Abstract
Importance:
The FDA uses the Manufacturer and User Facility Device Experience (MAUDE) database to evaluate the safety of urogynecologic meshes, however reports on individual meshes have not been characterized.
Objectives:
To compare complications among available urogynecologic meshes reported to the MAUDE database.
Study Design:
Cross-sectional analysis of medical device reports (MDRs) of urogynecologic mesh from 1/2004–3/2019, using the Reed Tech Navigator (LexisNexis), which codes MDRs. The percentage of reports containing specific complaints (not an adverse event rate) were compared with Chi2 tests with Dunn-Sidak correction. Correlations with time-on-market,mesh weight, stiffness, and porosity were determined.
Results:
The 34,485 reports examined included 6 transvaginal meshes, 4 sacrocolpopexy meshes, and 10 midurethral slings. Most reported events were pain, erosion, and infection. For transvaginal prolapse, <10% of Uphold Lite (Boston Scientific) reports contained pain or erosion vs >90% of Prolift/Prolift+M (Ethicon; p<0.001). For sacrocolpopexy mesh, >90% of Gynemesh (Ethicon; Prolift in vaginal form) reports included erosion and pain vs <60% for Artisyn (Ethicon), Restorelle (Colpoplast), and Upsylon (Boston Scientific, p<0.0001). For slings, Gynecare TVT Obturator had the highest proportion of erosion and pain complaints. Heavier sling meshes had more reports. When Ascend (Caldera Medical), an outlier with only 5 reports, was excluded, transvaginal mesh stiffness correlated strongly with number of reports. For transvaginal meshes, number of reports correlated with time-on-market (ρ=0.8, p=0.04).
Conclusions:
Individual meshes have different properties with different complication profiles which should inform mesh development and use. Gynemesh MDRs included pain and erosion more frequently than others. Comprehensive registries are needed.
Keywords: mesh complications, MAUDE database, pelvic organ prolapse, stress urinary incontinence, Gynemesh
Introduction
The Food and Drug Administration (FDA), policy makers, lawyers, and the public rely heavily on the Manufacturer and User Facility Device Experience database (MAUDE) to evaluate the safety of devices, including urogynecologic meshes.5 The database depends on mandatory (manufacturers and user facilities) and voluntary reporters (patients, caregivers, and health care professionals). The number of reports on a device is often what prompts the FDA to act. The 2008 FDA Public Health Notification regarding transvaginal urogynecologic mesh highlighted serious complications related to transvaginal meshes, based on over 1000 medical device reports (MDRs) and in 2011 these complications were labeled “not rare” based on 2,874 reports.6 Ultimately, transvaginal prolapse meshes were upgraded from Class II to Class III devices in 2018 and then, because the mandated additional post-market surveillance studies were not completed in time, suspended from distribution in 2019.
Criticism of the MAUDE database includes incomplete information, lack of true device denominator, and questionable clinical relevance of reported events.7 Previous studies have evaluated urogynecologic specific reports in the MAUDE database, but either looked at a limited number of complications or did not stratify by device.7–9 Importantly, structural, manufacturing, and material differences can drive complication profiles. 10,11 For mesh specifically, pore size, mesh weight, and mesh stiffness impact outcomes.12–14 However, comparative human studies are lacking.
We aimed to determine the number of MDRs for each currently available mesh product and the most commonly reported complications per total number of reports on each. We hypothesized that the number of reports for each device would be associated with time on the market, but that lighter weight, lower stiffness, and higher porosity mesh products would have lower percentages of reports that included erosion and pain.
Materials and Methods
We performed a cross-sectional analysis of MDRs on urogynecologic mesh products reported to the FDA from 1/2004–3/2019 using the Reed Tech Navigator (RTN; LexisNexis, Horsum PA). RTN codes MDRs from free text within MAUDE, using the FDA patient and device problem code hierarchies. These code hierarchies are standardized across all products and as such can lack terms specific to known mesh products (for example “erosion” is often used in place of “exposure”). Dates were chosen to include all available RTN data at the time of data collection. Duplicate reports were excluded. Mesh products are identified by name; therefore, RTN groups some products together that evolved from a progenitor with the same name, such as Prolift and Prolift+M (referred to here as a “mesh family” for clarity).
Using the RTN, the MAUDE database was searched for transvaginal prolapse mesh products (FDA product code OTO); sacrocolpopexy mesh products (OTP); and sling mesh products (OTN and PAH for mini-slings). We included all transvaginal prolapse mesh products. Due to the large number of possible comparisons, sacrocolpopexy mesh and midurethral slings were limited to currently available products at the time of data collection (March 2019). This excluded three sacrocolpopexy meshes (Polyform, Boston Scientific, 164 reports; Novasilk, Colpoplast, 45 reports; IntePro, American Medical Systems, 33 reports) and 5 slings (Ajust, C.R. Bard, 157 reports; Align, C.R. Bard, 815 reports; Monarc, American Medical Systems, 813 reports; T-sling, Colpoplast 42 reports; and Uretrex, C.R. Bard, 13 reports).
Unique patient and device problems were identified by searching the first 100 and most recent 100 MDRs for each product. Each mesh product was then interrogated for each identified problem to obtain the number of reports containing each problem. The number of reports listing each problem was divided by the total number of reports for that product to obtain the proportion of MDRs containing each problem for each mesh product. Within each mesh group, we compared the proportions of pain, exposure/erosion (which are listed together in the MAUDE database), and infection.
Finally, we compared time on the market, mesh weight, stiffness, and porosity with number of reports. Five slings and one transvaginal mesh product without percent porosity available were excluded from the porosity analysis (Table 2). Approval and withdrawal dates were identified using the FDA’s 510(k) Premarket Notification,15 Premarket Approval,16 Establishment Registration & Device Listing,17 Medical Device Recalls,18 and 522 Postmarket Surveillance Studies19 databases and manufacturer data (Table 2).
Table 2.
Mesh Characteristics That May Influence Number of Reports Per Mesh Product and Adverse Events
| Approved or Introduced | Withdrawn | Reports (n) | Time on Market (days)a | Weight (g/m2)17–20 | Stiffness (N/mm)b | Porosity (%) or pore size (μm) | Cut/Sealedc | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Transvaginal Prolapse Mesh Products | ||||||||
|
| ||||||||
| Prolift Family | 6334 | 4277d | ||||||
| Prolift | 03/01/2005 | 08/15/2012 | 2724 | 42 | 0.29–1.37 | 62% | ||
| Prolift+M | 05/15/2008 | 08/15/2012 | 1328 | 28 | 0.01–0.24 | 67% | ||
|
| ||||||||
| Uphold | 12/13/2012 | 04/16/2019 | 2027 | 2315 | 25 | 0.2 | 72% | |
|
| ||||||||
| Avaulta Family | 1115 | 3878 | ||||||
| Avaulta Solo | 03/12/2007 | 07/02/2012 | 1939 | 18 | 0.16 | 75% | ||
| Avaulta Plus/Biosynthetic | 03/12/2007 | 07/02/2012 | 1939 | 100 | ||||
|
| ||||||||
| Perigeee | 05/17/2004 | 01/03/2012 | 723 | 2787 | ||||
|
| ||||||||
| Exair | 05/08/2009 | 01/07/2014 | 26 | 1705 | 19 | 0.072 | 67% | |
|
| ||||||||
| Ascend | 03/31/2009 | 10/15/2012 | 5 | 1294 | 33 | 0.72–1.66 | 51% | |
|
| ||||||||
| Sacrocolpopexy Mesh Products | ||||||||
|
| ||||||||
| Gynecare Gynemesh | 01/08/2002 | 2056 | 6291 | 42 | 0.3–0.45 | 62% | ||
|
| ||||||||
| Restorelle | 08/04/2009 | 1168 | 3526 | 19 | 0.16–0.41 | 78% | ||
|
| ||||||||
| Upsylon | 12/18/2012 | 64 | 2294 | 25 | 0.2 | 72% | ||
|
| ||||||||
| Artisyn | 06/11/2012 | 31 | 2484 | 28 | 0.08–0.49 | 68% | ||
|
| ||||||||
| Midurethral Sling Mesh Products | ||||||||
|
| ||||||||
| Gynecare Obturator | 12/08/2013 | 11604 | 1939 | 100 | 0.09–2.0 | 54% | Laser or Mechanical | |
|
| ||||||||
| Gynecare TVT Combined | 10/26/2001 | 5444 | 6365 | 100 | 0.09–2.0 | 54% | Laser or Mechanical | |
|
| ||||||||
| Advantage Fit Blue | 12/20/2017 | 1063 | 466 | 100 | 0.05–1.9 | 58% | Mechanical; Heat Sealed | |
|
| ||||||||
| Desara Blueg | 09/23/2013 | 673 | 2015 | 103 | 0.23–2.23 | 33% | Heat Sealed | |
|
| ||||||||
| Gynecare TVT Exact | 03/16/2010 | 667 | 3302 | 100 | 0.09 | 1379 μm | Laser cut | |
|
| ||||||||
| Solyx | 12/20/2017 | 552 | 466 | 100 | 0.05–1.9 | 58% | Heat sealed | |
|
| ||||||||
| Gynecare TVT Abbrevo | 07/01/2010 | 437 | 3195 | 100 | 0.09 | 1379 μm | Laser cut | |
|
| ||||||||
| Altis | 11/05/2012 | 362 | 2337 | 70 | 1.5 | 375 μm | Heat sealed | |
|
| ||||||||
| Supris | 06/04/2011 | 86 | 2857 | 70 | 1.5 | 375 μm | Heat sealed | |
|
| ||||||||
| Aris | 03/09/2005 | 48 | 5135 | 70 | 1.5 | 375 μm | Heat sealed | |
Time on market was calculated through 3/31/2019, which was when this analysis was performed. Withdraw dates are from the manufacturer or FDA.
Stiffness is given as a range from low stiffness to high stiffness when mechanical testing throughout the stiffness curve is available. Lowest reported stiffness was used for linear regression in order to ensure comparable comparisons across meshes.
Cut technique and heat sealing has been shown to significantly influence stiffness for midurethral slings.
Cumulative days on the market were used for mesh families.
Information on Perigee was not available, despite contacting Boston Scientific, as it was manufactured by AMS but was not included in the company purchase.
Statistical analysis:
For each product group (transvaginal prolapse mesh, sacrocolpopexy mesh, and midurethral slings), the proportion of MDRs containing the most frequent complaints were compared using Chi2 and Fisher’s exact test. Dunn-Sidak correction resulted in significance if p<0.0034 for transvaginal mesh, p<0.0085 for sacrocolpopexy mesh, and p<0.0012 for slings. Spearman’s correlation was used to determine correlation between time on the market, weight, stiffness or porosity, and number of reports.
Results
Overall, there were six synthetic transvaginal prolapse mesh products with MDRs, four currently available sacrocolpopexy products, and ten currently available midurethral slings (Table 1), with 34,485 total reports. Characteristics and textile properties of available mesh products are listed in Table 2.20–23 Among the 20 products, there were 164 unique patient complaints, spanning multiple organ systems. The most frequent patient problems were the same for all mesh product groups: “unspecified injury,” pain, erosion, and infection.
Table 1.
Number of Medical Device Reports by Mesh Product Name
| Transvaginal Prolapse Mesh Products | Midurethral Sling Mesh Products | ||
|---|---|---|---|
| n= 10,275 | n (%) | n= 20,936 | n (%) |
| Gynecare Prolift and Prolift+Ma | 6334 (61.6%) | Transobturator | |
| Uphold Liteb | 2072 (20.2%) | Gynecare Obturatora | 11604 (55.4%) |
| Avaulta Solo and | 1115 (10.9%) | Gynecare Abbrevoa | 437 (2.1%) |
| Plus/Biosyntheticc | Arise | 48 (0.2%) | |
| Apogee/Perigeed | 723 (7.0%) | Retropubic | |
| Exair APRe | 26 (0.3%) | Gynecare TVTa | 5444 (26.0%) |
| Ascendf | 5 (<0.1%) | Advantage Fitb | 1063 (5.1%) |
| Gynecare TVT Exacta | 667 (3.2%) | ||
| Sacrocolpopexy Mesh Products | Suprise | 86 (0.4%) | |
| n= 3,319 | n (%) | Either approach | |
| Gynecare Gynemesha | 2056 (61.9%) | Desara Bluef | 673 (3.2%) |
| Restorellee | 1168 (35.2%) | Single Incision | |
| Upsylonb | 64 (1.9%) | Solyx SISb | 552 (2.6%) |
| Artisyna | 31 (0.9%) | Altis SISe | 362 (1.7%) |
Ethicon, Bridgewater NJ
Boston Scientific, Marlborough MA
Bard Medical, Covington GA
American Medical Systems, Minnetonka MN
Coloplast, Humlebaek Denmark
Caldera Medical, Agoura Hills CA
N describes number of reports in the Manufacturer and User Facility Device Experience database, and percentage given is out of all reports for a given mesh type (transvaginal; sacrocolpopexy; Midurethral slings).
Due to the large number of possible multiple comparisons, transabdominal prolapse mesh and midurethral slings were limited to currently available products.
Gynecare Prolift and Prolift+M, although different meshes, are grouped together by the Reed Tech Navigator, as are Avaulta Solo and Avaulta Plus/Biosynthetic.
Transvaginal Prolapse Mesh Group
Of the 10,275 transvaginal prolapse mesh reports, 7133 included pain, 6467 unspecified injury, 5907 erosion, and 1558 infection. Most reports contained multiple complaints, meaning percentages do not add up to 100%. Uphold Lite, a newer knitted lightweight, high porosity mesh with lower mesh burden than many of its predecessors, had a significantly lower proportion of MDRs that included pain, erosion, and infection, (p<0.003 except Ascend; Figure 1).
Figure 1.
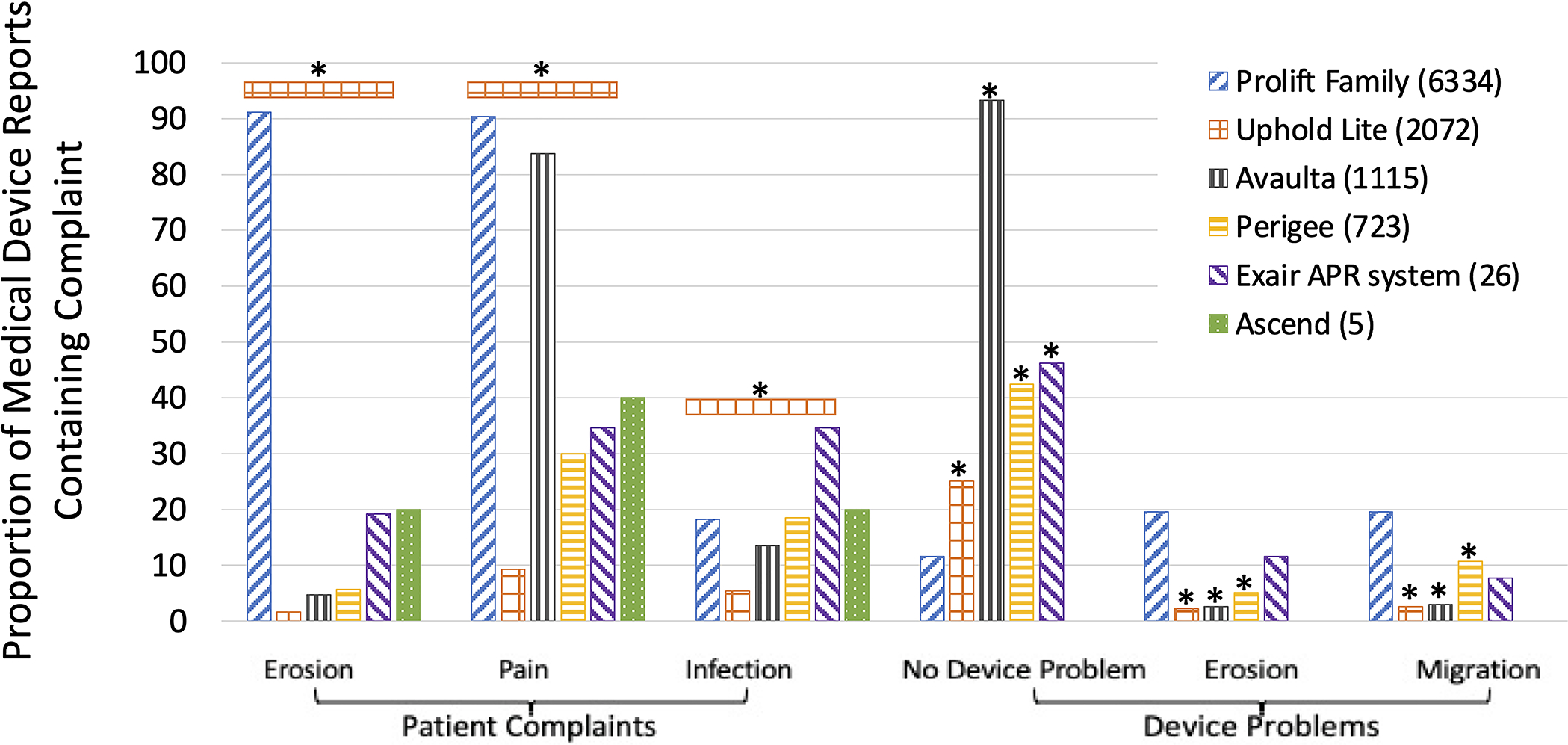
Proportion of common complaints in vaginal prolapse mesh Medical Device Reports by product. *P<0.0034. Device problems are compared to Gynecare Prolift.
2,907 transvaginal prolapse mesh MDRs included the most common device problem: “adverse event without identified device or use problem,” defined as patient harm without a problem with the device or the way it was used. The Prolift mesh family had the lowest proportion of this problem at 11.6% (735/6334 Prolift reports; p<0.0001). The Prolift family includes Prolift, the same mesh as sacrocolpopexy Gynemesh PS but designed for transvaginal prolapse repair, and Profift+M. Prolift is a heavier-weight (42g/m2 compared to Uphold Lite at 25g/m2), stiffer, knitted polypropylene mesh. Prolift+M is a 28-g/mm2, more porous material with a different knit pattern and with delayed-absorbable interwoven poliglecaprone fibers designed to reduce mesh burden.20
The next most common device problems were “migration or expulsion of device” (1411 reports) and “material erosion” (1354 reports). Uphold Lite and Avaulta had the lowest proportions of migration/expulsion and material erosion at 2–3%, significantly lower than the 19% seen for the Prolift family (p<0.0001).
Sacrocolpopexy Mesh Group
Most manufacturers fashion the same mesh with the same textile properties into a sacrocolpopexy form and a vaginal form (Table 3). Four sacrocolpopexy mesh products that are currently on the market had MDRs in MAUDE: 1. Gynemesh PS (Ethicon; Prolift); 2. Restorelle (Coloplast; Direct Fix); 3. Upsylon (Boston Scientific, Uphold Lite); 4. Artisyn (Ethicon, Prolift+M).20 Patient and device complaints were similar to transvaginal reports.
Table 3.
Meshes that are available with the same textile properties in both sacrocolpopexy and transvaginal mesh forms.
| Manufacturer | Sacrocolpopexy Mesh | Transvaginal Mesh |
|---|---|---|
| Ethicon | Gynemesh PS | Prolift |
| Boston Scientific | Upsylon | Uphold Lite |
| Bard Medical | Alyte | Avaulta |
| Ethicon | Artisyn (aka Ultrapro) | Prolift+M (aka Ultrapro) |
| Coloplast | Restorelle | Direct Fix |
| Coloplast | Novasilk | Exair |
| American Medical Systems | IntePro | Elevate |
See Table 1 for locations of manufacturers. Of note, some of these sacrocolpopexy meshes are not included in this study (shown in italics) if they are not currently available or do not have any reports in the MAUDE database.
Nearly all patient Gynemesh reports included erosion (93.8%; 1928/2056), a higher proportion (p<0.001) than all other mesh products (Artisyn 48.4% (15/31), Restorelle 13.6% (159/1168), Upsylon 1.6% (1/64), Figure 2). Gynemesh also had the highest proportion of reports with pain, 92.4% (1899/2056) compared to 59.9% for Restorelle, 41.9% for Artisyn, and 4.7% for Upsylon (p<0.0015). Restorelle had the highest proportion of reports with infection at 32.6% (381/1168) compared to the next highest Gynemesh at 17.0% (351/2056; p<0.0001, Figure 2). For device problems, Upsylon had a lower proportion of reports with erosion or migration than Gynemesh and Restorelle (p<0.002).
Figure 2.
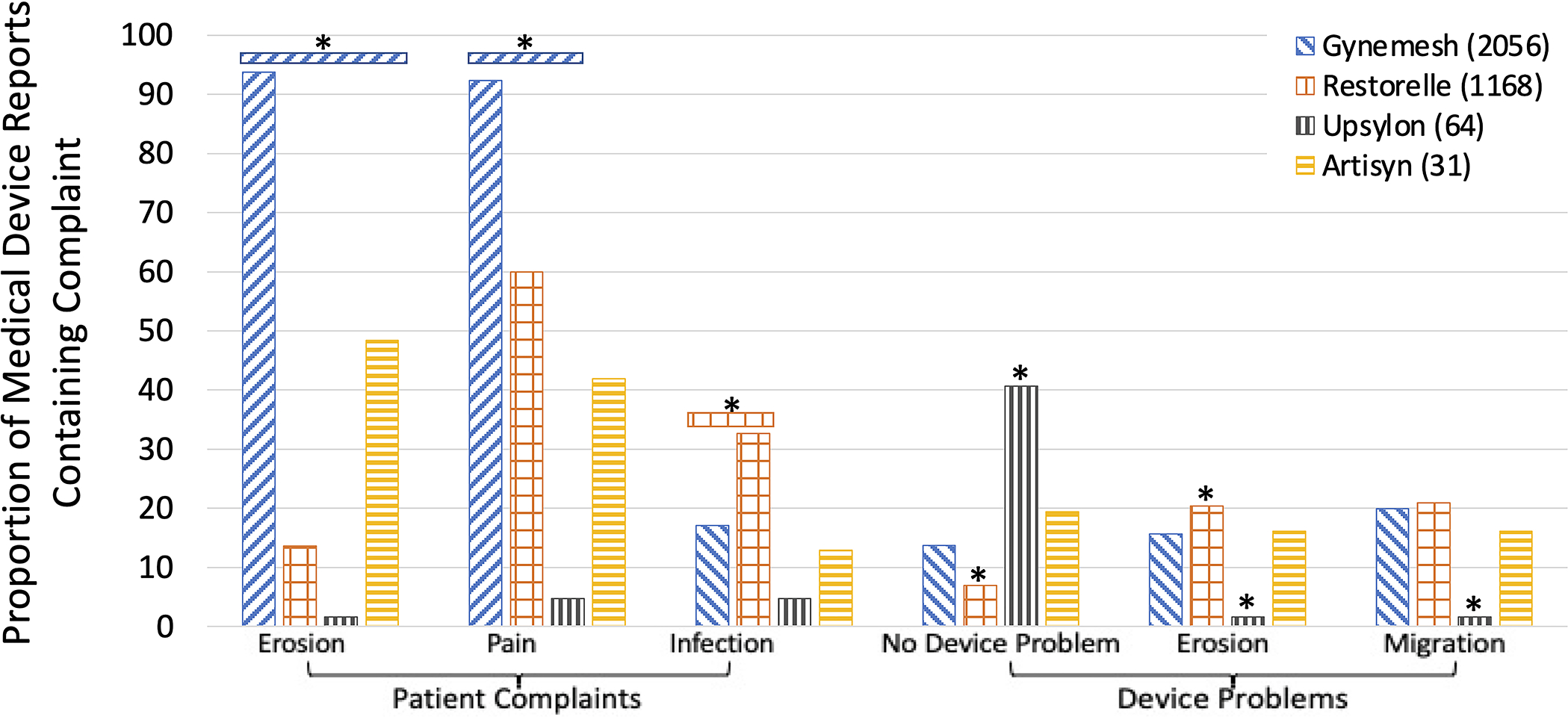
Proportion of common complaints in sacrocolpopexy mesh Medical Device Reports by product. *P<0.0085. Device problems are compared to Gynemesh.
Midurethral Sling Group
Midurethral slings currently on the market with MDRs included three transobturator slings, four retropubic slings, two single-incision slings, and one that can be placed via a retropubic or trans-obturator approach (Table 1). Gynecare TVT Obturator System, an “in to out” polypropylene mesh with mechanical and laser-cut versions, had the highest proportion of pain and erosion complaints (P<0.001, Figure 3). The three other Gynecare sling devices (Gynecare TVT Exact, laser-cut; Gynecare TVT Abbrevo, a mini-sling with laser-cut edges; and Gynecare TVT Retropubic, available in mechanical and laser-cut versions), had higher proportions of pain and erosion complaints than the other slings (P<0.001). Advantage Fit, a mechanically cut, heat-sealed sling of similar stiffness as the Gynecare TVT Obturator, had the lowest proportion of infection complaints (Figure 3). When combined, transobturator slings had a higher proportion of erosion and pain complaints compared to retropubic and single-incision slings (P<0.001). Retropubic slings had a higher proportion of infection complaints (P<0.001).
Figure 3.
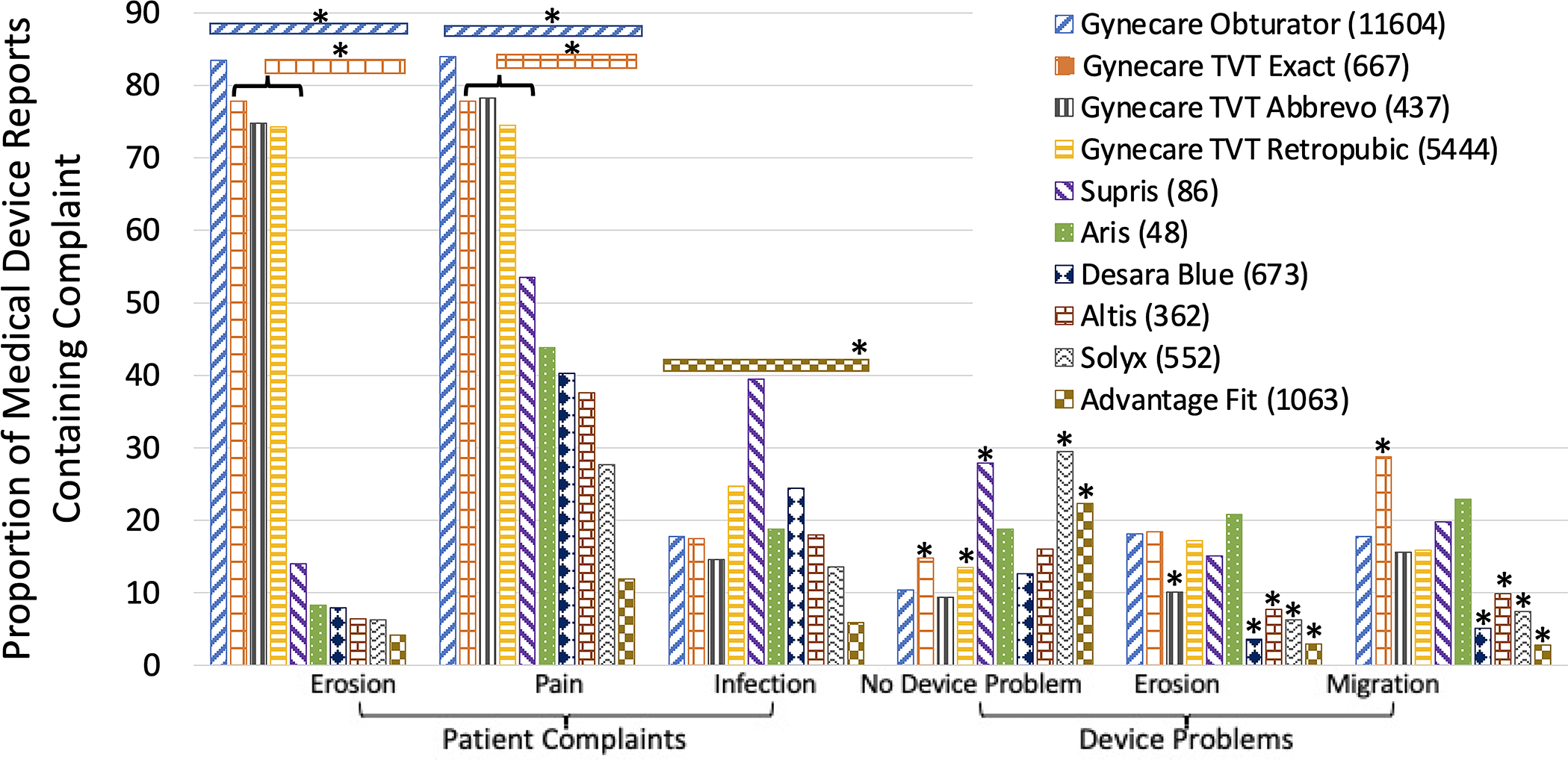
Proportion of common complaints in SUI mesh Medical Device Reports by product. *P<0.0012. Device problems are compared to Gynecare Obturator.
Textile properties
For slings, higher mesh weight was associated with more reports (ρ=0.7, p=0.02). Additionally, when Ascend, a visual outlier with only five reports, was excluded, there was a very strong correlation between transvaginal mesh stiffness and number of reports (ρ=0.99, p<0.001; Figure 4). Porosity at rest did not correlate with number of reports for any mesh type (P>0.05).
Figure 4.
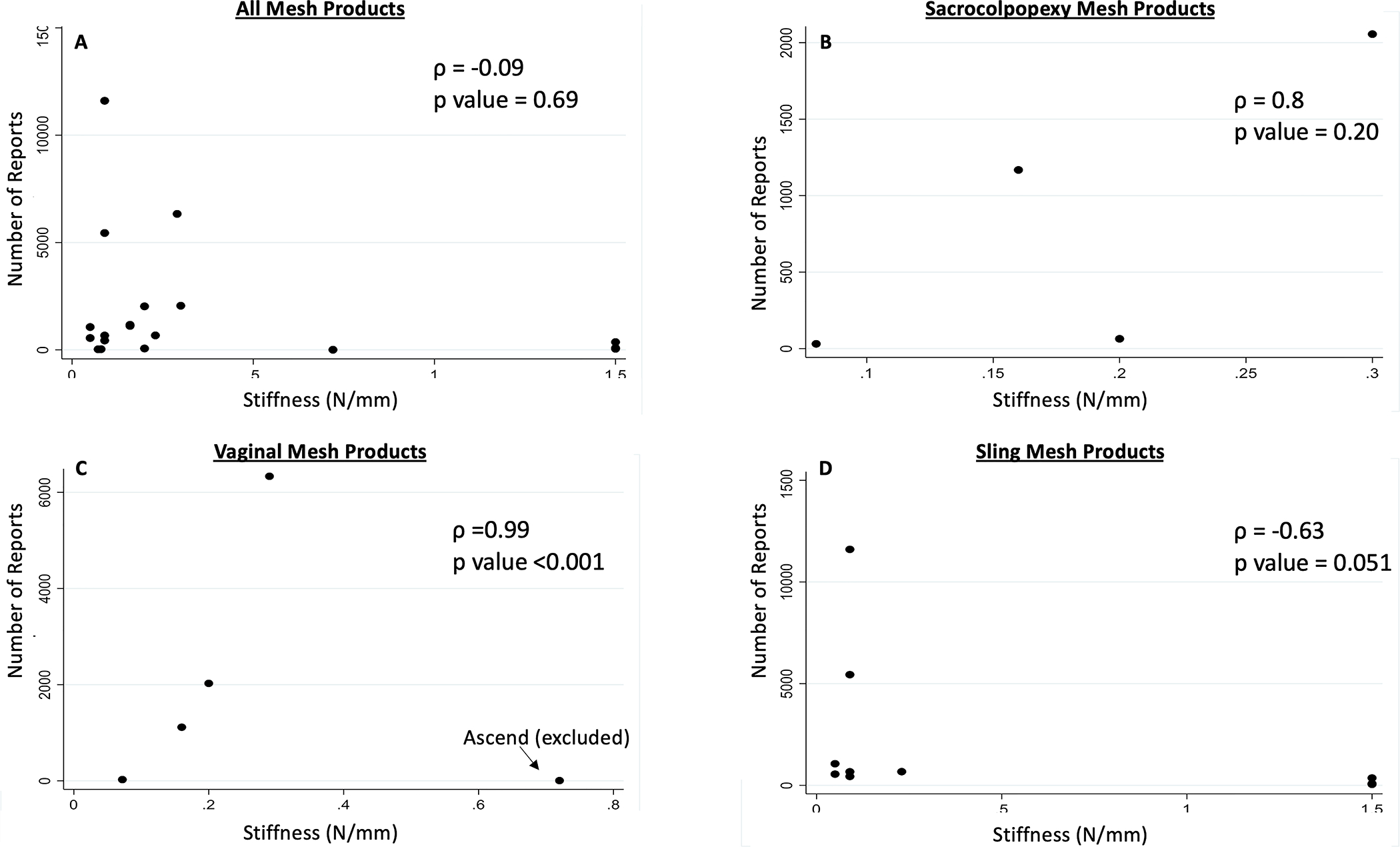
Number of MAUDE database reports on (A) urogynecologic mesh products, (B) sacrocolpopexy mesh products, (C) transvaginal mesh products, and (D) sling mesh products by stiffness. Correlation determined by Spearman’s Rho.
Time on Market
Of the six transvaginal meshes in MAUDE, Prolift, Avaulta, Ascend, and Perigee were withdrawn after the 2011 FDA notification without undergoing 522 studies (Table 2). Exair was withdrawn from the market in 2014 without completing a planned prospective cohort 522 study. Uphold was available until the 2019 FDA suspension of mesh distribution and was the second-longest-available mesh, after Perigee. Number of reports correlated with time on market for transvaginal meshes only (ρ=0.8, p=0.04; Supplemental Figure 1).
Discussion
The most important finding is that reports to the FDA describe a wide range of unadjucated adverse events with limited information and nonstandardized terminology. For transvaginal mesh products, Uphold Lite, one of the newer, lighter weight, lower stiffness products on the market had the fewest proportion of reports of pain, erosion, and infection. The Prolift family had the highest number of reports. Of the sacrocolpopexy meshes, Gynemesh, the same mesh used in Prolift, had the highest proportion of pain and erosion complaints. While these were the most widely used and distributed meshes on the market, our data did not support that the number of complications reported correlated with time on market. For midurethal slings, the Gynecare TVT Obturator System had the highest proportion of pain and erosion complaints, perhaps due to laser sealing of the mesh edges, which may make it substantially stiffer, or the “in to out” approach which may result in more lateral placement in the obturator space.
These proportions reflect the percentage of reports containing a specific complaint out of all MDRs for a particular mesh product. This does not reflect an adverse event rate. Because there are little data regarding the number of each product placed per year, we were unable to obtain a true denominator for each type of mesh. Large surgical databases, such as the American College of Surgeons’ NSQIP and Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample, do not include which mesh product is placed during a procedure, and data from manufacturers is not verifiable. We determined time each product spent on the market and found a correlation only for transvaginal mesh; however, market share is incompletely captured by this. Number of products distributed is not available. The proportions found here may also depend on mesh groupings, which is a function of the search technology used.
There is clear laboratory and animal data demonstrating the importance of biomechanical features of mesh, with more favorable host response seen with meshes that are lighter weight, more closely match the stiffness of the vagina, and have less pore collapse. 11,13,14,21,24–27 However, comparative human studies are lacking. These studies would be difficult to perform due to surgeon preference, regional variation, and hospital purchasing policies. Harnessing complication databases provides the most comprehensive information on relative proportions of complication types. Using MAUDE, we were able to demonstrate an association between mesh weight and number of reports for midurethral slings. Our analysis of the effect of porosity on number of reports for midurethral slings was limited by available porosity data, however, we did not find an association for transvaginal or sacrocolpopexy mesh products. It is likely that a complex combination of factors, including pore geometries, surgical tensioning of the mesh, and whether the mesh is deformed during placement, contributes to complications.
In animal studies on sacrocolpopexy mesh, Gynemesh PS, a heavier, less porous, and stiffer prolapse mesh, was shown to have a much more deleterious impact on the vagina compared to lighter, more porous, and less stiff meshes, Artisyn and Restorelle.14,27,28 This may underlie our findings that Gynemesh had a higher proportion of pain, erosion, and infection.
Among transvaginal meshes, the higher proportion of complications in the Prolift family (Prolift and Prolift+M) may be due to their unstable geometries; 22 however, it may also be due to its longest duration on the market. In addition, Prolift+M has more favorable biomechanical properties than Prolift and has a different knit pattern. It is difficult to determine the contribution of Prolift+M; the high number of reports in this family may be driven by Prolift alone but this cannot be accurately determined in this study.
Ascend is stiffer and less porous than Gynemesh PS24 but had lower proportions of pain, erosion, and infection in this study and appears to be an exception to a correlation between stiffness and number of reports. Similarly, the Aris sling is substantially stiffer, with smaller pores, than the Gynecare slings,21 but this does not match the expected proportions of complications in MAUDE. These discrepancies could be due to a small percentage of market share, properties of the mesh, such as processing, cutting, or sealing, or extrinsic factors, such as incomplete reporting, surgical trends, poor characterization, or media reporting.
Uphold Lite was chosen for the PFDN study “SUPeR” due to its favorable clinical and biomechanical properties and had the lowest proportion of pain, erosion, and infection in this study. In SUPeR, the number of exposures was 8%, and pain was 7%, over 36 months,4 corroborating the MAUDE findings. This mesh should be further studied to determine its possible risks and benefits, particularly compared to native tissue repairs.
Prior MAUDE studies have not provided more clarity. Sandberg et al. analyzed urogynecologic mesh products in MAUDE from 2005–2007.7 In this study, complications were categorized only by event type—“device malfunction,” “injury,” “death,” or “other” – not specific patient and device problem. Shah and Badlani and Brill et al. both discussed frequency of mesh complications reported to the FDA but did not stratify by device.8,9 Most studies also do not include information after the 2011 FDA Safety Communication, while 98% of medical device reports involving urogynecologic mesh occurred after this.29
Our data provide an interesting look at possible differences between mesh product outcomes but are limited in determining the relative safety of these products. There is great need for a comprehensive registry to compare efficacy and safety of devices used in urogynecologic procedures. The Pelvic Floor Disorder Mesh Registry, a multi-center, national effort to register and track outcomes of patients undergoing urogynecologic mesh procedures, is an excellent example. However, it primarily captures mesh placed at large academic centers, does not include number of each product placed annually, and excludes those being treated for stress urinary incontinence alone. Thus, the ability to compare mesh products within this registry is also limited.
This study highlights the heterogeneity in mesh products and the importance of considering individual mesh properties in mesh development, approval, and removal from the market. There are also meshes on the market without MDRs in the FDA database, suggesting either different complication profiles or incomplete reporting.
In conclusion, the quality of reports to the FDA varies, and event adjudication is needed prior to use of these reports in litigation and policy making. Individual mesh products have vastly different properties, with different complication profiles; this should be taken into consideration in mesh development and use. Gynemesh MDRs included pain and erosion more frequently than other meshes. A comprehensive, outcome-based registry of mesh products and standardization of reporting to the FDA are needed to determine the impact of additional mesh, patient, and surgical factors on complication profiles.
Supplementary Material
Supplementary Figure 1. Number of MAUDE database reports on (A) urogynecologic mesh products, (B) sacrocolpopexy mesh products, (C) transvaginal mesh products, and (D) sling mesh products by time on the market. Correlation determined by Spearman’s Rho.
Funding:
R01HD083383; AA is supported by NIH/ORWH Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) NIH K12HD043441.
Contributor Information
Amanda M. Artsen, Division of Urogynecology, University of Pittsburgh Medical Center, Pittsburgh, PA.
Jessica C. Sassani, Division of Urogynecology, University of Pittsburgh Medical Center, Pittsburgh, PA.
Pamela A. Moalli, Division of Urogynecology, University of Pittsburgh Medical Center, Pittsburgh, PA.
Megan S. Bradley, Division of Urogynecology, University of Pittsburgh Medical Center, Pittsburgh, PA.
Bibliography
- 1.Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–8. doi: 10.1016/j.ajog.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 2.Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014. doi: 10.1002/14651858.CD004014.pub5 [DOI] [PubMed] [Google Scholar]
- 3.Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024. doi: 10.1001/jama.2013.4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nager CW, Visco AG, Richter HE, et al. Effect of vaginal mesh hysteropexy vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: A randomized clinical trial. JAMA. 2019;322(11):1054–1065. doi: 10.1001/jama.2019.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Food and Drug Administration. FDA’s Activities: Urogynecologic Surgical Mesh. United States Food and Drug Administration. https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh-implants/fdas-activities-urogynecologic-surgical-mesh. Published October 24, 2019. Accessed February 23, 2020. [Google Scholar]
- 6.Obstetrics and Gynecology Devices Panel. Surgical Mesh for Transvaginal Repair ofPelvic Organ Prolapse in the Anterior Vaginal Compartment. FDA Executive Summary. February 2019. [Google Scholar]
- 7.Sandberg JM, Gray I, Pearlman A, Terlecki RP. An evaluation of the Manufacturer And User Facility Device Experience database that inspired the United States Food and Drug Administration’s Reclassification of transvaginal mesh. Investig Clin Urol. 2018;59(2):126–132. doi: 10.4111/icu.2018.59.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review. Indian J Urol. 2012;28(2):129–153. doi: 10.4103/0970-1591.98453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill AI. The hoopla over mesh: what it means for practice. 2012:14–15.
- 10.Bryan N, Battersby C, Smart N, Hunt J. A review of biocompatibility in hernia repair; considerations in vitro and in vivo for selecting the most appropriate repair material. Hernia. 2015;19(2):169–178. doi: 10.1007/s10029-014-1307-8 [DOI] [PubMed] [Google Scholar]
- 11.Feola A, Barone W, Moalli P, Abramowitch S. Characterizing the ex vivo textile and structural properties of synthetic prolapse mesh products. Int Urogynecol J. 2013;24(4):559–564. doi: 10.1007/s00192-012-1901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW. Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res. 2012;176(2):423–429. doi: 10.1016/j.jss.2011.09.031 [DOI] [PubMed] [Google Scholar]
- 13.Jallah Z, Liang R, Feola A, et al. The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG. 2016;123(7):1076–1085. doi: 10.1111/1471-0528.13514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang R, Abramowitch S, Knight K, et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG. 2013;120(2):233–243. doi: 10.1111/1471-0528.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. 510(k) Premarket Notification. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Published May 18, 2020. Accessed May 21, 2020. [Google Scholar]
- 16.Food and Drug Administration. Premarket Approval. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm. Published May 18, 2020. Accessed May 21, 2020.
- 17.Food and Drug Administration. Establishment Registration & Device Listing. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfrl/rl.cfm. Published May 18, 2020. Accessed May 21, 2020. [Google Scholar]
- 18.Food and Drug Administration. Medical Device Recalls. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm. Published May 18, 2020. Accessed May 21, 2020.
- 19.Food and Drug Administration. 522 Postmarket Surveillance Studies. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pss.cfm. Published May 18, 2020. Accessed May 21, 2020. [Google Scholar]
- 20.Liang R, Knight K, Abramowitch S, Moalli PA. Exploring the basic science of prolapse meshes. Curr Opin Obstet Gynecol. 2016;28(5):413–419. doi: 10.1097/GCO.0000000000000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moalli PA, Papas N, Menefee S, Albo M, Meyn L, Abramowitch SD. Tensile properties of five commonly used mid-urethral slings relative to the TVT. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):655–663. doi: 10.1007/s00192-007-0499-1 [DOI] [PubMed] [Google Scholar]
- 22.Barone WR, Moalli PA, Abramowitch SD. Textile properties of synthetic prolapse mesh in response to uniaxial loading. Am J Obstet Gynecol. 2016;215(3):326.e1–9. doi: 10.1016/j.ajog.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Maria C, Santoro V, Vozzi G. Biomechanical, topological and chemical features that influence the implant success of an urogynecological mesh: A review. Biomed Res Int. 2016;2016:1267521. doi: 10.1155/2016/1267521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shepherd JP, Feola AJ, Abramowitch SD, Moalli PA. Uniaxial biomechanical properties of seven different vaginally implanted meshes for pelvic organ prolapse. Int Urogynecol J. 2012;23(5):613–620. doi: 10.1007/s00192-011-1616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feola A, Pal S, Moalli P, Maiti S, Abramowitch S. Varying degrees of nonlinear mechanical behavior arising from geometric differences of urogynecological meshes. J Biomech. 2014;47(11):2584–2589. doi: 10.1016/j.jbiomech.2014.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones KA, Feola A, Meyn L, Abramowitch SD, Moalli PA. Tensile properties of commonly used prolapse meshes. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(7):847–853. doi: 10.1007/s00192-008-0781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feola A, Abramowitch S, Jallah Z, et al. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG. 2013;120(2):224–232. doi: 10.1111/1471-0528.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA. Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque. Am J Obstet Gynecol. 2015;212(2):174.e1–7. doi: 10.1016/j.ajog.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sassani JC, Artsen AM, Moalli PA, Bradley MS. Temporal trends of urogynecologic mesh reports to the U.S. food and drug administration. Obstet Gynecol. 2020;135(5):1084–1090. doi: 10.1097/AOG.0000000000003805 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Number of MAUDE database reports on (A) urogynecologic mesh products, (B) sacrocolpopexy mesh products, (C) transvaginal mesh products, and (D) sling mesh products by time on the market. Correlation determined by Spearman’s Rho.


