccharomyces cerevisiae. Acta Microbiol Pol. 1984;33:25–35. [PubMed] [Google Scholar]
- 38.Stam J C, Kwakman J, Meijer M, Stuitje A R. Efficient isolation of the linear DNA killer plasmid of Kluyveromyces lactis: evidence for location and expression in the cytoplasm and characterization of their terminally bound proteins. Nucleic Acids Res. 1986;14:6871–6884. doi: 10.1093/nar/14.17.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vondrejs V. A killer system in yeasts: applications to genetics and industry. Microbiol Sci. 1987;4:313–316. [PubMed] [Google Scholar]
- 40.Wang T T, Lee C F, Lee B H. The molecular biology of Schwanniomyces occidentalis klocker. Crit Rev Biotechnol. 1999;19:113–143. doi: 10.1080/0738-859991229215. [DOI] [PubMed] [Google Scholar]
- 41.Wilde J, Eiden J, Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990;28:1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson J J, Khachatourians G G, Ingledew W M. Schwanniomyces: SCP and ethanol from starch. Biotechnol Lett. 1982;4:333–338. [Google Scholar]
- 43.Woods D R, Bevan E A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968;51:115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]
- 44.Worsham P L, Bolen P L. Killer toxin production in Pichia acaciae is associated with linear DNA plasmids. Curr Genet. 1990;18:77–80. doi: 10.1007/BF00321119. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Imai M, Tachibana K, Mayumi M. Application of monoclonal antibodies to the isolation and characterization of a killer toxin secreted by Hansenula mrakii. FEBS Lett. 1986;195:253–257. doi: 10.1016/0014-5793(86)80170-3. [DOI] [PubMed] [Google Scholar]
- 46.Yokomori Y, Akiyama H, Shimizu K. Toxins of a wild Candida killer yeast with a novel killer property. Agric Biol Chem. 1988;52:2797–2801. [Google Scholar]
- 47.Young T W. The genetic manipulation of the killer character into brewing yeast. J Inst Brew. 1981;87:292–295. [Google Scholar]
- 48.Young T W, Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek. 1978;44:59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]
- 49.Zorg J, Kilian S, Radler F. Killer toxin producing strains of the yeasts Hanseniaspora uvarum and Pichia kluyveri. Arch Microbiol. 1988;149:261–267. [Google Scholar]