Abstract
Background.
Resistant hypertension (RH) is associated with increased risk of cognitive decline, stroke, and dementia. Lifestyle modification has been suggested to improve cognitive function through its salutary effects on vascular function.
Methods.
Participants included 140 patients with RH participating in the TRIUMPH trial. Participants were randomized to a cardiac rehabilitation-based lifestyle program (C-LIFE) or a standardized education and physician advice condition (SEPA). Participants completed a 45-minute cognitive test battery consisting of tests of Executive Functioning and Learning, Memory, and Processing Speed. Biomarkers of vascular (flow mediated dilation of the brachial artery [FMD]), microvascular, and cerebrovascular function were also collected, in addition to weight, fitness, and ambulatory blood pressure.
Results.
Participants averaged 63 years of age, 48% female, 59% Black, and obese (mean BMI = 36 kg/m2 [SD = 4]). Cognitive performance improved across the entire cohort during the 4-month trial (t-scores pre = 48.9 [48, 50] vs. post = 50.0 [49, 51], P<.001). Post-intervention Executive Function / Learning composite performance was higher for participants in C-LIFE compared to SEPA (d = 0.37, P = .039). C-LIFE intervention effects on Memory and Processing Speed were moderated by sex and baseline stroke risk, respectively (P = .026 and P = .043 for interactions), such that males and participants with greater stroke risk showed the greatest cognitive changes. FMD (C-LIFE: +0.3 % [-0.3, 1.0] vs. SEPA: -1.4 % [-2.5, -0.3], P = .022), and microvascular function (C-LIFE: 97 [65, 130] vs. SEPA: -25 [-75, 23], P<.001) were improved in C-LIFE compared to SEPA, whereas cerebrovascular reactivity was not (C-LIFE: -0.2 [-0.4, 0] vs. SEPA: 0.1 [-0.2, 0.4], P = .197). Mediation analyses suggested that increased Executive Function / Learning was associated with reduced ambulatory SBP levels secondary to weight loss (indirect effect: B = 0.25 [0.03, 0.71]).
Conclusions.
Lifestyle modification individuals with RH improves cognition, which appeared to be associated with reduced ambulatory SBP changes through weight loss. Cognitive improvements were accompanied by parallel improvements in endothelial and microvascular function.
Introduction
Hypertension is a significant and increasingly prevalent public health concern, impacting more than 1/3 of adults in the United States[1] and more than 1/2 of older adults ≥ 65 years of age.[2,3] An emerging body of evidence suggests that individuals with resistant hypertension (RH), defined as inadequately controlled blood pressure (BP) despite adherence to 3 or more optimally-dosed antihypertensive medications (including a diuretic), represent an important and growing subgroup of hypertensive adults at elevated risk for cardiovascular disease (CVD)[4] and stroke.[5] Because obesity and advancing age are two of the strongest risk factors for the development of RH,[4,6] the prevalence of RH is projected to continue increasing over the next several decades,[7] in parallel with increasing levels of obesity and a relatively larger proportion of older adults within the population of the United States .[8] Despite the increasing prevalence of RH and elevated risk of cognitive decline and Alzheimer’s Disease and Related Dementias (ADRD),[9–11] few studies have examined how lifestyle interventions among individuals with RH may enhance cognition.
Emerging evidence suggests that treatments to reduce BP among hypertensive patients may mitigate risk of cognitive impairment[12,13] and improve biomarkers of cognitive risk.[14] A number of randomized trials have demonstrated that lifestyle modifications targeting behavioral weight loss, aerobic exercise, and dietary modification improve cognitive outcomes.[15–17] The ENCORE study[18,19] demonstrated that the Dietary Approaches to Stop Hypertension (DASH) diet combined with caloric restriction and aerobic exercise conferred significant reductions in BP (~16/10 mm Hg),[19] improved CVD biomarkers,[18] and better cognitive functioning.[20] Although the mechanisms by which reduced BP improve cognition are not fully understood, improvements in endothelial[21,22] and cerebrovascular functioning[23,24] have both been suggested. The present ancillary study was designed to examine these additional hypotheses regarding cognitive performance in the context of the TRIUMPH trial,[25] which examined the impact of a combined behavioral weight loss intervention, using aerobic exercise, caloric restriction, and the DASH diet, on cognitive function and associated mechanisms among adults with RH.
METHODS
The TRIUMPH study was a randomized clinical trial (RCT) designed to evaluate whether an intensive, medically supervised lifestyle intervention, compared to an education and advice condition, can achieve greater clinically significant BP reductions in patients with RH.[25] Details of the primary trial have been reported previously and are described briefly below.[26] (NCT03001427 and NCT03001427) One hundred forty patients with RH were randomized with 2:1 allocation to either a Center-based Lifestyle Intervention (C-LIFE) or Standardized Education and Physician Advice (SEPA). The primary endpoint of the TRIUMPH cognitive ancillary study was Executive Function. Secondary outcomes included Memory and Processing Speed as well as both brachial artery and microvascular endothelial functioning and cerebrovascular reactivity.
Participants were considered for inclusion if they met criteria for RH, as defined by treatment for > 2 weeks with 3 or more antihypertensive medications of different classes, including a diuretic, with clinic systolic BP (SBP) ≥ 130 mm Hg or diastolic B(P) ≥ 80 mm Hg (previously 140/90 mm Hg), or the need for 4 or more drugs to achieve SBP ≤ 130 mm Hg and DBP ≤ 80 mm Hg, with SBP ≥ 120 mm Hg. Other inclusion criteria included BMI ≥ 25 kg/m2, lack of regular moderate or vigorous physical activity, and age 35–80 years; exclusions included moderate-severe kidney or ischemic cardiac disease, major psychiatric illness or substance dependence, or life-limiting comorbid medical conditions. High blood pressure was established by averaging 9 BP measurements acquired over a 3-week period following JNC 7 guidelines[27] and medication adherence was confirmed by self-report and and the Medication Event Monitoring System.[28] The intensity of antihypertensive medication burden was also quantified using the Daily Defined Dose (DDD).[29]
Treatment Conditions
Participants were randomized to one of two 4-month treatment arms using a 2:1 randomization and were encouraged to maintain their prescribed antihypertensive medications at the discretion of their physicians. The C-LIFE intervention was delivered by a registered dietitian and licensed clinical psychologist. The C-LIFE intervention consisted of (i) Dietary Modification: Participants received instruction on the DASH diet with caloric and sodium restriction (≤2300 mg/day). The DASH dietary eating plan was used in both the PREMIER [30] and ENCORE[19,31] studies; (ii) Behavioral Weight Management: Participants were provided with a target weight and underwent weekly 45-minute counseling sessions that emphasized changes in the initiation of eating behavior, individualized problem-solving, and maintenance of long-term behavior change; (iii) Supervised Exercise: Participants exercised at a state-certified CR facility 3 times per week for 30–45 min at a level of 70–85% of their initial heart rate reserve; (iv) Maintenance: Participants were coached to maintain all components that constitute the lifestyle intervention (See Appendix 1 for more details about the C-LIFE intervention).
Participants randomized to the medical management with Standardized Education and Physician Advice (SEPA) received a 1-hr educational session on BP management delivered by a health educator along with a workbook that outlined an individualized diet and exercise program, including instruction in the DASH diet with caloric restriction and the same exercise prescription received by C-LIFE.
Cognitive Performance.
We adopted a previously described neuropsychological battery,[32] similar to that recommended by the Neuropsychological Working Group for vascular cognitive disorders.[33] Assessments were selected to assess those domains most vulnerable to the effects of RH, including Executive Function / Learning, Processing Speed, and Memory. Executive Function refers to skills mediated by frontal-subcortical brain circuitry that are critical to the capacity of an individual to engage successfully in independent, purposive, self-regulated behaviors. Because Executive Function encompasses a broad range of skills, subdomains typically include self-monitoring, inhibitory control, working memory, cognitive flexibility, planning, mental abstraction, and problem-solving.[34] Assessments included tests of Executive Function (Trail Making Test Part B, Stroop Color-Word Section,[35] Animal Naming,[36] Digit Span,[37] COWA, [38] CVLT-II Discrimination Index,[39] BVMT-R Learning[40]), Processing Speed (Trail Making Test Part A, Stroop Word Section,[36] Stroop Color Section, Digit Symbol,[37] Ruff 2&7 Test[41]), and Memory (CVLT-II Total Recall, CVLT-II Free Recall, and CVLT Delayed Recall). The neuropsychological test battery took 45–60 minutes to complete and was administered in a fixed order of subtests using alternative forms.
Cognitive Complaints.
Participants also completed the Cognitive Difficulties Scale (CDS) to assess self-reported cognitive complaints.[42] The CDS is a 38-item self-report measure assessing subjective cognitive complaints in memory (immediate and delayed), attention / executive function, language, and psychomotor praxis. The CDS was obtained on 106 (76%) of participants at baseline.
Assessment of Brachial Artery and Microvascular Endothelial Function
Flow Mediated Dilation (FMD) of the brachial artery was obtained as a marker of conduit vessel endothelial function.[25] Microvascular Endothelial Function was assessed using hyperemic blood flow velocity change during the first 10 seconds following deflation of the forearm occlusion cuff that was inflated for 5-minutes as a part of the standardized FMD test protocol.[43,44]
Cerebrovascular Reserve was assessed non-invasively using functional Near Infrared Spectroscopy (fNIRS) using the NIRO-200NX (Hamatsu Inc., Japan). Our primary fNIRS measure of interest was cerebrovascular changes in tissue oxygen saturation during a standardized breath holding index (BHI).[45] Secondary outcomes included changes in oxy-Hb and total-Hb. The BHI has been used extensively as a marker of cerebrovascular reserve (CVR), has been shown to decrease with age,[46] is impaired among individuals with cerebrovascular disease[47,48] and cognitive impairment,[49] and is predictive of subsequent stroke.[50] Cerebrovascular changes were quantified as changes in tissue oxygen saturation (TOS) from baseline to BHI, divided by baseline TOS and total BHI time (BHI = [TOSBH − TOSBAS] × 100/ TOSBAS/DBH).[51] Voluntary breath holding was performed with a duration of 20 to 30 seconds, with subjects who were unable to hold their breath for at least 20 seconds excluded.
Fitness, Diet, and Obesity. Assessments of fitness, dietary patterns, and weight have been previously described in detail.[25,26] Assessment of aerobic fitness was obtained using a standard exercise treadmill test. DASH dietary patterns were assessed using a DASH diet score. Weight was assessed using a standard balance scale, which was used to calculated body mass index.
DATA ANALYSIS
Analyses were carried out using SAS 9.4 and R 4.1.2. Before modeling changes in cognition, subtests were first combined within domains of function following factor analyses of both pre and post-treatment test batteries to ensure a consistent factor structure. Factor analysis was accomplished using PROC FACTOR in SAS 9.4, residualized for age and education and an oblique rotation (Promax). Results revealed a three factor solution corresponding to Executive Function / Learning (TMT-B, Stroop Color-Word and Interference sections, Animal Naming, Ruff 2&7 Accuracy, Digit Span, CVLT Distraction, and BVMT Learning), Memory (CVLT-II Total, Immediate Recall, Delayed Recall, and Recognition, and BVMT Total, Recall, and Recognition), and Processing Speed (DSST, Stroop Word, COWA, TMT-A, TMT-B, and Ruff 2&7 speed). Clinical interpretation of baseline cognitive performance was characterized by using demographically-corrected t-scores, in which age, education, biological sex, and race are accounted for. Analyses of changes in cognition were examined using general linear models, controlling for age, education, race, creatinine, the Framingham Stroke Risk Profile score, and the pre-treatment level of the respective outcome. We also explored potential treatment moderation by age, education, biological sex, pretreatment stroke risk, and pretreatment cognitive function in all analyses of cognitive changes. In addition to our a priori testing of between group differences, we also examined longitudinal changes in cognition from pre-to-post treatment across the cohort. Time effects were tested using a repeated-measures, mixed model with mean, demographically-corrected t-scores across domains of function. A parallel analytic approach was utilized for the CDS.
Examination of secondary analyses included microvascular endothelial function (hyperemic blood flow response, expressed as percent change from resting baseline) and CVR. For these analyses, we controlled for the same covariates above as well as baseline resting level of the outcome (e.g. baseline arterial diameter and flow rate, or tissue oxygenation index). Tests of treatment mediation were examined using the PROCESS MACRO (template Model 6) within SAS for a priori identified mediators, including improvements in aerobic fitness, weight loss, ambulatory SBP reductions, improved FMD, and microvascular function. We also examined potential variations in treatment mechanisms between subgroups exhibiting differential improvements in cognitive function following treatment (i.e. biological sex and baseline cerebrovascular risk) (PROCESS MACRO template 14).
RESULTS
Demographic and Clinical Characteristics
As shown in Table 1, participants tended to be older, highly educated, and African-American. Cognitive performance was generally in the Average range, with approximately 20% of participants exhibiting performance that fell consistently below expectation based on demographic norms. Similarly, the average MoCA score for the sample fell at the conventional threshold (MoCA = 26) for identifying mild levels of cognitive impairment (mean MoCA = 25.4 [SD = 2.6]). Examination of baseline cognitive performance revealed variable levels of function across different domains.
Table 1.
Background and clinical characteristics of the sample. Values in parentheses represent % for categorical variables and standard deviations for continuous variables. Due to rounding, some category percentages do not sum to 100%.
| Variable | C-LIFE (n = 90) |
SEPA (n = 50) |
Total Cohort (n = 140) |
|---|---|---|---|
| Age, years | 62 (9) | 63 (9) | 63 (9) |
| Biological Sex Female Sex, n (%) Male Sex, n (%) |
43 (48%) 47 (52%) |
24 (48%) 26 (52%) |
67 (48%) 73 (52%) |
| Race / Ethnicity, n (%) African American White / Caucasian Asian Native American Hispanic Other |
47 (52%) 39 (43%) 1 (1%) 0 (0%) 1 (1%) 2 (2%) |
35 (70%) 11 (22%) 1 (2%) 2 (4%) 1 (2%) 0 (0%) |
82 (59%) 50 (36%) 2 (1%) 2 (1%) 2 (1%) 2 (1%) |
| Education, years | 16.1 (2.3) | 15.7 (2.4) | 15.9 (2.4) |
| Education Level, n (%) < High School High School Degree or Equivalent Some College College Degree Graduate Degree |
0 (0%) 6 (7%) 30 (33%) 25 (28%) 29 (32%) |
1 (2%) 5 (10%) 13 (26%) 17 (34%) 14 (28%) |
1 (1%) 11 (8%) 43 (31%) 42 (30%) 43 (31%) |
| Stroke History, n (%) | 6 (4) | 5 (4) | 11 (8) |
| Clinic SBP (mm Hg) | 139 (10) | 140 (10) | 139 (10) |
| Clinic DBP (mm Hg) | 79 (9) | 80 (8) | 79 (9) |
| Body Mass Index (kg/m2) | 36 (6) | 36 (5) | 36 (6) |
| Creatinine, umol/L | 81 (33) | 90 (45) | 84 (38) |
| Diabetes | 33 (37%) | 11 (22%) | 44 (31%) |
| Current smoker | 1 (1%) | 5 (10%) | 6 (4%) |
| Antihypertensive Medication Daily Defined Dose Sum | 5.1 (2) | 4.9 (2) | 5.0 (2) |
| Number of Antihypertensive Medications | 3.5 (0.7) | 3.4 (0.8) | 3.5 (0.7) |
| Vascular / Cerebrovascular Function | |||
| Flow Mediated Dilation, % | 2.0 (2.4) | 2.1 (2.1) | 2.0 (2.3) |
| Microvascular Endothelial Velocity, % Change | 220 (157) | 251 (159) | 231 (158) |
| Microvascular Endothelial Flow, % Change | 238 (178) | 269 (176) | 249 (177) |
| Intima Medial Thickness, mm | 0.77 (0.16) | 0.75 (0.14) | 0.76 (0.15) |
| Framingham Stroke Risk Profile Score | 10.0 (3.4) | 10.6 (3.2) | 10.2 (3.4) |
| Breath Holding Index, TOI | 1.7 (1.1) | 1.6 (1.3) | 1.7 (1.2) |
| Breath Holding Index, O2 | 1.1 (0.8) | 1.0 (0.8) | 1.0 (0.8) |
| Breath Holding Index, THI | 2.7 (6.8) | 3.4 (6.7) | 2.9 (6.8) |
| Cognitive Performance | |||
| Montreal Cognitive Assessment Battery Score | 25.6 (2.4) | 25.0 (3.0) | 25.4 (2.6) |
| Executive Function/Learning, Normative t-score, mean ≥ Normal Range Low Average Range Borderline / Impaired Range |
46.7 (5.7) 66 (73%) 19 (21%) 5 (6%) |
45.9 (6.4) 37 (74%) 8 (16%) 5 (10%) |
46.4 (5.9) 103 (74%) 27 (19%) 10 (7%) |
| Processing Speed, Normative t-score, mean ≥ Normal Range Low Average Range Borderline / Impaired Range |
46.6 (6.0) 63 (70%) 22 (24%) 5 (6%) |
45.9 (6.4) 36 (72%) 9 (18%) 5 (10%) |
46.4 (6.2) 99 (71%) 31 (22%) 10 (7%) |
| Memory, Normative t-score, mean ≥ Normal Range Low Average Range Borderline / Impaired Range |
50.9 (7.8) 77 (86%) 9 (10%) 4 (4%) |
49.3 (7.8) 39 (78%) 6 (12%) 5 (10%) |
50.4 (8.5) 116 (82%) 15 (11%) 9 (6%) |
Total antihypertensive medication burden was quantified using the World Health Organization’s Daily Defined Dose (https://www.who.int/tools/atc-ddd-toolkit/about-ddd). This allows for the standardization of antihypertensive dosages across a diverse array of medications. Scores therefore correspond to the number of antihypertensive medications of a ‘typical’ dose that a participant was taking at the time of baseline assessments.
Treatment group differences in weight, diet, and BP have been previously reported.[25] Briefly, C-LIFE participants showed substantial weight loss (–15.3 lbs [95% CI, –17.2 to –13.3]) compared with modest weight loss in SEPA (–8.5 lbs [95% CI, –11.4 to –5.6]). Both groups showed modest improvements in DASH eating plan scores (C-LIFE: 0.6 [95% CI, 0.3 to 0.8]; SEPA: 0.3 [95% CI, –0.1 to 0.6]). Clinic SBP changes were observed in both groups (C-LIFE: –12.5 [95% CI, –14.9 to –10.2] mm Hg; SEPA: –7.1 [95% CI, –10.4 to –3.7] mm Hg), with similar reductions in clinic DBP (C-LIFE: –5.9 [95% CI, –7.2 to –4.7] mm Hg; SEPA: –3.7 [95% CI, –5.6 to –1.8] mm Hg) (P=0.034). Substantial ambulatory BP reductions were also observed across both SBP (C-LIFE: –7.0 [95% CI, –8.5 to –4.0] mm Hg; SEPA: –0.3 [95% CI, –4.0 to 3.4] mm Hg) and DBP (C-LIFE: –3.9 [95% CI, –5.3 to –2.5] mm Hg; SEPA: 0.3 [95% CI, –2.4 to 2.8] mm Hg) (Supplemental Table 1).
Inspection of demographically corrected normative performance revealed that 102 participants (73%) exhibited at least one subtest score falling at or below -1.5 standard deviations compared to normative levels (‘Borderline / Impaired’ range). Compromises were most commonly observed on tests of Processing Speed (53% with ≥ 1 impairment) followed by Memory (31% with ≥ 1 impairment) and Executive Functioning / Learning (29% with ≥ 1 impairment). Across all domains, 38 individuals (27%) were free from any impairment, 46 (33%) exhibited one impairment, 22 exhibited two impairments (16%), and 34 individuals (24%) exhibited ≥ 3 impairments.
Cognitive Changes
Examination of longitudinal changes in cognition revealed that cognition improved from pre-to-post treatment across the cohort (pre overall t-score: 48.9 [47.9, 49.8], post overall t-score: 50.0 [49.0, 51.0], P<.001) (Supplemental Table 2). Examination of changes within each cognitive domain revealed improvements within each, including Processing Speed (pre t-score: 46.4 [45.4, 47.4], post t-score: 47.6 [46.6, 48.7], P<.001), Memory (pre t-score: 49.9 [48.5, 51.2], post t-score: 51.2 [49.9, 52.7], P=.011), and a trend for Executive Function / Learning (pre t-score: 50.3 [49.2, 51.4], post t-score: 51.0 [49.9, 52.2], P=.075).
Examination of treatment group comparisons are presented in Table 2. Results demonstrated that the C-LIFE group showed larger improvements in Executive Function / Learning compared to SEPA (d = 0.37, P = .039) but did not show improvements in Memory (P = .478) or Processing Speed (P = .763). Although we did not observe treatment improvements in Memory or Processing Speed, examination of potential treatment moderation revealed that treatment effects on Processing Speed were moderated by baseline stroke risk (P = .043), such that the C-LIFE group showed better Processing Speed compared to SEPA among those with higher baseline stroke risk (Figure 2a). In addition, the effects of treatment on Memory were moderated by sex (P = .026), such that males in C-LIFE experienced greater improvements in Memory relative to SEPA, with equivocal changes in females (Figure 2b). In contrast, we found no evidence to suggest this pattern of findings was moderated by baseline levels of cognitive function, age, or education.
Table 2.
Post-treatment levels of vascular and cerebrovascular function. Values for cognitive performance are presented as mean rank scores across subtests. Cognitive function scores are presented as model adjusted, mean rank scores within each outcome domain. Data for both baseline and posttreatment estimates were derived from the full, fitted regression models and may therefore differ slightly from raw values provided in Table 1.
| Outcome Measure (Primary / Secondary) |
C-LIFE | SEPA | ||
|---|---|---|---|---|
| Baseline | Post-Treatment | Baseline | Post-Treatment | |
| Executive Function / Learning, mean rank score (Primary)* | 64.2 (60.5, 67.8) | 66.8 (64.2, 69.4) | 65.1 (60.3, 70.0) | 62.6 (58.6, 66.6) |
| Memory, mean rank score (Secondary) | 63.9 (58.6, 69.3) | 66.3 (62.5, 70.0) | 66.1 (58.8, 73.3) | 65.5 (59.7, 71.2) |
| Processing Speed, mean rank score (Secondary) | 65.8 (60.8, 70.8) | 66.0 (63.9, 68.2) | 63.2 (56.4, 70.0) | 66.7 (63.4, 70.0) |
| Vascular and Microvascular Function | ||||
| Flow Mediated Dilation, % (Primary)* | 2.0 (1.5, 2.5) | 2.7 (2.1, 3.2) | 2.1 (1.5, 2.8) | 1.7 (0.8, 2.6) |
| Microvascular Flow (Secondary)** | 233 (195, 270) | 359 (320, 398) | 279 (228, 330) | 234 (174, 294) |
| Cerebrovascular Function | ||||
| TOI, % Oxygenation Change (Primary) | 1.68 (1.42, 1.94) | 1.44 (1.25, 1.64) | 1.64 (1.29, 2.00) | 1.64 (1.35, 1.93) |
| O2-Hb (Primary) | 1.04 (0.88, 1.21) | 0.80 (0.66, 0.94) | 1.05 (0.82, 1.27) | 0.91 (0.71, 1.10) |
| Framingham Stroke Risk Profile* | 10.1 (9.6, 10.5) | 8.3 (8.0, 8.7) | 10.4 (9.8, 11.0) | 9.0 (8.5, 9.5) |
| Subjective Cognitive Complaints | ||||
| Attention / Executive Function (Secondary)* | 1.91 (1.77, 2.04) | 1.88 (1.77, 1.99) | 1.84 (1.66, 2.01) | 2.04 (1.89, 2.19) |
| Psychomotor Praxis (Secondary)* | 1.34 (1.91, 1.48) | 1.41 (1.31, 1.50) | 1.48 (1.30, 1.66) | 1.56 (1.43, 1.69) |
| Memory (Secondary) | 2.05 (1.91, 2.19) | 2.02 (1.92, 2.12) | 2.00 (1.82, 2.17) | 2.15 (2.01, 2.28) |
P<.05 for adjusted, post-treatment comparisons of C-LIFE and SEPA
P < .01 for adjusted, post-treatment comparisons of C-LIFE and SEPA.
TOI = tissue oxygenation index from functional near infrared spectroscopy assessments; O2-Hb = oxygenated hemoglobin from functional near infrared spectroscopy assessments.
Figure 2.
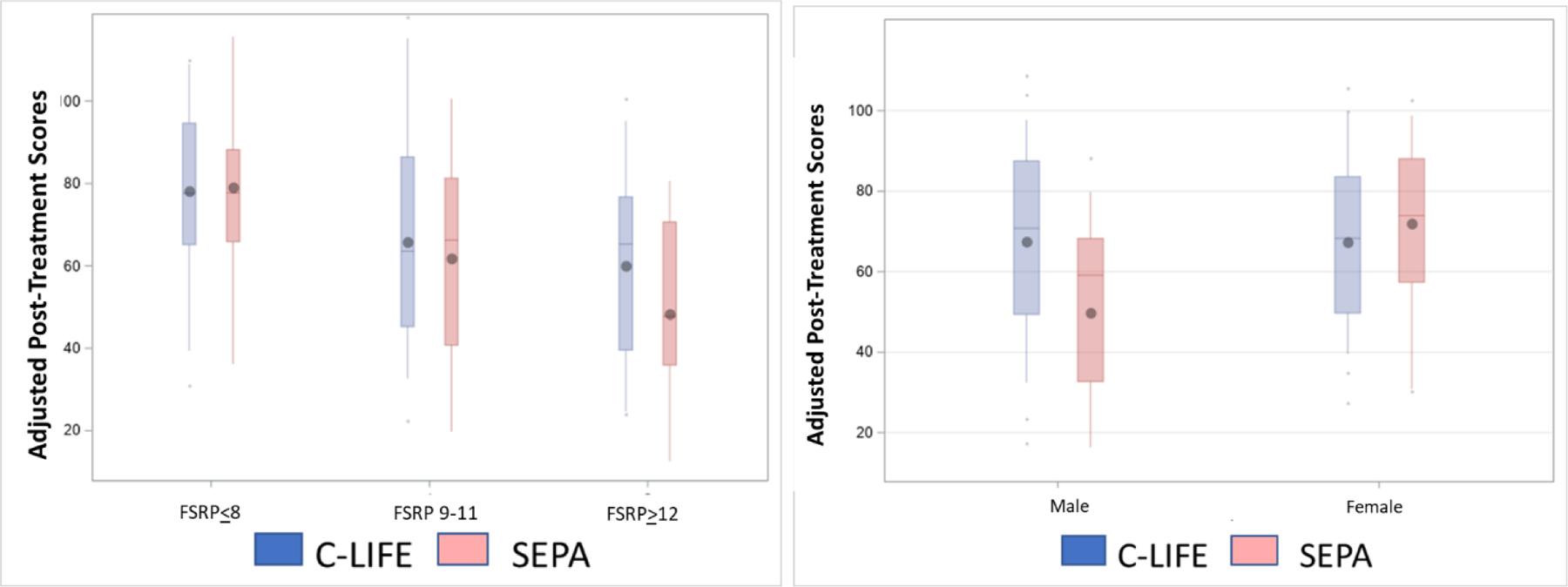
(A) Group differences in Processing Speed by baseline stroke risk (P = .043 for interaction). Using the Framingham Stroke Risk Profile, groups are subset into those with low (≤8%), moderate (9–11%), and high (≥12%) risk of stroke from baseline assessments. (B) Group differences in Memory by biological sex. Male participants in C-LIFE appeared to show greater Memory improvements compared to those in SEPA, with equivocal changes in female participants (P = .026 for interaction). Data are presented as a mean rank score within each cognitive domain.
Vascular Function Changes
As reported previously[25] C-LIFE showed improvements in FMD relative to SEPA, (C-LIFE: 0.3 % [-0.3, 1.0] vs. SEPA: -1.4 % [-2.5, -0.3], P = .022). Microvascular flow also improved in C-LIFE compared to SEPA (C-LIFE: 104 [69, 139], SEPA: -27 [-79, 25], P<.001).
Cerebrovascular Changes
Examination of cerebrovascular function changes focused on CVR during a breath holding challenge. Results suggested that tissue oxygenation index (TOI) changes tended to decrease from pre-to-post treatment across the cohort and did not differ between groups (-0.2 [-0.4, 0] vs. 0.1 [-0.2, 0.4], P = .197). Similar results were demonstrated for changes in oxygenated Hb and total hemoglobin index (THI), both of which decreased from pre-to-post treatment across the cohort (P = .004 and P = .019, respectively). Although both CVR indices decreased during the treatment period, neither oxygenated Hb (-0.3 [-0.4, -0.1] vs. 0.1 [-0.4, 0.0], P = .384) nor THI (-0.3 [-0.4, -0.1] vs. 0.1 [-0.4, 0.0], P = .615) differed between groups. Examination of treatment group differences in the Framingham Stroke Risk Profile score revealed reductions in stroke risk across both groups, with greater improvements in C-LIFE compared to SEPA (-1.8 [-2.2, -1.5] vs. -1.2 [-1.7, -0.7], P = .034).
Subjective Cognitive Complaints
C-LIFE participants also exhibited reduced subjective cognitive complaints in Attention / Executive Function (d = 0.27, P = .045) and Psychomotor Praxis (d = 0.28, P = .015), without improvements in Memory (P = .299).
Mechanisms of Lifestyle Change and Cognitive Function
Examination of associations between putative mechanisms of interest and improvements in Executive Function revealed that reductions in ambulatory SBP were associated with improved Executive Function / Learning (B = -0.18, P = .032). In contrast, we found no association between improvements in peak VO2 (B = 0.05, P = .336), weight loss (B = 0.01, P = .886), FMD (B = -0.06, P = .350), nor microvascular endothelial function (B = 0.03, P = .662) and changes in Executive Function. Results from sequential mediation analyses demonstrated that reductions in ambulatory SBP secondary to weight loss were most closely associated with higher post-treatment Executive Function / Learning scores (indirect effect: B = 0.25 [0.03, 0.71]) (Figure 3).
Figure 3.
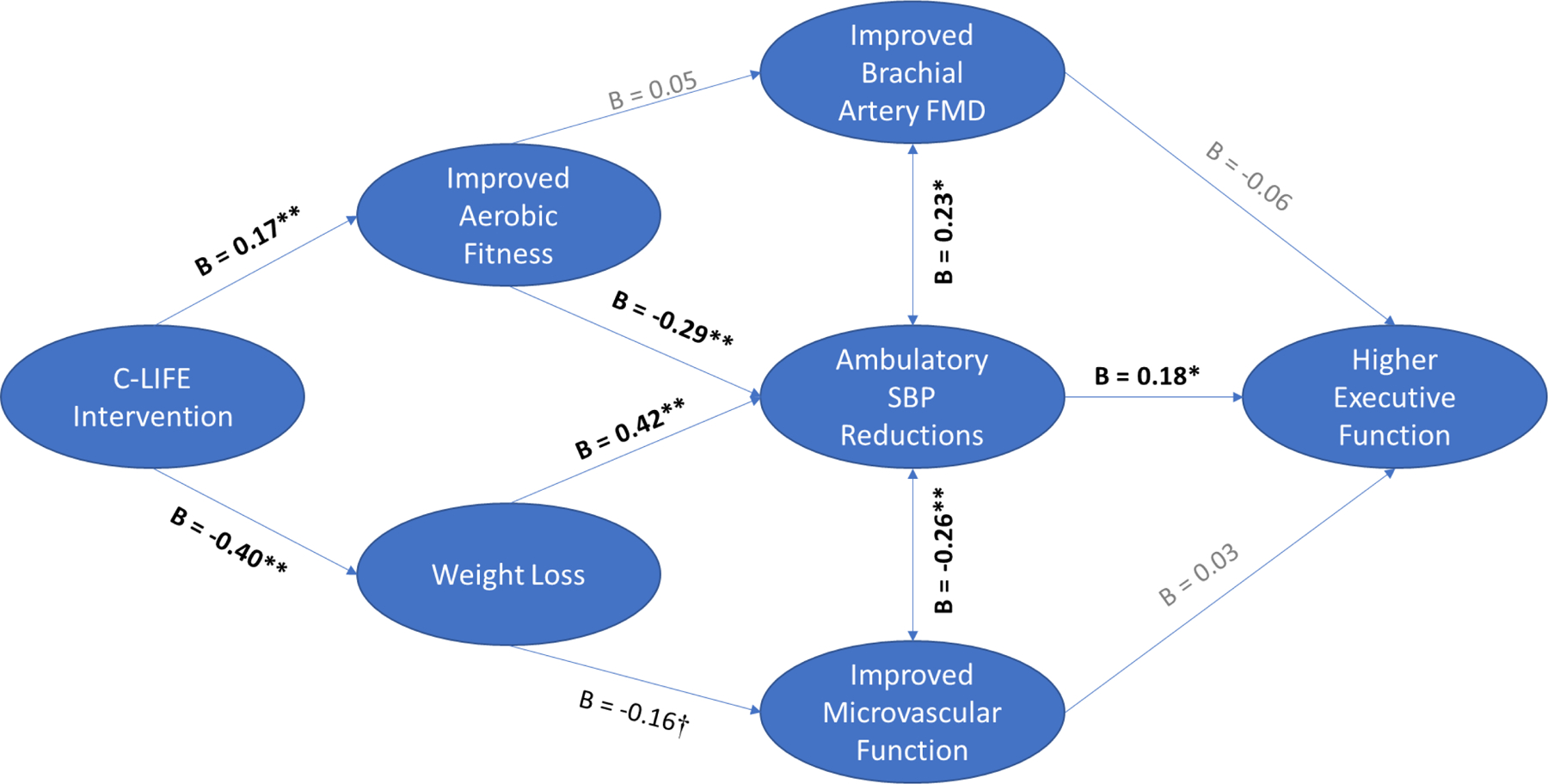
Mediators of higher post-treatment Executive Function / Learning. As shown, we found that reductions in ambulatory SBP through weight loss were the only pathway demonstrating a significant indirect effect by which treatment resulted in higher post-treatment Executive Function / Learning.
In addition to our analyses of treatment mechanisms, we also conducted explanatory analyses of mechanisms that differential affected changes in Memory and Processing Speed across subgroups that moderated treatment improvements. Examination of Memory demonstrated that improvements were differentially associated with changes in clinic SBP based on biological sex (P = .021 for interaction), which resulted in differential treatment improvements (test for equality of conditional indirect effect: B = 1.41 [0.10, 3.86]). Follow-up analyses revealed that reductions in clinic SBP associated with improved Memory in males (B = -0.18, P = .019) but not in females (B = 0.03, P = .717). Parallel explanatory analyses were conducted for changes in Processing Speed, which did not reveal any significant evidence that mechanistic associations varied consistently across levels of stroke risk.
DISCUSSION
Results from the present study suggest that a structured cardiac-rehabilitation-based lifestyle modification program, including aerobic exercise, weight loss, and dietary modification improve executive functioning and learning among individuals with RH. Our results demonstrated that the effects of lifestyle modification on cognitive function were explained by reductions in SBP and that these effects were most pronounced among men with greater levels of vascular risk. These results extend prior studies by demonstrating the efficacy of lifestyle treatments for improving cognitive functioning, particularly executive function,[52] among individuals with RH, who are at risk of CVD events,[53,54] as well as cognitive decline. Our results extend these prior studies by demonstrating that reduced ambulatory SBP due to weight loss may be an important mechanism of improved cognitive function. Our results also demonstrated that improvements in cognition were paralleled by improvements in endothelial and microvascular endothelial function, although these improvements did not appear to mediate treatment-related improvements in cognition.
Previous RCTs have demonstrated that lifestyle modification using behavioral weight loss can improve cognition among adults with hypertension. In the ENCORE trial, we previously demonstrated that a 4-month behavioral weight loss intervention improved cognitive performance on tests assessing executive functioning, memory, and learning.[20] Similarly, the ENLIGHTEN trial showed similar improvements in an aerobic exercise and dietary modification trial among older adults with cognitive impairment and CVD risk factors, many of whom had hypertension.[54] Collectively, these observations suggest that the lifestyle interventions improved executive functioning, without improvements in memory. In addition, improvements in executive functioning were associated with improvements in aerobic fitness, CVD risk profiles, dietary salt reduction, [54] and insulin sensitivity.[53] The FINGER trial previously demonstrated that a 2-year intervention utilizing a comprehensive intervention including exercise, diet, cognitive training, and vascular risk reduction improved a global marker of cognitive functioning, again especially executive function.[55] The present results therefore largely confirm prior study findings, by demonstrating that a 4-month intervention confers small-to-moderate improvements in cognition, especially for tests of frontal-subcortical functioning.
In addition to corroborating prior lifestyle trials, our study demonstrated that several important biobehavioral mechanistic markers were improved in parallel with cognition. Our findings that endothelial functioning within both conduit vessels and the microvasculature is potentially important, as hypertension has been widely associated with endothelial dysfunction, which occurs early over the course of hypertension development and typically precedes target organ damage. Endothelial cells are also among the most common cell types in the human brain,[56] constitute critical structural elements of the blood-brain-barrier, and are uniquely sensitive to the effects of cerebral hemodynamic perturbations.[57] Brain endothelial cells also have a prominent role in age-related neurogenesis, neuroinflammation, and cognition within animal models.[56]
Our finding that improvements in cognition were related to reduced ambulatory SBP but not to other treatment mechanisms is notable and warrants further exploration. This finding is consistent with recent results from the SPRINT-MIND trial,[13] which demonstrated that intensive pharmacological therapy to reduce BP was protective against the development of mild cognitive impairment,[13] despite a lack of domain-specific improvements on cognitive testing.[58] In our prior trials of lifestyle modification for cognition, treatment improvements have associated most closely with reductions in weight,[20] improved fitness,[20,54] and insulin sensitivity.[53] While endothelial functioning has been associated with cognition cross-sectionally,[59,60] it has been inconsistently improved in prior trials and not suggested to mediate observed improvements. There are several possibilities that may explain the lack of association between changes in aerobic fitness, weight loss, and cognitive changes in the current study. First, in contrast to prior studies, participants in the SEPA control condition in the present study were encouraged to alter their diet, exercise, and lose weight, but were not provided with a supervised training regimen. Individuals in the control group therefore differed from prior control groups in that they exhibited improvements in aerobic fitness (3.4% [-2.3, 9.2] VO2) and weight loss (-8.5 lbs [-11.4, -5.6]), although not to the extent observed in the structured intervention provided in C-LIFE group (14.8% [11.0, 18.6] VO2 and -15.3 lbs [-17.2, -13.3], respectively). This may partly explain why performance across the entire cohort improved, which was not observed in our prior trial among older adults with CVD risk factors.[54] In addition, multivariate regression analyses of changes in normative performance conducted across the entire cohort (irrespective of group) demonstrated that improvements in Executive Function and Processing Speed were indeed associated with improved fitness (B = 0.11, P = .049 and B = .09, P = .045, respectively), but not Memory (B = -.06, P = .744). Similarly, greater weight loss (B = -0.24, P = .022) and greater reductions in ambulatory SBP were strongly associated with reduced cerebrovascular oxygenation changes during our breath holding paradigm, which may suggest that both groups experienced compensatory relaxation in cerebrovascular reactivity indices in association with lifestyle changes.
Our findings that treatment group improvements in Memory and Processing Speed were moderated by baseline individual differences is also novel and warrants additional replication in other clinical trials. Sex differences in both the prevalence and rate of cognitive decline and dementia have been widely documented,[61] with women demonstrating a greater incidence and more rapid progression of ADRD. In addition, both sex and stroke risk have been shown to influence the efficacy of lifestyle modification on cognitive functioning.[62,63] Specifically, many studies have suggested that males[63,64] and individuals with modestly elevated stroke risk may experience the greatest cognitive gains.[65] Several mechanisms have been suggested for potential differential treatment responses by sex, including neurohormonal function, variations in sensitivity to central neurotrophins (e.g. BDNF), inflammatory function,[63,66] and lower small vessel disease burden.[67] Taken together, these individual differences have led some investigators to advocate for precision medicine approaches to tailor intervention training based on background characteristics.[68,69] In the present findings, we found that reductions in SBP associated with improvements in Memory among men but not women. It is possible that this difference was influenced by baseline differences between males and females in their vascular risk profiles, as we found that women had higher levels of stroke risk (FSRP score: 11.3 [SD = 3.2] vs. 9.1 [SD = 3.1], P<.001), although differential treatment effects were observed even after adjusting for baseline stroke risk. Moreover, male participants tended to exhibit larger SBP reductions compared to female participants, including ambulatory (C-LIFE males: -9.2 [-12.6, -5.8] vs. SEPA males: = +1.7 [-3.4, 6.8]; C-LIFE females: -4.3 [-7.8, -0.8] vs. SEPA females: -0.3 [-5.0, 4.3]) and clinic SBP (C-LIFE males: -14.7 [-18.1, -11.3] vs. SEPA males: -7.2 [-12.4, -1.9]; C-LIFE females: -10.5 [-14.0, -7.1] vs. SEPA females: -4.7 [-9.4, -0.1]), although weight loss was comparable across male and female participants (C-LIFE males: -14.6 [-19.3, -13.7] vs. SEPA males: -9.5 [-13.7, -5.4]; C-LIFE females: -13.7 [-16.6, -10.7] vs. SEPA females: -7.4 [-11.3, -3.6]). Future studies should attempt to expand upon our examination of individual differences to further delineate moderators of treatment response, particularly among individuals with elevated levels of vascular risk.
The present study had must be interpreted with its limitations in mind. First, cognitive improvements were observed over a relatively short time period of just 4-months. It is likely that the observed improvements may wane over time, particularly because most intensive lifestyle programs report a decline in behavioral compliance following the initial treatment period. The durability of the observed improvements will therefore need to be examined in future studies. Second, the present study is limited in its use of two active treatment groups, without the use of a ‘no lifestyle modification’ control. Third, our assessment of cerebrovascular mechanisms is somewhat limited in that measures were obtained entirely from peripheral and non-invasive techniques, which were prioritized to reduce participant burden. Future studies would therefore benefit from incorporating neuroimaging and/or cerebrospinal fluid assessments in order to delineate the effects of lifestyle modification and reductions in BP on central nervous system mechanisms linked with ADRD pathways of risk. This is particularly important as changes in our peripherally-assessed measures of cerebrovascular reactivity (oxygenation assessed by fNIRS) may have been confounded by restricted blood flow and reduced vasomotor tone, thereby constricting regional blood flow indices within the frontal lobe. Third, endothelial function was quantified by peripheral measures of both conduit and microvascular vessel functioning (brachial artery FMD and hyperemic flow indices). While these indices have been associated with global measures of small vessel disease obtained from neuroimaging, few studies have attempted to quantify the concordance between peripheral measures like microvascular endothelial function with the validated CNS measures of endothelial functioning.
Conclusions.
The present findings extend previous studies by demonstrating that lifestyle modification can not only reduce blood pressure, but also improve cognition among individuals with RH. Results also suggest that improvements in endothelial functioning and reductions in ambulatory SBP may be informative when delineating the role of lifestyle change in improving cognitive functioning. Future studies should examine cognitive changes from lifestyle using CNS mechanistic markers from either neuroimaging or CSF indices in order to more adequately delineate the causal associations between lifestyle interventions and improved cognitive outcomes.
Supplementary Material
Figure 1.
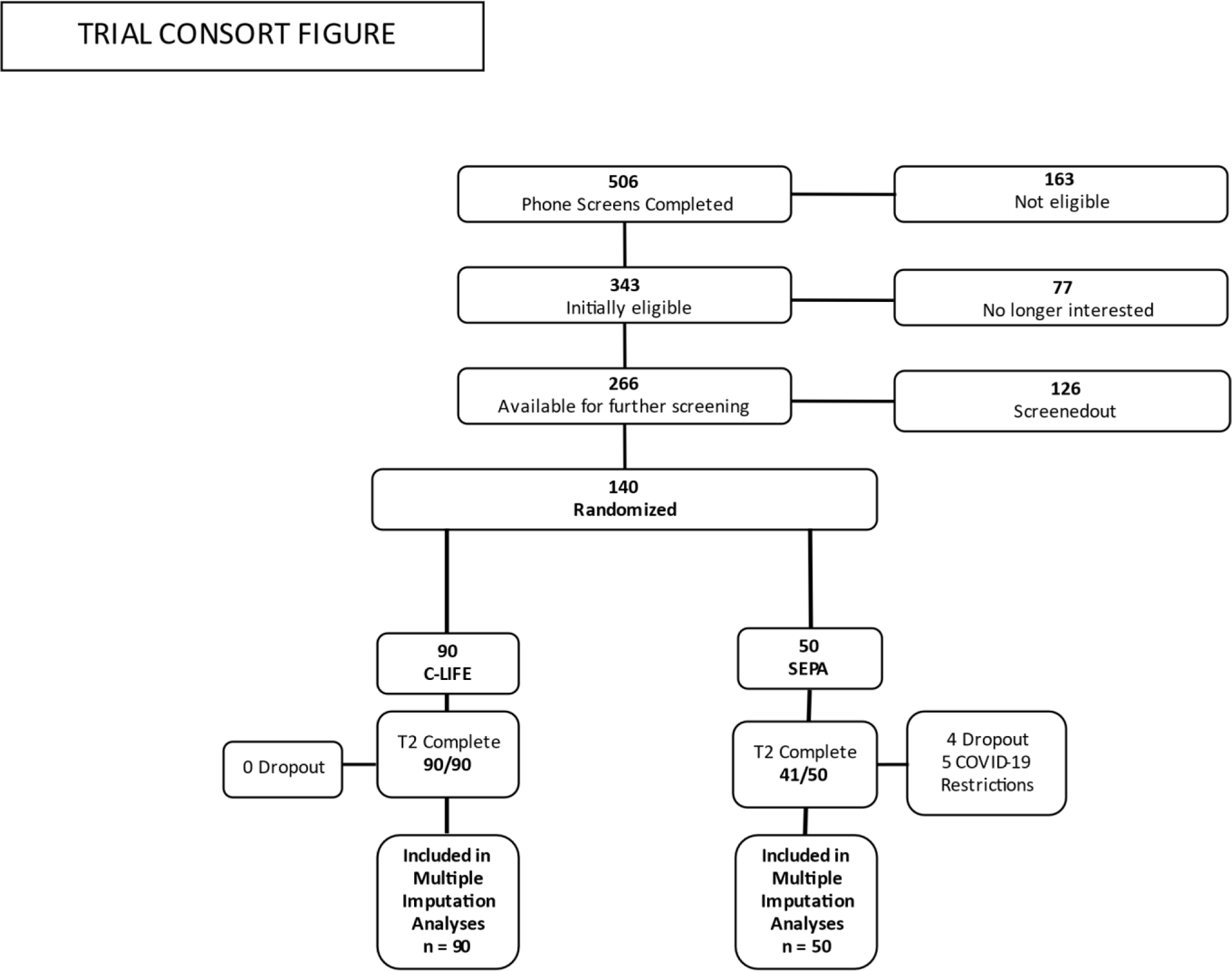
Consort chart showing participant flow.
Acknowledgements:
We would like to thank Michael Babyak for his guidance and oversight of our statistical analyses. We wish to express our appreciation to the members of our Data and Safety Monitoring Board, Diane Catellier Ph.D., David Sheps MD, and Robert Carney, PhD. We also thank our research staff including Nicholas Johnson MS, Margaret Deishmeister MS, Beth Drossman MS, Natalie Hamilton BA, Catherine Wu MS, Michael Ellis RDMAS, RVT, and Jeanne Schwartz PA-C
Funding:
Supported by Grants HL122836 and HL130237 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Disclosures: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–322. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; 125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Evans JC, Larson MG, Levy D. Treatment and control of hypertension in the community: a prospective analysis. Hypertension 2002; 40:640–646. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Zhao G, Li C, Pearson WS, Mokdad AH. Trends in obesity and abdominal obesity among hypertensive and nonhypertensive adults in the United States. Am J Hypertens 2008; 21:1124–1128. [DOI] [PubMed] [Google Scholar]
- 8.Assessing the Cost of Aging and Health Care. In: Aging NIo editor. 2014. [Google Scholar]
- 9.Skoog I, Gustafson D. Hypertension, hypertension-clustering factors and Alzheimer’s disease. NeurolRes 2003; 25:675–680. [DOI] [PubMed] [Google Scholar]
- 10.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens 2011; 29:1469–1477. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011; 10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamora Z, Williamson JD. The Effects of Lower BP Goals on Cognitive Function in the Elderly. Curr Cardiol Rep 2020; 22:63. [DOI] [PubMed] [Google Scholar]
- 13.Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasrallah IM, Gaussoin SA, Pomponio R, Dolui S, Erus G, Wright CB, et al. Association of Intensive vs Standard Blood Pressure Control With Magnetic Resonance Imaging Biomarkers of Alzheimer Disease: Secondary Analysis of the SPRINT MIND Randomized Trial. JAMA Neurol 2021; 78:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon A, Handels R, Wimo A, Antikainen R, Laatikainen T, Levalahti E, et al. Effect of a Multidomain Lifestyle Intervention on Estimated Dementia Risk. J Alzheimers Dis 2021; 82:1461–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponvel P, Shahar S, Singh DKA, Ludin AFM, Rajikan R, Rajab NF, et al. Multidomain Intervention for Reversal of Cognitive Frailty, Towards a Personalized Approach (AGELESS Trial): Study Design. J Alzheimers Dis 2021; 82:673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, et al. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimers Dement 2019; 15:729–741. [DOI] [PubMed] [Google Scholar]
- 18.Hinderliter AL, Babyak MA, Sherwood A, Blumenthal JA. The DASH diet and insulin sensitivity. Curr Hypertens Rep 2011; 13:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010; 170:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PJ, Blumenthal JA, Babyak MA, Craighead L, Welsh-Bohmer KA, Browndyke JN, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010; 55:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno RM, Stea F, Sicari R, Ghiadoni L, Taddei S, Ungar A, et al. Vascular Function Is Improved After an Environmental Enrichment Program: The Train the Brain-Mind the Vessel Study. Hypertension 2018; 71:1218–1225. [DOI] [PubMed] [Google Scholar]
- 22.Langeard A, Cloutier SO, Olmand M, Saillant K, Gagnon C, Gregoire CA, et al. High-intensity interval training vs. hydrochlorothiazide on blood pressure, cardiovascular health and cognition: Protocol of a non-inferiority trial. Contemp Clin Trials 2021; 102:106286. [DOI] [PubMed] [Google Scholar]
- 23.Kruger RL, Clark CM, Dyck AM, Anderson TJ, Clement F, Hanly PJ, et al. The Brain in Motion II Study: study protocol for a randomized controlled trial of an aerobic exercise intervention for older adults at increased risk of dementia. Trials 2021; 22:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White WB, Wakefield DB, Moscufo N, Guttmann CRG, Kaplan RF, Bohannon RW, et al. Effects of Intensive Versus Standard Ambulatory Blood Pressure Control on Cerebrovascular Outcomes in Older People (INFINITY). Circulation 2019; 140:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal JA, Hinderliter AL, Smith PJ, Mabe S, Watkins LL, Craighead L, et al. Effects of Lifestyle Modification on Patients With Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenthal JA, Sherwood A, Smith PJ, Mabe S, Watkins L, Lin PH, et al. Lifestyle modification for resistant hypertension: The TRIUMPH randomized clinical trial. Am Heart J 2015; 170:986–994 e985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 28.Olivieri NF, Matsui D, Hermann C, Koren G. Compliance assessed by the Medication Event Monitoring System. Arch Dis Child 1991; 66:1399–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014; 312:799–808. [DOI] [PubMed] [Google Scholar]
- 30.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010; 170:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith PJ, Blumenthal JA, Hinderliter AL, Mabe SM, Schwartz JE, Avorgbedor F, et al. Neurocognition in treatment-resistant hypertension: profile and associations with cardiovascular biomarkers. J Hypertens 2019; 37:1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke; a journal of cerebral circulation 2006; 37:2220–2241. [DOI] [PubMed] [Google Scholar]
- 34.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment, 5th ed. New York, NY, US: Oxford University Press; 2012. [Google Scholar]
- 35.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychiat 1935; 18:643–662. [Google Scholar]
- 36.Rosen WG. Verbal fluency in aging and dementia. J Clin Neuropsych 1980; 2:135–146. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale (WAIS-IV) San Antonio, TX: Harcourt Assessment; 2008. [Google Scholar]
- 38.Benton AL, Hamsher K. Multilingual aphasia examination Iowa City, Iowa: University of Iowa; 1978. [Google Scholar]
- 39.Delis DK J; Kaplan E; Ober B California Verbal Learning Test, 2nd Edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 40.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment 1996; 8:145–153. [Google Scholar]
- 41.Ruff RM, Niemann H, Allen CC. The Ruff 2 and 7 Selective Attention Test: A neuropsychological application. Perceptual and motor skills 1992; 75:1311–1319. [DOI] [PubMed] [Google Scholar]
- 42.Gunstad J, Cohen RA, Paul RH, Tate DF, Hoth KF, Poppas A. Understanding reported cognitive dysfunction in older adults with cardiovascular disease. Neuropsychiatr Dis Treat 2006; 2:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paine NJ, Hinderliter AL, Blumenthal JA, Adams KF Jr., Sueta CA, Chang PP, et al. Reactive hyperemia is associated with adverse clinical outcomes in heart failure. Am Heart J 2016; 178:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubin RF, Guajardo I, Ayer A, Mills C, Donovan C, Beussink L, et al. Associations of Macro- and Microvascular Endothelial Dysfunction With Subclinical Ventricular Dysfunction in End-Stage Renal Disease. Hypertension 2016; 68:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kastrup A, Li TQ, Glover GH, Moseley ME. Cerebral blood flow-related signal changes during breath-holding. AJNR Am J Neuroradiol 1999; 20:1233–1238. [PMC free article] [PubMed] [Google Scholar]
- 46.Zavoreo I, Demarin V. Breath holding index and arterial stiffness as markers of vascular aging. Curr Aging Sci 2010; 3:67–70. [DOI] [PubMed] [Google Scholar]
- 47.Apruzzese A, Silvestrini M, Floris R, Vernieri F, Bozzao A, Hagberg G, et al. Cerebral hemodynamics in asymptomatic patients with internal carotid artery occlusion: a dynamic susceptibility contrast MR and transcranial Doppler study. AJNR Am J Neuroradiol 2001; 22:1062–1067. [PMC free article] [PubMed] [Google Scholar]
- 48.Cupini LM, Diomedi M, Placidi F, Silvestrini M, Giacomini P. Cerebrovascular reactivity and subcortical infarctions. Arch Neurol 2001; 58:577–581. [DOI] [PubMed] [Google Scholar]
- 49.Zavoreo I, Kes VB, Morovic S, Seric V, Demarin V. Breath holding index in detection of early cognitive decline. J Neurol Sci 2010; 299:116–119. [DOI] [PubMed] [Google Scholar]
- 50.Reinhard M, Schwarzer G, Briel M, Altamura C, Palazzo P, King A, et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014; 83:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasdekis SN, Tsivgoulis G, Athanasiadis D, Andrikopoulou A, Voumvourakis K, Lazaris AM, et al. Cerebrovascular reacivity assessment in patients with carotid artery disease: a combined TCD and NIRS study. J Neuroimaging 2012; 22:261–265. [DOI] [PubMed] [Google Scholar]
- 52.Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 2020; 16:1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith PJ, Mabe SM, Sherwood A, Doraiswamy PM, Welsh-Bohmer KA, Burke JR, et al. Metabolic and Neurocognitive Changes Following Lifestyle Modification: Examination of Biomarkers from the ENLIGHTEN Randomized Clinical Trial. J Alzheimers Dis 2020; 77:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 2019; 92:e212–e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 56.Chen MB, Yang AC, Yousef H, Lee D, Chen W, Schaum N, et al. Brain Endothelial Cells Are Exquisite Sensors of Age-Related Circulatory Cues. Cell Rep 2020; 30:4418–4432 e4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Cao Y, Ma L, Pei H, Rausch WD, Li H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front Aging Neurosci 2018; 10:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rapp SR, Gaussoin SA, Sachs BC, Chelune G, Supiano MA, Lerner AJ, et al. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol 2020; 19:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith PJ, Blumenthal JA, Hinderliter AL, Watkins LL, Hoffman BM, Sherwood A. Microvascular Endothelial Function and Neurocognition Among Adults With Major Depressive Disorder. Am J Geriatr Psychiatry 2018; 26:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, et al. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med 2007; 69:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine DA, Gross AL, Briceno EM, Tilton N, Giordani BJ, Sussman JB, et al. Sex Differences in Cognitive Decline Among US Adults. JAMA Netw Open 2021; 4:e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sindi S, Kareholt I, Ngandu T, Rosenberg A, Kulmala J, Johansson L, et al. Sex differences in dementia and response to a lifestyle intervention: Evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement 2021; 17:1166–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barha CK, Hsu CL, Ten Brinke L, Liu-Ambrose T. Biological Sex: A Potential Moderator of Physical Activity Efficacy on Brain Health. Front Aging Neurosci 2019; 11:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu-Ambrose T, Barha C, Falck RS. Active body, healthy brain: Exercise for healthy cognitive aging. Int Rev Neurobiol 2019; 147:95–120. [DOI] [PubMed] [Google Scholar]
- 65.Barha CK, Dao E, Marcotte L, Hsiung GR, Tam R, Liu-Ambrose T. Cardiovascular risk moderates the effect of aerobic exercise on executive functions in older adults with subcortical ischemic vascular cognitive impairment. Sci Rep 2021; 11:19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu-Ambrose T, Barha CK, Best JR. Physical activity for brain health in older adults. Appl Physiol Nutr Metab 2018; 43:1105–1112. [DOI] [PubMed] [Google Scholar]
- 67.Dao E, Barha CK, Best JR, Hsiung GY, Tam R, Liu-Ambrose T. The Effect of Aerobic Exercise on White Matter Hyperintensity Progression May Vary by Sex. Can J Aging 2019; 38:236–244. [DOI] [PubMed] [Google Scholar]
- 68.Barha CK, Liu-Ambrose T. Sex differences in exercise efficacy: Is midlife a critical window for promoting healthy cognitive aging? FASEB J 2020; 34:11329–11336. [DOI] [PubMed] [Google Scholar]
- 69.Barha CK, Best JR, Rosano C, Yaffe K, Catov JM, Liu-Ambrose T. Sex-Specific Relationship Between Long-Term Maintenance of Physical Activity and Cognition in the Health ABC Study: Potential Role of Hippocampal and Dorsolateral Prefrontal Cortex Volume. J Gerontol A Biol Sci Med Sci 2020; 75:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


