Abstract
Background
Both viral infection and autoimmune diseases like dermatomyositis (DM) and polymyositis (PM) can cause myocarditis and inflammatory cardiomyopathy. It is of great importance to identify underlying etiologies and initiate appropriate treatment. This study aimed to describe the pattern of regional longitudinal strain (LS) and myocardial work in PM/DM patients with cardiac involvement and investigate the usefulness of the pattern to differentiate PM/DM from acute viral myocarditis (AVM) in the clinical setting.
Methods
A total of 46 PM/DM patients with cardiac involvement, 24 patients with AVM, and 30 healthy control participants (HCs) were included. Regional myocardial work and strain analyses were performed using two-dimensional (2D) echocardiography to calculate relative basal LS and myocardial work parameters and investigate their value for differential diagnosis.
Results
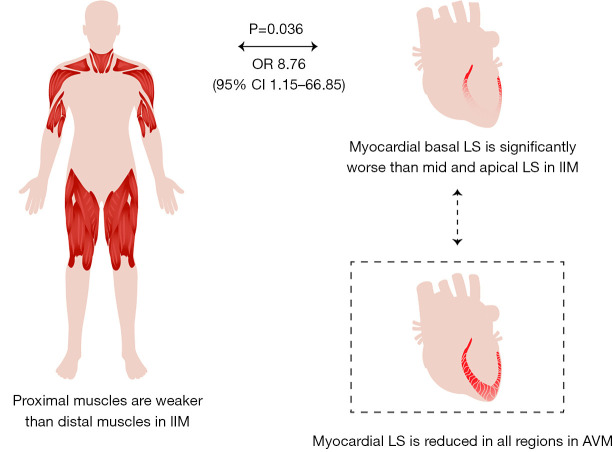
PM and DM are characterized by a pattern of basal myocardial weakness with LS (basal, mid, and apical segments: −15.0±4.4, −17.1±4.7, and −21.4±6.5, respectively), myocardial work index (basal, mid, and apical segments: 1,193±432, 1,272±394, and 1,431±451, respectively), and constructive work (basal, mid, and apical segments: 1,512±422, 1,628±413, and 1,912±433, respectively) that show a base-to-apex gradient in which the myocardium at the base is more severely injured that that of the apex. On cardiovascular magnetic resonance, the positive rate of late gadolinium enhancement was also significantly higher in the basal segments (64%) than the mid (44%) and apical (28%) segments (P=0.038). A relative basal LS of 0.43, defined using the equation [average basal LS/(average mid LS + average apical LS)], had an area under curve (AUC) of 0.88 with high sensitivity (88%) and specificity (78%) to differentiate PM/DM from AVM. Using multivariate logistic regression analysis, relative basal injury of myocardium and creatine kinase elevation were strongly correlated with proximal skeletal muscle weakness according to manual muscle testing (P=0.036 and P=0.010, respectively).
Conclusions
Similar to the typical proximal muscle weakness of limbs, PM/DM patients also presented with regionally decreased LS and myocardial work of the basal myocardium. A “basal weakness” pattern is easily recognizable and can be used to accurately differentiate PM/DM with cardiac involvement from AVM.
Keywords: Relative basal myocardial weakness, dermatomyositis and polymyositis (DM and PM), viral myocarditis, echocardiographic strain analysis, myocardial work
Introduction
Inflammatory cardiomyopathy refers to a broad group of acute or chronic inflammatory responses of the heart to environmental or endogenous triggers (1). The triggers can be classified as infectious or noninfectious and are most commonly caused by viral pathogenesis, namely acute viral myocarditis (AVM). Meanwhile, inflammatory activation occurs in other forms of myocardial injury, including autoimmune disorders (2). Connective tissue diseases break immune self-tolerance and induce cardiac autoimmunity, which results in progression from asymptomatic cardiac involvement to dilated cardiomyopathy and decompensatory heart failure (3). Regardless of the pathogenesis, cardiac symptoms in late-stage inflammatory cardiomyopathy are similar; they include arrhythmia, heart failure, and chest pain and are associated with myocardial injury, troponin, and creatine kinase elevation (4).
Dermatomyositis (DM) and polymyositis (PM) are inflammatory myopathies that are characterized by proximal muscle weakness, myalgia, dysphagia, and dyspnea due to peripheral or axial muscle involvement. Patients with DM also typically present with cutaneous manifestations, such as Gottron papules, heliotrope rash, and erythema of the anterior upper chest or the posterior neck (5,6). Although skeletal muscle is the main target of PM/DM, myocardium is also striated muscle like skeletal muscle, cardiac involvement can also occur. However, the prevalence of cardiac involvement is underestimated, and the diagnosis is often delayed (7). In addition, PM/DM can cause inflammatory cardiomyopathy, and the resultant heart failure, arrhythmia, and myocardial infarction are 3 main causes of cardiac mortality (8,9).
Echocardiography is a widely used, easily accessible, and cost-effective examination method to detect cardiac functional and structural abnormalities. Technical development of strain analysis and the more contemporary quantification of myocardial work has helped to improve the sensitivity of traditional echocardiographic parameters (10,11). However, reports of the characterization of PM/DM cardiac involvement using these new techniques are scarce. In systemic lupus erythematosus, global longitudinal strain (LS) could be a more sensitive marker of lupus myocarditis than left ventricular ejection fraction (LVEF) (12). However, the specificity of the global value of these techniques may be decreased because the indexes may be diminished in diverse myopathic processes.
Our study aimed to describe the pattern of regional LS and myocardial work in PM/DM patients with cardiac involvement, and (2) investigate the usefulness of the pattern to differentiate PM/DM from AVM in the clinical setting. We present the following article in accordance with the Standards for Reporting Diagnostic accuracy studies (STARD) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1098/rc).
Methods
Study population
This study retrospectively enrolled consecutive patients with newly diagnosed PM/DM or AVM in the Peking Union Medical College Hospital between January 2015 and April 2021, when echocardiographic videos were available for off-line analysis. The diagnosis of idiopathic inflammatory myopathy (IIM) was confirmed according to the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria. Subgroup identification was in accordance with the classification tree (13). Cardiac involvement was suspected if the PM/DM patients had one of the following manifestations: (I) elevated cardiac troponin I (cTnI) >0.056 pg/mL; (II) electrocardiogram (ECG) abnormalities, such as frequent atrial or ventricular premature beats, ventricular tachycardia, and conduction defects; and/or (III) pericardial effusion in echocardiography. We excluded patients for the following reasons: (I) inclusion body myositis, necrotizing autoimmune myositis, overlapping syndromes, drug-related myositis, and cancer-related myositis; (II) previous diagnosis of congenital heart disease, coronary heart disease, and valvular heart disease; (III) flu-like symptoms in the past 6 months; (IV) >2 weeks between laboratory tests and echocardiography examination; and (V) poor echocardiography image quality.
The instance of AVM was suspected based on medical history of the recent onset of chest pain, with or without flu-like symptoms, and gastroenteritis. Elevated cTnI, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) supported the diagnosis. The AVM was further confirmed if cardiac magnetic resonance (CMR) imaging revealed myocardial edema. Endomyocardial biopsy (EMB) was not routinely performed for the diagnosis of AVM in our center (14). Since EMB was not routinely conducted, other etiologies were cautiously excluded. If the patients had no dynamic changes on cTnI or ECG indicating acute coronary syndrome (ACS), but ACS was still suspected, coronary angiography was performed for exclusion. If the disease progression was fast and dramatic, EMB was conducted to exclude giant cell myocarditis. Patients were also checked to ensure that they did not have hypereosinophilia, they had no evidence of other autoinflammatory diseases besides PM/DM, they had not been vaccinated in the last 4 weeks, or that their echocardiography show the typical manifestation of Takotsubo disease or apical wall motion abnormality. Patients with >2 weeks between laboratory tests and echocardiography examination and poor echocardiography image quality were also excluded.
All healthy participants (HCs) had a normal ECG, no history of cardiovascular disease, no cardioactive medication, and a blood pressure (BP) of ≤140/90 at the time of inclusion and at the time of the echocardiography examination. All HCs had a body mass index (BMI) ≤30. This study was reviewed and approved by the Institutional Review Board of the Peking Union Medical College Hospital. Written informed consent for publication was provided by each patient before participation. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Clinical data
Demographic data, detailed symptoms, results of laboratory tests, and treatment strategies were collected based on a standard case report form. Manual muscle testing (MMT) proposed by the Medical Research Council (MRC) using the numeral grades 0–5 was adopted to determine proximal muscle weakness. If the proximal muscle strength was lower than the distal muscle strength, the patient was considered to have proximal muscle weakness. Laboratory tests included serum biomarkers of inflammation (including ESR and CRP), antinuclear antibody, and myositis autoantibodies. Creatine kinase (CK), CK-MB, cTnI, lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were selected as biochemical indices of muscle injury. A standard 12-lead ECG was performed for all participants on admission, and data from the Holter monitoring were also collected, if available. Interstitial lung disease was considered if the chest computed tomography (CT) scan indicated the presence of bibasilar interstitial fibrosis or alveolar infiltrates. Respiratory muscle involvement was considered if participants had ventilatory failure with decreased vital capacity.
Echocardiography
Echocardiographic examination was completed within 2 days after AVM or PM/DM when cardiac involvement was suspected. The authors were blind to the clinical data when analyzing the echocardiographic results. Standard echocardiography was performed using commercially available ultrasound machines GE Vivid 7 and 9 (GE Medical Systems, Waukesha, WI, USA) according to the American Society of Echocardiography/European Association of Cardiovascular imaging guidelines (15,16). The echocardiographic indexes, including morphologic parameters of inter-ventricular septal thickness (IVST) and left ventricular end-diastolic diameter (LVEDD), systolic and diastolic parameters of LVEF, peak early (E) and late (A) diastolic mitral inflow velocity and its ratio (E/A), and average of the medial and lateral mitral annular diastolic velocities (e’), were measured and recorded.
Images were digitally stored and analyzed offline for strain and myocardial work analysis using automated software (EchoPAC Version 203; GE Medical Systems, USA). The LS was measured using apical 2-, 3-, and 4-chamber views; each view was divided into 6 segments. The left ventricle (LV) endomyocardium was manually identified and tissue speckles were automatically tracked throughout the cardiac cycle. Global LS was determined by averaging the strain values of all segments in the 3 standard apical views. Non-invasive myocardial work calculation was first described by Russell et al. (10). By integrating LS and sphygmomanometrically-measured blood pressure on arteria brachialis, an LV pressure-strain loop was generated and adjusted according to the opening and closure of mitral and aortic valves. Myocardial work index (MWI) was defined as the total work within the area of the pressure-strain loop. Constructive work (CW) was defined as myocardial work performed during segmental shortening in the LV systolic phase and segmental lengthening in the LV diastolic phase, and for wasted work (WW), the definition was reversed. Work efficiency (WE) was calculated as CW/(CW + WW)×100%. Compared with strain analysis, quantification of myocardial work avoided the influence of LV afterload.
During strain and myocardial work analysis, the values for the 6 basal, 6 mid, and 6 apical segments were averaged to obtain the value at 3 ventricular levels. The segmental differences were further examined by calculating relative basal values. For example, relative basal LS was defined as: average basal LS/(average mid LS + average apical LS). Image quality was ensured to permit complete software analysis of all cardiac segments.
Cardiovascular magnetic resonance
For patients examined with cardiovascular magnetic resonance (CMR), CMR images were acquired using a 3.0 T scanner (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany). We obtained cine images via an ECG-gated two-dimensional (2D) balanced steady-state free precession sequence. Late gadolinium enhancement (LGE) images were collected 10 minutes after the injection of 0.1 mmol/kg gadopentetate dimeglumine using a 2D phase-sensitive inversion-recovery gradient-echo pulse sequence. Native and postcontrast T1 mapping was performed using a modified look-locker inversion recovery sequence in a 4-chamber long-axis slice and apical, middle, and basal short-axis slices (17). The CMR images were independently analyzed by 2 experienced investigators blind to echocardiographic and clinical outcome data. Visual assessment of LGE was performed according to standardized postprocessing recommendations for qualitative analysis (18). Extracellular volume (ECV) was analyzed semiautomatically via cvi42 software (version 5.3, Circle Cardiovascular Imaging, Calgary, Canada). The ECV was calculated using the T1 of myocardium and T1 of blood pool pre- and post-gadolinium contrast, along with the hematocrit value (19).
Statistical methods
Continuous variables were presented as mean ± standard deviation (SD) or median [interquartile range (IQR)] based on normal or nonnormal distribution, while categorical variables were expressed in frequency and percentages. Categorical variables were compared using an uncorrected chi-square test or Fisher’s exact test. Comparisons between 2 groups of continuous variables were carried out using independent samples t-test or the Mann-Whitney rank sum test. Comparisons across 3 groups were performed using one-way analysis of variance (ANOVA) or Kruskal-Wallis test with the Bonferroni post hoc analysis. Receiver operating characteristic (ROC) curve analysis and the Youden index were used to define cut-off value in diagnosing PM/DM from AVM. The sensitivity and specificity of this cut-off value to differentiate PM and DM separately from AVM were also assessed using ROC curve analysis. Multivariate logistic regression was applied for the assessment of the association between proximal muscle weakness and variables with P<0.1 in the univariate logistic regression (relative basal LS, global LS, and respiratory muscle involvement). Interobserver variability for LS was assessed by repeated measurements from 20 randomly chosen participants by a second observer (GTC) blind to the results of the first observer (LXH). Intraobserver variability was assessed by the first observer by performing repeated measurements that were blind to the first measurements. Repeatability was analyzed with Bland-Altman plots. All statistical analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Patient characteristics
In this study, a total of 476 PM/DM patients were initially screened; 49 (27 PM patients and 19 DM patients) that fulfilled the inclusion criteria for cardiac involvement were included, while 3 (1 PM patient and 2 DM patients) were further excluded because of inadequate image quality (Figure 1). Among the 46 PM/DM patients finally included, 24 were “definite idiopathic inflammatory myopathy (IIM)” and 22 were “probable IIM” (13). A total of 26 patients with AVM and 30 HCs were included, while 2 patients with AVM were excluded due to unsatisfying image quality for strain or myocardial work analysis.
Figure 1.
Flow diagram of PM/DM patients included in the study. DM, dermatomyositis; PM, polymyositis; IIMs, idiopathic inflammatory myopathies; CVD, cardiovascular disease.
Baseline characteristics of the study population are shown in Table 1. The mean age of HCs (44±16 years) was between the mean age of PM/DM patients and AVM patients, and the gender distribution (9 males, 21 females) was comparable to that of patients. The PM/DM patients and AVM patients had similar cardiovascular risk profiles except for age and hypertension. The cTnI levels were not significantly different between AVM and PM/DM patients, while N-terminal pro-B-type natriuretic peptide (NT-proBNP) was significantly higher in AVM patients. The increase of muscular enzyme levels in PM/DM patients tended to be greater than that in patients with AVM, but the differences were not statistically significant. The inflammatory biomarkers of both ESR and CRP were above the upper normal limits in the 2 groups, with CRP significantly higher in AVM patients.
Table 1. Baseline characteristics for patients with IIM and AVM.
| Variables | IIM (n=46) | AVM (n=24) | P value |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 48±16 | 28±12†‡ | <0.001 |
| Male | 14 (30.4%) | 12 (50%) | 0.108 |
| BMI (kg/m2), mean ± SD | 20.8±6.0 | 23.7±3.6 | 0.046 |
| Smoking | 11 (23.9%) | 6 (25%) | 0.920 |
| Hypertension | 16 (34.7%) | 0 (0%)† | 0.001 |
| Diabetes mellitus | 5 (10.8%) | 0 (0%) | 0.235 |
| Hyperlipidemia | 10 (21.7%) | 2 (8.3%) | 0.281 |
| Clinical characteristics | |||
| Interstitial lung disease | 22 (47.8%) | 0 (0%)†‡ | <0.001 |
| Dysphagia | 9 (19.5%) | 0 (0%)‡ | 0.052 |
| Respiratory muscular involvement | 4 (8.6%) | 0 (0%) | 0.344 |
| Raynaud phenomenon | 4 (8.6%) | 0 (0%) | 0.344 |
| Shortness of breath | 27 (58.6%) | 18 (75.0%)‡ | 0.177 |
| Palpitation | 16 (34.7%) | 6 (25.0%)† | 0.403 |
| Nonproductive cough | 4 (8.6%) | 2 (8.3%) | 0.959 |
| Chest pain | 3 (6.5%) | 12 (50%)†‡ | <0.001 |
| Arrhythmias | 21 (45.6%) | 6 (25.0%) | 0.092 |
| Muscle strength grading of MMT | |||
| Proximal upper extremities, mean ± SD | 4.2±0.8 | NA | NA |
| Distal upper extremities, mean ± SD | 4.9±0.3 | NA | NA |
| Proximal lower extremities, mean ± SD | 4.0±0.8 | NA | NA |
| Distal lower extremities, mean ± SD | 4.9±0.4 | NA | NA |
| Proximal muscle weakness, mean ± SD | 31 (67.3%) | NA | NA |
| Laboratory tests | |||
| ALT (U/L), median [IQR] | 65 [124] | 161 [340] | 0.041 |
| AST (U/L), median [IQR] | 68 [113] | 65 [104] | 0.877 |
| LDH (U/L), median [IQR] | 419 [388] | 376 [408] | 0.584 |
| Creatinine (µmol/L), median [IQR] | 50 [29] | 99 [41]† | 0.007 |
| ESR (mm/h), median [IQR] | 16 [27] | 14 [25] | 0.314 |
| CRP (mg/L), median [IQR] | 7 [17] | 21 [31] | 0.033 |
| Creatine kinase (U/L), median [IQR] | 1,293 [3,497] | 314 [436] | 0.863 |
| Creatine kinase-MB (U/L), median [IQR] | 39 [140] | 22 [67] | 0.369 |
| cTnI (pg/mL), median [IQR] | 1.8 [3.3] | 4.1 [5.6] | 0.611 |
| NT-proBNP (pg/mL), median [IQR] | 1,081 [2,067] | 2,147 [3,762]† | 0.014 |
| Antinuclear antibody | 31 (67.3%) | NA | NA |
| Myositis specific antibodies | |||
| Anti-Jo-1/PL-7/EJ/OJ | 13 (28.2%) | NA | NA |
| Anti-Mi-2/TIF1γ/MDA5/NXP2 | 10 (21.7%) | NA | NA |
| Anti-SRP | 4 (8.7%) | NA | NA |
| Myositis associated antibodies | |||
| Anti-Ro52 | 21 (45.6%) | NA | NA |
| Anti-Ku | 5 (10.9%) | NA | NA |
| Anti-PM-Scl 100/PM-Scl 75 | 0 (0%) | NA | NA |
| Treatment | |||
| Long-term glucocorticoids | 46 (100%) | NA | NA |
| Glucocorticoids pulse-treatment | 14 (30.4%) | NA | NA |
| Immunosuppressive agents | 41 (89.1%) | NA | NA |
| Intravenous immune globulin | 16 (34.8%) | NA | NA |
†, P<0.05 versus PM; ‡, P<0.05 versus DM. IIM, idiopathic inflammatory myopathies; AVM, acute viral myocarditis; BMI, body mass index; MMT, Manual Muscle Testing; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PM, polymyositis; DM, dermatomyositis.
Conventional echocardiographic parameters
Conventional echocardiographic parameters are presented in Table 2. The proportion of patients with pericardial effusion was higher in both AVM and PM/DM than HCs. As indicated by a higher LVEF, the systolic function was better preserved in HCs, but the LVEF was still within normal range in the PM/DM and AVM patients. Likewise, other routinely-used conventional indexes assessing cardiac function and structure were generally normal and none of them were significantly different between PM/DM and AVM patients.
Table 2. Echocardiographic characteristics for IIM patients, AVM patients, and healthy controls.
| Variables | Control (n=30) | IIM (n=46) | AVM (n=24) | P value (IIM vs. AVM) |
|---|---|---|---|---|
| IVST (mm) | 9.3±2.0 | 8.5±1.7 | 8.5±1.7 | 0.953 |
| LVEDD (mm) | 45±4 | 48±10 | 51±5 | 0.205 |
| LVEF (%) | 68±7 | 58±14 | 54±11 | 0.154 |
| E(m/s) | 0.74±0.16 | 0.79±0.25 | 0.72±0.20 | 0.293 |
| E/A | 1.50±0.53 | 1.12±0.47 | 1.38±0.49 | 0.046 |
| e' (m/s) | 0.10±0.02 | 0.07±0.03 | 0.09±0.03 | 0.123 |
| Pericardial effusion | 0 (0) | 13 (28.2) | 6 (25.0) | 0.771 |
| Global LS | −22.8±2.0 | −17.8±4.9 | −14.5±2.4 | <0.001 |
| Regional basal LS | −20.1±1.7 | −15.0±4.4 | −14.2±2.3 | 0.379 |
| Regional mid LS | −21.9±2.0 | −17.1±4.7 | −14.1±2.5 | 0.001 |
| Regional apical LS | −26.2±3.7 | −21.4±6.5 | −15.3±3.2 | <0.001 |
Values are mean ± SD or n (%). IIM, idiopathic inflammatory myopathies; AVM, acute viral myocarditis; IVST, inter-ventricular septal thickness; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; E, peak early; A, peak late; e', average of the medial and lateral mitral annular diastolic velocities; LS, longitudinal strain; PM, polymyositis; DM, dermatomyositis.
Absolute LS
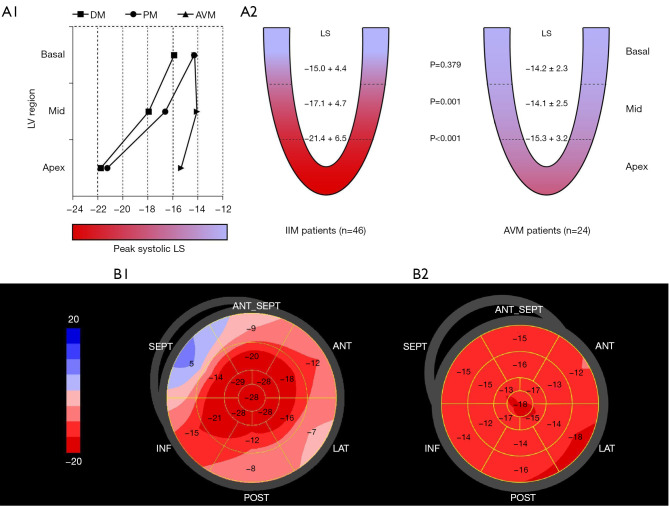
In contrast to conventional echocardiography, deformation imaging with strain analysis revealed significant differences between the PM/DM group and AVM group (Table 2). Patients with PM/DM had greater absolute value of global LS than AVM patients (−17.8 in PM/DM vs. −14.5 in AVM, P<0.001). Of note, as shown in Figure 2, the LS was equally damaged at all 3 ventricular levels in AVM patients, but PM/DM patients showed a base-to-apex gradient, with basal LS comparable to AVM patients (−15.0 in PM/DM vs. −14.2 in AVM, P=0.379) and mid and apical LS significantly better in PM/DM patients (mid: −17.1 in PM/DM vs. −14.1 in AVM, P=0.001; apex: −21.4 in PM/DM vs. −15.3 in AVM, P<0.001). The pattern of more severely damaged basal LS was seen in 45 (97.8%) of the PM/DM patients.
Figure 2.
Regional LS and differences between groups. Line graphs of DM, PM and AVM represented differences in regional basal, mid, and apical LS of left ventricle (A1). Mid and apical LS were significantly better in PM/DM patients (A2). Examples of bull’s-eye plots of peak systolic LS obtained from patients with PM/DM (B1) and AVM (B2). LV, left ventricle; DM, dermatomyositis; PM, polymyositis; AVM, acute viral myocarditis; LS, longitudinal strain; IIM, idiopathic inflammatory myopathy; PM/DM, idiopathic inflammatory myopathies.
Myocardial work
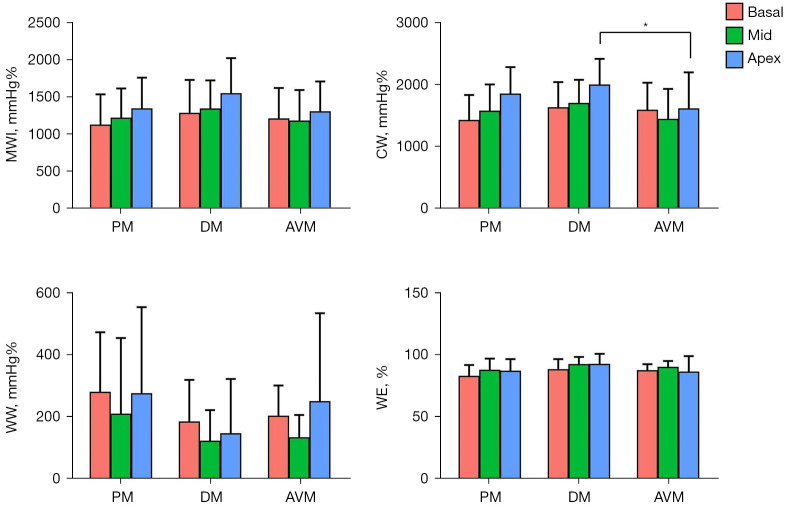
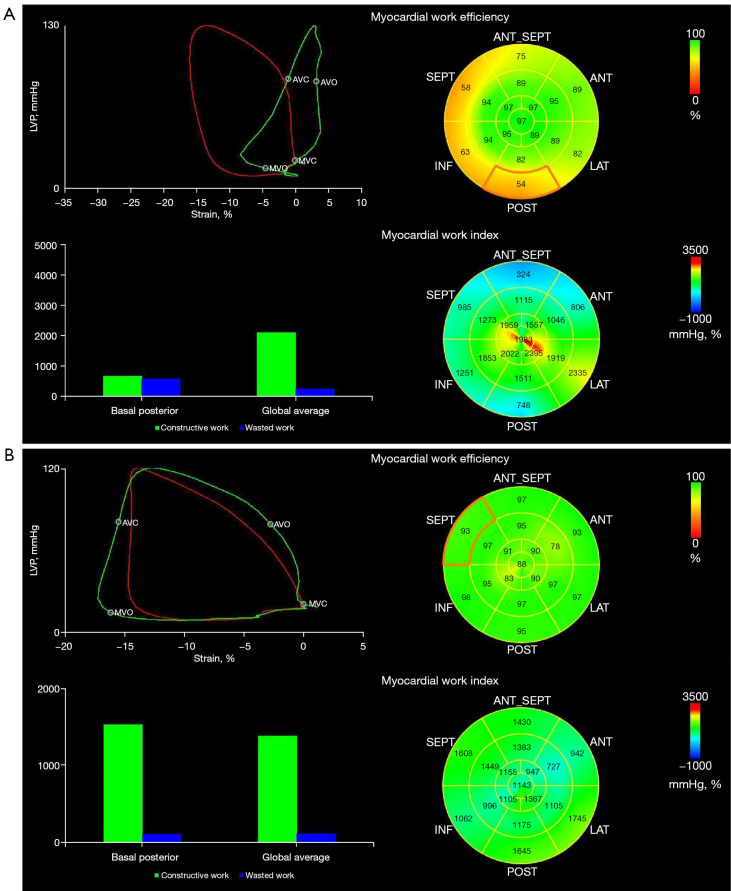
The 4 main myocardial work indexes of MWI, CW, WW, and WE in PM, DM, and AVM patients are shown in Figure 3. Both PM/DM and AVM patients had significantly reduced LV MWI, CW, and WE and increased WW compared with HCs (Table S1). However, when comparing PM/DM patients to AVM patients, these indexes were not statistically different, except for higher apical CW in DM patients. Similar to LS, for patients with PM/DM, the regional differences from apex to base in MWI and CW were also observed. None of the myocardial work indexes in AVM patients showed the pattern of more severely injured basal myocardium. Figure 4 displays examples of 17-segment bull’s-eye plots of myocardial work obtained from a PM/DM patient and an AVM patient.
Figure 3.
Myocardial work for PM, DM, and AVM patients. *P<0.05 between DM and AVM patients. MWI, myocardial work index; CW, constructive work; WW, wasted work; WE, work efficiency; PM, polymyositis; DM, dermatomyositis; AVM, acute viral myocarditis.
Figure 4.
Seventeen-segment bull’s-eye plots of myocardial work obtained from a PM/DM patient (A) and an AVM patient (B). PM, polymyositis; DM, dermatomyositis; AVM, acute viral myocarditis.
Relative basal parameters
The relative basal LS was significantly lower in the PM/DM group than AVM group (0.39 in PM/DM vs. 0.49 in AVM, P<0.001; Table 3). Relative myocardial work indexes were also significantly different in distinguishing the 2 groups, which was manifested in the lower relative basal CW (P<0.001), relative basal WE (P=0.013), and relative basal MWI (P=0.045) of PM/DM patients.
Table 3. Relative basal parameters for patients with IIM and AVM.
| Variables | IIM (n=46) | AVM (n=24) | P value |
|---|---|---|---|
| Relative basal LS | 0.39±0.07 | 0.49±0.08†‡ | <0.001 |
| Relative basal MWI | 0.44±0.08 | 0.49±0.10 | 0.045 |
| Relative basal CW | 0.43±0.07 | 0.54±0.11†‡ | <0.001 |
| Relative basal WW | 0.98±1.04 | 0.73±0.46 | 0.291 |
| Relative basal WE | 0.48±0.03 | 0.50±0.05† | 0.013 |
Values are mean ± SD. †, P<0.05 versus PM; ‡, P<0.05 versus DM. IIM, idiopathic inflammatory myopathies; AVM, acute viral myocarditis; LS, longitudinal strain; MWI, myocardial work index; CW, constructive work; WW, wasted work; WE, work efficiency; PM, polymyositis; DM, dermatomyositis.
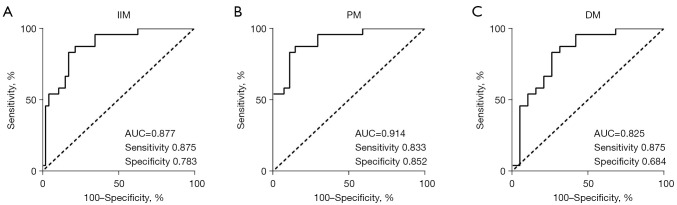
The ROC curves demonstrated the ability of relative basal LS, myocardial work indexes, and traditional echocardiographic parameters to differentiate PM/DM from AVM (Figures 5,6). With a cut-off value of 0.43, relative basal LS performed as the best indicator with the highest area under curve (AUC) of 0.877 (sensitivity 0.875, specificity 0.783). Specifically, to differentiate PM from AVM, relative basal LS had an AUC of 0.914 (sensitivity 0.833, specificity 0.852), and to differentiate DM from AVM, the relative basal LS had an AUC of 0.825 (sensitivity 0.875, specificity 0.684).
Figure 5.
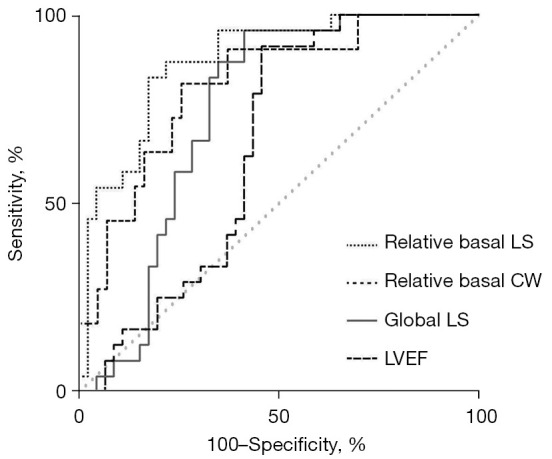
Comparison of ROC curves of both non-deformation and deformation echocardiographic parameters to diagnose PM/DM. LS, longitudinal strain; CW, constructive work; LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic; PM, polymyositis; DM, dermatomyositis.
Figure 6.
ROC curves of relative basal LS for the detection of PM/DM, PM and DM. ROC curves using a cut-off value of 0.43 for the relative basal LS to differentiate (A) PM/DM, (B) PM, and (C) DM from AVM. IIM, idiopathic inflammatory myopathies; PM, polymyositis; DM, dermatomyositis; AUC, area under curve; ROC, receiver operating characteristic; LS, longitudinal strain.
The AUC of global LS and relative basal CW were also over 0.8 (0.81 and 0.80, respectively), but were less useful than relative basal LS. Compared with traditional echocardiographic parameters, relative basal LS demonstrated larger AUC for detecting PM/DM, including LVEF (AUC 0.75), E/A (AUC 0.64), e' (AUC 0.63), and LVEDD (AUC 0.63).
CMR characteristics
In our study, 25 PM/DM patients had available CMR data. A total of 16 (64%) patients had LGE in the basal segments of the myocardium, and this was significantly higher than the mid segments (11/25, 44%) and apical segments (7/25, 28%) (P=0.038). The results of T1 mapping and ECV did not have a base-to-apex gradient, with the highest values in the basal segments and the lowest values in the mid segments. The differences were not statistically significant for T1 mapping and ECV (Table 4).
Table 4. CMR findings in 25 patients with IIM.
| Variables | Basal segments | Mid segments | Apical segments | P value |
|---|---|---|---|---|
| LGE | 16 (64%) | 11 (44%) | 7 (28%) | 0.038 |
| ECV, % | 33.5±4.3 | 32.3±4.2 | 33.0±4.2 | 0.634 |
| T1 mapping, ms | 1,373±71 | 1,359±62 | 1,367±64 | 0.740 |
Values are mean ± SD or n (%). CMR, cardiac magnetic resonance; IIM, idiopathic inflammatory myopathies; LGE, late gadolinium enhancement; ECV, extracellular volume.
Differences between subtypes of PM/DM
The pattern of worse myocardial basal segment was seen in both PM and DM patients, according to the similar relative basal LS (0.38 in PM vs. 0.41 in DM, P=0.123) (Table S2). Besides, there were no differences between the two subtypes of PM/DM in neither global LS (P=0.444) nor regional LS (including basal, mid, and apical segments; P=0.224, P=0.356, P=0.797, respectively). In terms of myocardial work, no differences were seen between them in any myocardial work indexes (MWI P=0.204; WE P=0.054; CW P=0.192; WW P=0.094).
Correlation between proximal muscle weakness and basal myocardial weakness in PM/DM
We analyzed the correlations between proximal skeletal muscle weakness according to MMT and relative basal myocardial weakness in patients with PM/DM. In this analysis, patients with relative basal LS lower than the cut-off value of 0.43 were defined as having basal myocardial weakness. Among all clinical and echocardiographic variables, relative basal LS, CK ≥1,000 U/L, dysphagia, and respiratory muscle involvement had a P value less than 0.1 in the univariate logistic regression. Among them, relative basal LS and CK elevation were independent predictors of proximal skeletal muscle weakness (P=0.036 and P=0.010, respectively) using multivariate logistic regression analysis (Figure 7; Table S3). Furthermore, using ANOVA and t-test, patients with proximal skeletal muscle weakness had significantly lower relative basal LS, especially in the lower extremities (Table S4).
Figure 7.
Association of proximal muscle weakness and basal myocardial weakness in PM/DM. OR, odds ratio; CI, confidence interval; LS, longitudinal strain; PM, polymyositis; DM, dermatomyositis; AVM, acute viral myocarditis.
Inter- and intra-observer variability
The LS measurements were reproducible. Interobserver agreement was high, with only marginal bias (−0.01±0.21). Intraobserver variability was low, with minor bias (−0.6±5.6).
Discussion
This study used 2D speckle tracking and LV myocardial work parameters to show the relative muscle weakness in the basal segments of myocardium in PM/DM patients with cardiac involvement, similar to proximal muscle weakness in the limbs. Furthermore, in these patients, relatively decreased basal LS and MWI differentiated PM/DM with cardiac involvement from AVM patients with better sensitivity and specificity than traditional indexes. To our knowledge, this is the first study to assess regional cardiac function in PM/DM patients using recently developed echocardiographic techniques and compare AVM to PM/DM myocarditis with imaging evaluation solely of the heart.
Clinically, PM/DM is characterized by progressive proximal weakness. Patients describe difficulty in reaching for objects above their head due to weakness of upper extremities or difficulty rising from a seated position due to weakness of lower extremities (20). Inflammatory cell infiltrates were thought to be the primary pathological process causing myositis-induced muscle weakness. In recent years, growing evidence has indicated that non-inflammatory factors, such as reactive oxygen species, may also be involved (21). As for myocardium, patients in our cohort also presented a diverse extent of muscle weakness in different heart regions, and basal segments were more severely damaged than apex segments. Meanwhile, basal segment damage was correlated with proximal skeletal muscle weakness in these patients. This pattern was discovered by strain and myocardial work analysis, which are advanced echocardiographic techniques and can provide more insights into the heart function than traditional parameters. The accuracy and clinical application of strain measurement have been repeatedly verified. The measurement of LS has been recognized as a more sensitive index in the early detection of subclinical LV systolic dysfunction than LVEF and it has superior prognostic value (22). Russell et al. (10) first introduced non-invasive and load-independent quantification of myocardial work in 2012, and it has been gradually implemented in clinical practice in recent years. To date, myocardial work has been assessed in patients with hypertension, hypertrophic cardiomyopathy, and chronic kidney disease (23-25), but data on connective tissue diseases are limited (26). In CMR, in accordance with strain and myocardial work in echo, the LGE positive rate had a base-to-apex gradient which was significantly higher in the basal segments than the mid and apical segments. Although T1 mapping and ECV were also highest in the basal myocardium, they did not reveal a base-to-apex gradient in PM/DM patients with available CMR data in our study. In the future, CMR should be performed in a larger PM/DM patient population.
We first quantified myocardial work in PM/DM and identified relative proximal muscle weakness, but further basic research is required to investigate the underlying mechanism. From pattern recognition to mechanism discovery, the significance of regional analysis was well demonstrated in cardiac amyloidosis. In 2012, “apical sparing” in cardiac amyloidosis patients was first identified by Phelan et al. (27) when referring to the relatively spared strain value in the apex. Later, Bravo et al. (28) explained this pattern and suggested that the statistically significant base-to-apex gradient was attributed to the distribution of total amyloid volume. The segmental differences in terms of amyloid deposition were also supported by histology, CMR, bone scintigraphy, and positron emission tomography (PET) (29). In PM/DM, the reason for proximal, rather than distal, muscle involvement of the extremities has not been fully investigated. For further investigation into the relative basal myocardial weakness, studies providing pathological and multi-modality cardiovascular imaging evidence are required, and the similarities and differences between skeletal muscle and heart muscle injuries in PM/DM could be more comprehensively compared.
More importantly, the relative basal myocardial weakness by strain analysis could differentiate PM/DM from AVM patients with good sensitivity and specificity, better than all other traditional echocardiographic indexes. Identifying different underlying etiologies of myocardial inflammation is of great importance, because they imply different treatment pathways. For AVM, current therapies largely focus on supportive care with attention to guideline-directed treatment for heart failure and arrhythmia. Proposed options have included anti-viral therapies in the viral active replication phase and modulating the immune response with glucocorticoids or immunosuppressive agents in the activation of adaptive immunity phase. However, their efficacy did not reach a consensus among published trials. Confirming an infection negative status is critical before initiating a safe treatment with steroid and immunosuppressive agents in AVM (4). By contrast, for PM/DM, glucocorticoids or immunosuppressive agents are cornerstones during treatment without standard cardiovascular therapy if the patients were suspected having myocardial involvement. Although EMB could confirm the type of inflammation, it is preferably performed in the early course of disease in life-threatening presentations by an experienced team, considering its invasive nature and possible sampling error (30,31). Accordingly, the role of CMR in the diagnosis of inflammatory cardiomyopathy continues to increase thanks to its unique advantage in tissue characterization (32). Newly developed sequences and T2 mapping help identify edema, and native T1 and ECV help detect inflammatory injury. However, CMR cannot distinguish between specific causes of myocardial inflammation (33). Huber et al. (34) successfully detected cardiac inflammation in PM/DM, but failed to differentiate PM/DM from AVM using CMR myocardial mapping. Interestingly, they found CMR parameters in the thoracic skeletal muscles had satisfying ability for differential diagnosis. In our opinion, it was an innovative idea to adopt skeletal muscle data during CMR scanning, but it is not surprising because skeletal muscle was originally involved in PM/DM. The finding of basal myocardial weakness in the present study combines both the advantage of non-invasiveness of CMR and the differentiation value for infectious or non-infectious etiology of EMB. The ratio of relative basal LS can be easily calculated and measured in both clinically stable patients at low risk and urgent patients at high risk because echocardiography can also be done at the patient’s bedside.
Our study has several limitations. First, it is a single-center observational study with a relatively small number of patients. However, the incidence of PM/DM is low, with 2–10 cases per million persons (35). The sample size in our research compared favorably with a previously published study (34). The differentiation between PM/DM with cardiac involvement and AVM had already been found to be highly significant in basal myocardial weakness. Second, because it is a retrospective study, only about half of the PM/DM patients underwent CMR. Despite this, if we refer to the “apical sparing” in cardiac amyloidosis, even though the segmental differences were not completely confirmed by CMR (36), the diagnostic value of this pattern by LS still stands (28). Third, to date, there is no widely accepted definition for cardiac involvement in PM/DM. Therefore, the severity of cardiac dysfunction may differ between studies. We established our inclusion criteria to mirror those used in published literature (37) and modified it according to the clinical procedure of our center. Fourth, the AVM cohort was significantly younger than the IIM cohort and HCs due to the characteristics of the diseases themselves. However, the base-to-apex gradient is not dependent on age, as shown in a previous study (38). Fifth, although we found the basal segments were more severely injured in PM/DM, we do not know whether the base was affected first. Serial echocardiographic data could provide dynamic changes in different stages of disease development and progression. Finally, patients with immune-mediated necrotizing myopathy (IMNM) could not be distinguished from patients with PM in the subclassification tree established in the 2017 EUALR-ACR criteria (13). Some patients with IMNM might have been misdiagnosed as having PM in our study population. However, their diagnosis of IIM was accurate, and differentiation between different subtypes of IIMs was not the main purpose of this study.
Conclusions
To identify the underlying mechanism earlier than is currently possible and to initiate the appropriate treatment in suspected myositis patients is of great importance in clinical practice. In this study, we demonstrated a pattern of basal myocardial weakness in PM/DM patients with cardiac involvement, which can be clearly recognized via 2D echocardiography. Furthermore, this method can help differentiate PM/DM from AVM in patients in both stable and life-threatening conditions because echocardiography is an easily accessible and time saving examination. More studies are required to confirm and investigate the mechanism of relative basal injury.
Acknowledgments
We appreciate Dr. Chao-Qun Zheng for the help on the design of this study, and Dr. Xiao Li and Dr. Yi-Ning Wang for the provision and analysis of CMR data.
Funding: This work was supported by the Beijing Natural Science Foundation (No. 7192156); the Capital’s Funds for Health Improvement and Research, CFH (No. 2020-2-40110); and the CAMS Innovation Fund for Medical Sciences (No. CIFMS,2020-I2M-C&T-B-006 to W.C).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Institutional Review Board of the Peking Union Medical College Hospital. Written informed consent for publication was provided by each patient before taking part. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1098/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1098/coif). The authors have no conflicts of interest to declare.
References
- 1.Heymans S, Eriksson U, Lehtonen J, Cooper LT, Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol 2016;68:2348-64. 10.1016/j.jacc.2016.09.937 [DOI] [PubMed] [Google Scholar]
- 2.Trachtenberg BH, Hare JM. Inflammatory Cardiomyopathic Syndromes. Circ Res 2017;121:803-18. 10.1161/CIRCRESAHA.117.310221 [DOI] [PubMed] [Google Scholar]
- 3.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581-90. 10.1161/CIRCULATIONAHA.105.606509 [DOI] [PubMed] [Google Scholar]
- 4.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-48, 2648a-2648d. [DOI] [PubMed]
- 5.Selva-O'Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol 2018;17:816-28. 10.1016/S1474-4422(18)30254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt J. Current Classification and Management of Inflammatory Myopathies. J Neuromuscul Dis 2018;5:109-29. 10.3233/JND-180308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu XH, Feng XJ, Shi JY, Jia FW, Liu YX, Zhu YL, Li X, Wang YN, Huo L, Wang Q, Chen W. The quest for diagnostic approaches of cardiac involvement in polymyositis and dermatomyositis. Ann Palliat Med 2020;9:2256-70. 10.21037/apm-19-650 [DOI] [PubMed] [Google Scholar]
- 8.Schiopu E, Phillips K, MacDonald PM, Crofford LJ, Somers EC. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: effect of corticosteroids, methotrexate and azathioprine. Arthritis Res Ther 2012;14:R22. 10.1186/ar3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz T, Diederichsen LP, Lundberg IE, Sjaastad I, Sanner H. Cardiac involvement in adult and juvenile idiopathic inflammatory myopathies. RMD Open 2016;2:e000291. 10.1136/rmdopen-2016-000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, Edvardsen T, Smiseth OA. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J 2012;33:724-33. 10.1093/eurheartj/ehs016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 2000;1:154-70. 10.1053/euje.2000.0031 [DOI] [PubMed] [Google Scholar]
- 12.Du Toit R, Herbst PG, van Rensburg A, Snyman HW, Reuter H, Doubell AF. Speckle tracking echocardiography in acute lupus myocarditis: comparison to conventional echocardiography. Echo Res Pract 2017;4:9-19. 10.1530/ERP-17-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol 2017;69:2271-82. 10.1002/art.40320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao XX, Yuan WF. The 4D B-spline method of calculating left ventricular functional parameters of cardiac MRI to evaluate myocardial injury of the apical segment in patients with myocarditis: a case-controlled observational study. Quant Imaging Med Surg 2020;10:2133-43. 10.21037/qims-20-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:1301-10. 10.1093/ehjci/jex244 [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD; Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321-60. 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Li X, Feng J, Shen KN, Tian Z, Sun J, Mao YY, Cao J, Jin ZY, Li J, Selvanayagam JB, Wang YN. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J Cardiovasc Magn Reson 2018;20:2. 10.1186/s12968-017-0419-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 2020;22:19. 10.1186/s12968-020-00610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treibel TA, Fridman Y, Bering P, Sayeed A, Maanja M, Frojdh F, Niklasson L, Olausson E, Wong TC, Kellman P, Miller CA, Moon JC, Ugander M, Schelbert EB. Extracellular Volume Associates With Outcomes More Strongly Than Native or Post-Contrast Myocardial T1. JACC Cardiovasc Imaging 2020;13:44-54. 10.1016/j.jcmg.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 20.Suresh E, Wimalaratna S. Proximal myopathy: diagnostic approach and initial management. Postgrad Med J 2013;89:470-7. 10.1136/postgradmedj-2013-131752 [DOI] [PubMed] [Google Scholar]
- 21.Lightfoot AP, McArdle A, Jackson MJ, Cooper RG. In the idiopathic inflammatory myopathies (IIM), do reactive oxygen species (ROS) contribute to muscle weakness? Ann Rheum Dis 2015;74:1340-6. 10.1136/annrheumdis-2014-207172 [DOI] [PubMed] [Google Scholar]
- 22.Dohi K, Sugiura E, Ito M. Utility of strain-echocardiography in current clinical practice. J Echocardiogr 2016;14:61-70. 10.1007/s12574-016-0282-8 [DOI] [PubMed] [Google Scholar]
- 23.Tadic M, Cuspidi C, Pencic B, Grassi G, Celic V. Myocardial work in hypertensive patients with and without diabetes: An echocardiographic study. J Clin Hypertens (Greenwich) 2020;22:2121-7. 10.1111/jch.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemstra YL, van der Bijl P, El Mahdiui M, Bax JJ, Delgado V, Marsan NA. Myocardial Work in Nonobstructive Hypertrophic Cardiomyopathy: Implications for Outcome. J Am Soc Echocardiogr 2020;33:1201-8. 10.1016/j.echo.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 25.Ke QQ, Xu HB, Bai J, Xiong L, Li MM. Evaluation of global and regional left ventricular myocardial work by echocardiography in patients with chronic kidney disease. Echocardiography 2020;37:1784-91. 10.1111/echo.14864 [DOI] [PubMed] [Google Scholar]
- 26.Boe E, Skulstad H, Smiseth OA. Myocardial work by echocardiography: a novel method ready for clinical testing. Eur Heart J Cardiovasc Imaging 2019;20:18-20. 10.1093/ehjci/jey156 [DOI] [PubMed] [Google Scholar]
- 27.Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442-8. 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 28.Bravo PE, Fujikura K, Kijewski MF, Jerosch-Herold M, Jacob S, El-Sady MS, Sticka W, Dubey S, Belanger A, Park MA, Di Carli MF, Kwong RY, Falk RH, Dorbala S. Relative Apical Sparing of Myocardial Longitudinal Strain Is Explained by Regional Differences in Total Amyloid Mass Rather Than the Proportion of Amyloid Deposits. JACC Cardiovasc Imaging 2019;12:1165-73. 10.1016/j.jcmg.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapezzi C, Fontana M. Relative Left Ventricular Apical Sparing of Longitudinal Strain in Cardiac Amyloidosis: Is it Just Amyloid Infiltration? JACC Cardiovasc Imaging 2019;12:1174-6. 10.1016/j.jcmg.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 30.Dominguez F, Kühl U, Pieske B, Garcia-Pavia P, Tschöpe C. Update on Myocarditis and Inflammatory Cardiomyopathy: Reemergence of Endomyocardial Biopsy. Rev Esp Cardiol (Engl Ed) 2016;69:178-87. 10.1016/j.rec.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 31.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, Pedrotti P, Rimoldi OE, Schultheiss HP, Tschöpe C, Cooper LT, Jr, Camici PG. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail 2020;13:e007405. 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc Imaging 2020;13:1221-34. 10.1016/j.jcmg.2019.06.030 [DOI] [PubMed] [Google Scholar]
- 33.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol 2018;72:3158-76. 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 34.Huber AT, Bravetti M, Lamy J, Bacoyannis T, Roux C, de Cesare A, Rigolet A, Benveniste O, Allenbach Y, Kerneis M, Cluzel P, Kachenoura N, Redheuil A. Non-invasive differentiation of idiopathic inflammatory myopathy with cardiac involvement from acute viral myocarditis using cardiovascular magnetic resonance imaging T1 and T2 mapping. J Cardiovasc Magn Reson 2018;20:11. 10.1186/s12968-018-0430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazzani C, Cavazzana I, Ceribelli A, Vizzardi E, Dei Cas L, Franceschini F. Cardiological features in idiopathic inflammatory myopathies. J Cardiovasc Med (Hagerstown) 2010;11:906-11. 10.2459/JCM.0b013e32833cdca8 [DOI] [PubMed] [Google Scholar]
- 36.Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, Wintersperger BJ, Thavendiranathan P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson 2017;19:61. 10.1186/s12968-017-0376-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Wang GC, Ma L, Zu N. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol 2012;35:686-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menting ME, McGhie JS, Koopman LP, Vletter WB, Helbing WA, van den Bosch AE, Roos-Hesselink JW. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography 2016;33:1665-75. 10.1111/echo.13323 [DOI] [PubMed] [Google Scholar]