Summary
Background
Data on the rate and severity of SARS-CoV-2 reinfections in real-world settings are scarce and the effects of vaccine boosters on reinfection risk are unknown.
Methods
In a population-level observational study, registered SARS-CoV-2 laboratory-confirmed Vojvodina residents, between March 6, 2020 and October 31, 2021, were followed for reinfection ≥90 days after primary infection. Data were censored at the end of follow-up (January 31, 2022) or death. The reinfection risk was visualized with Kaplan-Meier plots. To examine the protective effect of vaccination, the subset of individuals with primary infection in 2020 (March 6–December 31) were matched (1:2) with controls without reinfection.
Findings
Until January 31, 2022, 13,792 reinfections were recorded among 251,104 COVID-19 primary infections (5.49%). Most reinfections (86.77%, 11,967/13,792) were recorded in January 2022. Reinfections were mostly mild (99.17%, 13,678/13,792). Hospitalizations were uncommon [1.08% (149/13,792) vs. 3.66% (505/13,792) in primary infection] and COVID-19 deaths were very rare (20/13,792, case fatality rate 0.15%). The overall incidence rate of reinfections was 5.99 (95% CI 5.89–6.09) per 1000 person-months. The reinfection risk was estimated as 0.76% at six months, 1.36% at nine months, 4.96% at 12 months, 16.68% at 15 months, and 18.86% at 18 months. Unvaccinated (OR=1.23; 95%CI=1.14–1.33), incompletely (OR=1.33; 95%CI=1.08–1.64) or completely vaccinated (OR=1.50; 95%CI=1.37–1.63), were modestly more likely to be reinfected compared with recipients of a third (booster) vaccine dose.
Interpretation
SARS-CoV-2 reinfections were uncommon until the end of 2021 but became common with the advent of Omicron. Very few reinfections were severe. Boosters may modestly reduce reinfection risk.
Funding
No specific funding was obtained for this study.
Keywords: SARS-CoV-2, Reinfection risk, Reinfection severity, COVID-19 vaccines, Boosters
Research in context.
Evidence before this study
We performed a (non-language-specific) search of PubMed with (re-infection* OR reinfection*) and COVID-19 which retrieved 1063 items as of April 4, 2022. There is evidence from single observational studies and from reviews thereof that reinfection had been uncommon until late 2021 and some evidence that reinfections have become more common with the advent of Omicron variant. However, there is limited evidence on the exact frequency and the severity of these reinfections overall and as compared with the primary infection, the risk of hospitalization and death following reinfection, and the effectiveness of vaccination boosters to reduce reinfections.
Added value of this study
Our study provided population-level data on the incidence and severity of reinfections over a two-year period in 2020–2022. We show that reinfections increased sharply in January 2022 to account for 15% of documented infections. The vast majority of reinfections are mild, hospitalizations are very uncommon (four times lower risk than with primary infection) and deaths are distinctively uncommon. Booster vaccination (third dose) was associated with a very modest reduction of reinfection risk.
Implications of all the available evidence
Reinfection risk is no longer negligible with the Omicron variant, but severe consequences are rare. Estimated absolute risks can be considered in the setting of active epidemic waves to decide on best management choices.
Alt-text: Unlabelled box
Introduction
Reinfections with SARS-CoV-2 are an important aspect of COVID-19 and its potential transition to endemicity.1, 2, 3 Current challenges include the absence of widespread genomic surveillance, the durability of immunity after primary infection, and the need, timing and variant target of booster doses.4
Reinfections, commonly defined by a positive RT-PCR test ≥90 days from the first episode, were rare (absolute rate 0–1.1%) in the early stages of pandemic.5, 6, 7 As mass vaccination proceeded, while the virus continued to adapt to humans diversifying into variants with increased infectivity and ability to evade immune responses, and longer follow-up accrued, reinfection parameters, particularly pertaining to severity, are important to evaluate.8 Recent studies showed that reinfection is less likely in vaccinated individuals who have recovered from a previous infection.9,10
Previous systematic reviews of reinfection have covered data until March 202111 or September 202112 and included studies with criteria that would not qualify for reinfections currently (e.g. <3 months from primary infection). Nevertheless, the authors concluded that reinfection, the causes and risk factors of which are not fully understood at present, is not specific to any particular virus strain.12
This study aimed to assess the SARS-CoV-2 reinfection rate and associated severity in the population of the Autonomous Province of Vojvodina, which comprises almost a third of Serbia's population. A secondary aim was to determine the potential reinfection prevention benefit of vaccination in persons who have recovered from an initial episode.
Methods
Study setting, objectives, and data collection
The study was conducted at the Institute of Public Health of Vojvodina (IPHV), an integrated health institution responsible for the oversight of disease control and prevention for ∼1.9 million Vojvodina inhabitants. Reinfection was defined as the detection of SARS-CoV-2 RNA or antigen in nasopharyngeal swab specimens after ≥90 days from the first episode (primary infection), regardless of the presence of symptoms.
Our primary objective was to determine the risk of reinfection and associated severity in Vojvodina residents with primary infection (first positive SARS-CoV-2 test) between March 6, 2020 and October 31, 2021. Data were censored at the end of follow-up (January 31, 2022) or death. A secondary objective was to examine whether vaccination protected from reinfection. For this purpose, the risk of reinfection was estimated for the subset of participants with primary infection in 2020 (March 6–December 31), before vaccines were available. The parallel distribution of SARS-CoV-2 variants in Europe during the six pandemic waves is presented in SI File 1.
Socio-demographic and epidemiological data were retrieved from the IPHV surveillance database which contains data for all registered COVID-19 cases in Vojvodina from the beginning of the pandemic (March 6, 2020). All cases were residents of Vojvodina. Data originated from epidemiological questionnaires and mandatory notification forms. The minimum set of data for each laboratory-confirmed case included demographics, occupation, date of symptom(s) onset and list of symptoms, severity of COVID-19 disease, date and type of positive laboratory diagnostic test, date of case registration, number of comorbidities, hospitalization status (hospitalized vs. non-hospitalized), disease outcome with a specified date (active/recovered/death outcome) and COVID-19 vaccination status (type of vaccine, number of doses and date(s) of vaccination(s)). Regarding disease severity, for surveillance purposes, COVID-19 cases were classified into three groups: 1) mild, if there were no symptoms or COVID-19-related symptoms were present but without confirmed pneumonia by chest imaging; 2) severe, if COVID-19 pneumonia was confirmed by chest imaging; and 3) critical, if COVID-19 pneumonia required mechanical ventilation and/or admission to the intensive care unit.
Laboratory testing
For the purpose of SARS-CoV-2 detection, posterior nasopharingeal swab samples were collected as part of case-based surveillance protocol for COVID-19 or due to other indications, regardless of reason for testing. Different semi-quantitative RT-PCR tests were used, depending on availability; results were interpretated in accordance with the manufacturer's instructions.13, 14, 15, 16 Antigen rapid diagnostic tests (Ag-RDT), for the qualitative detection of SARS-CoV-2 antigens in nasopharyngeal swabs, were introduced in November 2020. Patients with COVID-19-related symptoms within five days of symptom(s) onset were mostly tested using the STANDARD Q COVID-19 Ag Test (96.52% sensitivity/99.68% specificity, SD Biosensor, Gyeonggi-do, South Korea).17,18 The following Ag-RDTs were also employed during the first five days of COVID-19-related symptoms onset: Abbott Panbio™ COVID-19 Ag rapid test (98.1% sensitivity/99.8% specificity), and AMP rapid test SARS-CoV-2 Ag cassette (97.3% sensitivity/100% specificity).17,18 Patients with COVID-19-related symptoms and negative Ag-RDT results were subsequently tested by RT-PCR in a repeated nasopharyngeal swab for a definitive diagnosis.19 A COVID-19 laboratory-confirmed case was defined as a subject with detected SARS-CoV-2 RNA or antigen in a nasopharyngeal swab by a positive RT-PCR test or Ag-RDT, respectively.
Assessment of the protective effect of vaccination towards reinfection
Eligibility criteria and case/control definitions
The study case was a resident of Vojvodina, with laboratory-confirmed SARS-CoV-2 infection reported in the period March 6, 2020 to December 31, 2020, with confirmed reinfection from January 1, 2021 to January 31, 2022. The study control was a resident of Vojvodina with a laboratory-confirmed SARS-CoV-2 infection reported in the period March 6 to December 31, 2020 and without laboratory confirmation of reinfection from January 1, 2021 to January 31, 2022.
Exclusion criteria: To examine the relationship between vaccination and SARS-CoV-2 reinfections, 25 detected reinfection cases in 2020, when vaccination was still unavailable, were excluded. For the same reason, the status of all study cases and controls was checked in the provincial mortality database and in case of death outcome (regardless of cause) before January 31, 2022, were excluded from analysis (12 study cases and 2784 controls). Since children did not have the same chances for vaccination as adults (recommendations for vaccination of children aged 12–17 years were issued in June 2021), 2051 children and adolescents aged <18 years were excluded from analysis.
Matching of study cases and controls
The IPHV electronic database was searched to assess the SARS-CoV-2 reinfection rate in the study period and to identify study cases and controls, according to eligibility criteria and case definitions. To document reinfection, we used the Unique Master Citizen Number (ID number) that accompanies all reported cases. For 5.6% of persons for whom the ID number was missing, the search was performed by patient name, surname, date of birth and residence. Matching of study cases and controls was done in a ratio of 1:2. Each study case was paired individually with a control in relation to gender, date of initial SARS-CoV-2 positive test (±10 days) and corresponding age (±3 years). Random selection was applied if several controls corresponded to a study case by using a random number generator. Of 7103 study-cases ≥18 years, 32 (0.45%) were excluded due to unsuccessful matching in relation to applied criteria.
Classification of study cases and controls by vaccination status
The following definitions were used: Unvaccinated status was considered if no vaccine dose was received or if a single (or the first of a two-dose scheme) dose was received ≤14 days before the reinfection date, which was the date of reinfection symptoms onset (or positive test, in the absence of symptoms). Incomplete (partial) vaccination was considered if a single vaccine dose was received and >14 days had passed since the vaccination day; if the vaccination schedule had not been completed; or if the final dose was given ≤14 days or >6 months before the reinfection date. Fully vaccinated status was considered if two vaccine doses were administered and the final dose was received >14 days and ≤6 months before the reinfection date. Boosted were considered patients who received the third (booster) dose and >7 days had passed since receiving the vaccine before the reinfection date. The same definitions were applied to controls.
Statistical analysis
Descriptive analysis of socio-demographic, epidemiological and clinical features, including reinfections severity, was conducted. Cochran–Armitage test was used to assess trend of proportions of primary infection that were reinfected in the observed period. We assessed the risk of suspected reinfection, using time-to-event Kaplan-Meier analysis to estimate the reinfection risk during follow-up. Only patients who survived three months after primary infection were considered for analysis (since, by definition, there can be no reinfection risk in the first three months). We also show Kaplan-Meir plots separately for primary infections that occurred in time periods defining each pandemic wave. Each of these curves were plotted for those who were first infected in that time interval. Using the same analysis, we additionally estimated the risk of severe reinfection or worse outcome (severe/critical/death) over time, again starting at three months after primary infection. We also show Kaplan-Meier plots stratified by gender and age category. Pearson's chi-squared test or Fisher's exact test, as appropriate, was used to compare differences between groups of categorical set of data. For variables with multiple ordered categories, we used analyses adjusted for trend. Binary logistic regression was applied to assess the association between different factors and reinfection severity. Conditional logistic regression was applied to compare unvaccinated, incomplete and full vaccination with boosted vaccination status as a reference variable among study-cases and controls. McNemar test was used to test difference in proportions between paired data. A p-value <0.005 was considered statistically significant and p-values <0.05 were considered suggestive.20 Stata v.16 (College Station, TX: StataCorp LLC. 2019) and TIBCO Statistica™ 14.0.0 (license for University of Novi Sad) were used for matching and statistical analyses.
Ethics statement
This case-control study was performed in the frame of national public health surveillance. Sample collection for laboratory diagnosis of COVID-19 formed part of the standard patient management, thus, only oral informed patient consent was required. Access to patients’ data was restricted for employees directly involved in COVID-19 diagnosis, treatment, and reporting. Patients’ data were anonymized before any analysis was conducted. According to the law, no approval by the Ethics Committee for the retrospective analysis of anonymized data is required in Serbia.
Role of the funding source
There was no funder of the study and, thus, no sponsor had a role in study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit for publication.
Results
SARS-CoV-2 reinfections over time
A total of 362,650 COVID-19 cases were registered among the population of Vojvodina from the beginning of the pandemic (March 6, 2020) until January 31, 2022 (Table 1). The cohort, described in detail in SI Figure 1, included a subset of 251,104 patients with primary infection in the period March 6, 2020–October 31, 2021, of which 13,792 (5.49%) experienced reinfection (Table 2). Before August 2021, reinfections were sporadically registered (rate <1%). Most reinfections (86.77%) were recorded in January 2022, when the reinfection rate abruptly increased up to 15.32%. The highest proportion of primary infections that were reinfected was in October and November 2020 (11.05% and 10.67%, respectively), with decreasing linear trend after October 2020 (SI Figure 2). The average time duration from primary infection to reinfection was 340±101 days (min 90 days–max 662 days).
Table 1.
Proportion of patients with SARS-CoV-2 reinfection in relation to overall registered COVID-19 cases in Vojvodina, Serbia, March 6, 2020–January 31, 2022.
| Months | Overall registered COVID-19 casesa |
Proportion of reinfectionsb |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 2020 | March-June | 1570 | 0.43 | - | - |
| July | 4597 | 1.27 | 0 | 0 | |
| August | 1750 | 0.48 | 0 | 0 | |
| September | 330 | 0.09 | 0 | 0 | |
| October | 1547 | 0.43 | 1 | 0.06 | |
| November | 30,659 | 8.45 | 9 | 0.03 | |
| December | 38,392 | 10.59 | 15 | 0.04 | |
| Total | 78,845 | - | 25 | 0.03 | |
| 2021 | January | 11,441 | 3.15 | 7 | 0.06 |
| February | 11,868 | 3.27 | 11 | 0.09 | |
| March | 30,012 | 8.27 | 47 | 0.16 | |
| April | 21,929 | 6.05 | 47 | 0.21 | |
| May | 5084 | 1.40 | 21 | 0.41 | |
| June | 586 | 0.16 | 5 | 0.85 | |
| July | 860 | 0.24 | 8 | 0.93 | |
| August | 7029 | 1.94 | 80 | 1.14 | |
| September | 35,515 | 9.79 | 351 | 0.99 | |
| October | 47,935 | 13.22 | 567 | 1.18 | |
| November | 28,194 | 7.78 | 474 | 1.68 | |
| December | 5217 | 1.44 | 182 | 3.49 | |
| Total | 205,670 | - | 1800 | 0.63 | |
| 2022 | January | 78,135 | 21.55 | 11,967 | 15.32 |
| TOTAL | 362,650 | 100.00 | 13,792 | 3.80 | |
by month of first episode registration.
by month of reinfection registration.
Table 2.
Overall registered SARS-CoV-2 primary infections (March 6, 2020–October 31,2021), SARS-CoV-2 reinfections and related hospitalization rates, in Vojvodina, Serbia, in the period March 6, 2020–January 31, 2022.
| Overall SARS-CoV-2 primary infectionsa |
Proportion of hospitalized primary infectionsa |
SARS-CoV-2 reinfectionsb |
Proportion of hospitalized reinfectionsb |
Primary SARS-CoV-2 infections that were reinfected |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | n | % | N | % | n | % | n | % | N | % | |
| 2020 | March-June | 1570 | 0.63 | 1539 | 98.02 | - | - | - | - | 80 | 5.10 |
| July | 4597 | 1.83 | 2102 | 45.73 | 0 | 0 | - | - | 296 | 6.44 | |
| August | 1750 | 0.70 | 420 | 24.00 | 0 | 0 | - | - | 92 | 5.26 | |
| September | 330 | 0.13 | 66 | 20.00 | 0 | 0 | - | - | 29 | 8.79 | |
| October | 1547 | 0.62 | 196 | 12.67 | 1 | 0.01 | 0 | 0 | 171 | 11.05 | |
| November | 30,659 | 12.21 | 1796 | 5.86 | 9 | 0.07 | 1 | 11.11 | 3272 | 10.67 | |
| December | 38,392 | 15.29 | 2138 | 5.57 | 15 | 0.11 | 1 | 6.67 | 3353 | 8.73 | |
| Total | 78,845 | - | 8257 | 10.47 | 25 | - | 2 | 8.00 | 7293 | 9.25 | |
| 2021 | January | 11,441 | 4.56 | 868 | 7.59 | 7 | 0.05 | 0 | 0.00 | 858 | 7.50 |
| February | 11,868 | 4.73 | 831 | 7.00 | 11 | 0.08 | 3 | 27.27 | 925 | 7.79 | |
| March | 30,012 | 11.95 | 2089 | 6.96 | 47 | 0.34 | 2 | 4.25 | 1874 | 6.24 | |
| April | 21,929 | 8.73 | 1691 | 7.71 | 47 | 0.34 | 2 | 4.25 | 1211 | 5.52 | |
| May | 5084 | 2.02 | 491 | 9.66 | 21 | 0.15 | 0 | 0.00 | 287 | 5.65 | |
| June | 586 | 0.23 | 70 | 11.95 | 5 | 0.04 | 1 | 20.00 | 43 | 7.34 | |
| July | 860 | 0.34 | 71 | 8.26 | 8 | 0.06 | 0 | 0.00 | 39 | 4.53 | |
| August | 7029 | 2.80 | 442 | 6.28 | 80 | 0.58 | 6 | 7.50 | 187 | 2.66 | |
| September | 35,515 | 14.14 | 2094 | 5.90 | 351 | 2.54 | 13 | 3.70 | 657 | 1.85 | |
| October | 47,935 | 19.09 | 3061 | 6.39 | 567 | 4.11 | 19 | 3.35 | 418 | 0.87 | |
| November | - | - | - | - | 474 | 3.44 | 13 | 2.74 | - | - | |
| December | - | - | - | - | 182 | 1.32 | 6 | 3.30 | - | - | |
| Total | 172,259 | - | 11,708 | 6.80 | 1800 | - | 65 | 3.61 | 6499 | 3.77 | |
| 2022 | January | - | - | - | - | 11,967 | 86.77 | 82 | 0.69 | - | - |
| TOTAL | 251,104 | 100.00 | 19,965 | 7.95 | 13,792 | 100.00 | 149 | 1.08 | 13,792 | 5.49 | |
by month of first episode registration.
by month of reinfection registration.
The SARS-CoV-2 infection rate during the first, second, third, fourth, fifth and sixth pandemic wave was 0.08%, 0.44%, 4.39%, 4.02%, 7.37%, and 7.96%, respectively, for documented infections.
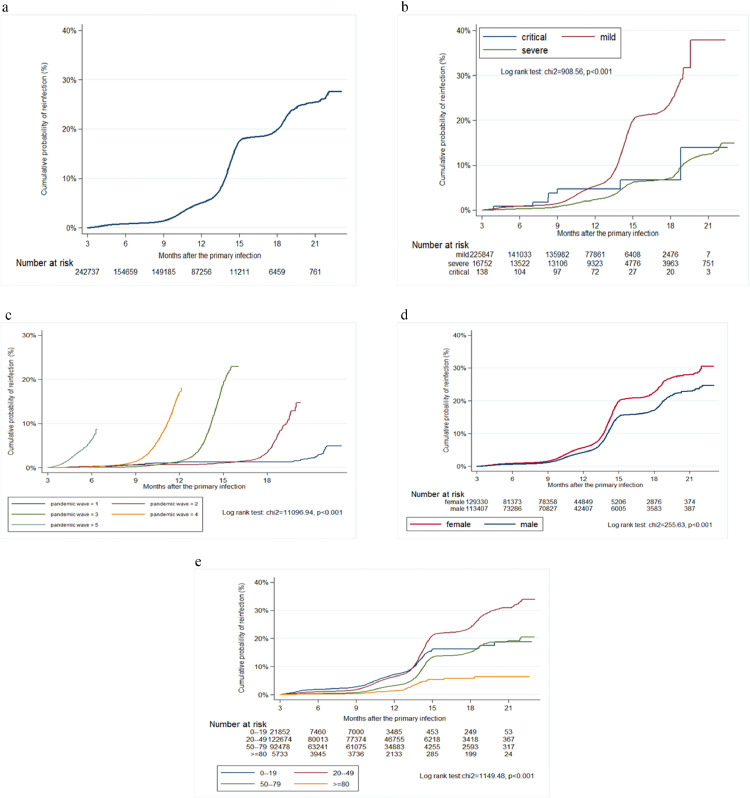
The overall incidence rate of documented SARS-CoV-2 reinfections was 5.99 (CI 5.89–6.09) per 1000 person-months. Figure 1A shows the Kaplan-Meier plot for the reinfection risk for patients who survived three months after primary infection (n=242,737). The risk becomes 0.76% at six months, 1.36% at nine months, 4.96% at 12 months, 16.68% at 15 months, 18.86% at 18 months (Figure 1A). The risk of reinfection for patients who suffered from severe or critical clinical forms of primary infections at these time points was 0.25%, 0.74%, 2.26%, 6.14%, 7.10%, and 0.82%, 3.74%, 4.87%, 6.78%, 6.78%, respectively (Figure 1B). The probability of reinfection was low before, and substantially higher, after the advent of Omicron in the sixth pandemic wave (Figure 1C). The reinfection risk for patients who survived three months after primary infection was higher in females (Figure 1D) and middle-aged groups (Figure 1E).
Figure 1.
Kaplan-Meier curve showing the cumulative probability of reinfection in the overall cohort (A), according to severity of primary infection (B), pandemic waves (C), gender (D), and age category (E) in Vojvodina, Serbia, March 2020–January 2022. The duration of pandemic waves was as follows: First pandemic wave: March 6–June 1, 2020; Second pandemic wave: June 2–October 6, 2020; Third pandemic wave: October 7, 2020–January 31, 2021; Fourth pandemic wave: February 1–July 23, 2021; Fifth pandemic wave: July 24–December 31, 2021.
The dynamics of testing markedly varied during the pandemic. Most SARS-CoV-2 tests were performed in the period October–December 2021 (28.89%) and January 2022 (16.04%), and only 3.09% of all tests were conducted since the beginning of pandemic until October 2020. The frequency of testing by assay type in the study period is shown in SI Table 1.
Patients’ characteristics and risk factors for reinfection and for severe reinfection
Compared to patients without reinfection, reinfected patients were significantly younger (mean age 41.61±14.43 vs. 46.16 ±18.87 years), with more pronounced female dominance (57.98% vs. 52.65%), more frequently employed as health-care workers (HCWs) (10.90% vs. 4.37%), and with lower vaccination coverage (2.51% vs. 10.26% who received ≥2 doses) at the day of laboratory confirmation of primary infection. Other main characteristics of both groups of patients are shown in Table 3. Older age (≥70 years), ≥1 comorbidities and severe (OR 7.35, CI 4.84–11.17, p<0·001) or critical primary infection (OR 221.40, CI 48.74–1005.65, p<0·001) were significantly associated with severe reinfections (Table 4).
Table 3.
Demographic characteristics and vaccination status of COVID-19 cases and SARS-CoV-2 reinfections in Vojvodina, Serbia, March 2020-October 2021.a
| Characteristic | Overall COVID-19 cases n=251,104 |
Reinfection casesbn=13,792 |
Cases without reinfectionbn=237,312 |
P value | |||
|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | ||
| Gender | |||||||
| Male | 118,152 | 47.05 | 5795 | 42.02 | 112,357 | 47.35 | <0.001 |
| Female | 132,952 | 52.95 | 7997 | 57.98 | 124,955 | 52.65 | |
| Age (years), mean ± SD | 45.91± 18.68 | 41.61±14.43 | 46.16±18.87 | <0.001 | |||
| Age category | n | % | N | % | n | % | |
| 0-9 | 4220 | 1.68 | 105 | 0.76 | 4115 | 1.73 | <0.001 |
| 10-19 | 17,633 | 7.02 | 661 | 4.79 | 16,972 | 7.15 | |
| 20-29 | 29,490 | 11.74 | 1976 | 14.33 | 27,514 | 11.59 | |
| 30-39 | 45,235 | 18.01 | 3693 | 26.78 | 41,542 | 17.51 | |
| 40-49 | 48,262 | 19.22 | 3516 | 25.49 | 44,746 | 18.86 | |
| 50-59 | 40,687 | 16.20 | 2189 | 15.87 | 38,498 | 16.22 | |
| 60-69 | 36,702 | 14.62 | 1189 | 8.62 | 35,513 | 14.96 | |
| 70-79 | 20,450 | 8.14 | 361 | 2.62 | 20,089 | 8.47 | |
| ≥80 | 8425 | 3.36 | 102 | 0.74 | 8323 | 3.51 | |
| Occupation | |||||||
| Healthcare workers (HCWs) | 11,872 | 4.73 | 1504 | 10.9 | 10,368 | 4.37 | <0.001 |
| Non-HCWsc | 187,924 | 74.84 | 11,215 | 81.32 | 176,709 | 74.46 | |
| Retired | 51,308 | 20.43 | 1073 | 7.78 | 50,235 | 21.17 | |
| Comorbidities | |||||||
| 0 | 198,017 | 78.86 | 11,514 | 83.48 | 186,503 | 78.59 | <0.001 |
| 1 | 39,828 | 15.86 | 1803 | 13.07 | 38,025 | 16.02 | |
| 2 | 10,123 | 4.03 | 372 | 2.7 | 9751 | 4.11 | |
| ≥3 | 3136 | 1.25 | 103 | 0.75 | 3033 | 1.28 | |
| Vaccination status | |||||||
| Unvaccinated | 213,268 | 84.93 | 13,207 | 95.76 | 200,061 | 84.30 | <0.001 |
| Partially vaccinated | 13,154 | 5.24 | 238 | 1.73 | 12,916 | 5.44 | |
| Fully vaccinated | 21,846 | 8.7 | 325 | 2.35 | 21,521 | 9.10 | |
| Boosted | 2836 | 1.13 | 22 | 0.16 | 2814 | 1.16 | |
Indicators of significance between groups using Pearson's chi-squared test and unpaired t-test.
At the time of laboratory confirmation of primary infection.
Non-HCWs included professions that provide various services, other employment categories as well as retired individuals.
Table 4.
Associations between different factors and the severity of the reinfection (i.e. severe+critical vs. mild) in Vojvodina, Serbia, Mar 2020–Jan 2022.
| Univariate Models |
Multivariate Modelsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Occupation | ||||||||
| Health-care workers (HCWs) | 0.81 | 0.37 | 1.78 | 0.608 | 0.87 | 0.40 | 1.93 | 0.737 |
| Non-HCWsb | Reference group | Reference group | ||||||
| Retired | 7.27 | 4.92 | 10.76 | <0.001 | 3.00 | 1.63 | 5.52 | <0.001 |
| Number of comorbidities | ||||||||
| 0 | Reference group | Reference group | ||||||
| 1 | 2.81 | 1.82 | 4.32 | <0.001 | 1.58 | 1.00 | 2.50 | 0.051 |
| 2 | 5.53 | 2.97 | 10.30 | <0.001 | 2.59 | 1.35 | 4.99 | 0.004 |
| ≥3 | 4.98 | 1.54 | 16.07 | 0.007 | 2.10 | 0.64 | 6.93 | 0.224 |
| Severity of primary infection | ||||||||
| Mild | Reference group | Reference group | ||||||
| Severe | 7.35 | 4.84 | 11.17 | <0.001 | 5.01 | 3.26 | 7.71 | <0.001 |
| Critical | 221.40 | 48.74 | 1005.65 | <0.001 | 189.00 | 39.13 | 912.95 | <0.001 |
adjusted for age (continuous) and gender.
Non-HCWs included professions that provide various services, other employment categories as well as retired individuals.
Severity of reinfections versus primary infections
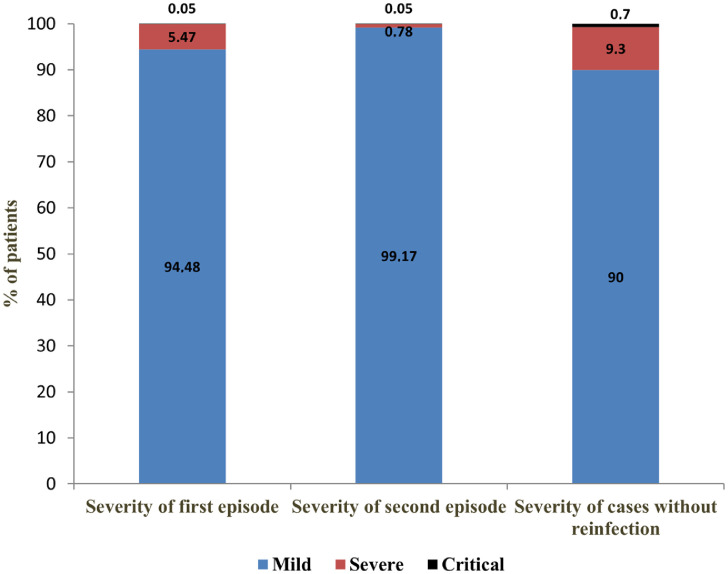
Most reinfections were mild (99.17%), and only a minority were severe (0.78%) or critical (0.05%). Reinfections were milder than primary infections. The share of severe clinical forms decreased from 5.47% in initial episodes to 0.78% in reinfections, while critical forms remained the same (0.05%). The proportion of severe and critical disease forms was higher in cases without reinfection (9.30% and 0.70%, respectively) (Figure 2).
Figure 2.
Severity of COVID-19 in patients with reinfection (first and second episode) vs. patients without reinfection, in Vojvodina, Serbia, March 6, 2020–January 31, 2022.
Hospitalizations after reinfection
Overall, the hospitalization rate of COVID-19 patients in the observed period was 7.95%. At the beginning of the pandemic (March–June 2020) almost all patients were hospitalized (98·02%). The proportion of hospitalized gradually decreased from 10.47% in 2020 to 6.80% in 2021 (Table 2). Reinfected patients were rarely hospitalized (1.08% vs. 3.66% during initial infection). Reinfected patients were 4.2 times more likely to be hospitalized during initial infection compared to reinfection (McNemar OR=4.21, 95%CI 3.41–5.22, p<0.001). Overall, 38 patients were hospitalized in both the primary infection and reinfection. The proportion of hospitalized reinfections until January 2022 (pre-Omicron period) was higher (3.67%) and decreased to 0.69% in January 2022 (Omicron period), when most reinfections were recorded.
Deaths
Among reinfected patients, 31 deaths occurred, of which 20 were classified as COVID-19 deaths (case fatality ratio 0.15% among 13,792 reinfections); the rest were caused by non-COVID causes. COVID-19 deaths occurred mainly in reinfected patients ≥60 years (70.0%), with severe (75.0%) or critical disease (25.0%). Most of them died during the fifth (50.00%) or sixth (35.00%) pandemic wave (SI Table 2). Kaplan-Meier curves for the risk of death for the entire cohort of 251,104 positive patients and for reinfected patients appear in SI Figure 3.
Second reinfections
In total, 34 cases (0.01%) with three consecutive SARS-CoV-2 reinfections were recorded (SI Table 3). Cases of second reinfection ≥90 days after the previous one were registered in December 2021 (n=1) and January 2022 (n=33). Overall, 14.7% of patients were hospitalized due to severe primary infection, while first and second reinfections were mild. None of these reinfections led to hospitalization or death. On the day of laboratory confirmation of the second reinfection, most patients (55.9%) were unvaccinated.
Case control study
A total of 13,189 reinfections were recorded among adults (>18 years) in the period January 1, 2021, through January 31, 2022, of which 7071 (53.6%) met the eligibility criteria for study cases and were matched with 14,142 controls. Women (56.8%) and middle-age groups (55.0%) predominated in the study population, while most participants (91.4%) were initially infected during October–December 2020 (SI Table 4). In relation to vaccination status of study cases and controls, the distribution of unvaccinated (∼52%) and incompletely vaccinated (∼2%) was similar but there were more fully vaccinated (24.7% vs. 20.3%), and less boosted (21.3% vs. 25.9%) (p<0.001) among study cases. All three categories of cases, regardless of whether they were unvaccinated (OR=1.23; 95%CI=1.14–1.33), incompletely (OR=1.33; 95% CI=1.08–1.64) or completely vaccinated (OR=1.50; 95% CI=1.37–1.63), were modestly more likely to be reinfected compared with those who received a booster dose (SI Table 5).
Discussion
In this large population-based study, we have documented that the risk of reinfection remained exceedingly low before the emergence of Omicron and increased substantially thereafter, accounting for 15% of the infections during January 2022. Reinfections were generally mild, and their severity was much lower compared with primary infections. Accordingly, hospitalizations were uncommon and fatal outcomes were distinctly rare, much lower than in primary infections. Vaccination, in particular a booster dose, diminished modestly the probability of reinfection in a case-control analysis.
The relatively low rates of SARS-CoV-2 infection recorded in Vojvodina refer to patients who sought medical help and tested positive for SARS-CoV-2. However, the prevalence of antibodies against SARS-CoV-2 in the population, estimated at 16.67% (95%CI=14.45–19.08) corresponding to 322,033 infections in total by the end of September 2020. implies a much more widespread infection than indicated by the number of confirmed cases.21 Under-ascertainment was more prominent in 2020 than in 2021–2022; therefore, the observed risks of reinfection may be under-estimates by many-fold for the first and second waves and may still be substantially underestimated even for the most recent waves.
In the early stages of the pandemic, accumulated evidence supported the effectiveness of natural immunity after infection in protecting against reinfection with different SARS-CoV-2 variants for at least one year.3 A recent study from Sweden showed that natural immunity after three months was associated with a 95% lower risk of SARS-CoV-2 reinfection and an 87% risk of hospitalization due to COVID-19, compared to the cohort with no immunity, for up to 20 months.22 Omicron, however, showed substantial ability to evade natural and vaccine-induced immunity, leading to reduced protection against reinfection, but similar protection against hospitalization or death due to reinfection.23 Unlike the Beta and Delta periods of predominance when there was no evidence of population-level immune escape, the explosive spread of Omicron in South Africa led to a dramatic increase of reinfections in mid-November 2021.24 Similar findings have also been reported in Qatar and the United Kingdom and are relevant to the dominance of Omicron worldwide by early 2022.23,25
Repeated infections should be anticipated from viruses like SARS-CoV-2 that infect mucosal surfaces without a viremic phase, which typically result in relatively short-lived antibody responses.26 Accordingly, we registered 34 patients with three consecutive infections, almost all in January 2022. An increased risk of a third infection was also documented in South Africa, with 1788 individuals with at least three and 18 with four suspected infections.24 Almost all third infections occurred after October 2021, during the period of Omicron circulation.
The global epidemiology of SARS-CoV-2 in January 2022 was characterized by the emergence of Omicron (B.1.1.529), declining prevalence of Delta (B.1.617.2), and very low-level circulation of previous VOCs.27 The only published genomic surveillance data from Serbia thus far revealed the circulation of clades 20A and 20B in the period April–July 2020 and the dominance of 20D in the summer of 2020.28,29 The clades circulating during the fifth pandemic wave (Jul–Dec, 2021) were 21J (Delta) and 21K (Omicron).30 Despite the limited availability of sequencing data from Serbia, it seems reasonable to assume that the increase in reinfections after August 2021 was due to introduction and spread of Delta, while the abrupt increase in January 2022 was due to the explosive spread of Omicron.
The higher estimated probability of reinfection among the younger adults and the socially active, working-age groups (more often employed as HCWs), support the hypothesis that the reinfection risk is a function of the risk of exposure.3 However, we cannot exclude the possibility that some of these population groups may also have been tested more intensively than others. In a large population-based study conducted in England before the Delta variant became dominant, the median age for reinfection was 48 years, and both prior infection and vaccination were protective against severe disease; in addition, men were less likely to experience SARS-CoV-2 reinfection compared to women.31 If this gender difference is not due to bias (e.g. differential testing) or differential exposure, it may reflect behavioural, genetic, and/or hormonal differences.31 Older age groups, especially retirees, were less exposed due to lockdowns in 2020 and generally more compliant with the recommendations of mask wearing, distancing, and accepting vaccination. In our study, being older ≥70 years, having ≥1 comorbidities and a severe or critical primary infection were significantly associated with severe reinfections. In a retrospective cohort study of SARS-CoV-2-positive HCWs in 2020, reinfections were uncommon and more likely in women, adults, immunocompromised and previously hospitalized for COVID-19.32
Testing may also markedly affect the documentation of both infections and reinfections. Testing was not uniform throughout the pandemic. As a result of implementation of different testing criteria in Serbia during the pandemic, it is likely that many asymptomatic and mild infections were underreported.21 At the beginning of the pandemic (March–May 2020), the RT-PCR testing capacity was sufficient to test mostly severe cases of COVID-19 (only ∼3% of all tests were performed in the period March–October 2020). In late 2020, RT-PCR testing become more readily available for mild cases as well. Ag-RDTs were officially introduced in November 2020 and enabled mass testing under different settings (e.g. for travel, admission to hospitals or nursing homes, or contact tracing). Testing protocols have changed over time depending on many factors, including occupation (HCWs and nursing-home employees), health status (chronic patients and people who permanently live in long-term facilities), and the need for follow-up testing of recovered patients. Given that many infections were probably missed, it is likely that the risk of reinfection is much larger than shown in the Kaplan-Meier curves and the risk of second reinfection must also be substantially higher than recorded. The much higher rate of reinfections in January 2022 may not just reflect a genuinely higher risk of reinfection with Omicron, but also some under-estimation of the reinfection risk with prior variants. Most reinfections are likely to be missed if they are asymptomatic, or have very mild symptoms.
Clinical manifestations of SARS-CoV-2 reinfections compared to primary infections vary from mild to severe and even life-threatening, yet factors contributing to changes in severity remain largely unknown. The severity of SARS-CoV-2 reinfections, also reflected in the hospitalization rates, was five times higher in the “pre-Omicron period“ than in January 2022, when it dropped to 0.69% during the Omicron surge while Delta variants continued to circulate. This finding could indicate the circulation of potentially more virulent variants of SARS-CoV-2 in the “pre-Omicron period”, but also differences in diagnostic capacities and applied criteria for hospitalization. Testing was far more extensive in January 2022 than in earlier phases of the pandemic, thus the proportion of infections requiring hospitalization may not have changed much over time. Several investigations suggest that Omicron is not necessarily less virulent.33,34 In our study, reinfections tended to be milder compared to primary infections. Similarly, reinfections had 90% lower odds of resulting in hospitalization or death compared to primary infections in Qatar and were generally rare and mild.35 A plausible explanation for this finding could be a primed immune system after the initial infection, which may not suffice to prevent additional infections especially by different viral strains, but which raises hope that the disease course could be milder when the virus becomes endemic. The massive surges of Omicron in late 2021 and 2022 that were often accompanied by very mild disease and disproportionately few severe cases may be consistent with a transition to endemicity. Perhaps as exceptions to the general rule, severe reinfections in different age and racial/ethnic groups have also been reported.33,34
A recent systematic review showed wide variations between the severity of primary infection compared to reinfection, with increases in asymptomatic cases.11 In a study by Wang et al., the majority (69%) of reinfections with a genetically distinct SARS-CoV-2 strain, were of similar severity; 18.8% had worse and 12.5% milder symptoms with the second episode.36 Additionally, in a pre-print of a cohort study on the immune correlates of natural infection, seropositivity was associated with 69.2% protection from symptomatic infection mostly against Gamma and Delta variants with higher protection against moderate or severe infection and reinfections were less severe than first infections.37
The low vaccination rate in Serbia (47.78%, as of May 10, 2022), which has increased only slightly since mid-2021, may have possibly contributed to relatively high reinfection rates.38 Vaccine coverage rates, particularly for booster doses, were higher among the elderly compared to younger age groups. This could increase the risk of infection and reinfection for younger people. Moreover, the effectiveness of used vaccines (BNT162b2, BBIBP-CorV, Gam-COVID-Vac, and ChAdOk1 nCoV-19) in the prevention of symptomatic, severe and mild COVID-19 proved to be high in the elderly (≥60 years), but has not been adequately assessed in middle-aged groups that are particularly susceptible to reinfections.39 We acknowledge that given the observational nature of the data, estimates of effectiveness of booster doses for reinfection need to be considered with caution.40 The frequency and timeliness of required vaccination doses (typically for protection against severe COVID-19) have not yet been determined with certainty. Vaccination after previous infection may result in an additional risk reduction against reinfection and hospitalization for up to 9 months, although with small differences in absolute numbers.22 We observed a slightly decreased reinfection risk in boosted versus unvaccinated, partially and fully vaccinated people. This result, however, needs to be interpreted with caution since individuals may vary in other factors that were not accounted for in our analysis.40 For example, boosted people may be more health conscious in general and have a heightened sense of protection, resulting in lower reinfection rates. Several studies have shown vaccinated people to be in better general health, e.g. having three-fold lower mortality risk from non-COVID-19 causes than non-vaccinated.41 Still, our data are compatible with the potential benefits of hybrid immunity, generated from the combination of prior infection plus vaccination. If true, the modest benefit of a booster dose on a relative risk scale needs to be examined also in terms of absolute risk reduction, a task that might not be trivial currently, in the absence of an active epidemic wave in most countries. Randomized trials should be considered to evaluate booster doses in the future.
We must emphasize some limitations. Both primary infections and reinfections were diagnosed mainly by Ag-RDT testing without subsequent confirmative RT-PCR testing of positive results. While no test is perfect, the test performance characteristics of RT-qPCR tests are superior theoretically compared to Ag-RDTs. In clinical and epidemiologic practice, however, the goals of the testing strategy should be considered, particularly within the context of a pandemic when pathogen containment is a priority.42 Although we cannot exclude the possibility that some of the recorded infections might have been due to false positive results, we anticipate that these cases represented only a small fraction of results with negligible overall impact on study findings and conclusions. The reasons that render this plausible include the positive predictive value of tests in a high prevalence setting, the combination of testing with clinical presentation with a short time frame (five days) of COVID-19-related symptom(s) onset, and the strong agreement between the performance of the principally used STANDARD Q COVID-19 Ag test and PCR in the early disease stages that justifies the chosen national diagnostic protocol.17 There is a lack of evidence of genotyping variance, threshold cycle values as well as at least one negative between two positive RT-PCR tests in patients with suspected reinfection.43 Therefore, the possibility that reinfection was caused by genetically different SARS-CoV-2 variants compared to primary infection could not be investigated. Subjects who tested negative for SARS-CoV-2 were not included in the study. Thus, lack of a control group without previous infection is also a limitation. Most asymptomatic patients and those who did not seek testing were not captured, particularly in the early pandemic days. Also, if previously infected people were tested less due to their presumed natural immunity, the reinfection rate could have been underestimated. Given missed asymptomatic reinfections, the proportion of severe reinfections is certainly substantially over-estimated many-fold. Matching was not implemented to control for differences in race, nationality and education level that might influence the decision to be vaccinated or vaccine choice.
Our study offers large-scale population-level evidence on reinfections over a two-year period. Since spatiotemporal differences are relevant to SARS-CoV-2 reinfections, longer prospective population-based studies with well characterized virologic and immunologic data are needed to assess the risk of reinfection in the future and whether low severity remains a key feature.
Contributors
SM, CA and JPAI conceived and designed the study. SM, VV, ND, VP, MR, TP and ZG processed laboratory results and collected data, SM, CA, ZLC, ND, VV, AT analyzed and interpreted data, SM and CA supervised and validated data. SM, CA, ZLC, ND, VV, TP and JPAI were involved in data visualization, presentation and formal analysis. SM, ZLC, VV, ND and VP accessed and verified underlying data. SM, CA, AT and JPAI drafted the Article. All authors critically reviewed the article. All authors had access to aggregated data reported in the study and had final responsibility for the decision to submit the publication.
Data sharing statement
The data that support the findings of this study are available upon request with approval needed from the Center for Disease Control and Prevention, Institute of Public Health of Vojvodina. The data are not publicly available due to restrictions pertaining to contained information that could compromise the privacy of patients.
Declaration of interests
None.
Acknowledgements
The authors would like to thank all epidemiologists and health-care workers who were involved in the study. We also wish to thank computer engineer Dušan Krstić for the excellent technical assistance. The work of Zagorka Lozanov-Crvenković was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant numbers: 451-03-68/2022-14/200032 and 451-03-68/2022-14/200125).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100453.
Appendix. Supplementary materials
References
- 1.European Centre for Disease Prevention and Control E. Reinfection with SARSCoV-2: considerations for public health response 2020. Available at:https://www.ecdc.europa.eu/sites/default/files/documents/Re-infection-and-viral-shedding-threat-assessment-brief.pdf. Accessed 12 March 2022.
- 2.Yahav D, Yelin D, Eckerle I, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. 2021;27(3):315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209 doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397:1421–1423. doi: 10.1016/S0140-6736(21)00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O Murchu E, Byrne P, Carty PG, et al. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. 2022;32(1):e2260. doi: 10.1002/rmv.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilz S, Chakeri A, Ioannidis JP, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51(4):e13520. doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tay JH, Porter AF, Wirth W, Duchene S. The emergence of SARS-CoV-2 variants of concern is driven by acceleration of the substitution rate. Mol Biol Evol. 2022;39(2):msac013. doi: 10.1093/molbev/msac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination - Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronchini C, Gandini S, Pasqualato S, et al. Lower probability and shorter duration of infections after COVID-19 vaccine correlate with anti-SARS-CoV-2 circulating IgGs. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0263014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon RA, Qamar MA, Gilani JA, et al. The mystery of COVID-19 reinfections: a global systematic review and meta-analysis. Ann Med Surg (Lond) 2021;72 doi: 10.1016/j.amsu.2021.103130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren X, Zhou J, Guo J, et al. Reinfection in patients with COVID-19: a systematic review. Glob Health Res Policy. 2022;7(1):12. doi: 10.1186/s41256-022-00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bio-Tech Corp, Ltd . Bio-Tech Corp, Ltd; Shanghai: 2020. Liferiver Novel Coronavirus (2019-nCoV) Real Time Multiplex RT-PCR Kit.https://www.who.int/diagnostics_laboratory/eual/eul_0486_139_00_novel_coronavirus_sars_cov_2_real_time_multiplex_rt_pcr_kit_ifu.pdf Accessed 30 December 2021. [Google Scholar]
- 14.OSANG Healthcare Corp, Ltd . OSANG Healthcare Corp, Ltd; Anyang-si: 2020. GeneFinder COVID-19 Plus RealAmp Kit - Instructions for Use.https://www.fda.gov/media/137116/download BioMérieux, Inc. ARGENE® SARS-COV-2 R-GENE. Accessed 31 December 2021. [Google Scholar]
- 15.Durham, North Carolina: BioMérieux, Inc; 2020. https://www.fda.gov/media/137742/download. Accessed 29 December 2021.
- 16.BGI GenomicsCorp Ltd . BGI Genomics Corp Ltd; Shenzhen: 2021. Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2 - Instructions for Use.https://www.fda.gov/media/136472/download [Google Scholar]
- 17.Ristić M, Nikolić N, Čabarkapa V, Turkulov V, Petrović V. Validation of the STANDARD Q COVID-21 antigen test in Vojvodina, Serbia. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European commision directorate-general for health and food safety. EU health preparedness: a common list of COVID-19 rapid antigen tests. Last update: 8 April 2022. Available at: https://ec.europa.eu/health/system/files/2022-05/covid-19_rat_common-list_en.pdf. Accessed 18 May 2022.
- 19.World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. Interim guidance. 11 September 2020. Geneva; 2020. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays. Accessed 11 February 2022.
- 20.Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 21.Ristić M, Milosavljević B, Vapa S, Marković M, Petrović V. Seroprevalence of antibodies against SARS-CoV-2 virus in Northern Serbia (Vojvodina): a four consecutive sentinel population-based survey study. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–790. doi: 10.1016/S1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022:eabn4947. doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson N, Ghani A, Cori A, Hogan A, Hinsley W, Volz E, Imperial College COVID-19 Response Team, “Report 49: Growth, population distribution and immune escape of Omicron in England” (WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis, Jameel Institute, Imperial College London, 2021). https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-16-COVID19-Report-49.pdf. Accessed 1 April 2022.
- 26.Cohen JI, Burbelo PD. Reinfection with SARS-CoV-2: implications for vaccines. Clin Infect Dis. 2021;73(11):e4223–e4228. doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation: COVID-19 Weekly Epidemiological Update, Edition 74, published 11 January 2022. https://apps.who.int/iris/bitstream/handle/10665/351044/CoV-weekly-sitrep11Jan22-eng.pdf?sequence=1&isAllowed=y. Accessed 22 February 2022.
- 28.Miljanovic D, Milicevic O, Loncar A, Abazovic D, Despot D, Banko A. The first molecular characterization of Serbian SARS-CoV-2 isolates from a unique early second wave in Europe. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.691154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidanović D, Tešović B, Volkening J, et al. First whole-genome analysis of the novel coronavirus (SARS-CoV-2) obtained from COVID-19 patients from five districts in Western Serbia. Epidemiol Infect. 2021;149:E246. [Google Scholar]
- 30.Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mensah AA, Lacy J, Stowe J, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. 2022;84(4):542–550. doi: 10.1016/j.jinf.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slezak J, Bruxvoort K, Fischer H, Broder B, Ackerson B, Tartof S. Rate and severity of suspected SARS-Cov-2 reinfection in a cohort of PCR-positive COVID-19 patients. Clin Microbiol Infect. 2021;27(12):1860.e7–1860.e10. doi: 10.1016/j.cmi.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor CA, Whitaker M, Anglin O, et al. COVID-19–Associated hospitalizations among adults during SARS-CoV-2 delta and omicron variant predominance, by race/ethnicity and vaccination status — COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:466–473. doi: 10.15585/mmwr.mm7112e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tso WWY, Kwan M, Wang YL, et al. Intrinsic severity of SARS-CoV-2 omicron BA.2 in uninfected, unvaccinated children: a population-based, case-control study on hospital complications. Lancet. 2022 doi: 10.2139/ssrn.4063036. published online March 21. (preprint) [DOI] [Google Scholar]
- 35.Abu-Raddad LJ, Chemaitelly H, Bertollini R. National study group for COVID-19 epidemiology. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021;385(26):2487–2489. doi: 10.1056/NEJMc2108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Kaperak C, Sato T, Sakuraba A. COVID-19 reinfection: a rapid systematic review of case reports and case series. J Investig Med. 2021;69(6):1253–1255. doi: 10.1136/jim-2021-001853. [DOI] [PubMed] [Google Scholar]
- 37.Maier H.E, Balmaseda A, Ojeda S, et al. An immune correlate of SARS-CoV-2 infection and severity of reinfections. medRxiv. 2021 doi: 10.1101/2021.11.23.21266767. published online November 24. (preprint) [DOI] [Google Scholar]
- 38.Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus Pandemic (COVID- 19); 2022. Available at: https://ourworldindata.org/coronavirus/country/serbia#what-share-of-the-population-has-completed-the-initial-vaccination-protocol. Accessed 17 May 2022.
- 39.Petrović V, Vuković V, Marković M, Ristić M. Early effectiveness of four SARS-CoV-2 vaccines in preventing COVID-19 among adults aged ≥60 Years in Vojvodina, Serbia. Vaccines (Basel) 2022;10(3):389. doi: 10.3390/vaccines10030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ioannidis JPA. Factors influencing estimated effectiveness of COVID-19 vaccines in non-randomised studies. BMJ Evid Based Med. 2022 doi: 10.1136/bmjebm-2021-111901. bmjebm-2021-111901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520–1524. doi: 10.15585/mmwr.mm7043e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity—a strategy for containment. N Engl J Med. 2020;383(22):e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 43.Sciscent BY, Eisele CD, Ho L, King SD, Jain R, Golamari RR. COVID-19 reinfection: the role of natural immunity, vaccines, and variants. J Commun Hosp Intern Med Perspect. 2021;11(6):733–739. doi: 10.1080/20009666.2021.1974665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.