Abstract
Background:
Limited studies have functionally evaluated the heterogeneity in early ex vivo immune responses during sepsis. Our aim was to characterize early sepsis ex vivo functional immune response heterogeneity by studying whole blood endotoxin responses and derive a transcriptional metric of ex vivo endotoxin response.
Methods:
Blood collected within 24 hours of hospital presentation from 40 septic patients was divided into two fractions and incubated with media (unstimulated) or endotoxin. Supernatants and cells were isolated, and responses measured using: supernatant cytokines, lung endothelial permeability after supernatant exposure, and RNA expression. A transcriptomic signature was derived in unstimulated cells to predict the ex vivo endotoxin response. The signature was tested in a separate cohort of 191 septic patients to evaluate for association with clinical outcome. Plasma biomarkers were quantified to measure in vivo host inflammation.
Results:
Ex vivo response to endotoxin varied and was unrelated to immunosuppression, white blood cell count, or the causative pathogen. 35% of patients demonstrated a minimal response to endotoxin, suggesting early immunosuppression. High ex vivo cytokine production by stimulated blood cells correlated with increased in vitro pulmonary endothelial cell permeability and was associated with attenuated in vivo host inflammation. A 4 gene signature of endotoxin response detectable without the need for a functional assay was identified. When tested in a separate cohort of septic patients, its expression was inversely associated with hospital mortality.
Conclusions:
An attenuated ex vivo endotoxin response in early sepsis is associated with greater host in vivo inflammation and a worse clinical outcome.
Keywords: Sepsis, immune system, endotoxin tolerance, RNA expression
Introduction
Sepsis is a dysregulated host response to infection that results in life-threatening organ dysfunction [1] associated with overwhelming early immune activation followed by immunoparalysis days to weeks later [2, 3]. Due to substantial heterogeneity in sepsis physiology [4, 5], this concept is controversial.
One of the indicators of the dysregulated immune response in sepsis is a diminished response to endotoxin [6, 7], a highly conserved lipopolysaccharide (LPS) found in the outer lipid bilayer of Gram-negative bacteria. Endotoxin is the most potent microbial mediator implicated in the pathogenesis of sepsis [8] that binds to Toll-like receptor 4 (TLR4) expressed on innate immune cells [9], initiating an inflammatory signaling cascade, including the production of tumor necrosis factor (TNF) α and interleukin (IL)-6. Tolerance to repeated challenge by a pathogen was first reported in 1946 [10] and is observed after recurrent in vitro and in vivo microbial exposure [11]. This tolerant state is due to cellular reprogramming that blunts the response to further stimulation, rather than an anti-inflammatory response [12], which has been demonstrated in both Gram-negative and Gram-positive [13] infections. While a blunted response to endotoxin (endotoxin tolerance), may protect against excessive inflammation during states of colonization and chronic infection, when present during sepsis, it may be associated with greater disease severity and higher mortality [14, 15].
Although immunosuppression is a feature of late sepsis [16-18], its presence in early sepsis remains incompletely understood. Many reports rely on quantifying proteins in plasma and transcriptomic analyses in whole blood [4, 14, 15, 19], but few describe the functional state of immune cells in patients with early sepsis [20, 21], the focus of this study.
To examine immune cell function in early sepsis, we studied 40 critically ill patients with early sepsis from our university hospital-based sepsis Early Assessment of Renal and Lung Injury (EARLI) cohort [22-24] that enrols patients who present to the emergency department with suspected sepsis and require intensive care unit (ICU) admission. We measured immune responses to an ex vivo endotoxin challenge in unstimulated and endotoxin-stimulated whole blood using three techniques. First, cytokine levels were quantified in supernatants derived from endotoxin-stimulated cells. Second, pulmonary endothelial cell permeability was measured after in vitro exposure to the same supernatants. Third, RNA sequencing was employed to study gene expression in stimulated and unstimulated cells. These detailed ex vivo phenotypic data were subsequently used to develop a gene expression endotoxin response signature in unstimulated cells, which was applied to a separate subset of 191 patients with early sepsis from the EARLI cohort to test its association with hospital mortality. The signature was also compared to previously published transcriptomic signatures of endotoxin tolerance. Lastly, we tested the association between the host inflammatory state (plasma cytokines) and the magnitude of the ex vivo endotoxin response. The overall goal was to potentially identify a clinically relevant ex vivo immune function molecular signature defined by ex vivo endotoxin responses associated with clinical outcome in early sepsis.
Materials and Methods
Patient selection
Patients with early sepsis were prospectively enrolled if admitted to the ICU from the emergency department of a tertiary care hospital (University of California, San Francisco (USCF) Parnassus), as part of the ongoing EARLI cohort, as previously described [22-24]. Please refer to supplementary methods for description of 40 patient included in ex vivo immune response studies and 191 patients included in endotoxin response gene expression signature validation. Controls included 22 healthy subjects. The study was approved by the UCSF Institutional Review Board. Informed consent was obtained as previously described [24].
Ex vivo biological assays
A schematic of sample preparation is presented in Supplementary Figure 1. Detailed methods are presented in the online supplement. Briefly, plasma was removed from whole blood, the cell pellet was diluted 1:5 and incubated for 4h at 37°C in media alone (unstimulated control) or with 5 ng/mL of endotoxin (stimulated condition; List Biological Laboratories, Inc., Ultra-Pure LPS from Escherichia coli 0111:B4), approximating circulating endotoxin levels [25]. The culture supernatant and the cell pellet fractions were separated and cryopreserved at −80°C.
Eleven proteins were quantified in plasma using the Luminex® platform, as previously described [26]. 26 cytokines from culture supernatants of unstimulated and matched endotoxin-stimulated whole blood were also quantified on the Luminex® platofrm using an inflammation cytokine kit (BioRad). To determine the fold-change in cytokine concentration, endotoxin-stimulated supernatant cytokine concentrations were normalized by subtracting the concentration in the unstimulated sample, then divided by the unstimulated cytokine concentration.
To measure in vitro endothelial permeability, Primary Human Pulmonary Microvascular Endothelial cells (PromoCell, Heidelberg, Germany) were grown on Electric cell-substrate impedance sensing (ECIS) culture arrays, followed by co-culture with culture supernatants, as previously described [22]. The absolute area under the curve (AUC) during the 5-hour experiment represented endothelial permeability.
Gene expression in unstimulated and endotoxin-stimulated samples was measured in RNA extracted from whole blood cell pellets or whole blood Paxgene (Qiagen) tubes (for gene expression signature validation). RNA sequencing was performed on the Illumina Novaseq 6000 instrument. Gene expression analysis is described in the supplementary material.
Statistical analysis
The Wilcoxon ranksum test was used to compare supernatant cytokine concentrations in unstimulated relative to stimulated samples and the fold change in supernatant cytokines in patients with sepsis relative to healthy controls. Spearman rank correlation was used to test for associations between supernatant cytokines and in vitro endothelial permeability. Statistical differences were considered significant if P values were <0.05. Analysis and graphical presentation of supernatant and plasma cytokines and endothelial permeability was done using STATA v14.1 (StataCorp 2015).
The overall change in gene expression due to endotoxin stimulation was quantified by representing each subject’s change in expression as a vector in gene expression space and computing its dot product with the average change in expression (see supplementary methods). To identify transcriptional signatures, differential gene expression mixed-effects models were fit with the limma-voom function in the R package limma (CITE). Two differential expression models were fit (see supplementary methods). A false discovery rate (FDR) of <0.1 was used for all differential expression results.
The relationship between differentially expressed genes associated with the magnitude of the endotoxin response in unstimulated samples and hospital mortality was studied by examining gene expression in whole blood of 191 patients from the EARLI cohort. Statistical significance was assessed individually for each gene and was performed using DESeq2, as well as for total expression of the four genes (sum of normalized gene expression), performed using the Wilcoxon ranksum test.
Results
Patient characteristics
Forty patients with early sepsis were recruited from the EARLI cohort for detailed phenotypic physiological analysis of whole blood responses to an ex vivo endotoxin challenge. Patients had a high severity of illness with a mean APACHE III score of 87.4 ± 35.4 (SD) and a hospital mortality of 15%. The microbial etiology of sepsis and the anatomic source of sepsis were similar to previous reports [27], with lung followed by intraabdominal source of infection being most common. Patient clinical characteristics are summarized in Table 1.
Table 1:
Clinical characteristics of patients with early sepsis included in studies of ex vivo immune cell response to endotoxin.
| Whole cohort (n=40) |
|
|---|---|
| Clinical characteristics | |
| Age | 66 (±18) |
| Sex (male, %) | 25 (63) |
| WBC (109/mL) | 13.6 (10.3, 22.5) |
| Immunosuppression (%) | 14 (35) |
| Shock (%) | 14 (35) |
| APACHE III | 87 (±35) |
| SAPS II | 46 (±16) |
| Hospital death (%) | 6 (15) |
| Source of sepsis (%) | |
| Pulmonary | 23 (58) |
| Intraabdominal | 9 (23) |
| Urinary tract | 2 (5) |
| Skin and soft tissue | 2 (5) |
| Other | 4 (10) |
| Causative organism (%) | |
| Gram positive | 13 (33) |
| Gram negative | 11 (28) |
| Virus | 6 (15) |
| Negative cultures | 16 (40) |
Abbreviations: WBC, white blood cell count; APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score.
Elevated plasma biomarker levels in early sepsis
The host inflammatory state was evaluated by quantifying eleven plasma proteins representative of immune, endothelial, and epithelial activation. Levels of these proteins varied among patients with early sepsis (Supplementary Figure 2A) and the variability was greater relative to healthy controls (Supplementary Figure 2B). All the biomarker levels were significantly higher in patients with early sepsis relative to healthy controls (Supplementary Table 1), with the greatest differences noted for IL-6, MMP8, and CXCL9/MIG.
Heterogeneity in endotoxin response: cytokine production in cell culture supernatants
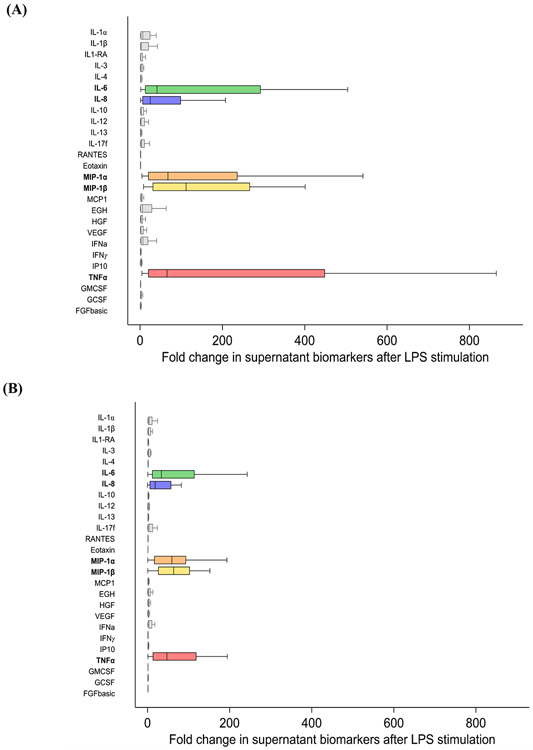
After the 4-hour endotoxin stimulation, the levels of all 26 cytokines in cell culture supernatants increased (Supplementary Table 2). The five cytokines with the greatest fold change after exposure to endotoxin were IL-6, IL-8, MIP-1α, MIP-1β, and TNFα, reaching an increase of over a 2,270-fold for TNFα (Figure 1A). However, there was significant response heterogeneity and some patients did not mount a response to endotoxin, demonstrated by a minimal change in the five most altered cytokines (Figure 1A).
Figure 1.
Distribution of the fold change in cell culture supernatant cytokines after ex vivo endotoxin stimulation in (A) patients with early sepsis and in (B) healthy controls.
The five most altered cytokines were used to generate a 5-cytokine signature to classify patients into endotoxin response groups. Subjects with minimal change in production of at least one of the five cytokines (first quartile of the distribution of the cytokine change after endotoxin stimulation) were classified as minimal responders (14/40 patients, 35%). Subjects with maximal change in production of at least one of the five cytokines (fourth quartile of the distribution of the cytokine change after endotoxin stimulation) were classified as maximal responders (15/40 patients, 38%). Patients meeting neither criterion were classified as intermediate responders (11/40 patients, 28%).
Clinical characteristics stratified by the ex vivo endotoxin response are presented in Table 2. There was no association between endotoxin response magnitude and white blood cell count, history of immunosuppression, severity of illness, anatomic source of infection, or causative organism. Characteristics of patients with clinical immunosuppression are displayed in Supplementary Table 3.
Table 2:
Characteristics of patients with early sepsis classified by endotoxin response*
| Minimal endotoxin response (n=14) |
Intermediate endotoxin response (n=11) |
Maximal endotoxin response (n=15) |
P-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 67 (±14.6) | 74.7 (±20.7) | 57.9 (±15.1) | 0.05 |
| Sex (male, %) | 9 (64%) | 8 (73%) | 8 (53%) | 0.7 |
| WBC (109/mL) | 13.4 (7, 24.3) | 12.2 (10.8, 15.4) | 14.5 (10.6, 28) | 0.6 |
| Immunosuppression (%) | 4 (28.6%) | 3 (27.3%) | 7 (46.7%) | 0.5 |
| Shock (%) | 6 (42.9%) | 3 (27.3%) | 5 (33.3%) | 0.7 |
| APACHE III | 86 (±35.6) | 92.5 (±41.5) | 84.8 (±31.8) | 0.9 |
| SAPS II | 46.7 (±18.2) | 45.2 (±16.3) | 46.6 (±14.9) | 1 |
| Hospital death | 3 (21.4%) | 1 (9.1%) | 2 (13.3%) | 0.7 |
| Source of sepsis (%) | 0.9 | |||
| Pulmonary | 8 (57%) | 8 (73%) | 7 (46.7%) | |
| Intraabdominal | 3 (21.4%) | 2 (18.2%) | 4 (26.7%) | |
| Urinary tract | 1 (7.1%) | 0 (0%) | 1 (6.7%) | |
| Skin and soft tissue | 0 (0%) | 0 (0%) | 2 (13.3%) | |
| Other | 2 (14.3%) | 1 (9.1%) | 1 (6.7%) | |
| Causative organism (%) | ||||
| Gram positive | 4 (28.6%) | 4 (36.4%) | 5 (33.3%) | 1 |
| Gram negative | 4 (28.6%) | 4 (36.4%) | 3 (20%) | 0.7 |
| Virus | 3 (21.4%) | 1 (9.1%) | 2 (13.3%) | 0.7 |
| Negative cultures | 5 (35.7%) | 6 (54.6%) | 5 (33.3%) | 0.5 |
| Biologic in vivo, ex vivo, and in vitro variables | ||||
| Plasma CXCL9/MIG (pg/mL) | 2130 (1976, 3911) | 1123 (571, 1659) | 700 (391, 2778) | 0.004 |
| Plasma MMP8 (pg/mL) | 11985 (6120, 32904) | 2899 (2424, 10584) | 4044 (1804, 14420) | 0.03 |
| Plasma IL-6 (pg/mL) | 203 (39, 658) | 31 (21, 418) | 46 (4, 151) | 0.2 |
| Supernatant TNFα fold change | 12.3 (8.7, 25.4) | 55.6 (30.2, 138) | 559 (369, 1,118) | 0.0001 |
| Endothelial permeability group (%) | <0.0001 | |||
| Minimal | 9 (64.3%) | 0 (0%) | 0 (0%) | |
| Intermediate | 5 (35.7%) | 11 (100%) | 6 (40%) | |
| Maximal | 0 (0%) | 0 (0%) | 9 (60%) | |
| Endothelial permeability (AUC) | 0.13 (0.07, 0.28) | 0.4 (0.35, 0.49) | 0.71 (0.63, 0.82) | 0.0001 |
Endotoxin response group was defined by fold change in 5 most deranged cytokines in cell culture supernatants. Values presented as median and interquartile range (IQR) for non-normally distributed variables and means ± standard deviation for normally distributed variables. P-values computed using Fisher’s exact test and Kruskal Wallis test.
Abbreviations: WBC, white blood cell count; AUC, Sum of the absolute Area Under the Curve after 5-hours of continual measurement of in vitro pulmonary endothelial permeability using Electric cell-substrate impedance sensing (ECIS).
The median endotoxin response (fold change in the five most altered cytokines after endotoxin stimulation) in healthy controls was similar to patients with early sepsis and the most deranged cytokines in culture supernatants were also IL-6, IL-8, MIP-1α, MIP-1β, and TNFα (Figure 3B). Healthy control endotoxin response was less heterogenous and resembled the intermediate endotoxin response generated by patients with sepsis (Supplementary Table 4).
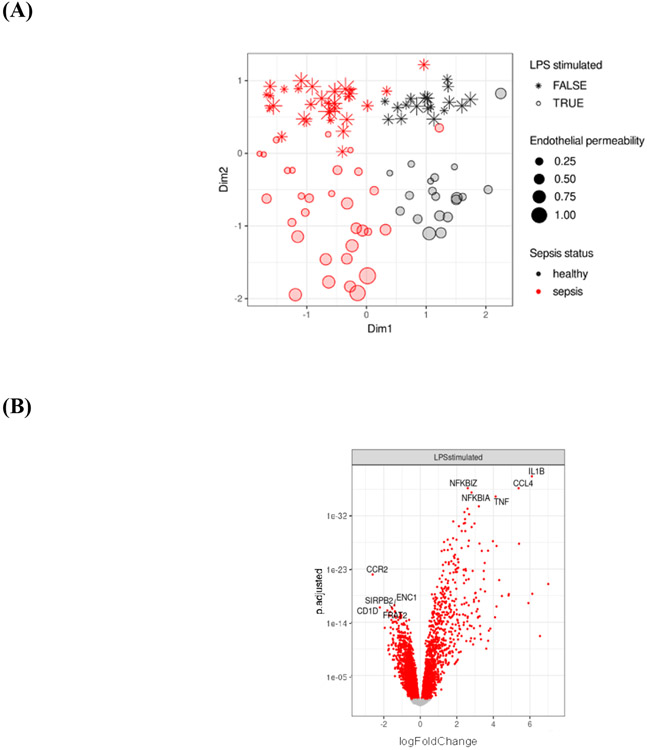
Figure 3.
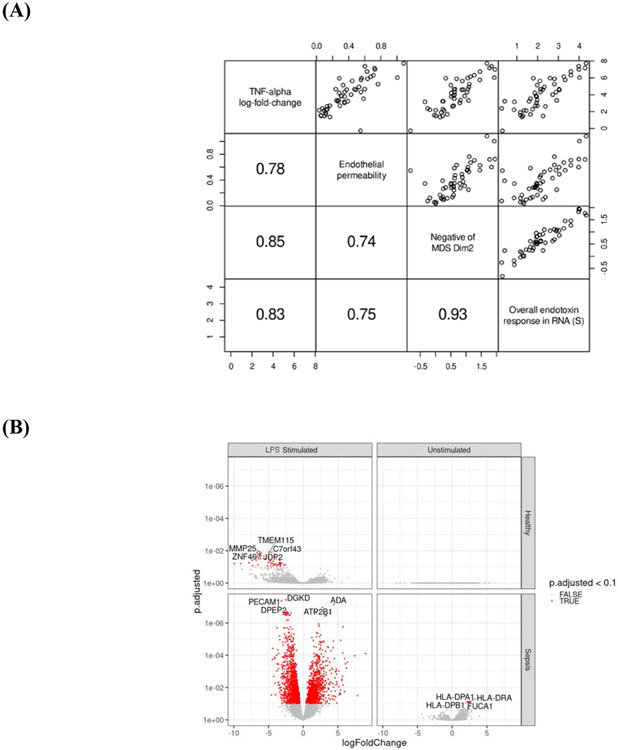
Heterogeneity in endotoxin response: gene expression. (A) Multidimensional-scaling (MDS) clusters samples by sepsis status (dimension 1) and by endotoxin stimulation (dimension 2).
* MDS dimension 2 can be interpreted as a measure of the endotoxin response, with positive values corresponding to minimal endotoxin responses and negative values corresponding to maximal endotoxin responses. LPS stimulated FALSE and TRUE refer to unstimulated and stimulated experimental conditions. Within endotoxin-stimulated cells from patients with sepsis, more negative values along dimension 2 correspond to higher in vitro pulmonary endothelial permeability.
(B) Differential gene expression among unstimulated and endotoxin-stimulated cells from patients with sepsis and from healthy controls,
*4594 differentially expressed genes (1920 genes upregulated, 2674 genes downregulated)
(C) Heatmap of the union of top 30 differentially expressed genes related to endotoxin stimulation,
*Each column represents an individual sample. Classification by LPS stimulation, blue: stimulated, purple: unstimulated. Classification by sepsis status green: healthy control, orange: sepsis.
(D) Heatmap of most significant genes associated with in vitro pulmonary endothelial permeability in endotoxin-stimulated cells from patients with sepsis.
*Each column represents an individual patient.
(E) Heatmap of most significant genes associated with in vitro pulmonary endothelial permeability in endotoxin-stimulated cells from healthy controls.
*Each column represents an individual sample.
Heterogeneity in endotoxin response: pulmonary endothelial cell permeability
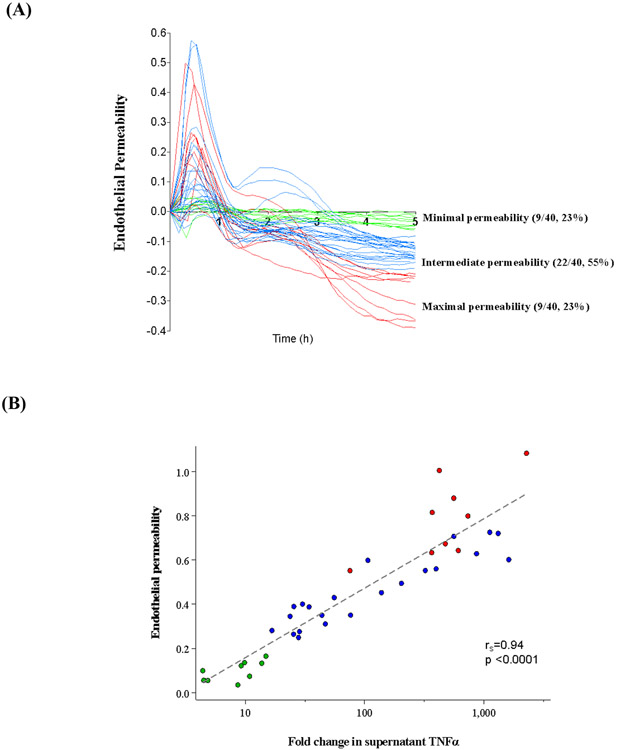
Culture supernatants from unstimulated and endotoxin-stimulated whole blood were added to a monolayer of Human Pulmonary Microvascular Endothelial Cells (HPMECs) and resistance was continually measured. There was heterogeneity in the in vitro endothelial cell permeability after exposure to endotoxin stimulated whole blood cell culture supernatants (Figure 2A), as previously reported [22]. There was a consistent significant association between in vitro endothelial permeability and the ex vivo production of all measured supernatant cytokines (Supplementary Table 5), with strongest association for TNFα (Figure 2B).
Figure 2.
Heterogeneity in endotoxin response measured by pulmonary endothelial cell permeability. (A) Electric cell-substrate impedance sensing (ECIS) tracings of in vitro pulmonary endothelial cell permeability after exposure to culture supernatants from ex vivo endotoxin-stimulated whole blood of patients with sepsis.
*A decrease in resistance across the Human Pulmonary Microvascular Endothelial Cell (HPMEC) monolayer suggested increased permeability and the absolute area under the curve during the 5-hours of ECIS measurements was subsequently used to represent endothelial permeability, as described previously. Marker color represents the magnitude of in vitro endothelial permeability (green: minimal, blue: intermediate, red: maximal).
(B) Relationship between fold change in culture supernatant TNFα and in vitro pulmonary endothelial permeability.
Heterogeneity in endotoxin response: transcriptional signature of endotoxin response
RNAseq recapitulated the heterogeneity in endotoxin responses measured as supernatant cytokine levels of ex vivo endotoxin-stimulated blood cells, and in vitro pulmonary endothelial permeability. Transcriptional differences based on endotoxin-stimulation and sepsis status were revealed by multidimensional-scaling (MDS) (Figure 3A), which clustered samples by sepsis status (dimension 1) and endotoxin response (dimension 2).
4,594 differentially genes were identified between unstimulated and endotoxin-stimulated cells (Figure 3B). Unsupervised clustering identified heterogeneity in endotoxin responses in patients with sepsis and in healthy controls (Figure 3C). The most significant genes associated with in vitro pulmonary endothelial permeability in endotoxin-stimulated cells were different in patients with sepsis (Figure 3D) relative to healthy controls (Figure 3E), inferring that the response to endotoxin is different during critical illness and in health. Differential expression analysis identified 40 genes with a significantly different response to endotoxin between patients with sepsis and healthy controls (Supplementary Figure 3).
Ex vivo endotoxin response metrics were strongly correlated, including (1) fold-change culture supernatant TNFα, (2) pulmonary endothelial permeability, (3) dimension 2 from the RNAseq MDS (from Figure 3A), and (4) a summary statistic S of the overall gene expression endotoxin response magnitude (Figure 4A). Given the strong association between all metrics of the ex vivo endotoxin response, in vitro pulmonary endothelial permeability, a metric we previously described [22], was used in transcriptomic analysis to assess for differential gene expression among unstimulated and endotoxin-stimulated cells.
Figure 4.
Association between response to endotoxin and gene expression. (A) Pairwise scatter plots demonstrating a linear correlation between complimentary metrics of ex vivo response to endotoxin.
*R2 values in the lower left panels and are significant at p <1x10−9 for every pair of variables, based on linear regression. MDS Dim2 refers to Multidimensional-scaling dimension 2 (Figure 3A). Within endotoxin-stimulated cells from patients with sepsis, more negative values along dimension 2 correspond to higher in vitro pulmonary endothelial permeability.
(B) Differential gene expression for response to endotoxin.
*Response to endotoxin was measured by pulmonary endothelial cell permeability. 2720 significant genes were differentially expressed in stimulated cells from patients with sepsis (1328 upregulated, 1392 downregulated), 47 significant genes in stimulated cells from healthy controls (0 upregulated, 47 downregulated), 4 significant genes in unstimulated cells from patients with sepsis (4 upregulated, 0 downregulated), and no significant genes in unstimulated cells from healthy controls.
Differential expression analysis identified 2720 genes significantly associated with in vitro pulmonary endothelial permeability in endotoxin-stimulated cells from patients with early sepsis. Gene set enrichment analysis demonstrated that these genes were associated with cytokine signalling and cell chemotaxis (Supplementary Figure 4). Interestingly, we identified a 4-gene (HLA-DRA, HLA-DPA1, HLA-DPB1, FUCA1) transcriptional signature of endotoxin tolerance that existed in unstimulated cells from patients with sepsis (Figure 4B).
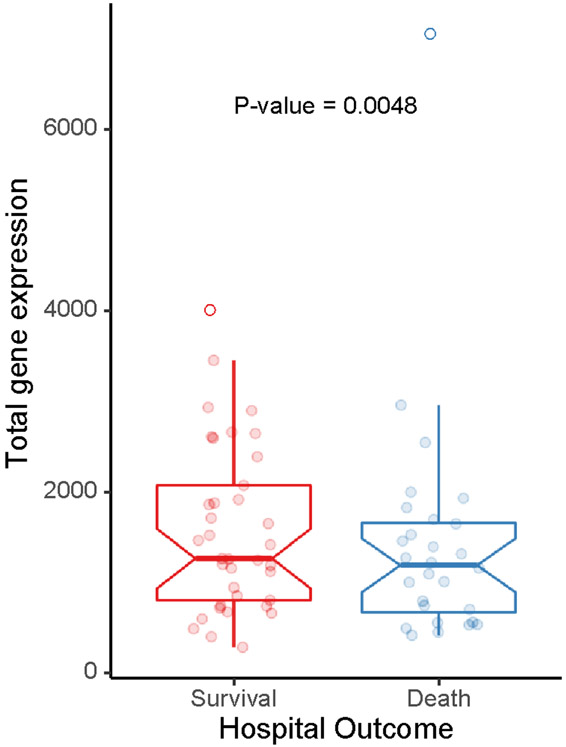
To assess the clinical significance of the 4-gene endotoxin tolerance signature, we evaluated their expression in a different subset of 191 patients with sepsis from the EARLI cohort (Supplementary Table 6). Lower expression these genes was significantly associated with in-hospital death (Figure 5). This was true for individual genes as well as a composite of the four genes (Supplementary Table 7).
Figure 5.
Relationship between the 4 gene signature associated with the endotoxin response and hospital mortality in an external validation cohort of 191 patients with early sepsis.
*The four genes included: HLA-DRA, HLA-DPA1, HLA-DPB1, and FUCA1. Values on Y axis represent combined metric for total expression of the four genes (sum of normalized gene expression for each gene). Host gene counts were normalized using DESeq2. All samples included in the analysis had >1 million host gene counts and expression of >10,000 unique genes.
Three whole blood transcriptomic studies in patients with sepsis used hierarchical clustering to identify subgroups and subsequently derived gene expression signatures suggestive of endotoxin tolerance [14, 15, 19]. When the three signatures were tested for an association with our functional ex vivo endotoxin response RNAseq analysis, none of the signatures were present (Supplementary Table 8).
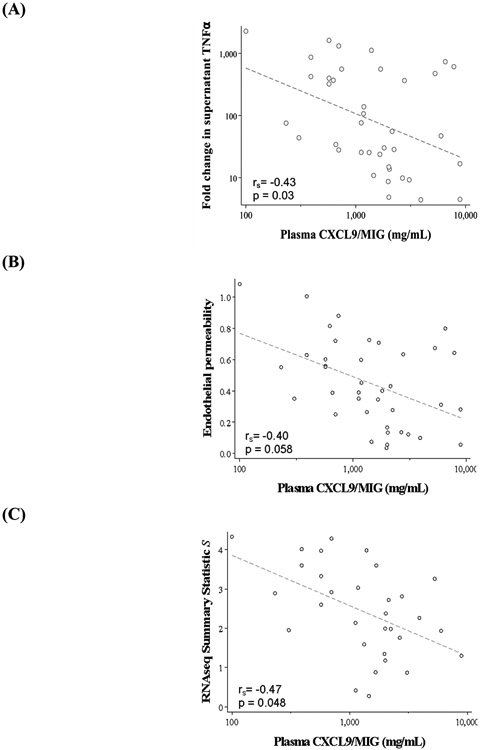
High host inflammatory state is associated with low ex vivo response to endotoxin
The host inflammatory state, as reflected by plasma cytokine levels, may influence the ability to respond to ex vivo stimulation. The three most differentially abundant plasma cytokines in patients with sepsis relative to healthy controls were IL-6, MMP8, and CXCL9/MIG. These three plasma proteins were tested against ex vivo metrics of endotoxin response, including: (1) fold change in culture supernatant TNFα, (2) in vitro endothelial permeability, and (3) summary statistic S for the RNAseq endotoxin response size. An inverse relationship was identified between plasma inflammatory cytokines (strongest for CXCL9/MIG) and all metrics of endotoxin response (Figure 6A-C); when plasma inflammation was high, response to endotoxin was low.
Figure 6.
Association between host inflammatory state (plasma CXCL9/MIG) and ex vivo response to endotoxin measured by (A) the fold change in TNFα in cell culture supernatant, (B) in vitro pulmonary endothelial permeability, and (C) the summary statistic S for the RNAseq endotoxin response size.
*Spearman rank correlation was used to compute p-values and included a Bonferroni correction for multiple testing.
Discussion
Through functional studies and comprehensive host inflammatory assessment, this work advances our understanding of immune response heterogeneity in early sepsis. Endotoxin stimulation has been used to study sterile inflammation in healthy volunteers [11, 28, 29] as well as to characterize immune competence in critically ill adults [21, 30, 31] and children [32, 33]. This is the first study to use three independent approaches to investigate the ex vivo immune response to an endotoxin challenge in very early sepsis among critically ill adults and build a molecular metric of immune responsiveness. We derived a 4-gene transcriptional signature of endotoxin tolerance and tested it in an independent subgroup of patients with early sepsis, demonstrating greater hospital mortality among patients with lower endotoxin response gene expression.
Substantial heterogeneity was present in the immune response to an ex vivo endotoxin challenge in patients with early sepsis. Approximately 1 in 3 patients demonstrated a minimal response, suggesting early immunosuppression. This is particularly striking as all specimens were collected within 24 hours of hospital admission, representing the earliest phase of illness. The ex vivo endotoxin response was inversely associated with the in vivo inflammatory state (plasma cytokines). This suggests that critically ill patients who are exposed to low in vivo inflammation have a preserved immune response to secondary stimuli, while those with high inflammation may have a less competent immune system and an inferior response to further stimulation.
The lack of association between the causative infectious organism and endotoxin response suggests that the magnitude of the ex vivo endotoxin response may be independent of prior in vivo endotoxin exposure. It is possible that a blunted ex vivo endotoxin response represents cross-tolerance due to gene expression reprogramming, as demonstrated in Gram-positive [13] and Gram-negative infections, as well as in sterile inflammation [34].
When exposed to ex vivo endotoxin, some patients with sepsis exhibit overly hyporesponsive (tolerant) responses while others generate extremely high responses. The former group may be experiencing repression of inflammatory gene expression while the latter group may represent activation of inflammatory genes and/or de-repression of anti-inflammatory genes. These extreme response groups warrant further investigation to determine the clinical implications of ex vivo immune dysregulation.
In earlier studies, unsupervised clustering of whole blood gene expression of patients with sepsis [14], pneumonia [15], and fecal peritonitis [19] classified patients into groups with different clinical outcomes. Pathway analysis in patients with poor outcome identified genes related to endotoxin tolerance. However, these studies did not ascertain whether altered gene expression suggestive of endotoxin tolerance corresponded to an ex vivo endotoxin response. Our data suggest that determining which patients are endotoxin tolerant requires ex vivo exposure to endotoxin. Therefore, unstimulated whole blood transcriptomic analysis may be inadequate to draw conclusions about ex vivo immune function in sepsis.
Differential gene expression in unstimulated cells from patients with sepsis identified associations with altered MHC class II genes expression (HLA-DRA, HLA-DPA1, HLA-DPB1). The downregulation of monocyte human leukocyte antigen DR (mHLA-DR) is a surrogate of immunosuppression in critically ill patients with sepsis [35-37]. The impact of altered antigen presentation by phagocytic immune cells with an endotoxin tolerant phenotype on the adaptive immune response is inadequately understood, but may contribute to dysregulated antigen-specific responses [38] and predispose patients to secondary infection [36, 39].
Some questions remain regarding the heterogeneity in the ex vivo endotoxin response in early sepsis and its clinical relevance. The impact of pathogen type or burden on the ex vivo endotoxin response is unknown. While monocytes from patients with cystic fibrosis [40] and neutrophils from healthy volunteers [41] have diminished endotoxin responses, in acute critical illness the contribution of specific immune cell types is yet to be determined. The mechanisms of the refractory state, its duration, and its link to unfavorable outcome need additional prospective studies. Cellular reprogramming linked to endotoxin responses may cause maladaptive immune responses and imprint increased risk for nosocomial infection [12, 42], but this was not the case when the response to endotoxin was studied in ICU patients with and without sepsis [31]. The lack of association in these studies may suggest that the inability to mount an ex vivo response to endotoxin is a form of self-regulation rather than immune suppression per se [43]. In our validation in 191 patients with early sepsis, a significant association was identified between a lower expression of genes related to the ex vivo endotoxin response and increased hospital mortality, suggesting that septic patients with a blunted immune response have a worse clinical outcome, a finding that warrants testing in larger cohorts.
There are some limitations to our study. First, as detailed ex vivo characterization of immune responses is time intensive, a modest sample size was studied. To increase sample size and the generalizability of our findings, future functional assays will need to be conducted at multiple sites. Second, we focused on a single antigen to study ex vivo immune responses and future studies should consider additional antigens [21]. Third, whole blood was used to study the response to endotoxin and as such, a mixed cell type population was examined. Using single cell RNAseq may provide additional insight. Fourth, a single time point was studied and it is possible that some patients develop immunosuppression later in the course of critical illness. Since we found that the ex vivo response to endotoxin can stratify patients by risk of hospital mortality, studying samples from even a single time point during early sepsis has major clinical relevance.
Overall, this study establishes that a diminished ex vivo endotoxin response, and thereby immunosuppression, is present very early in many patients with sepsis. While prior studies reported a gene signature for endotoxin tolerance, to our knowledge this is the first study to test the derivation of this phenotype using both ex vivo physiologic and protein data. Low expression of the 4-gene endotoxin response signature was significantly associated with in-hospital death in an independent subset of patients with early sepsis. This surrogate measure of immune dysregulation in critically ill patients may assist in predictive enrichment for patients who may benefit from immunomodulatory therapies and may identify modifiable molecular treatment targets. The inability to mount a strong ex vivo response to endotoxin in early sepsis may be maladaptive, and our study supports investigating this response in larger cohorts of patients with early sepsis.
Supplementary Material
Acknowledgments:
We are grateful for patients and their family for participation in the EARLI cohort. We thank the EARLI cohort coordinators (Serena Ke, Thomas Deiss, Matthew Lippi, Tom Liu) for their dedication to the longitudinal cohort patient enrollment.
Sources of support:
University of Toronto Clinician Investigator Program and the Clinician Scientist Training Program (A.L.), Chan-Zuckerberg Biohub (J.K.), Chan Zuckerberg Initiative (K.K.), the National Institutes of Health (HL146753 to A.P.; K23HL116800 to K.N.K.; HL140026 to K.D.L.; HL140026 to C.S.C.; K23HL138461-01A1 to C.L.; and HL51856 to M.A.M.)
References:
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13(12):862–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG: The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17(7):407–420, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Leligdowicz A, Matthay MA: Heterogeneity in sepsis: new biological evidence with clinical applications. Crit Care 23(1):80, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg NM, Leligdowicz A, Slutsky AS, Friedrich JO, Lee WL: Is nosocomial infection really the major cause of death in sepsis? Crit Care 18(5):540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas SK, Lopez-Collazo E: Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30(10):475–487, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Collazo E, del Fresno C: Pathophysiology of endotoxin tolerance: mechanisms and clinical consequences. Crit Care 17(6):242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opal SM: Endotoxins and other sepsis triggers. Contrib Nephrol 2010, 167:14–24. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B, Rietschel ET: Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3(2):169–176, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Beeson PB: Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med 61:248–250, 1946. [DOI] [PubMed] [Google Scholar]
- 11.Kox M, de Kleijn S, Pompe JC, Ramakers BP, Netea MG, van der Hoeven JG, Hoedemaekers CW, Pickkers P: Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Crit Care Med 39(8):1866–1870, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Cavaillon JM, Adib-Conquy M: Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care 10(5):233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley JM, Wang JH, Redmond HP: Cellular reprogramming by gram-positive bacterial components: a review. J Leukoc Biol 80(4):731–741, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Pena OM, Hancock DG, Lyle NH, Linder A, Russell JA, Xia J, Fjell CD, Boyd JH, Hancock RE: An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 1(1):64–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, Rautanen A, Gordon AC, Garrard C, Hill AV et al. : Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 4(4):259–271, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS et al. : Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306(23):2594–2605, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arens C, Bajwa SA, Koch C, Siegler BH, Schneck E, Hecker A, Weiterer S, Lichtenstern C, Weigand MA, Uhle F: Sepsis-induced long-term immune paralysis--results of a descriptive, explorative study. Crit Care 20(1):93, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, Bauer M, Riedemann NC: The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care 15(4):R183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnham KL, Davenport EE, Radhakrishnan J, Humburg P, Gordon AC, Hutton P, Svoren-Jabalera E, Garrard C, Hill AVS, Hinds CJ et al. : Shared and Distinct Aspects of the Sepsis Transcriptomic Response to Fecal Peritonitis and Pneumonia. Am J Respir Crit Care Med 196(3):328–339, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, La Rosee P, Monneret G, Venet F, Bauer M et al. : Current gaps in sepsis immunology: new opportunities for translational research. The Lancet infectious diseases 2019. [DOI] [PubMed] [Google Scholar]
- 21.Albert Vega C, Oriol G, Bartolo F, Lopez J, Pachot A, Rimmele T, Venet F, Leray V, Monneret G, Delwarde B et al. : Deciphering heterogeneity of septic shock patients using immune functional assays: a proof of concept study. Sci Rep 10(1):16136, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leligdowicz A, Chun LF, Jauregui A, Vessel K, Liu KD, Calfee CS, Matthay MA: Human pulmonary endothelial cell permeability after exposure to LPS-stimulated leukocyte supernatants derived from patients with early sepsis. Am J Physiol Lung Cell Mol Physiol 315(5):L638–L644, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, Rogers A, Seeley EJ, Chu J, Liu T et al. : Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol 308(11):L1102–1113, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS: Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med 187(7):736–742, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN: Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care 6(4):342–348, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leligdowicz A, Conroy AL, Hawkes M, Zhong K, Lebovic G, Matthay MA, Kain KC: Validation of two multiplex platforms to quantify circulating markers of inflammation and endothelial injury in severe infection. PloS one 12(4):e0175130, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A, Co-operative Antimicrobial Therapy of Septic Shock Database Research G: Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med 189(10):1204–1213, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Segre E, Fullerton JN: Stimulated Whole Blood Cytokine Release as a Biomarker of Immunosuppression in the Critically Ill: The Need for a Standardized Methodology. Shock 45(5):490–494, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wurfel MM, Park WY, Radella F, Ruzinski J, Sandstrom A, Strout J, Bumgarner RE, Martin TR: Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J Immunol 175(4):2570–2578, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Appoloni O, Vincent JL, Duchateau J: Response of tumour necrosis factor-alpha to delayed in vitro monocyte stimulation in patients with septic shock is related to outcome. Clin Sci (Lond) 102(3):315–320, 2002. [PubMed] [Google Scholar]
- 31.van Vught LA, Wiewel MA, Hoogendijk AJ, Scicluna BP, Belkasim-Bohoudi H, Horn J, Schultz MJ, van der Poll T: Reduced Responsiveness of Blood Leukocytes to Lipopolysaccharide Does not Predict Nosocomial Infections in Critically Ill Patients. Shock 44(2):110–114, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, Carcillo JA: Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 37(3):525–532, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bline KE, Moore-Clingenpeel M, Hensley J, Steele L, Greathouse K, Anglim L, Hanson-Huber L, Nateri J, Muszynski JA, Ramilo O et al. : Hydrocortisone treatment is associated with a longer duration of MODS in pediatric patients with severe sepsis and immunoparalysis. Crit Care 24(1):545, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Fresno C, Soler-Rangel L, Soares-Schanoski A, Gomez-Pina V, Gonzalez-Leon MC, Gomez-Garcia L, Mendoza-Barbera E, Rodriguez-Rojas A, Garcia F, Fuentes-Prior P et al. : Inflammatory responses associated with acute coronary syndrome up-regulate IRAK-M and induce endotoxin tolerance in circulating monocytes. Journal of endotoxin research 13(1):39–52, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P: Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32(8):1175–1183, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D: Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med 37(10):2746–2752, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohe J, Vanhems P, Monneret G: Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med 36(11):1859–1866, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM: A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care 16(3):R112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drewry AM, Ablordeppey EA, Murray ET, Beiter ER, Walton AH, Hall MW, Hotchkiss RS: Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit Care 20(1):334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.del Fresno C, Garcia-Rio F, Gomez-Pina V, Soares-Schanoski A, Fernandez-Ruiz I, Jurado T, Kajiji T, Shu C, Marin E, Gutierrez del Arroyo A et al. : Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 182(10):6494–6507, 2009. [DOI] [PubMed] [Google Scholar]
- 41.de Kleijn S, Kox M, Sama IE, Pillay J, van Diepen A, Huijnen MA, van der Hoeven JG, Ferwerda G, Hermans PW, Pickkers P: Transcriptome kinetics of circulating neutrophils during human experimental endotoxemia. PloS one 7(6):e38255, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denstaedt SJ, Singer BH, Standiford TJ: Sepsis and Nosocomial Infection: Patient Characteristics, Mechanisms, and Modulation. Front Immunol 9:2446, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansonetti PJ, Medzhitov R: Learning tolerance while fighting ignorance. Cell 138(3):416–420, 2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.